Automated Estimation of Mammary Gland Content Ratio Using Regression Deep Convolutional Neural Network and the Effectiveness in Clinical Practice as Explainable Artificial Intelligence
Abstract
:Simple Summary
Abstract
1. Introduction
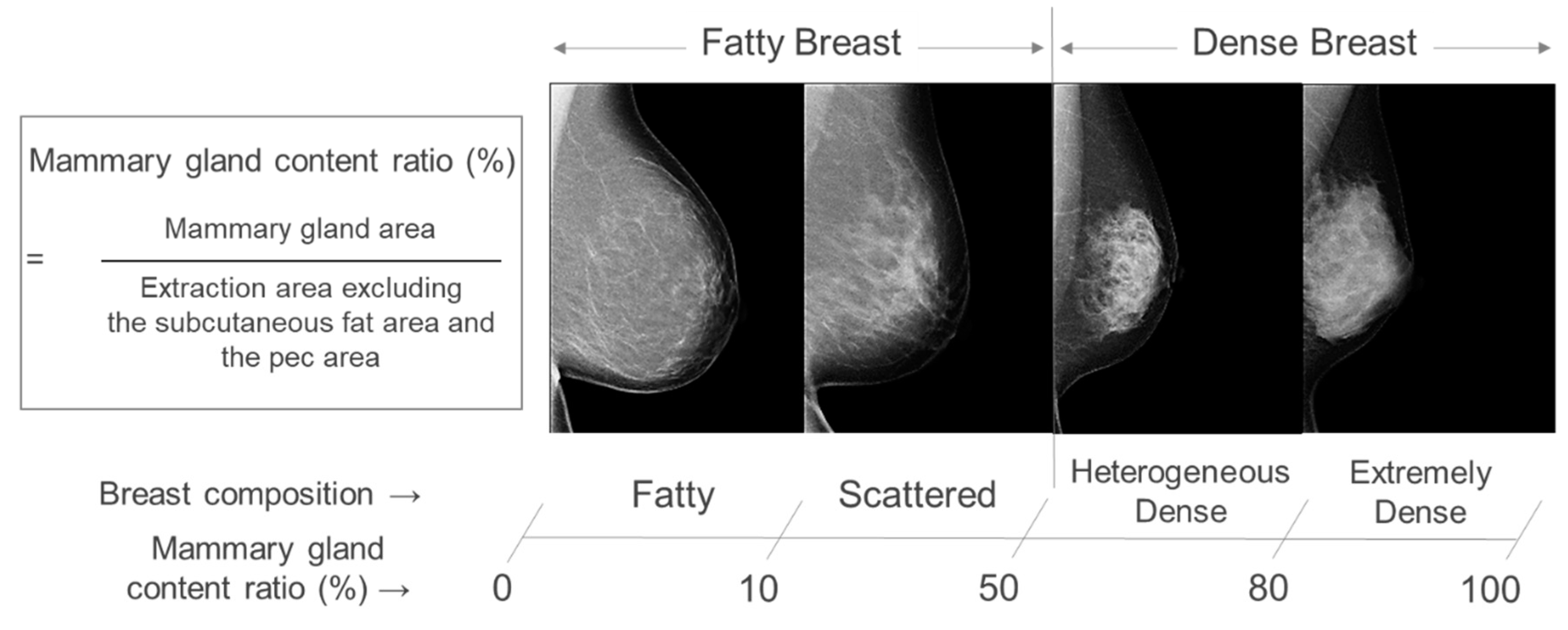
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Center. Cancer Information Service: Latest Cancer Statistics 2018. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary.html (accessed on 5 July 2022).
