Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pleomorphic Lobular Breast Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Quantification of sTILs
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. sTIL Distribution and PD-L1 Expression (22C3–SP142)
3.3. sTIL Distribution and PD-L1 (22C3–SP142) Expression According to Patient Characteristics
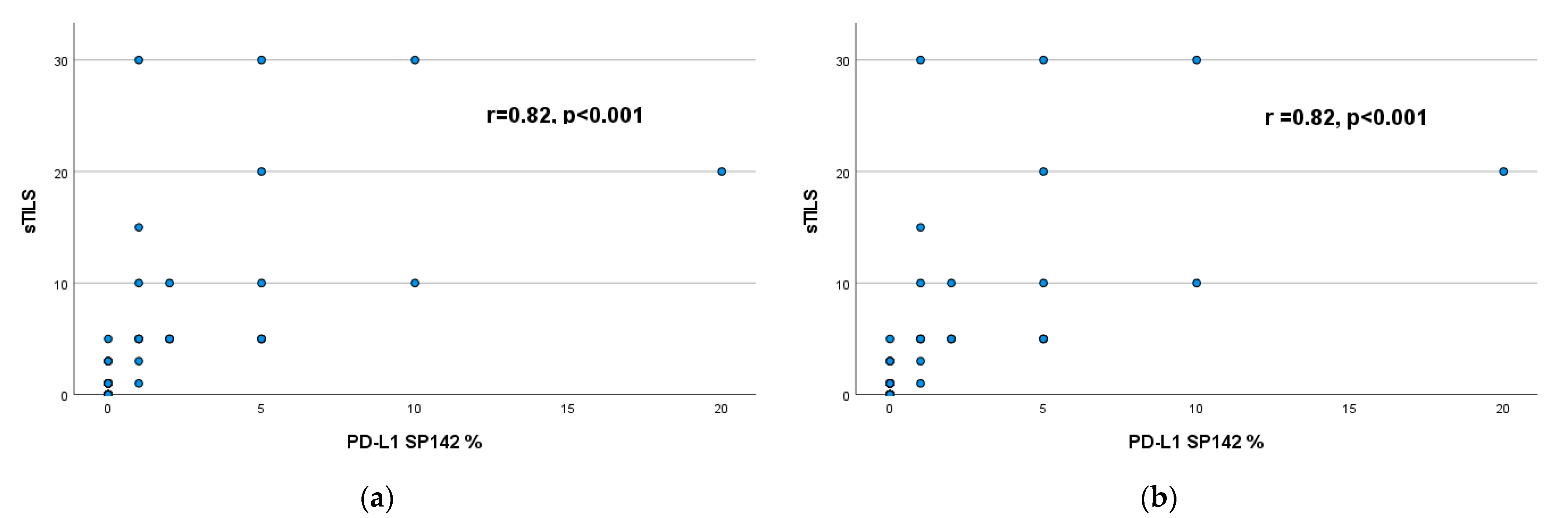
3.4. Correlation of sTILs with PD-L1 Expression (22C3–SP142)
3.5. Correlation between sTILs and PD-L1 (22C3–SP142) Expression and Patient Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.I.; Anderson, B.O.; Daling, J.R.; Moe, R.E. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 2003, 289, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Breast Tumours. In WHO Classification of Tumours, 5th ed.; WHO Classification of Tumours Series; WHO: Geneva, Switzerland, 2019; Volume 2, Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Breast-Tumours-2019 (accessed on 9 May 2023).
