Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
3. Results
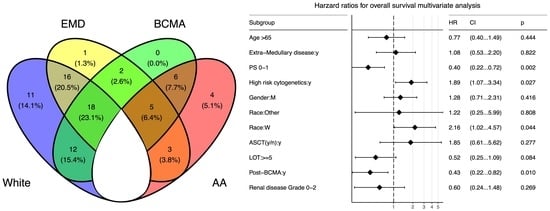
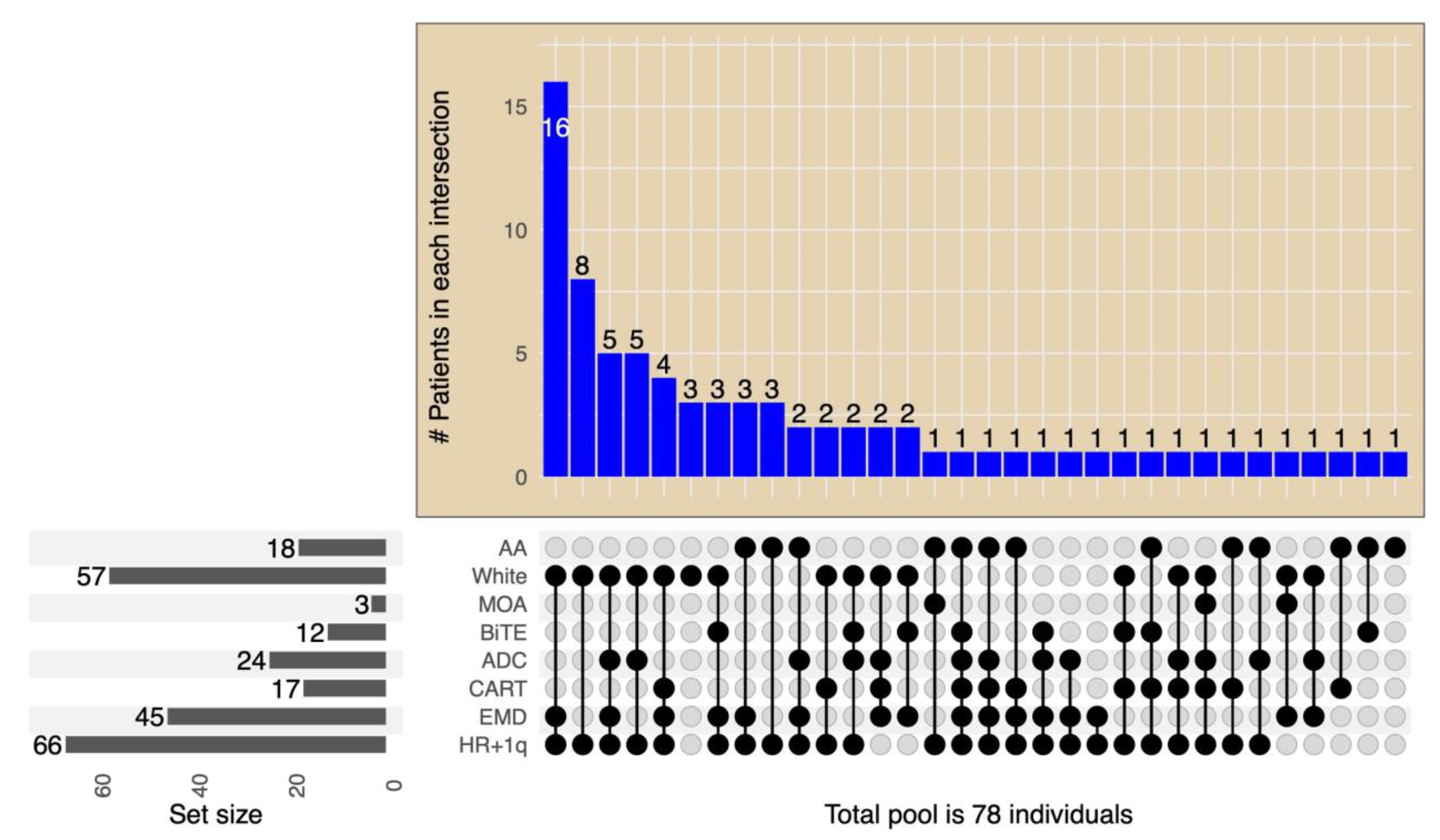
3.1. Patients Characteristics
| Characteristics n (%) | All Patients (n = 78) | Received BCMA-Targeting Therapy (n = 43) | Did Not Receive BCMA-Targeting Therapy (n = 35) | p-Value |
|---|---|---|---|---|
| Male | 44 (56%) | 28 (65%) | 19 (54%) | 0.36 |
| Age, years, median (range) | 65 (42–83) | 65 (42–83) | 65 (44–83) | 1 |
| Race, no of patients (%) | ||||
| Caucasian | 57 (73%) | 30 (70%) | 27(77%) | 0.68 |
| African American | 18 (23%) | 11 (26%) | 7 (20%) | 0.6 |
| Hispanic | 2 (3%) | 2 (5%) | 0 | |
| Asian | 1 (1%) | 0 | 1 (3%) | |
| Performance status | ||||
| PS 1 | 48 | 31 (72%) | 17 (48%) | 0.04 |
| PS 2 | 27 | 11 (26%) | 16 (46%) | 0.09 |
| PS 3 | 3 | 1 (2%) | 2 (6%) | 0.58 |
| MM paraprotein, no. of patients (%) | ||||
| IgG | 44 (56%) | 24 (56%) | 20 (57%) | 1 |
| Non-IgG | 21 (27%) | 10 (23%) | 11 (31%) | 0.86 |
| Light chain | 13 (17%) | 9 (21%) | 4 (12%) | 0.73 |
| Baseline R-ISS stage, no of patients (%) | ||||
| Stage III | 29 (37%) | 11(26%) | 18 (51%) | 0.27 |
| Stage II | 26 (33%) | 20 (47%) | 6 (17%) | 0.28 |
| Stage I | 18 (23%) | 11 (26%) | 7 (20%) | 0.86 |
| Unknown | 5 (7%) | 1 (2%) | 4 (11%) | |
| Cytogenetics, no of patients (%) | ||||
| High-risk | 63 (81%) | 35 (81%) | 28 (80%) | 1 |
| High-risk + 1q gain | 66 (84%) | 35 (81%) | 31 (88%) | 0.54 |
| Standard risk | 15 (19%) | 8 (19%) | 7 (20%) | 1 |
| Extramedullary disease | 45 (58%) | 25 (58%) | 20 (57%) | 1 |
| Previous autologous SCT | 67 (85%) | 34 (81%) | 32 (91%) | 0.34 |
| Median LOT prior to T0 | 5 (3–12) | 5 (3–8) | 6 (3–12) | |
3.2. Survival Outcomes
4. Discussion
| Target | N | LOT | TCRRMM | PCRRMM | RR (All Doses) | RR (Rec Dose) | mPFS (Months) | mDOR (Months) | |
|---|---|---|---|---|---|---|---|---|---|
| ABBV-383 [33] | BCMA | 124 | 5 (3–15) | 82% | 35% | 59% | 57% | 10.4 (5.0–19.2) | NR (12.9–NR) |
| Linvoseltamab * [34] | BCMA | 252 | 5 (1–16) | 81% | 37% | 50% | 64% | n/a | n/a |
| Elrantamab * [35] | BCMA | 55 | 5 (2–14) | 91% | n/a | 64% | 64% | 12m: 60% | 17.1 (10.6–NE) |
| Teclistimab [10] | BCMA | 165 | 5 (2–14) | 78% | 30% | 63% | n/a | 11.3 (8.8–1.1) | 18.4 (14.9–NE) |
| HPN-217 * [36] | BCMA | 62 | 6 (2–19) | 76% | 42% | 57% | 77% | n/a | 12 (6–NE) * |
| Alnuctamab * [37] | BCMA | 68 | 4 (3–11) | 63% | 28% | 53% | 65% | n/a | n/a |
| Talquetamab [28] | GPRC5D | 288 | 5 (2–13) | 72% | 26% | 73% | 73% | 7.5 (5.7–9.2) | 9.3 (6.6–20) |
| RG6234 [38] | GPRC5D | 107 | 5 (2–15) | 67% | 39% | 67% | 67% | n/a | 10.5/12.5 month |