- Moss, S.M.; Cuckle, H.; Evans, A.; Johns, L.; Waller, M.; Bobrow, L.; Trial Management Group. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years’ follow-up: A randomised controlled trial. Lancet 2006, 368, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.M.; Wale, C.; Smith, R.; Evans, A.; Cuckle, H.; Duffy, S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years’ follow-up: A randomised controlled trial. Lancet Oncol. 2015, 16, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Prorok, P.C.; O’Malley, A.J.; Kramer, B.S. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N. Engl. J. Med. 2016, 375, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Fontham, E.T.; Etzioni, R.; Herzig, A.; Michaelson, J.S.; Shih, Y.C.; Walter, L.C.; Church, T.R.; Flowers, C.R.; LaMonte, S.J.; et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015, 314, 1599–1614. [Google Scholar] [CrossRef]
- Destounis, S.V.; Santacroce, A.; Arieno, A. Update on breast density, risk estimation, and supplemental screening. Am. J. Roentgenol. 2020, 214, 296–305. [Google Scholar] [CrossRef]
- Vachon, C.M.; van Gils, C.H.; Sellers, T.A.; Ghosh, K.; Pruthi, S.; Brandt, K.R.; Pankratz, V.S. Mammographic density, breast cancer risk and risk prediction. Breast Cancer Res. 2007, 9, 217. [Google Scholar] [CrossRef]
- American College of Radiology; BI-RADS Committee. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Kerlikowske, K.; Scott, C.G.; Mahmoudzadeh, A.P.; Ma, L.; Winham, S.; Jensen, M.R.; Wu, F.F.; Malkov, S.; Pankratz, V.S.; Cummings, S.R. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: A case–control study. Ann. Intern. Med. 2018, 168, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Kawaguchi, Y.; Sugiyama, Y.; Saji, S.; Kashiki, Y. Relationship between mammographic density and the risk of breast cancer in Japanese women: A case-control study. Breast Cancer 2003, 10, 228–233. [Google Scholar] [CrossRef]
- Nagata, C.; Matsubara, T.; Fujita, H.; Nagao, Y.; Shibuya, C.; Kashiki, Y.; Shimizu, H. Mammographic density and the risk of breast cancer in Japanese women. Br. J. Cancer. 2005, 92, 2102–2106. [Google Scholar] [CrossRef]
- Kotsuma, Y.; Tamaki, Y.; Nishimura, T.; Tsubai, M.; Ueda, S.; Shimazu, K.; Kim, S.J.; Miyoshi, Y.; Tanji, Y.; Taguchi, T.; et al. Quantitative assessment of mammographic density and breast cancer risk for Japanese women. Breast 2008, 17, 27–35. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.; Graff, R.E.; Ursin, G.; Santos Silva, I.D.; McCormack, V.; Baglietto, L.; Vachon, C.; Bakker, M.F.; Giles, G.G.; Chia, K.S.; et al. Mammographic density phenotypes and risk of breast cancer: A metaanalysis. J. Natl. Cancer Inst. 2014, 106, dju078. [Google Scholar] [CrossRef] [PubMed]
- Yaghjyan, L.; Colditz, G.A.; Rosner, B.; Tamimi, R.M.J. Mammographic breast density and breast cancer risk: Interactions of percent density, absolute dense, and non-dense areas with breast cancer risk factors. Breast Cancer Res. Treat. 2015, 150, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shieh, Y.; Scott, C.G.; Jensen, M.R.; Norman, A.D.; Bertrand, K.A.; Pankratz, V.S.; Brandt, K.R.; Visscher, D.W.; Shepherd, J.A.; Tamimi, R.M.; et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019, 21, 48. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.X.M.; Moon, S.G.; Kim, S.; Park, B. Association of the interaction between mammographic breast density, body mass index, and menopausal status with breast cancer risk among Korean women. JAMA Netw. Open 2021, 4, e2139161. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.X.M.; Kim, S.; Song, H.; Park, B. Mammographic breast density, body mass index and risk of breast cancer in Korean women aged 75 years and older. Int. J. Cancer 2022, 151, 869–877. [Google Scholar] [CrossRef]