- Pramod, N.; Nigam, A.; Basree, M.; Mawalkar, R.; Mehra, S.; Shinde, N.; Tozbikian, G.; Williams, N.; Majumder, S.; Ramaswamy, B. Comprehensive Review of Molecular Mechanisms and Clinical Features of Invasive Lobular Cancer. Oncologist 2021, 26, e943–e953. [Google Scholar] [CrossRef] [PubMed]
- Delpech, Y.; Coutant, C.; Hsu, L.; Barranger, E.; Iwamoto, T.; Barcenas, C.H.; Hortobagyi, G.N.; Rouzier, R.; Esteva, F.J.; Pusztai, L. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br. J. Cancer 2013, 108, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Marmor, S.; Hui, J.Y.C.; Huang, J.L.; Kizy, S.; Beckwith, H.; Blaes, A.H.; Rueth, N.M.; Tuttle, T.M. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer 2017, 123, 3015–3021. [Google Scholar] [CrossRef]
- Pestalozzi, B.C.; Zahrieh, D.; Mallon, E.; Gusterson, B.A.; Price, K.N.; Gelber, R.D.; Holmberg, S.B.; Lindtner, J.; Snyder, R.; Thürlimann, B. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: Combined results of 15 International Breast Cancer Study Group clinical trials. J. Clin. Oncol. 2008, 26, 3006–3014. [Google Scholar] [CrossRef]
- Adachi, Y.; Ishiguro, J.; Kotani, H.; Hisada, T.; Ichikawa, M.; Gondo, N.; Yoshimura, A.; Kondo, N.; Hattori, M.; Sawaki, M. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 2016, 16, 248. [Google Scholar] [CrossRef]
- Korhonen, T.; Kuukasjärvi, T.; Huhtala, H.; Alarmo, E.L.; Holli, K.; Kallioniemi, A.; Pylkkänen, L. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast 2013, 22, 1119–1124. [Google Scholar] [CrossRef]
- Desmedt, C.; Salgado, R.; Fornili, M.; Pruneri, G.; Van den Eynden, G.; Zoppoli, G.; Rothé, F.; Buisseret, L.; Garaud, S.; Willard-Gallo, K. Immune Infiltration in Invasive Lobular Breast Cancer. J. Natl. Cancer Inst. 2018, 110, 768–776. [Google Scholar] [CrossRef]
- Tille, J.-C.; Vieira, A.F.; Saint-Martin, C.; Djerroudi, L.; Furhmann, L.; Bidard, F.-C.; Kirova, Y.; Tardivon, A.; Reyal, F.; Carton, M. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod. Pathol. 2020, 33, 2198–2207. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559. [Google Scholar] [CrossRef]
- Gao, G.; Wang, Z.; Qu, X.; Zhang, Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; De Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–455. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Salgado, R.; Sirtaine, N.; Jose, V.; Fumagalli, D.; Kellokumpu-Lehtinen, P.L.; Bono, P.; Kataja, V.; Desmedt, C. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Narendra, S.; Jenkins, S.M.; Khoor, A.; Nassar, A. Clinical outcome in pleomorphic lobular carcinoma: A case-control study with comparison to classic invasive lobular carcinoma. Ann. Diagn. Pathol. 2015, 19, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Karatas, F.; Erdem, G.U.; Hacioglu, B.; Altundag, K. Invasive Pleomorphic Lobular Histology Is an Adverse Prognostic Factor on Survival in Patients with Breast Cancer. Am. Surg. 2017, 83, 359–364. [Google Scholar] [CrossRef]
