Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Sample Extraction
2.4. Determination of Secondary Metabolites
2.5. Determination of Cytotoxic Activity Using MTT Assay
2.6. Determination of Anti-Migration Effect Using Scratch Wound Healing Assay
2.7. Secondary Metabolites Profiling Using Ultra-High Performance Liquid Chromatography Mass Spectrophotometry (UHPLC-MS)
2.8. Molecular Docking
2.9. Statistical Analysis
3. Results
3.1. Extraction Process and Secondary Metabolites Analysis
3.2. Cytotoxic Activity of Kaempferia parviflora
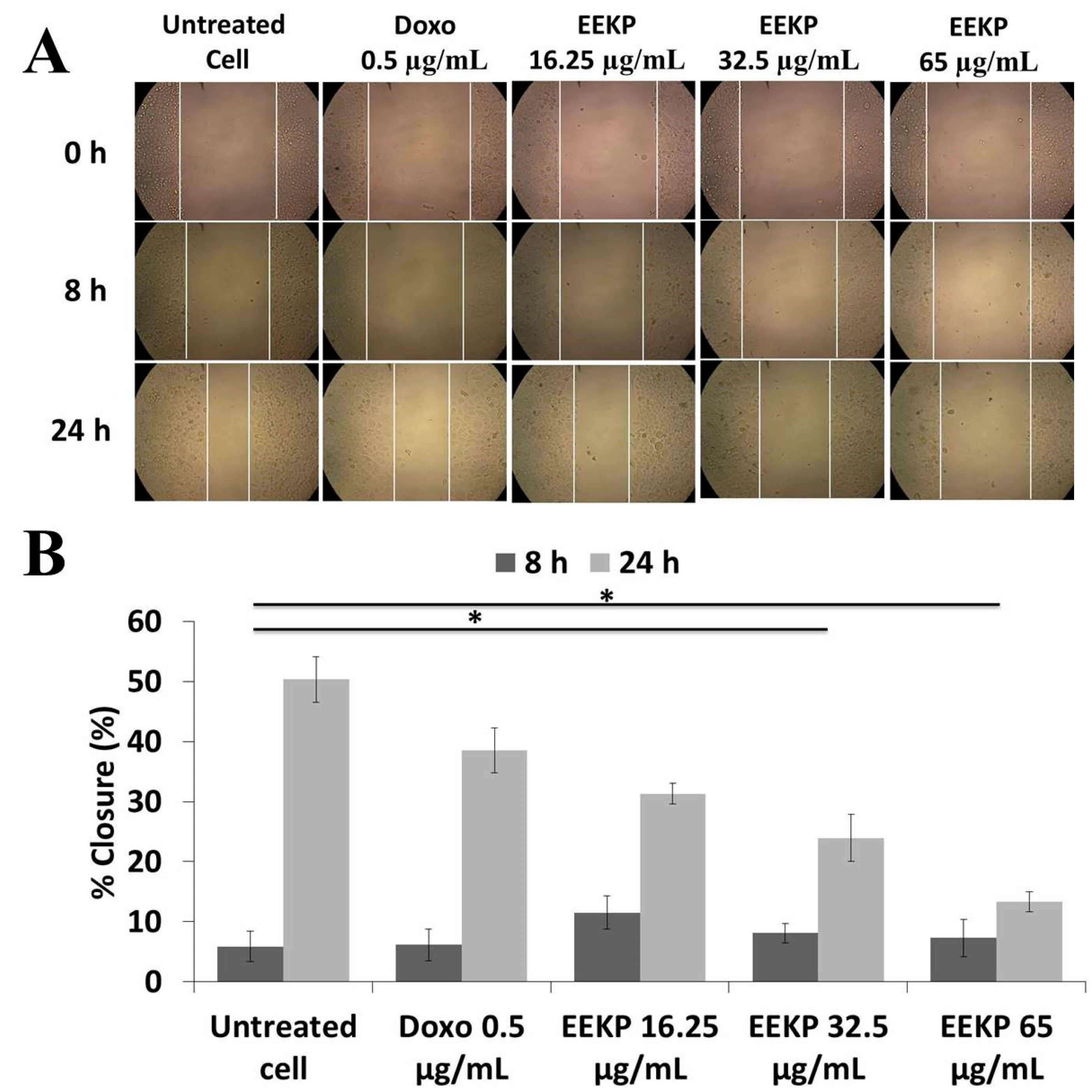
3.3. Anti-Migration Activity on 4T1 Breast Cancer Cell Line
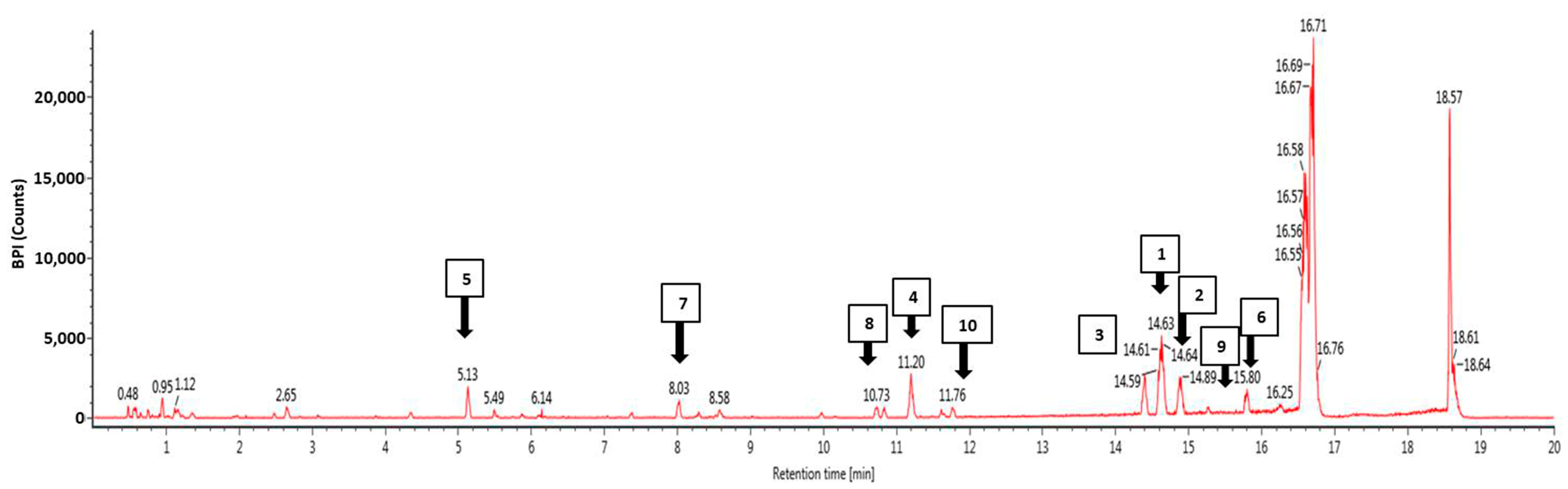
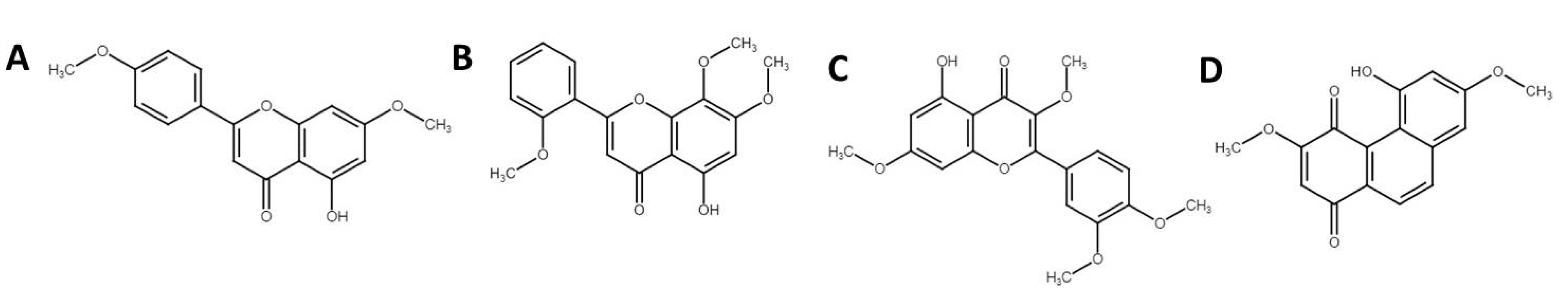
3.4. Phytochemical Profiling Using Ultra-High Performance Liquid Chromatography Mass Spectrophotometry (UHPLC-MS)
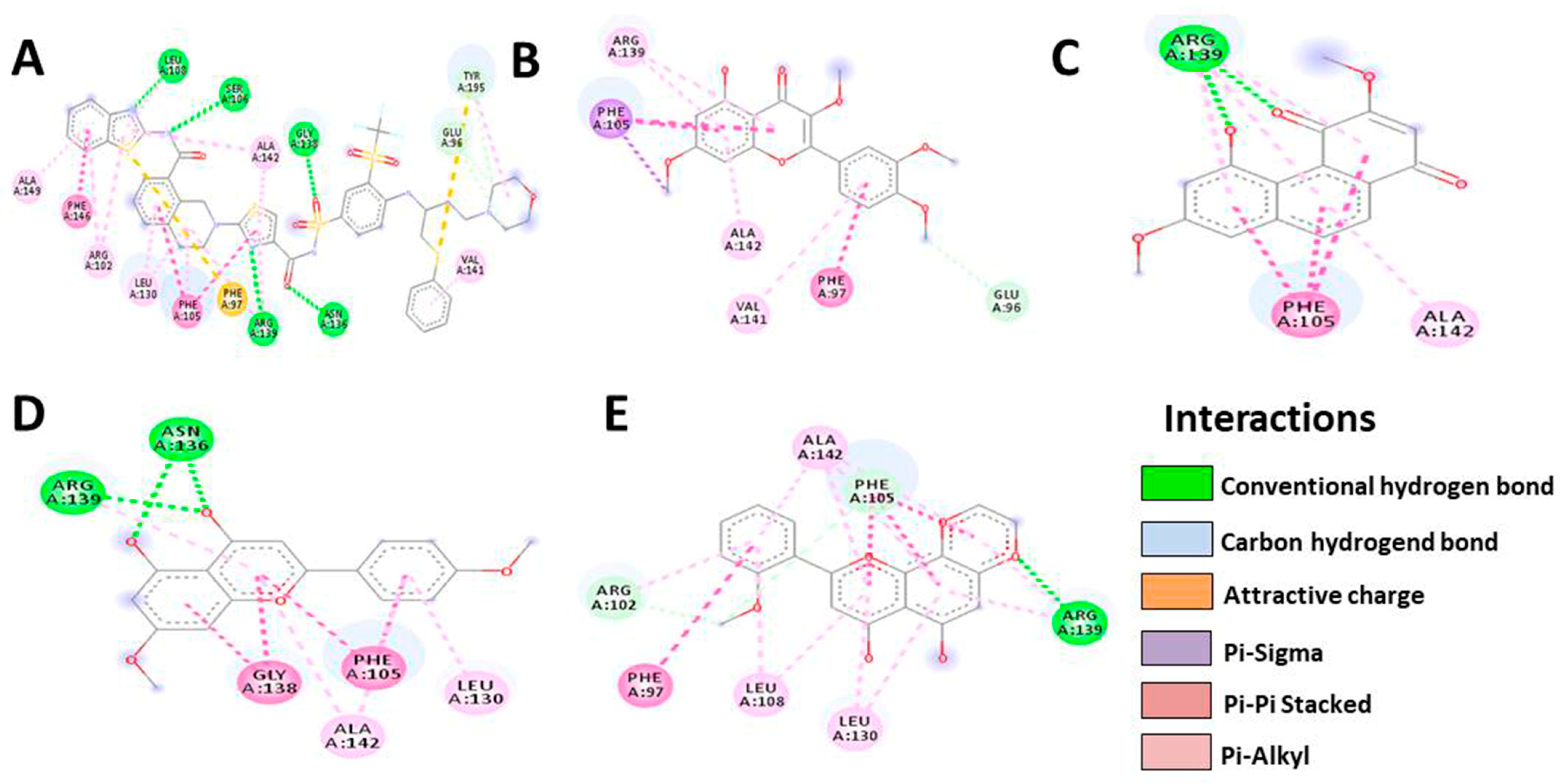
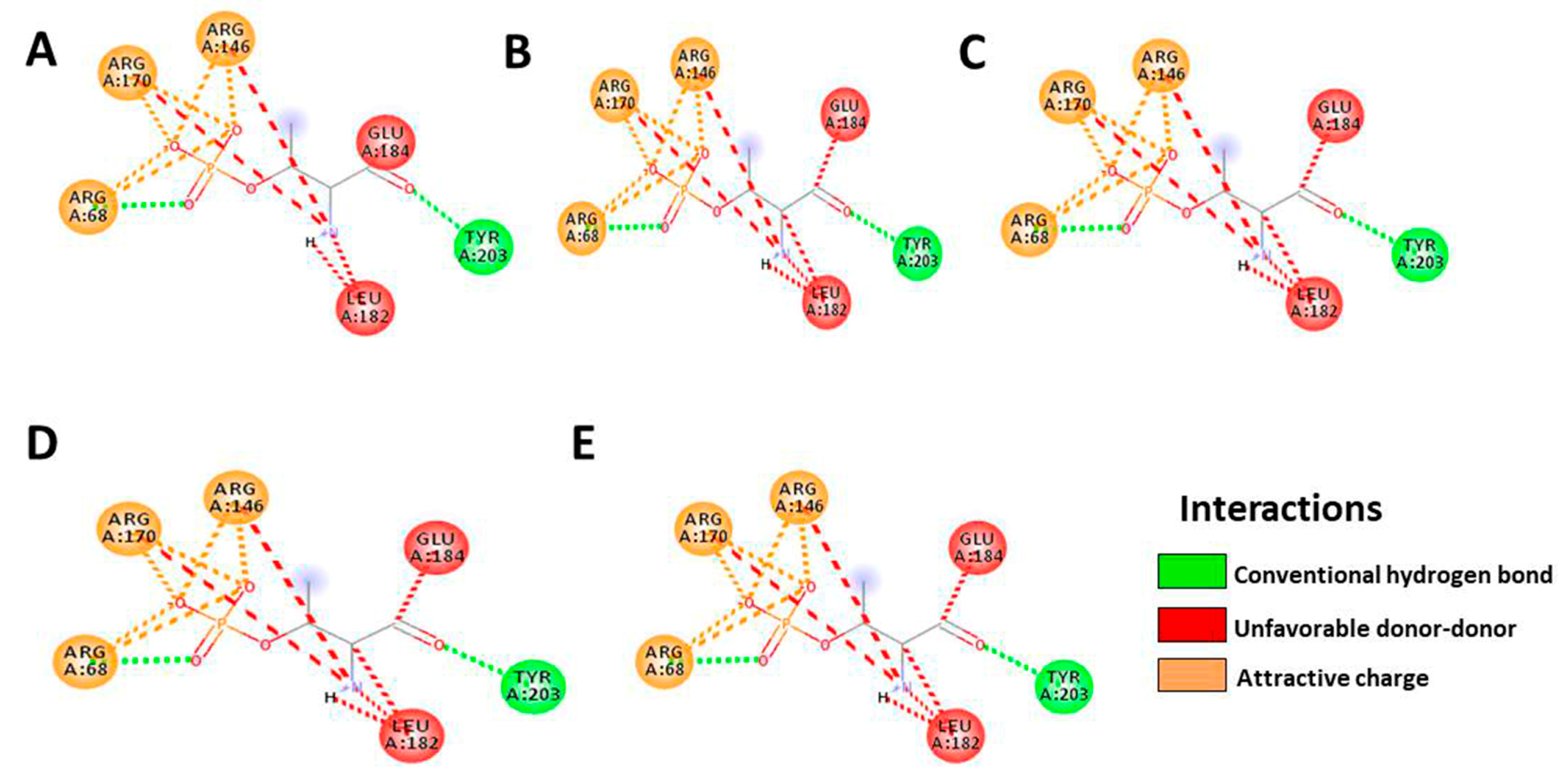
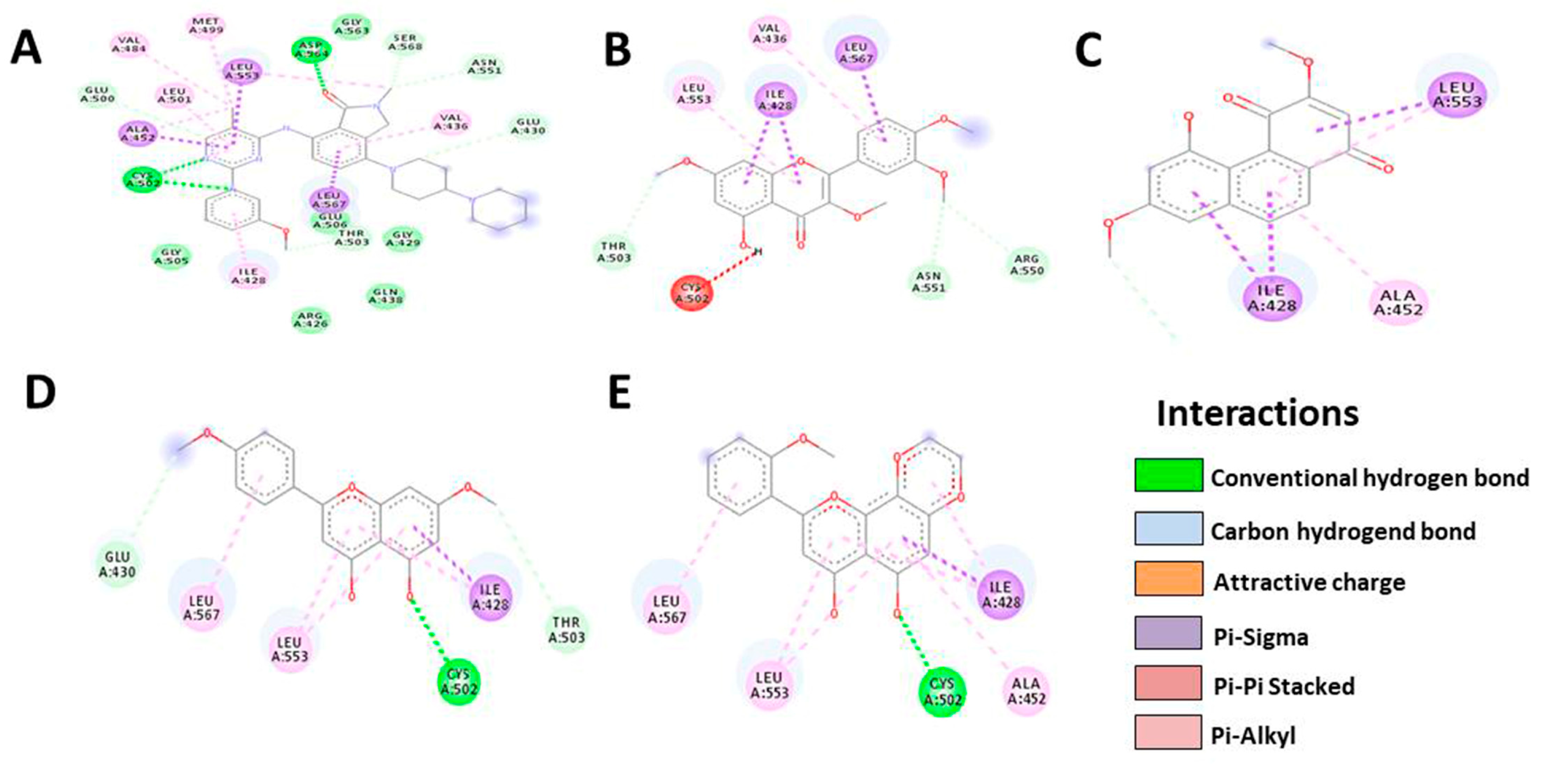
3.5. In Silico Molecular Docking
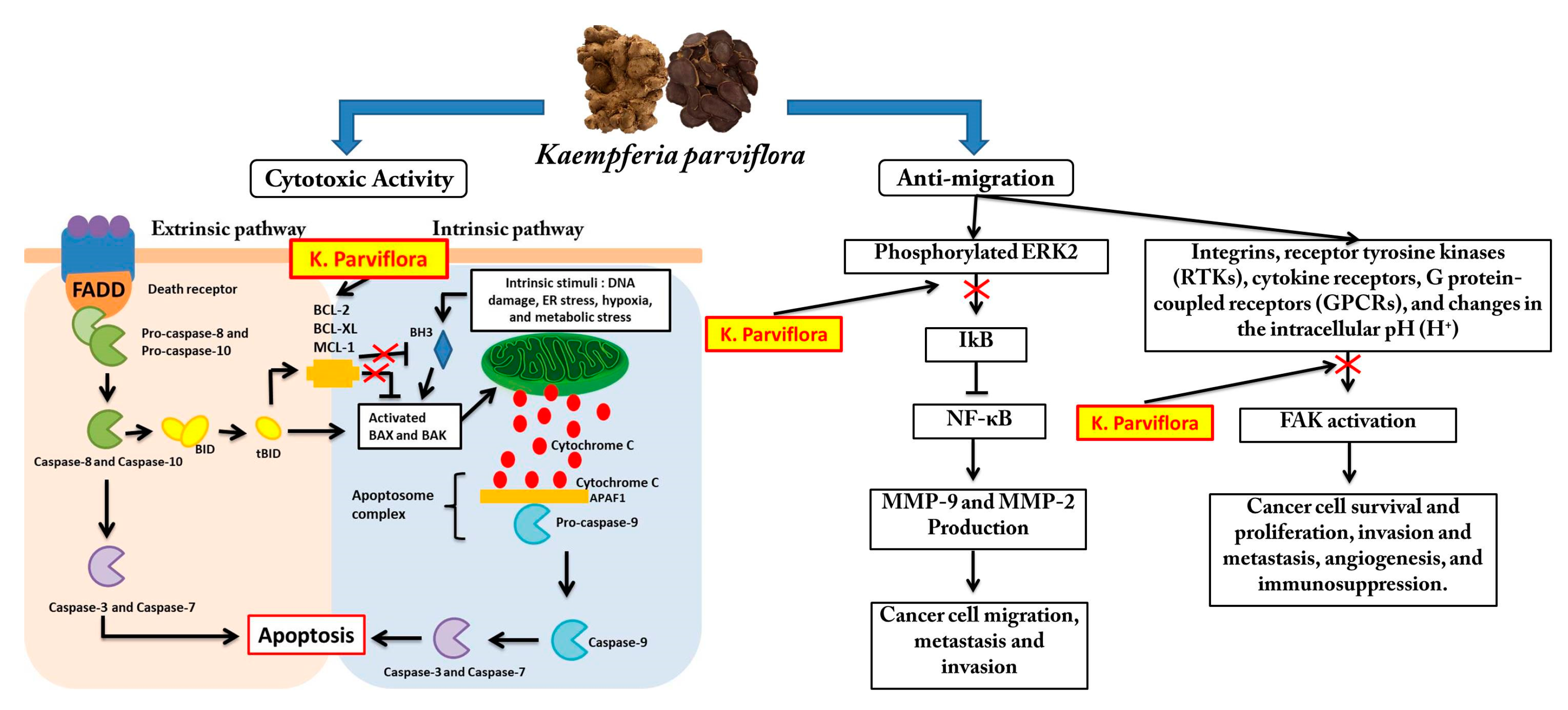
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding Breast Cancer as a Global Health Concern. Br. J. Radiol. 2022, 95, 7–9. [Google Scholar] [CrossRef] [PubMed]
- European Cancer Information System. ECIS Breast Cancer Burden in EU-27 2020 New Cases (Incidence) and Deaths (Mortality) Estimates New Cases Deaths 1 in 11. Jt. Res. Cent. 2020, 44–45. Available online: https://ecis.jrc.ec.europa.eu/pdf/Breast_cancer_factsheet-Oct_2020.pdf (accessed on 1 March 2023).
- Lim, Y.X.; Lim, Z.L.; Ho, P.J.; Li, J. Breast Cancer in Asia: Incidence, Mortality, Early Detection, Mammography Programs, and Risk-Based Screening Initiatives. Cancers 2022, 14, 4218. [Google Scholar] [CrossRef] [PubMed]