| Cevostamab [30] | FcRH5 | 53 | 6 (2–15) | 72% | 45% | 53% | 61% | n/a | n/a |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mikala, G.; Varga, G. Myeloma: A Lot of Progress, Still a Long Way to Go. Cancers 2021, 13, 6087. [Google Scholar] [CrossRef] [PubMed]
- Nunnelee, J.; Cottini, F.; Zhao, Q.; Faisal, M.S.; Elder, P.; Rosko, A.; Bumma, N.; Khan, A.; Devarakonda, S.; Benson, D.M.; et al. Improvement in Post-Autologous Stem Cell Transplant Survival of Multiple Myeloma Patients: A Long-Term Institutional Experience. Cancers 2022, 14, 2277. [Google Scholar] [CrossRef] [PubMed]
- Chacon, A.; Leleu, X.; Bobin, A. 30 Years of Improved Survival in Non-Transplant-Eligible Newly Diagnosed Multiple Myeloma. Cancers 2023, 15, 1929. [Google Scholar] [CrossRef] [PubMed]
- Seefat, M.R.; Cucchi, D.G.J.; Dirven, S.; Groen, K.; Zweegman, S.; Blommestein, H.M. A Systematic Review of Cost-Effectiveness Analyses of Novel Agents in the Treatment of Multiple Myeloma. Cancers 2021, 13, 5606. [Google Scholar] [CrossRef] [PubMed]
- Ackley, J.; Ochoa, M.A.; Ghoshal, D.; Roy, K.; Lonial, S.; Boise, L.H. Keeping Myeloma in Check: The Past, Present and Future of Immunotherapy in Multiple Myeloma. Cancers 2021, 13, 4787. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of Patients with Multiple Myeloma Refractory to CD38-Targeted Monoclonal Antibody Therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-F.; Anderson, K.C.; Tai, Y.-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef]
- Krejcik, J.; Barnkob, M.B.; Nyvold, C.G.; Larsen, T.S.; Barington, T.; Abildgaard, N. Harnessing the Immune System to Fight Multiple Myeloma. Cancers 2021, 13, 4546. [Google Scholar] [CrossRef]
- Martino, M.; Canale, F.A.; Alati, C.; Vincelli, I.D.; Moscato, T.; Porto, G.; Loteta, B.; Naso, V.; Mazza, M.; Nicolini, F.; et al. CART-Cell Therapy: Recent Advances and New Evidence in Multiple Myeloma. Cancers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Moreau, P.; Garfall, A.L.; van de Donk, N.W.C.J.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Martin, T.; Usmani, S.Z.; Berdeja, J.G.; Agha, M.; Cohen, A.D.; Hari, P.; Avigan, D.; Deol, A.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. J. Clin. Oncol. 2022, 41, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab Mafodotin for Relapsed or Refractory Multiple Myeloma (DREAMM-2): A Two-Arm, Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Boquoi, A.; Rings, V.; Mohring, A.; Savickaite, I.; Zukovs, R.; Strapatsas, J.; Nachtkamp, K.; Kobbe, G.; Germing, U.; Fenk, R. Opportunities for Participation in Randomized Controlled Trials for Patients with Multiple Myeloma: Trial Access Depends on Restrictive Eligibility Criteria and Patient Expectations. Cancers 2022, 14, 2147. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “Ggplot2”; 2021; Available online: https://CRAN.R-project.org/package=survminer (accessed on 3 May 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Hoffman, J.E.; Lipe, B.; Melear, J.; Liedtke, M.; Schroeder, M.A.; Niesvizky, R.; Strouse, C.; Yasenchak, C.A.; Green, D.J.; Sauer, J.; et al. Sea-BCMA Mono- and Combination Therapy in Patients with Relapsed/Refractory Multiple Myeloma: Updated Results of a Phase 1 Study (SGNBCMA-001). Blood 2022, 140, 10160–10162. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural History of Relapsed Myeloma, Refractory to Immunomodulatory Drugs and Proteasome Inhibitors: A Multicenter IMWG Study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Gonsalves, W.I. Melflufen for Multiple Myeloma: A Promise Unfulfilled? Lancet Haematol. 2022, 9, e82–e84. [Google Scholar] [CrossRef]
- Weisel, K.; Hopkins, T.G.; Fecteau, D.; Bao, W.; Quigley, C.; Jewell, R.C.; Nichols, M.; Opalinska, J. Dreamm-3: A Phase 3, Open-Label, Randomized Study to Evaluate the Efficacy and Safety of Belantamab Mafodotin (GSK2857916) Monotherapy Compared with Pomalidomide Plus Low-Dose Dexamethasone (Pom/Dex) in Participants with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 1900. [Google Scholar] [CrossRef]
- Atieh, T.; Atrash, S.; Ahmed, N.; Mohan, M.; Cui, W.; Shune, L.; Hajjar, S.; Mahmoudjafari, Z.; Quick, J.; Wishna, A.; et al. Belantamab in Combination with Dexamethasone in Patients with Triple-Class Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2022, 22, 912–919. [Google Scholar] [CrossRef]
- Iula, R.; De Novellis, D.; Trastulli, F.; Della Pepa, R.; Fontana, R.; Carobene, A.; Di Perna, M.; D’Ambrosio, A.; Romano, M.; Leone, A.; et al. Efficacy and Safety of Belantamab-Mafodotin in Triple-Refractory Multiple Myeloma Patients: A Multicentric Real-Life Experience. Front. Oncol. 2022, 12, 1026251. [Google Scholar] [CrossRef]
- Vaxman, I.; Abeykoon, J.; Dispenzieri, A.; Kumar, S.K.; Buadi, F.; Lacy, M.Q.; Dingli, D.; Hwa, Y.; Fonder, A.; Hobbs, M.; et al. “Real-Life” Data of the Efficacy and Safety of Belantamab Mafodotin in Relapsed Multiple Myeloma-the Mayo Clinic Experience. Blood Cancer J. 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.K.; Sidana, S.; Peres, L.C.; Colin Leitzinger, C.; Shune, L.; Shrewsbury, A.; Gonzalez, R.; Sborov, D.W.; Wagner, C.; Dima, D.; et al. Idecabtagene Vicleucel for Relapsed/Refractory Multiple Myeloma: Real-World Experience From the Myeloma CAR T Consortium. J. Clin. Oncol. 2023, 41, 2087–2097. [Google Scholar] [CrossRef]