- Segar, J.M.; Pandey, R.; Farr, K.J.; Nagle, R.; Lebeau, L.; Gonzalez, V.J.; Chalasani, P. Clinicopathological and Molecular Characteristics of Pleomorphic Invasive Lobular Carcinoma. Int. J. Breast Cancer 2020, 2020, 8816824. [Google Scholar] [CrossRef]
- Haque, W.; Arms, A.; Verma, V.; Hatch, S.; Brian Butler, E.; Teh, B.S. Outcomes of pleomorphic lobular carcinoma versus invasive lobular carcinoma. Breast 2019, 43, 67–73. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- Stovgaard, E.S.; Dyhl-Polk, A.; Roslind, A.; Balslev, E.; Nielsen, D. PD-L1 expression in breast cancer: Expression in subtypes and prognostic significance: A systematic review. Breast Cancer Res. Treat. 2019, 174, 571–584. [Google Scholar] [CrossRef]
- Sabatier, R.; Finetti, P.; Mamessier, E.; Adelaide, J.; Chaffanet, M.; Raza Ali, H.; Viens, P.; Caldas, C.; Birnbaum, D.; Bertucci, F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449–5464. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Ryan, É.; Davey, M.S.; Lowery, A.J.; Miller, N.; Kerin, M.J. Clinicopathological and prognostic significance of programmed cell death ligand 1 expression in patients diagnosed with breast cancer: Meta-analysis. Br. J. Surg. 2021, 108, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Cirqueira, M.B.; Rodrigues Mendonça, C.; Noll, M.; Soares, L.R.; Auxiliadora, M.; Cysneiros, P.C.; Paulinelli, R.R.; Amaral, M.; Moreira, R.; Freitas-Junior, R. Prognostic Role of PD-L1 Expression in Invasive Breast Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 6090. [Google Scholar] [CrossRef] [PubMed]
- Dill, E.A.; Gru, A.A.; Atkins, K.A.; Friedman, L.A.; Moore, M.E.; Bullock, T.N.; Cross, J.V.; Dillon, P.M.; Mills, A.M. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am. J. Surg. Pathol. 2017, 41, 334–342. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an International TILS Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Droeser, R.; Zlobec, I.; Kilic, E.; Güth, U.; Heberer, M.; Spagnoli, G.; Oertli, D.; Tapia, C. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 2012, 12, 134. [Google Scholar] [CrossRef]
- Iorfida, M.; Maiorano, E.; Orvieto, E.; Maisonneuve, P.; Bottiglieri, L.; Rotmensz, N.; Montagna, E.; Dellapasqua, S.; Veronesi, P.; Galimberti, V. Invasive lobular breast cancer: Subtypes and outcome. Breast Cancer Res. Treat. 2012, 133, 713–723. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Adams, S.; Loibl, S.; Budczies, J.; Denkert, C.; Salgado, R. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: Clinical utility in an era of checkpoint inhibition. Ann. Oncol. 2021, 32, 1236–1244. [Google Scholar] [CrossRef]
- Park, J.H.; Jonas, S.F.; Bataillon, G.; Criscitiello, C.; Salgado, R.; Loi, S.; Viale, G.; Lee, H.J.; Dieci, M.V.; Kim, S.B. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1941–1949. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A. Association between CD8+ T-cell infiltration and breast cancer survival in 12439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef]
