Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design and Patient Selection
2.3. Evaluations
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Changes in Serum Markers ALP and PSA, and aBSI
3.3. Correlation of Changes in Serum Markers ALP and PSA with aBSI
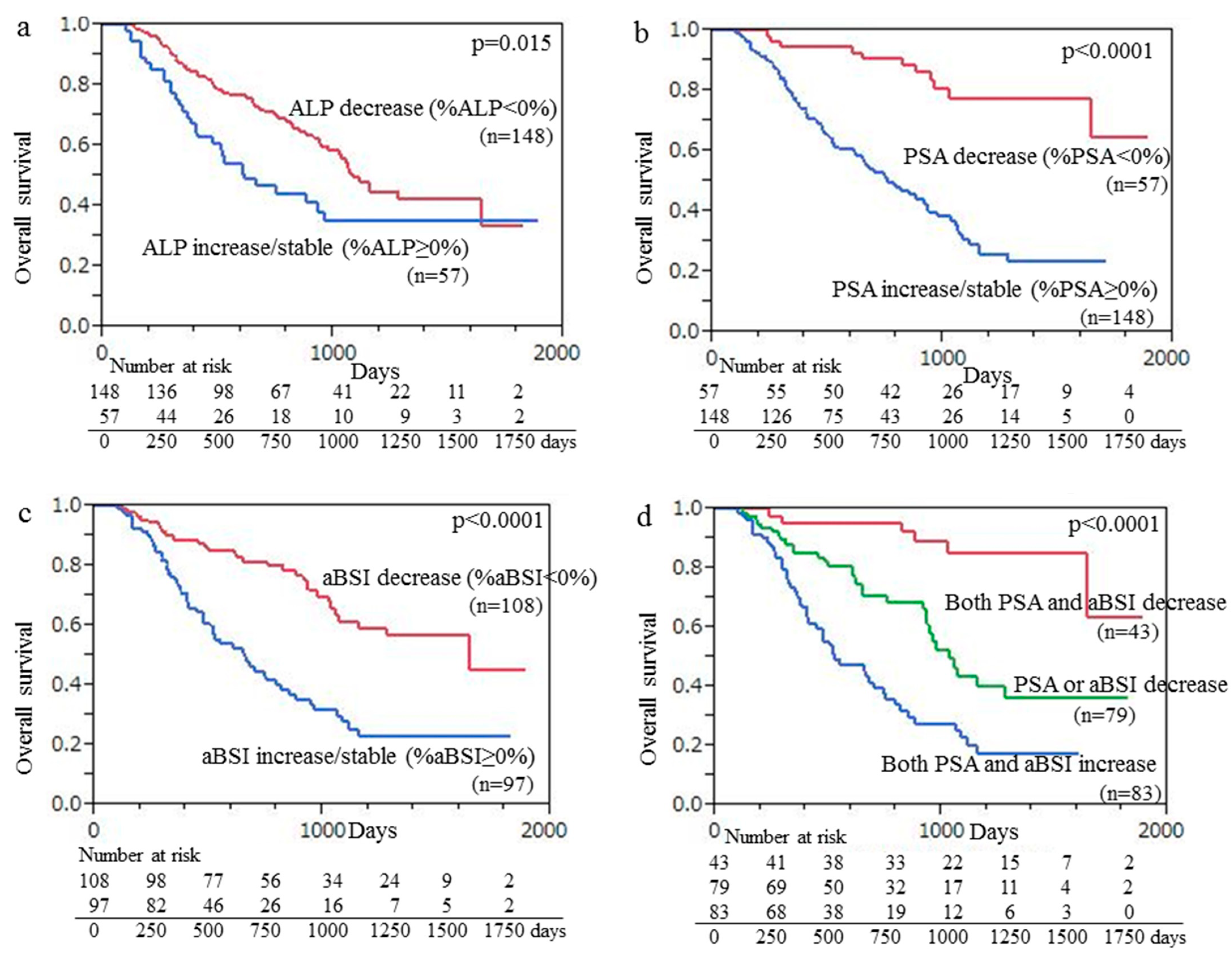
3.4. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aBSI | automated bone scan index |
| mCRPC | metastatic castration-resistant prostate cancer |
| OS | overall survival |
| HR | hazard ratio |
| CI | confidence interval |
| ALP | alkaline phosphatase |
| PSA | prostate-specific antigen |
| PCWG3 | Prostate Cancer Clinical Trials Working Group3 |
| 99mTc-MDP | technetium-99m methylene diphosphonate |
| CTCAE | Common Terminology Criteria for Adverse Events |
| SD | standard deviation |
| FDG | fluorodeoxyglucose |
| PET | positron emission tomography |
| PSMA | prostate-specific membrane antigen |
References
- Bruland, Ø.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Heidenreich, A.; Nilsson, S.; Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 2018, 21, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations from the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Ulmert, D.; Kaboteh, R.; Fox, J.J.; Savage, C.; Evans, M.J.; Lilja, H.; Abrahamsson, P.A.; Björk, T.; Gerdtsson, A.; Bjartell, A.; et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the bone scan index. Eur. Urol. 2012, 62, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Morris, M.J.; Kaboteh, R.; Reza, M.; Trägårdh, E.; Matsunaga, N.; Edenbrandt, L.; Bjartell, A.; Larson, S.M.; Minarik, D. A preanalytic validation study of automated bone scan index: Effect on accuracy and reproducibility due to the procedural variabilities in bone scan image acquisition. J. Nucl. Med. 2016, 57, 1865–1871. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Anand, A.; Edenbrandt, L.; Bondesson, E.; Bjartell, A.; Widmark, A.; Sternberg, C.N.; Pili, R.; Tuvesson, H.; Nordle, Ö.; et al. Phase 3 assessment of the automated bone scan index as a prognostic imaging biomarker of overall survival in men with metastatic castration-resistant prostate cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018, 4, 944–951. [Google Scholar] [CrossRef]
- Alva, A.; Nordquist, L.; Daignault, S.; George, S.; Ramos, J.; Albany, C.; Isharwal, S.; McDonald, M.; Campbell, G.; Danchaivijitr, P.; et al. Clinical correlates of benefit from Radium-223 therapy in metastatic castration resistant prostate cancer. Prostate 2017, 77, 479–488. [Google Scholar] [CrossRef]