- Leblay, N.; Maity, R.; Barakat, E.; McCulloch, S.; Duggan, P.; Jimenez-Zepeda, V.; Bahlis, N.J.; Neri, P. Cite-Seq Profiling of T Cells in Multiple Myeloma Patients Undergoing BCMA Targeting CAR-T or Bites Immunotherapy. Blood 2020, 136, 11–12. [Google Scholar] [CrossRef]
- Krishnan, A.; Nooka, A.K.; Chari, A.; Garfall, A.L.; Martin, T.G.; Nair, S.; Lin, X.; Qi, K.; Londhe, A.; Pei, L.; et al. Teclistamab versus Real-World Physician’s Choice of Therapy in Triple-Class Exposed Relapsed/Refractory Multiple Myeloma. J. Comp. Eff. Res. 2023, e220186. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.W.C.J.; Rodríguez-Otero, P.; Askari, E.; Mateos, M.-V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef]
- Mailankody, S.; Devlin, S.M.; Landa, J.; Nath, K.; Diamonte, C.; Carstens, E.J.; Russo, D.; Auclair, R.; Fitzgerald, L.; Cadzin, B.; et al. GPRC5D-Targeted CAR T Cells for Myeloma. N. Engl. J. Med. 2022, 387, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Richter, J.; Trudel, S.; Cohen, A.D.; Spencer, A.; Forsberg, P.A.; Laubach, J.P.; Thomas, S.K.; Bahlis, N.J.; Costa, L.J.; et al. Enduring Responses after 1-Year, Fixed-Duration Cevostamab Therapy in Patients with Relapsed/Refractory Multiple Myeloma: Early Experience from a Phase I Study. Blood 2022, 140, 4415–4417. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, Lenalidomide, Bortezomib, & Dexamethasone for Transplant-Eligible Newly Diagnosed Multiple Myeloma: GRIFFIN. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Costa, L.J.; Godby, K.N.; Ravi, G.; Giri, S.; Bal, S. Drug Class Refractoriness, Not Number of Prior Lines of Therapy (LOT), Properly Classify Patients with Relapsed and Refractory Multiple Myeloma (RRMM) Receiving Contemporary Regimens. Blood 2022, 140, 10084–10085. [Google Scholar] [CrossRef]
- D’Souza, A.; Shah, N.; Rodriguez, C.; Voorhees, P.M.; Weisel, K.; Bueno, O.F.; Pothacamury, R.K.; Freise, K.J.; Yue, S.; Ross, J.A.; et al. A Phase I First-in-Human Study of ABBV-383, a B-Cell Maturation Antigen × CD3 Bispecific T-Cell Redirecting Antibody, in Patients With Relapsed/Refractory Multiple Myeloma. J. Clin. Oncol. 2022, 40, 3576–3586. [Google Scholar] [CrossRef]
- Bumma, N.; Richter, J.; Brayer, J.; Zonder, J.A.; Dhodapkar, M.; Shah, M.R.; Hoffman, J.E.; Mawad, R.; Maly, J.J.; Lentzsch, S.; et al. Updated Safety and Efficacy of REGN5458, a BCMAxCD3 Bispecific Antibody, Treatment for Relapsed/Refractory Multiple Myeloma: A Phase 1/2 First-in-Human Study. Blood 2022, 140, 10140–10141. [Google Scholar] [CrossRef]