- Sanft, T.; Berkowitz, A.; Schroeder, B.; Hatzis, C.; Schnabel, C.A.; Brufsky, A.; Gustavsen, G.; Pusztai, L.; Londen, G. van A prospective decision-impact study incorporating Breast Cancer Index into extended endocrine therapy decision-making. Breast Cancer Manag. 2019, 8, BMT22. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.A.; Hitre, E. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sobral-Leite, M.; Van de Vijver, K.; Michaut, M.; van der Linden, R.; Hooijer, G.K.J.; Horlings, H.M.; Severson, T.M.; Mulligan, A.M.; Weerasooriya, N.; Sanders, J. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7, e1509820. [Google Scholar] [CrossRef] [PubMed]
- Ades, F.; Zardavas, D.; Bozovic-Spasojevic, I.; Pugliano, L.; Fumagalli, D.; De Azambuja, E.; Viale, G.; Sotiriou, C.; Piccart, M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J. Clin. Oncol. 2014, 32, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Uhercik, M.; Sanders, A.J.; Owen, S.; Davies, E.L.; Sharma, A.K.; Jiang, W.G.; Mokbel, K. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res. 2017, 37, 4249–4254. [Google Scholar]
- Beckers, R.K.; Selinger, C.I.; Vilain, R.; Madore, J.; Wilmott, J.S.; Harvey, K.; Holliday, A.; Cooper, C.L.; Robbins, E.; Gillett, D. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016, 69, 25–34. [Google Scholar] [CrossRef]
- Ali, H.R.; Glont, S.-E.; Blows, F.M.; Provenzano, E.; Dawson, S.-J.; Liu, B.; Hiller, L.; Dunn, J.; Poole, C.J.; Bowden, S. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann. Oncol. 2015, 26, 1488–1493. [Google Scholar] [CrossRef]
- Bae, S.B.; Cho, H.D.; Oh, M.H.; Lee, J.H.; Jang, S.H.; Hong, S.A.; Cho, J.; Kim, S.Y.; Han, S.W.; Lee, J.E. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J. Breast Cancer 2016, 19, 242–251. [Google Scholar] [CrossRef]
- Qin, T.; Zeng, Y.D.; Qin, G.; Xu, F.; Lu, J.B.; Fang, W.F.; Xue, C.; Zhan, J.H.; Zhang, X.K.; Zheng, Q.F. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015, 6, 33972–33981. [Google Scholar] [CrossRef]
- Mori, H.; Kubo, M.; Yamaguchi, R.; Nishimura, R.; Osako, T.; Arima, N.; Okumura, Y.; Okido, M.; Yamada, M.; Kai, M. The combination of PD-L1 expression and decreased tumorinfiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–15592. [Google Scholar] [CrossRef]
- Thompson, E.D.; Taube, J.M.; Asch-Kendrick, R.J.; Ogurtsova, A.; Xu, H.; Sharma, R.; Meeker, A.; Argani, P.; Emens, L.A.; Cimino-Mathews, A. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod. Pathol. 2017, 30, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.G.; Kim, S.K.; Shepherd, J.H.; Cha, Y.J.; Bae, S.J.; Kim, C.; Jeong, J.; Perou, C.M. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res. Treat. 2021, 188, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.S.; Ed Erique Penault-Llorca, F.; Reis-Filho, J.S.; Deurloo, R.; Siziopikou, K.P.; D’arrigo, C.; Viale, G. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. J. Natl. Cancer Inst. 2021, 114, 664–674. [Google Scholar] [CrossRef]