- Tungsukruthai, S.; Petpiroon, N.; Chanvorachote, P. Molecular Mechanisms of Breast Cancer Metastasis and Potential Anti-Metastatic Compounds. Anticancer Res. 2018, 38, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Woditschka, S.; Reed, L.T.; Nakayama, J.; Mayer, M.; Wetzel, M.; Steeg, P.S. Breast Cancer Metastasis: Issues for the Personalization of Its Prevention and Treatment. Am. J. Pathol. 2013, 183, 1084–1095. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rubovszky, G.; Kocsis, J.; Boér, K.; Chilingirova, N.; Dank, M.; Kahán, Z.; Kaidarova, D.; Kövér, E.; Krakovská, B.V.; Máhr, K.; et al. Systemic Treatment of Breast Cancer. 1st Central-Eastern European Professional Consensus Statement on Breast Cancer. Pathol. Oncol. Res. 2022, 28, 1610383. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA—J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Lim, B.; Hortobagyi, G.N. Current Challenges of Metastatic Breast Cancer. Cancer Metastasis Rev. 2016, 35, 495–514. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Natsume, N.; Yamano, A.; Watanabe, A.; Yonezawa, T.; Woo, J.T.; Yamakuni, T.; Teruya, T. Effect of Methoxyflavones Contained in Kaempferia Parviflora on CRE-Mediated Transcription in PC12D Cells. Bioorg. Med. Chem. Lett. 2020, 30, 127606. [Google Scholar] [CrossRef] [PubMed]
- Tangjitjaroenkun, J.; Yahayo, W.; Supabphol, S.; Supabphol, R. Selective Cytotoxicity of Kaempferia Parviflora Extracts in Human Cell Lines. Asian Pac. J. Cancer Prev. 2021, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Suradej, B.; Sookkhee, S.; Panyakaew, J.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Kaempferia Parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in Hela Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4226. [Google Scholar] [CrossRef] [PubMed]
- Paramee, S.; Sookkhee, S.; Sakonwasun, C.; Na Takuathung, M.; Mungkornasawakul, P.; Nimlamool, W.; Potikanond, S. Anti-Cancer Effects of Kaempferia Parviflora on Ovarian Cancer SKOV3 Cells. BMC Complement. Altern. Med. 2018, 12, 12. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Burapan, S.; Han, J.; Cho, S.K. Induction of ER Stress-Mediated Apoptosis by the Major Component 5,7,4′-Trimethoxyflavone Isolated from Kaempferia Parviflora Tea Infusion. Nutr. Cancer 2018, 70, 984–996. [Google Scholar] [CrossRef]