- Fosbøl, M.Ø.; Petersen, P.M.; Kjaer, A.; Mortensen, J. 223Ra therapy of advanced metastatic castration-resistant prostate cancer: Quantitative assessment of skeletal tumor burden for prognostication of clinical outcome and hematologic toxicity. J. Nucl. Med. 2018, 59, 596–602. [Google Scholar] [CrossRef]
- Nakashima, K.; Makino, T.; Kadomoto, S.; Iwamoto, H.; Yaegashi, H.; Iijima, M.; Kawaguchi, S.; Nohara, T.; Shigehara, K.; Izumi, K.; et al. Initial experience with Radium-223 chloride treatment at the Kanazawa University Hospital. Anticancer Res. 2019, 39, 2607–2614. [Google Scholar] [CrossRef]
- Frantellizzi, V.; Pani, A.; Ippoliti, M.D.; Farcomeni, A.; Aloise, I.; Colosi, M.; Polito, C.; Pani, R.; Vincentis, G. Scintigraphic load of bone disease evaluated by DASciS software as a survival predictor in metastatic castration-resistant prostate cancer patients candidates to 223RaCl treatment. Radiol. Oncol. 2019, 54, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Trägårdh, E.; Edenbrandt, L.; Beckman, L.; Svensson, J.H.; Thellenberg, C.; Widmark, A.; Kindblom, J.; Ullén, A.; Bjartell, A. Assessing radiographic response to 223Ra with an automated bone scan index in metastatic castration-resistant prostate cancer patients. J. Nucl. Med. 2020, 61, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Tsutsumi, S.; Kawahara, T.; Yasui, M.; Uemura, K.; Yoneyama, S.; Yokomizo, Y.; Hayashi, N.; Yao, M.; Uemura, H. Prognostic value of automated bone scan index for predicting overall survival among bone metastatic castration resistant prostate cancer patients treated with radium-223. BJUI Compass 2021, 2, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Igeta, M.; Kuyama, J.; Kawahara, T.; Suga, T.; Otani, T.; Sugawara, S.; Kono, Y.; Tamaki, Y.; Seko-Nitta, A.; et al. Novel nomogram developed for determining suitability of metastatic castration resistant prostate cancer patients to receive maximum benefit from radium-223 dichloride treatment—Japanese Ra-223 Therapy in Prostate Cancer using Bone Scan Index (J-RAP-BSI) Trial. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1487–1498. [Google Scholar] [PubMed]
- Soloway, M.S.; Hardeman, S.W.; Hickey, D.; Raymond, J.; Todd, B.; Soloway, S.; Moinuddin, M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988, 61, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Wagatsuma, K.; Miyaji, N.; Murata, T.; Miwa, K.; Takiguchi, T.; Makino, T.; Koyama, M. Evaluation of a computer-assisted diagnosis system, BONENAVI version 2, for bone scintigraphy in cancer patients in a routine clinical setting. Ann. Nucl. Med. 2015, 29, 138–148. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (accessed on 4 April 2023).