- Raje, N.; Bahlis, N.J.; Costello, C.; Dholaria, B.; Solh, M.; Levy, M.Y.; Tomasson, M.H.; Damore, M.A.; Jiang, S.; Basu, C.; et al. Elranatamab, a BCMA Targeted T-Cell Engaging Bispecific Antibody, Induces Durable Clinical and Molecular Responses for Patients with Relapsed or Refractory Multiple Myeloma. Blood 2022, 140, 388–390. [Google Scholar] [CrossRef]
- Abdallah, A.-O.; Cowan, A.J.; Leleu, X.; Touzeau, C.; Lipe, B.; Medvedova, E.; Costello, C.; Hillengass, J.; Bergsagel, P.L.; Mawad, R.; et al. Updated Interim Results from a Phase 1 Study of HPN217, a Half-Life Extended Tri-Specific T Cell Activating Construct (TriTAC®) Targeting B Cell Maturation Antigen (BCMA) for Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2022, 140, 7284–7285. [Google Scholar] [CrossRef]
- Wong, S.W.; Bar, N.; Paris, L.; Hofmeister, C.C.; Hansson, M.; Santoro, A.; Mateos, M.-V.; Rodríguez-Otero, P.; Lund, J.; Encinas, C.; et al. Alnuctamab (ALNUC; BMS-986349; CC-93269), a B-Cell Maturation Antigen (BCMA) x CD3 T-Cell Engager (TCE), in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Results from a Phase 1 First-in-Human Clinical Study. Blood 2022, 140, 400–402. [Google Scholar] [CrossRef]
- Carlo-Stella, C.; Mazza, R.; Manier, S.; Facon, T.; Yoon, S.-S.; Koh, Y.; Harrison, S.J.; Er, J.; Pinto, A.; Volzone, F.; et al. RG6234, a GPRC5DxCD3 T-Cell Engaging Bispecific Antibody, Is Highly Active in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Updated Intravenous (IV) and First Subcutaneous (SC) Results from a Phase I Dose-Escalation Study. Blood 2022, 140, 397–399. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrash, S.; Mammadzadeh, A.; Peng, F.; Alkharabsheh, O.; Afrough, A.; Cui, W.; Mahmoudjafari, Z.; Abdallah, A.-O.; Hashmi, H. Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy. Cancers 2023, 15, 2891. https://doi.org/10.3390/cancers15112891
Atrash S, Mammadzadeh A, Peng F, Alkharabsheh O, Afrough A, Cui W, Mahmoudjafari Z, Abdallah A-O, Hashmi H. Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy. Cancers. 2023; 15(11):2891. https://doi.org/10.3390/cancers15112891
Chicago/Turabian StyleAtrash, Shebli, Aytaj Mammadzadeh, Fulei Peng, Omar Alkharabsheh, Aimaz Afrough, Wei Cui, Zahra Mahmoudjafari, Al-Ola Abdallah, and Hamza Hashmi. 2023. "Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy" Cancers 15, no. 11: 2891. https://doi.org/10.3390/cancers15112891
APA StyleAtrash, S., Mammadzadeh, A., Peng, F., Alkharabsheh, O., Afrough, A., Cui, W., Mahmoudjafari, Z., Abdallah, A.-O., & Hashmi, H. (2023). Outcomes of Penta-Refractory Multiple Myeloma Patients Treated with or without BCMA-Directed Therapy. Cancers, 15(11), 2891. https://doi.org/10.3390/cancers15112891