- Huang, X.; Ding, Q.; Guo, H.; Gong, Y.; Zhao, J.; Zhao, M.; Sui, D.; Wu, Y.; Chen, H.; Liu, H. Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum. Pathol. 2021, 108, 42–50. [Google Scholar] [CrossRef]
- Shah, A.N.; Flaum, L.; Helenowski, I.; Santa-Maria, C.A.; Jain, S.; Rademaker, A.; Nelson, V.; Tsarwhas, D.; Cristofanilli, M.; Gradishar, W. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J. Immunother. Cancer 2020, 8, e000173. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Barroso-Sousa, R.; Keenan, T.; Li, T.; Trippa, L.; Vaz-Luis, I.; Wulf, G.; Spring, L.; Sinclair, N.F.; Andrews, C. Effect of Eribulin With or Without Pembrolizumab on Progression-Free Survival for Patients With Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1598–1605. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef]
| All Cases | ER+/HER2- | TNBC | HER2+ | p | |
|---|---|---|---|---|---|
| Total number n (%) | 66 | 54 (82) | 5 (8) | 7 (10) | |
| Age (years) | 0.61 | ||||
| Mean | 58 | 58 | 61 | 62 | |
| Median | 59 | 59.5 | 57 | 62 | |
| Tumor size n (%) | 0.58 | ||||
| pT1 | 22 (33) | 16 (30) | 2 (40) | 4 (57) | |
| pT2 | 33 (50) | 29 (54) | 2 (40) | 2 (29) | |
| pT3 | 11 (17) | 9 (6) | 1 (20) | 1 (14) | |
| Tumor size (mm) | 34 | 35 | 42 | 26 | |
| Grade (%) | 0.17 | ||||
| G2 | 30 (45) | 27 (50) | 2 (40) | 1 (15) | |
| G3 | 36 (55) | 27 (50) | 3 (60) | 6 (85) | |
| LVSI n (%) | 0.33 | ||||
| Negative | 40 (61) | 35 (65) | 2 (40) | 3 (43) | |
| Positive | 26 (39) | 19 (35) | 3 (60) | 4 (57) | |
| Lymph node metastases n (%) | 0.014 | ||||
| pN0 | 38 (60) | 31 (57) | 1 (20) | 6 (85) | |
| pN1 | 17 (25) | 15 (28) | 2 (40) | 0 | |
| pN2 | 7 (10) | 7 (13) | 0 | 0 | |
| pN3 | 4 (5) | 1 (2) | 2 (40) | 1 (15) | |
| Ki67 n (%) | 0.21 | ||||
| <15 | 18 (37) | 17 (42) | 0 (0) | 1 (16) | |
| ≥15 | 31 (63) | 23 (58) | 3 (100) | 5 (84) | |
| Missing | 17 | ||||
| Distant metastasis (%) | |||||
| Bone | 5 | 3 (50) | 1 (50) | 1 (50) | 0.3 |
| Liver | 4 | 2 (34) | 1 (50) | 1 (50) | |
| Brain | 1 | 1 (16) | 0 | 0 | |
| All Cases | ER+/HER2- | TNBC | HER2+ | p | |
|---|---|---|---|---|---|
| sTILs n (%) | 0.26 | ||||
| 0 | 24 (36) | 18 (33) | 3 (60) | 3 (43) | |
| <5 | 19 (29) | 18 (33) | 0 | 1 (14) | |
| 5–9 | 11 (17) | 10 (18) | 1 (20) | 0 | |
| ≥10 | 12 (18) | 8 (16) | 1 (20) | 3 (43) | |
| PD-L1 SP142 ICs n (%) | 0.48 | ||||
| <1% | 38 (64) | 32 (68) | 3 (60) | 3 (43) | |
| ≥1% | 21 (36) | 15 (32) | 2 (40) | 4 (57) | |
| Non-assessable | 7 | ||||
| PD-L1 22C3 CPS n (%) | 0.58 | ||||
| <1 | 42 (72) | 35 (76) | 3 (60) | 4 (57) | |
| ≥1 | 13 (23) | 9 (20) | 2 (40) | 2 (29) | |
| ≥10 | 3 (5) | 2 (4) | 0 | 1 (14) | |
| Non-assessable | 8 |
| sTILs | PD-L1 SP142 | PD-L1 22C3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 1–4% | 5–9% | ≥10% | <1% | ≥1% | <1 | ≥1 | ≥10 | |
| Age (years) | |||||||||
| Mean | 57 | 61 | 57 | 58.5 | 57 | 60 | 58 | 56 | 66 |
| Tumor (mm) | 33 | 33 | 35 | 36 | 34 | 34.3 | 36 | 30 | 3 |
| Stage, n (%) | |||||||||
| pT1 | 9 (37) | 6 (32) | 4 (36) | 3 (25) | 13 (34) | 8 (38) | 14 (33) | 6 (46) | 0 |
| pT2 | 12 (50) | 9 (47) | 5 (46) | 7 (58) | 19 (50) | 9 (43) | 20 (48) | 5 (38) | 3 (100) |