- Leardkamolkarn, V.; Tiamyuyen, S.; Sripanidkulchai, B.O. Pharmacological Activity of Kaempferia Parviflora Extract against Human Bile Duct Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2009, 10, 695–698. [Google Scholar]
- Potikanond, S.; Sookkhee, S.; Takuathung, M.N.; Mungkornasawakul, P.; Wikan, N.; Smith, D.R.; Nimlamool, W. Kaempferia Parviflora Extract Exhibits Anti-Cancer Activity against HeLa Cervical Cancer Cells. Front. Pharmacol. 2017, 8, 630. [Google Scholar] [CrossRef]
- Hairunisa, I.; Utomo, R.Y.; Ertanto, Y.; Jenie, R.I.; Meiyanto, E. Pentagamaboronon-0 Fructose Inhibited Migration and Overexpression of Matrix Metalloproteinases 9 on MCF-7/HER2 Breast Cancer Cells. Indones. J. Cancer Chemoprevention 2018, 9, 134. [Google Scholar] [CrossRef]
- Asamenew, G.; Kim, H.W.; Lee, M.K.; Lee, S.H.; Kim, Y.J.; Cha, Y.S.; Yoo, S.M.; Kim, J.B. Characterization of Phenolic Compounds from Normal Ginger (Zingiber officinale Rosc.) and Black Ginger (Kaempferia parviflora Wall.) Using UPLC-DAD-QToF-MS. Eur. Food Res. Technol. 2019, 245, 653–665. [Google Scholar] [CrossRef]
- Miyata, Y.; Tatsuzaki, J.; Yang, J.; Kosano, H. Potential Therapeutic Agents, Polymethoxylated Flavones Isolated from Kaempferia Parviflora for Cataract Prevention through Inhibition of Matrix Metalloproteinase-9 in Lens Epithelial Cells. Biol. Pharm. Bull. 2019, 42, 1658–1664. [Google Scholar] [CrossRef]
- Health Ministry of Republic Indonesia Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/MENKES/187/2017 Tentang Formularium Ramuan Obat Tradisional Indonesia. 2017. Available online: http://hukor.kemkes.go.id/uploads/produk_hukum/KMK_No._HK_.01_.07-MENKES-187-2017_ttg_Formularium_Ramuan_Obat_Tradisional_Indonesia_.pdf (accessed on 22 October 2022).
- Saifudin, A. Senyawa Alam Metabolit Sekunder (Teori, Konsep Dan Teknik Pemurnian); Deepublish: Yogyakarta, Indonesia, 2014; ISBN 9786022804727. [Google Scholar]