- Kim, S.; Tran, T.X.M.; Song, H.; Ryu, S.; Chang, Y.; Park, B. Mammographic breast density, benign breast disease, and subsequent breast cancer risk in 3.9 million Korean women. Radiology 2022, 304, 534–541. [Google Scholar] [CrossRef]
- Harris, E. FDA Updates Breast Density Reporting Standards, Other Mammogram Rules. JAMA 2023, 329, 1142–1143. [Google Scholar] [CrossRef]
- Tsunoda, H. Current status on evaluation of breast composition. J. Jpn. Assoc. Breast Cancer Screen. 2021, 30, 1. [Google Scholar] [CrossRef]
- Gastounioti, A.; Desai, S.; Ahluwalia, V.S.; Conant, E.F.; Kontos, D. Artificial intelligence in mammographic phenotyping of breast cancer risk: A narrative review. Breast Cancer Res. 2022, 24, 14. [Google Scholar] [CrossRef]
- Wu, N.; Geras, K.J.; Shen, Y.; Su, J.; Kim, S.G.; Kim, E.; Wolfson, S.; Moy, L.; Cho, K. Breast density classification with deep convolutional neural networks. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Calgary, AB, Canada, 15–20 April 2018; pp. 6682–6686. [Google Scholar]
- Lehman, C.D.; Yala, A.; Schuster, T.; Dontchos, B.; Bahl, M.; Swanson, K.; Barzilay, R. Mammographic breast density assessment using deep learning: Clinical implementation. Radiology 2019, 290, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Beers, A.L.; Brink, L.; Patel, J.B.; Singh, P.; Arun, N.T.; Hoebel, K.V.; Gaw, N.; Shah, M.; Pisano, E.D. Multi-institutional assessment and crowdsourcing evaluation of deep learning for automated classification of breast density. J. Am. Coll. Radiol. 2020, 17, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ma, Y.; Li, D.; Zhao, J.; Liu, Y.; Zhang, H. Classification of breast density categories based on SE-attention neural networks. Comput. Methods Programs Biomed. 2020, 193, 105489. [Google Scholar] [CrossRef]
- Yagishita, K.; Tsunoda, H. Objective estimation of mammographic density—Breast cancer detection. J. Jpn. Assoc. Breast Cancer Screen. 2021, 30, 79. [Google Scholar] [CrossRef]
- SONY Neural Network Console. Available online: https://dl.sony.com/ja/ (accessed on 8 August 2022).
- Ohuchi, N.; Suzuki, A.; Sobue, T.; Kawai, M.; Yamamoto, S.; Zheng, Y.F.; Narikawa-Shiono, Y.; Saito, H.; Kuriyama, S.; Tohno, E.; et al. J-START investigator groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): A randomized controlled trial. Lancet 2016, 387, 341–348. [Google Scholar] [CrossRef]
- Harada-Shoji, N.; Suzuki, A.; Ishida, T.; Zheng, Y.F.; Narikawa-Shiono, Y.; Sato-Tadano, A.; Ohta, R.; Ohuchi, N. Evaluation of adjunctive ultrasonography for breast cancer detection among women aged 40–49 years with varying breast density undergoing screening mammography: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2021, 4, e2121505. [Google Scholar] [CrossRef]
- Tohno, E.; Unemoto, T.; Ito, A.; Kujiraoka, Y.; Koshikawa, K.; Fukuda, Y.; Mori; Takahashi, H. Interobserver agreement in evaluation of breast composition and differences in sensitivity according to breast composition. J. Jpn. Assoc. Breast Cancer Screen. 2015, 24, 113–121. [Google Scholar]