- Prelaj, A.; Rebuzzi, S.E.; Buzzacchino, F.; Pozzi, C.; Ferrara, C.; Frantellizzi, V.; Follacchio, G.A.; Civitelli, L.; De Vincentis, G.; Tomao, S.; et al. Radium-223 in patients with metastatic castration-resistant prostate cancer: Efficacy and safety in clinical practice. Oncol. Lett. 2019, 17, 1467–1476. [Google Scholar] [CrossRef]
- Nilsson, S.; Franzén, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernäs, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007, 8, 587–594. [Google Scholar] [CrossRef]
- Nilsson, S.; Strang, P.; Aksnes, A.K.; Franzèn, L.; Olivier, P.; Pecking, A.; Staffurth, J.; Vasanthan, S.; Andersson, C.; Bruland, Ø.S. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 678–686. [Google Scholar] [CrossRef]
- Dizdarevic, S.; Jessop, M.; Begley, P.; Main, S.; Robinson, A. 223Ra-Dichloride in castration-resistant metastatic prostate cancer: Improving outcomes and identifying predictors of survival in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2264–2273. [Google Scholar] [CrossRef]
- Sartor, O.; Coleman, R.E.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Vogelzang, N.J.; Bruland, Ø.; Kobina, S.; Wilhelm, S.; et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann. Oncol. 2017, 28, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Carles, J.; Gillessen, S.; Heidenreich, A.; Heinrich, D.; Gratt, J.; Lévy, J.; Miller, K.; Nilsson, S.; Petrenciuc, O.; et al. Radium-223 International Early Access Program Investigators. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: An international, early access, open-label, single-arm phase 3b trial. Lancet Oncol. 2016, 17, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Larsen, R.H.; Fosså, S.D.; Balteskard, L.; Borch, K.W.; Westlin, J.E.; Salberg, G.; Bruland, O.S. First clinical experience with α-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005, 11, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.A.; George, D.J. Pain, PSA flare, and bone scan response in a patient with metastatic castration-resistant prostate cancer treated with radium-223, a case report. BMC Cancer 2015, 15, 371. [Google Scholar] [CrossRef]
- Keizman, D.; Fosboel, M.O.; Reichegger, H.; Peer, A.; Rosenbaum, E.; Desax, M.C.; Neiman, V.; Petersen, P.M.; Mueller, J.; Cathomas, R.; et al. Imaging response during therapy with radium-223 for castration-resistant prostate cancer with bone metastases-analysis of an international multicenter database. Prostate Cancer Prostatic Dis. 2017, 20, 289–293. [Google Scholar] [CrossRef]