- Ramírez-Salinas, G.L.; García-Machorro, J.; Rojas-Hernández, S.; Campos-Rodríguez, R.; de Oca, A.C.M.; Gomez, M.M.; Luciano, R.; Zimic, M.; Correa-Basurto, J. Bioinformatics Design and Experimental Validation of Influenza A Virus Multi-Epitopes That Induce Neutralizing Antibodies. Arch. Virol. 2020, 165, 891–911. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Syamsul, E.S.; Umar, S.; Wahyuni, F.S.; Martien, R.; Hamidi, D. Anti-Aging Activity, In Silico Modeling and Molecular Docking from Sonneratia Caseolaris. Open Access Maced. J. Med. Sci. 2022, 10, 1471–1477. [Google Scholar] [CrossRef]
- Raikwar, S.; Jain, K.S. Opportunities and Challenges in Breast Cancer. Int. J. Pharm. Life Sci. 2020, 11, 6858–6873. [Google Scholar]
- Kilmister, E.J.; Koh, S.P.; Weth, F.R.; Gray, C.; Tan, S.T. Cancer Metastasis and Treatment Resistance: Mechanistic Insights and Therapeutic Targeting of Cancer Stem Cells and the Tumor Microenvironment. Biomedicines 2022, 10, 2988. [Google Scholar] [CrossRef] [PubMed]
- Barillé-Nion, S.; Lohard, S.; Juin, P.P. Targeting of Bcl-2 Family Members during Anticancer Treatment: A Necessary Compromise between Individual Cell and Ecosystemic Responses? Biomolecules 2020, 10, 1109. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Mohamed, T.A.; Essa, A.F.; Abd-Elgawad, A.M.; Alqahtani, A.S.; Shahat, A.A.; Yoneyama, T.; Farrag, A.R.H.; Noji, M.; El-Seedi, H.R.; et al. Recent Advances in Kaempferia Phytochemistry and Biological Activity: A Comprehensive Review. Nutrients 2019, 11, 2396. [Google Scholar] [CrossRef]
- Obeng, E. Apoptosis (Programmed Cell Death) and Its Signals—A Review. Braz. J. Biol. 2021, 81, 1133–1143. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 Family Proteins in Apoptosis: Apoptosomes or Mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Kirby, R. Apoptosis: A Review of pro-Apoptotic and Anti-Apoptotic Pathways and Dysregulation in Disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Sun, Y.L.; Jiang, W.Q.; Luo, Q.Y.; Yang, D.J.; Cai, Y.C.; Huang, H.Q.; Sun, J. A Novel Bcl-2 Inhibitor, BM-1197, Induces Apoptosis in Malignant Lymphoma Cells through the Endogenous Apoptotic Pathway. BMC Cancer 2019, 20, 1. [Google Scholar] [CrossRef]