| pT3 | 3 (13) | 4 (21) | 2 (18) | 2 (17) | 6 (16) | 4 (19) | 8 (19) | 2 (15) | 0 |
| Grade, n (%) | |||||||||
| G2 | 12 (50) | 9 (47) | 3 (27) | 6 (50) | 19 (50) | 6 (28) | 18 (43) | 5 (38) | 1 (33) |
| G3 | 12 (50) | 10 (53) | 8 (73) | 6 (50) | 19 (50) | 15 (72) | 24 (57) | 8 (62) | 2 (67) |
| LVSI, n (%) | |||||||||
| Negative | 16 (66) | 11 (58) | 7 (64) | 6 (50) | 24 (63) | 11 (52) | 27 (64) | 5 (38) | 2 (66) |
| Positive | 8 (34) | 8 (42) | 4 (36) | 6 (50) | 14 (37) | 10 (48) | 15 (34) | 8 (63) | 1 (34) |
| Lymph node metastasis, n (%) | |||||||||
| pN0 | 16 (67) | 10 (53) | 6 (60) | 5 (42) | 22 (58) | 11 (55) | 25 (60) | 5 (42) | 2 (66) |
| pN1 | 4 (17) | 6 (32) | 3 (30) | 4 (33) | 9 (24) | 6 (30) | 10 (24) | 4 (33) | 1 (34) |
| pN2 | 3 (12) | 2 (10) | 1 (10) | 1 (8) | 5 (13) | 1 (5) | 5 (12) | 1 (8) | 0 |
| pN3 | 1 (4) | 1 (5) | 0 | 2 (17) | 2 (5) | 2 (10) | 2 (5) | 2 (17) | 0 |
| HER2, n (%) | |||||||||
| Negative | 21 (87) | 18 (95) | 11 (100) | 9 (75) | 35 (92) | 17 (81) | 38 (90) | 11 (85) | 2 (67) |
| Positive | 3 (13) | 1 (5) | 0 | 3 (25) | 3 (8) | 4 (19) | 4 (10) | 2 (15) | 1 (33) |
| Ki67, n (%) | |||||||||
| <15 | 7 (50) | 7 (41) | 2 (18) | 2 (25) | 13 (45) | 2 (12) * | 13 (41) | 3 (18) | 0 |
| ≥15 | 7 (50) | 10 (59) | 8 (82) | 6 (75) | 16 (55) | 15 (88) * | 19 (59) | 9 (82) | 3 (100) |
| (a) | ||||
| <1% | ≥1% | |||
| n(%) | n(%) | |||
| sTILs | 0% | 21 (55) | 0 | |
| <5% | 16 (42) | 2 (10) | ||
| 5–9% | 1 (3) | 9 (43) | ||
| ≥10% | 0 | 10 (47) | ||
| (b) | ||||
| <1 | ≥1 | ≥10 | ||
| n(%) | n(%) | n(%) | ||
| sTILs | 0% | 20 (48) | 0 | 0 |
| <5% | 16 (38) | 2 (15) | 0 | |
| 5–9% | 4 (9) | 5 (39) | 1 (34) | |
| ≥10% | 2 (5) | 6 (46) | 2 (66) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göker, M.; Deblaere, S.; Denys, H.; Vergauwen, G.; Naert, E.; Veldeman, L.; Monten, C.; Van den Broecke, R.; Van Dorpe, J.; Braems, G.; et al. Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pleomorphic Lobular Breast Carcinoma. Cancers 2023, 15, 2894. https://doi.org/10.3390/cancers15112894
Göker M, Deblaere S, Denys H, Vergauwen G, Naert E, Veldeman L, Monten C, Van den Broecke R, Van Dorpe J, Braems G, et al. Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pleomorphic Lobular Breast Carcinoma. Cancers. 2023; 15(11):2894. https://doi.org/10.3390/cancers15112894
Chicago/Turabian StyleGöker, Menekse, Stephanie Deblaere, Hannelore Denys, Glenn Vergauwen, Eline Naert, Liv Veldeman, Chris Monten, Rudy Van den Broecke, Jo Van Dorpe, Geert Braems, and et al. 2023. "Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pleomorphic Lobular Breast Carcinoma" Cancers 15, no. 11: 2894. https://doi.org/10.3390/cancers15112894
APA StyleGöker, M., Deblaere, S., Denys, H., Vergauwen, G., Naert, E., Veldeman, L., Monten, C., Van den Broecke, R., Van Dorpe, J., Braems, G., & Van de Vijver, K. (2023). Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pleomorphic Lobular Breast Carcinoma. Cancers, 15(11), 2894. https://doi.org/10.3390/cancers15112894