- Castello, A.; Macapinlac, H.A.; Lopci, E.; Santos, E.B. Prostate-specific antigen flare induced by 223RaCl2 in patients with metastatic castration-resistant prostate cancer. Eur. J. Nul. Med. Mol. Imaging 2018, 45, 2256–2263. [Google Scholar] [CrossRef]
- Bauckneht, M.; Capitanio, S.; Donegani, M.I.; Zanardi, E.; Miceli, A.; Murialdo, R.; Raffa, S.; Tomasello, L.; Vitti, M.; Cavo, A.; et al. Role of Baseline and Post-Therapy 18F-FDG PET in the Prognostic Stratification of Metastatic Castration-Resistant Prostate Cancer (mCRPC) Patients Treated with Radium-223. Cancers 2019, 12, 31. [Google Scholar] [CrossRef]
- Uprimny, C.; Kroiss, A.; Nilica, B.; Buxbaum, S.; Decristoforo, C.; Horninger, W.; Virgolini, I.J. 68Ga-PSMA ligand PET versus 18F-NaF PET: Evaluation of response to 223Ra therapy in a prostate cancer patient. Eur. J. Nul. Med. Mol. Imaging 2015, 42, 362–363. [Google Scholar] [CrossRef]
- De Feo, M.S.; Frantellizzi, V.; Bauckneht, M.; Farcomeni, A.; Filippi, L.; Rizzini, E.L.; Lavelli, V.; Stazza, M.L.; Raimondo, T.D.; Fornarini, G.; et al. The DASciS Software for BSI Calculation as a Valuable Prognostic Tool in mCRPC Treated with 223RaCl2, A Multicenter Italian Study. Biomedicines 2023, 11, 1103. [Google Scholar] [CrossRef]
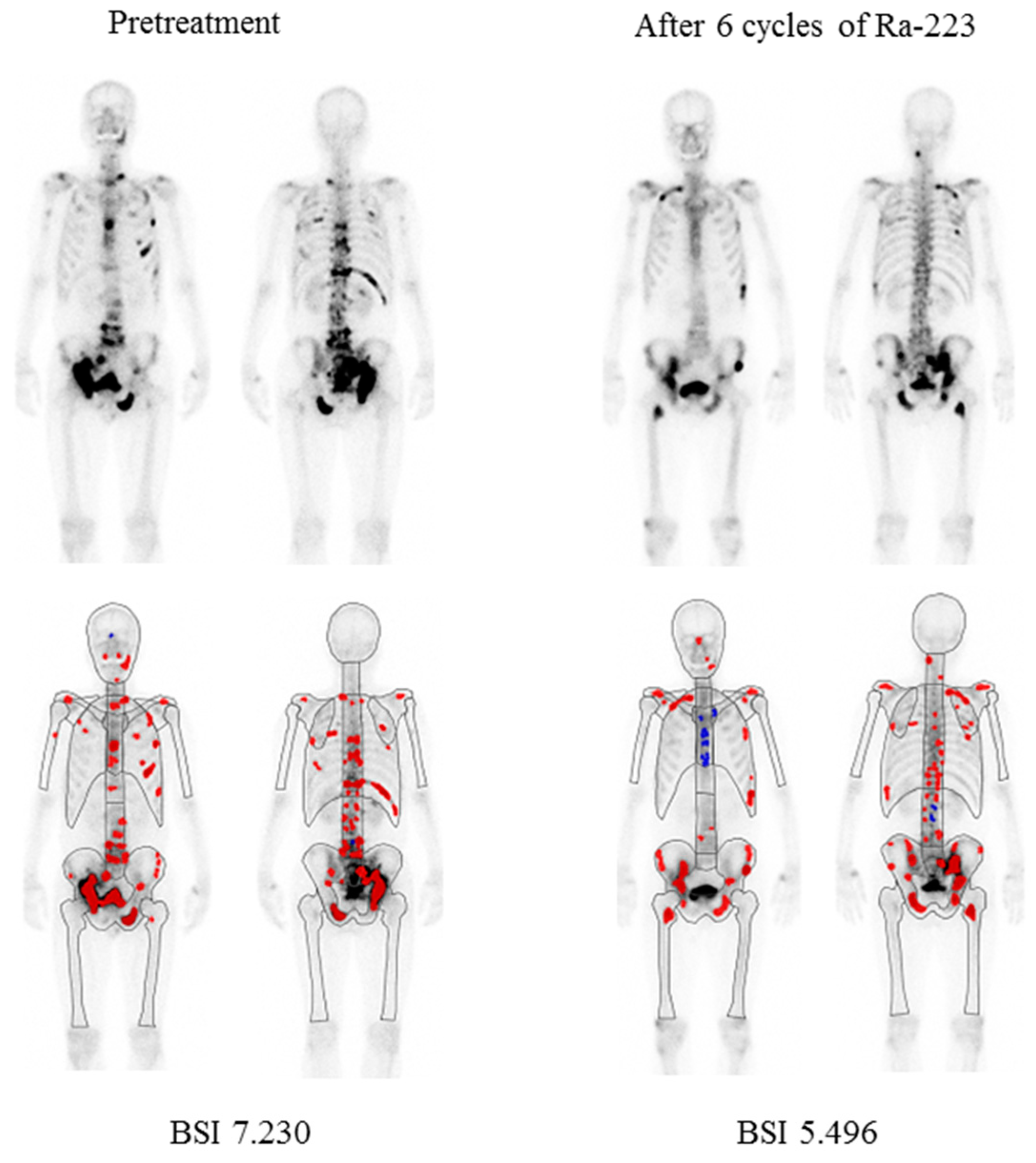

| Characteristics | Value (Range) | % |
|---|---|---|
| Median age (years) | 73 (54–88) | |
| Initial stage | ||
| II | 19 | 9.3 |
| III | 29 | 14.1 |
| IV | 157 | 76.6 |
| Initial median PSA (ng/mL) | 94.9 (0.103–22412) | |
| Initial Gleason score | ||
| 6 | 6 | 29.3 |
| 7 | 23 | 11.2 |
| 8 | 50 | 24.4 |
| 9 | 97 | 47.3 |
| 10 | 29 | 14.1 |
| Prior external radiotherapy for primary prostate cancer | 50 | 24.4 |
| Prior external radiotherapy for bone metastasis | 58 | 28.3 |
| Prior ARTA therapy | 158 | 77.1 |
| Prior taxane-based chemotherapy | 91 | 44.4 |
| Prior systemic therapy line for mCRPC | ||
| 0 | 8 | 3.9 |
| 1 | 26 | 12.7 |
| 2 | 125 | 61.0 |
| 3 | 28 | 13.7 |
| 4 | 18 | 8.8 |
| Prior bisphosphonate/denosumab | 144 | 70.2 |
| ECOG performance status | ||
| 0 | 131 | 63.9 |
| 1 | 65 | 31.1 |
| 2 | 7 | 3.4 |
| 3 | 2 | 0.96 |
| Median Hemoglobin (g/dl) | 12.5 (8.4–18.1) | |
| Median Neutrophil count (/μL) | 4127 (1512–12470) | |
| Median Platelet count (×104/μL) | 22.3 (10.1–53.4) | |
| Median ALP (U/L) | 285 (68–3494) | |
| Median LDH (U/L) | 201 (100–952) | |
| Median PSA (ng/mL) | 15.48 (0.002–3630.56) | |