- Wen, M.; Deng, Z.K.; Jiang, S.L.; Guan, Y.D.; Wu, H.Z.; Wang, X.L.; Xiao, S.S.; Zhang, Y.; Yang, J.M.; Cao, D.S.; et al. Identification of a Novel Bcl-2 Inhibitor by Ligand-Based Screening and Investigation of Its Anti-Cancer Effect on Human Breast Cancer Cells. Front. Pharmacol. 2019, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P. Apoptosis Regulators Bcl-2. Encylopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef]
- Wang, T.; Jin, X.; Liao, Y.; Sun, Q.; Luo, C.; Wang, G.; Zhao, F.; Jin, Y. Association of NF-ΚB and AP-1 with MMP-9 Overexpression in 2-Chloroethanol Exposed Rat Astrocytes. Cells 2018, 7, 96. [Google Scholar] [CrossRef]
- Yoon, H.; Dehart, J.P.; Murphy, J.M.; Lim, S.T.S. Understanding the Roles of FAK in Cancer: Inhibitors, Genetic Models, and New Insights. J. Histochem. Cytochem. 2015, 63, 114–128. [Google Scholar] [CrossRef]
- Chuang, H.H.; Zhen, Y.Y.; Tsai, Y.C.; Chuang, C.H.; Hsiao, M.; Huang, M.S.; Yang, C.J. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Wongsrikaew, N.; Kim, H.; Vichitphan, K.; Cho, S.K.; Han, J. Antiproliferative Activity and Polymethoxyflavone Composition Analysis of Kaempferia Parviflora Extracts. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 813–817. [Google Scholar] [CrossRef]
- Sutthanut, K.; Lu, X.; Jay, M.; Sripanidkulchai, B. Solid Lipid Nanoparticles for Topical Administration of Kaempferia parviflora Extracts. J. Biomed. Nanotechnol. 2009, 5, 224–232. [Google Scholar] [CrossRef]