- Redondo, A.; Comas, M.; Macià, F.; Ferrer, F.; Murta-Nascimento, C.; Maristany, M.T.; Molins, E.; Sala, M.; Castells, X. Inter- and intraradiologist variability in the BI-RADS assessment and breast density categories for screening mammograms. Br. J. Radiol. 2012, 85, 1465–1470. [Google Scholar] [CrossRef]






| Characteristic | Training Set | Test Set |
|---|---|---|
| All Images | 1076 | 400 |
| Breast Composition | ||
| Fatty | 74 | 28 |
| Scattered | 528 | 214 |
| Heterogeneous Dense | 450 | 152 |
| Extremely Dense | 24 | 6 |
| System (Screening Period) | ||
| FPD/Cannon (2020/1–2020/8) | 598 | 230 |
| CR/Konica Minolta (2007/11–2008/3) | 62 | 34 |
| FPD/Siemens (2020/11–2021/5) | 416 | 136 |
| Prediction | |||||
| Truth | Fatty | Scattered | Heterogeneous Dense | Extremely Dense | |
| Fatty | 9 | 19 | 0 | 0 | |
| Scattered | 2 | 203 | 9 | 0 | |
| Heterogeneous Dense | 0 | 23 | 127 | 2 | |
| Extremely Dense | 0 | 0 | 6 | 0 | |
| Accuracy: 84.8% (339/400) | |||||
| Prediction | |||
| Truth | FattyBreast | Dense Breast | |
| FattyBreast | 233 | 9 | |
| DenseBreast | 23 | 135 | |
| Accuracy: 92.0% (368/400) | |||
| (a) Breast Composition four Categories | (b) Breast Composition Two Categories | ||||||||
| Prediction | Prediction | ||||||||
| Fatty | Scattered | Heterogeneous Dense | Extremely Dense | FattyBreast | Dense Breast | ||||
| Truth | Fatty | 23 | 4 | 1 | 0 | Truth | Fatty Breast | 233 | 9 |
| Scattered | 15 | 191 | 8 | 0 | |||||
| Heterogeneous Dense | 0 | 34 | 117 | 1 | Dense Breast | 34 | 124 | ||
| Extremely Dense | 0 | 0 | 3 | 3 | |||||
| Accuracy: 83.5% (334/400) | Accuracy: 89.0% (357/400) | ||||||||
| <5% | <10% | <15% | |
|---|---|---|---|
| Fatty |
57.9% (11/19) |
94.7% (18/19) |
100% (19/19) |
| Scattered |
45.5% (5/11) |
81.8% (9/11) |
100% (11/11) |
| Heterogeneous Dense |
84% (21/25) |
100% (25/25) |
100% (25/25) |
| Extremely Dense |
16.7% (1/6) |
66.7% (4/6) |
100% (6/6) |
| total |
62% (38/61) |
91.8% (56/61) |
100% (61/61) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kai, C.; Ishizuka, S.; Otsuka, T.; Nara, M.; Kondo, S.; Futamura, H.; Kodama, N.; Kasai, S. Automated Estimation of Mammary Gland Content Ratio Using Regression Deep Convolutional Neural Network and the Effectiveness in Clinical Practice as Explainable Artificial Intelligence. Cancers 2023, 15, 2794. https://doi.org/10.3390/cancers15102794
Kai C, Ishizuka S, Otsuka T, Nara M, Kondo S, Futamura H, Kodama N, Kasai S. Automated Estimation of Mammary Gland Content Ratio Using Regression Deep Convolutional Neural Network and the Effectiveness in Clinical Practice as Explainable Artificial Intelligence. Cancers. 2023; 15(10):2794. https://doi.org/10.3390/cancers15102794
Chicago/Turabian StyleKai, Chiharu, Sachi Ishizuka, Tsunehiro Otsuka, Miyako Nara, Satoshi Kondo, Hitoshi Futamura, Naoki Kodama, and Satoshi Kasai. 2023. "Automated Estimation of Mammary Gland Content Ratio Using Regression Deep Convolutional Neural Network and the Effectiveness in Clinical Practice as Explainable Artificial Intelligence" Cancers 15, no. 10: 2794. https://doi.org/10.3390/cancers15102794
APA StyleKai, C., Ishizuka, S., Otsuka, T., Nara, M., Kondo, S., Futamura, H., Kodama, N., & Kasai, S. (2023). Automated Estimation of Mammary Gland Content Ratio Using Regression Deep Convolutional Neural Network and the Effectiveness in Clinical Practice as Explainable Artificial Intelligence. Cancers, 15(10), 2794. https://doi.org/10.3390/cancers15102794