| Extent of skeletal disease | ||
| <6 metastases | 77 | 37.6 |
| 6–20 metastases | 63 | 30.7 |
| >20 metastases | 57 | 27.8 |
| Superscan | 8 | 3.9 |
| Median aBSI | 1.07 (0.02–18.58) | |
| Number of 223Ra applications | ||
| 1 injection | 3 | 1.5 |
| 2 injections | 3 | 1.5 |
| 3 injections | 13 | 6.3 |
| 4 injections | 9 | 4.4 |
| 5 injections | 12 | 5.9 |
| 6 injections | 165 | 80.5 |
| Concomitany use of enzalutamide | 57 | 27.8 |
| Concomitany use of Bisphosphonate/denosumab | 120 | 58.5 |
| Item | Number of | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| Patients | p (Log-Rank) | Hazard Ratio (95% CI) | p (Log-Rank) | Hazard Ratio (95% CI) | |
| ALP | |||||
| Decrease (%ALP < 0%) | 148 | 0.028 | 1.72 (1.09–2.64) | 0.0008 | 2.06 (1.35–3.14) |
| Increase/stable (%ALP ≥ 0%) | 57 | ||||
| PSA | |||||
| Decrease (%PSA < 0%) | 57 | <0.0001 | 5.20 (2.86–10.4) | <0.0001 | 4.30 (2.32–8.77) |
| Increase/stable (%PSA ≥ 0%) | 148 | ||||
| aBSI | |||||
| Decrease (%aBSI < 0%) | 108 | <0.0001 | 2.94 (1.92–4.60) | 0.0003 | 2.22 (1.43–3.50) |
| Increase/stable (%aBSI ≥ 0%) | 97 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitajima, K.; Kuyama, J.; Kawahara, T.; Suga, T.; Otani, T.; Sugawara, S.; Kono, Y.; Tamaki, Y.; Seko-Nitta, A.; Ishiwata, Y.; et al. Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial. Cancers 2023, 15, 2784. https://doi.org/10.3390/cancers15102784
Kitajima K, Kuyama J, Kawahara T, Suga T, Otani T, Sugawara S, Kono Y, Tamaki Y, Seko-Nitta A, Ishiwata Y, et al. Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial. Cancers. 2023; 15(10):2784. https://doi.org/10.3390/cancers15102784
Chicago/Turabian StyleKitajima, Kazuhiro, Junpei Kuyama, Takashi Kawahara, Tsuyoshi Suga, Tomoaki Otani, Shigeyasu Sugawara, Yumiko Kono, Yukihisa Tamaki, Ayumi Seko-Nitta, Yoshinobu Ishiwata, and et al. 2023. "Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial" Cancers 15, no. 10: 2784. https://doi.org/10.3390/cancers15102784
APA StyleKitajima, K., Kuyama, J., Kawahara, T., Suga, T., Otani, T., Sugawara, S., Kono, Y., Tamaki, Y., Seko-Nitta, A., Ishiwata, Y., Ito, K., Toriihara, A., Watanabe, S., Hosono, M., Miyake, H., Yamamoto, S., Sasaki, R., Narita, M., & Yamakado, K. (2023). Assessing Therapeutic Response to Radium-223 with an Automated Bone Scan Index among Metastatic Castration-Resistant Prostate Cancer Patients: Data from Patients in the J-RAP-BSI Trial. Cancers, 15(10), 2784. https://doi.org/10.3390/cancers15102784