- Mekjaruskul, C.; Yang, Y.T.; Leed, M.G.D.; Sadgrove, M.P.; Jay, M.; Sripanidkulchai, B. Novel Formulation Strategies for Enhancing Oral Delivery of Methoxyflavones in Kaempferia Parviflora by SMEDDS or Complexation with 2-Hydroxypropyl-β- Cyclodextrin. Int. J. Pharm. 2013, 445, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mekjaruskul, C.; Jay, M.; Sripanidkulchai, B. Pharmacokinetics, Bioavailability, Tissue Distribution, Excretion, and Metabolite Identification of Methoxyflavones in Kaempferia parviflora Extract in Rats. Drug Metab. Dispos. 2012, 40, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Song, J.I.; Kang, Y.J.; Yong, H.Y.; Kim, Y.C.; Moon, A. Denbinobin, a Phenanthrene from Dendrobium Nobile, Inhibits Invasion and Induces Apoptosis in SNU-484 Human Gastric Cancer Cells. Oncol. Rep. 2012, 27, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Uen, Y.H.; Suk, F.M.; Liang, Y.C.; Wang, Y.J.; Ho, Y.S.; Li, I.H.; Lin, S.Y. Molecular Mechanisms of Denbinobin-Induced Anti-Tumorigenesis Effect in Colon Cancer Cells. World J. Gastroenterol. 2005, 11, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Peng, C.Y.; Pai, H.C.; Teng, C.M.; Chen, C.C.; Yang, C.R. Denbinobin Suppresses Breast Cancer Metastasis through the Inhibition of Src-Mediated Signaling Pathways. J. Nutr. Biochem. 2011, 22, 732–740. [Google Scholar] [CrossRef]
- Zhanzhaxina, A.; Suleimen, Y.; Metwaly, A.M.; Eissa, I.H.; Elkaeed, E.B.; Suleimen, R.; Ishmuratova, M.; Akatan, K.; Luyten, W. In Vitro and in Silico Cytotoxic and Antibacterial Activities of a Diterpene from Cousinia alata Schrenk. J. Chem. 2021, 2021, 5542455. [Google Scholar] [CrossRef]



| Kaempferia parviflora | ||
|---|---|---|
| Ethanol | Water | |
| Yield (%) | 12.98 | 12.49 |
| Organoleptic | ||
| Odor | Odorless | Smoky |
| Color | Black | Black |
| Type of Secondary Metabolites | Kaempferia parviflora | Type of the Test | |
|---|---|---|---|
| Ethanol | Water | ||
| Saponin | −ve | +ve | Saponin test |
| Tannin | +ve | −ve | Tannin test |
| Steroid | −ve | +ve | Lieberman Buchard’s test |
| Alkaloid | |||
| Mayer | +ve | +ve | Mayer’s test |
| Dragendorf | +ve | +ve | Dragendorf’s test |
| Buchardat | +ve | −ve | Buchardat’s test |
| Flavonoid | +ve | +ve | Lead acetate test |
| Glycosides | −ve | −ve | Keller–Kiliani’s test |
| Quinone | −ve | +ve | Quinone test |
| Polyphenol | +ve | +ve | Polyphenol test |
| No | Component Name | Formula | Observed m/z | Observed RT (min) | Response |
|---|---|---|---|---|---|
| 1 | 5-Hydroxy-7,4′-dimethoxyflavanone | C17H14O5 | 297.0764 | 14.62 | 81,895 |
| 2 | 1,3-Dihydroxy-2-ethoxymethyl-anthraquinone | C17H14O5 | 297.0762 | 14.88 | 36,111 |
| 3 | Retusine | C19H18O7 | 357.0975 | 14.4 | 35,908 |
| 4 | Denbinobin | C16H12O5 | 283.0605 | 11.2 | 26,614 |
| 5 | Tectoruside | C21H30O13 | 489.1611 | 5.13 | 24,405 |
| 6 | 5-Hydro-7,8,2′-trimethoxyflavanone | C18H16O6 | 327.0869 | 15.79 | 24,310 |
| 7 | Acacetin-7-galactoside | C22H22O10 | 445.1137 | 8.02 | 17,504 |
| 8 | Viscumneoside Ⅵ | C24H26O12 | 505.1346 | 10.83 | 11,985 |
| 9 | 5-Hydro-7,8,2′-trimethoxyflavanone | C18H16O6 | 327.0869 | 15.27 | 10,317 |
| 10 | 3′,5-Dihydroxy-7,4′-dimethoxy flavone | C17H14O6 | 313.0711 | 11.77 | 8933 |
| Bcl-2 | Native | Denbinobin | Retusine | HDMF | HTMF |
|---|---|---|---|---|---|
| Docking score | −9.1 | −8.8 | −8.4 | −8.1 | −9.4 |
| Residue | GLU325 | GLU325 | GLU326 | ARG304 | TYR29 |
| TYR215 | GLU326 | TRP30 | ASP305 | PRO216 | |
| TRP30 | ARG304 | GLU217 | TRP308 | ASP305 | |
| GLU217 | TRP308 | PRO216 | ASP356 | TRP30 | |
| ASP356 | ASP305 | TYR215 | TYR215 | TYR215 | |
| LYS355 | TRP30 | TYR29 | |||
| GLU259 | GLU217 | ||||
| ASP305 | TYR29 | ||||
| TRP308 | PRO216 | ||||
| ARG304 | |||||
| Number of H-bonds | 10 | 9 | 5 | 6 | 5 |
| Bcl-XL | Native | Denbinobin | Retusine | HDMF | HTMF |
|---|---|---|---|---|---|
| Docking score | −13.4 | −8.2 | −8.1 | −7.9 | −8.0 |
| Residue | LEU108 | ARG139 | PHE105 | ARG139 | ALA142 |
| SER106 | PHE105 | ARG139 | ASN136 | PHE105 | |
| ALA142 | ALA142 | ALA142 | GLY138 | ARG139 | |
| GLY138 | VAL141 | ALA142 | LEU130 | ||
| GLU96 | PHE97 | PHE105 | LEU108 | ||
| TYR195 | GLU96 | LEU130 | PHE97 | ||
| VAL141 | ARG102 | ||||
| Number of H-bonds | 7 | 3 | 6 | 6 | 7 |
| ERK2 | Native | Denbinobin | Retusine | HDMF | HTMF |
|---|---|---|---|---|---|
| Docking score | −8.7 | −9.0 | −8.8 | −7.5 | −7.9 |
| Residue | ARG68 | ARG68 | ARG68 | ARG68 | ARG68 |
| ARG170 | ARG170 | ARG170 | ARG170 | ARG170 | |
| ARG146 | ARG146 | ARG146 | ARG146 | ARG146 | |
| GLU184 | GLU184 | GLU184 | GLU184 | GLU184 | |
| LEU182 | LEU182 | LEU182 | LEU182 | LEU182 | |
| TYR203 | TYR203 | TYR203 | TYR203 | TYR203 | |
| number of H-bonds | 6 | 6 | 6 | 6 | 6 |
| FAK | Native | Denbinobin | Retusine | HDMF | HTMF |
|---|---|---|---|---|---|
| Docking score | −9.8 | −7.9 | −7.8 | −7.3 | −8.1 |
| Residue | MET499 | LEU553 | VAL436 | GLU430 | LEU567 |
| VAL484 | ALA452 | LEU567 | LEU567 | LEU553 | |
| LEU501 | ILE428 | ILE428 | LEU553 | CYS502 | |
| LEU553 | THR503 | LEU553 | CYS502 | ALA452 | |
| ASP564 | THR503 | ILE428 | ILE428 | ||
| GLY563 | CYS502 | THR503 | |||
| SER568 | ASN551 | ||||
| ASN551 | ARG550 | ||||
| Number of H-bonds | 8 | 4 | 8 | 6 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hairunisa, I.; Bakar, M.F.A.; Da’i, M.; Bakar, F.I.A.; Syamsul, E.S. Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell. Cancers 2023, 15, 2785. https://doi.org/10.3390/cancers15102785
Hairunisa I, Bakar MFA, Da’i M, Bakar FIA, Syamsul ES. Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell. Cancers. 2023; 15(10):2785. https://doi.org/10.3390/cancers15102785
Chicago/Turabian StyleHairunisa, Indah, Mohd Fadzelly Abu Bakar, Muhammad Da’i, Fazleen Izzany Abu Bakar, and Eka Siswanto Syamsul. 2023. "Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell" Cancers 15, no. 10: 2785. https://doi.org/10.3390/cancers15102785
APA StyleHairunisa, I., Bakar, M. F. A., Da’i, M., Bakar, F. I. A., & Syamsul, E. S. (2023). Cytotoxic Activity, Anti-Migration and In Silico Study of Black Ginger (Kaempferia parviflora) Extract against Breast Cancer Cell. Cancers, 15(10), 2785. https://doi.org/10.3390/cancers15102785






