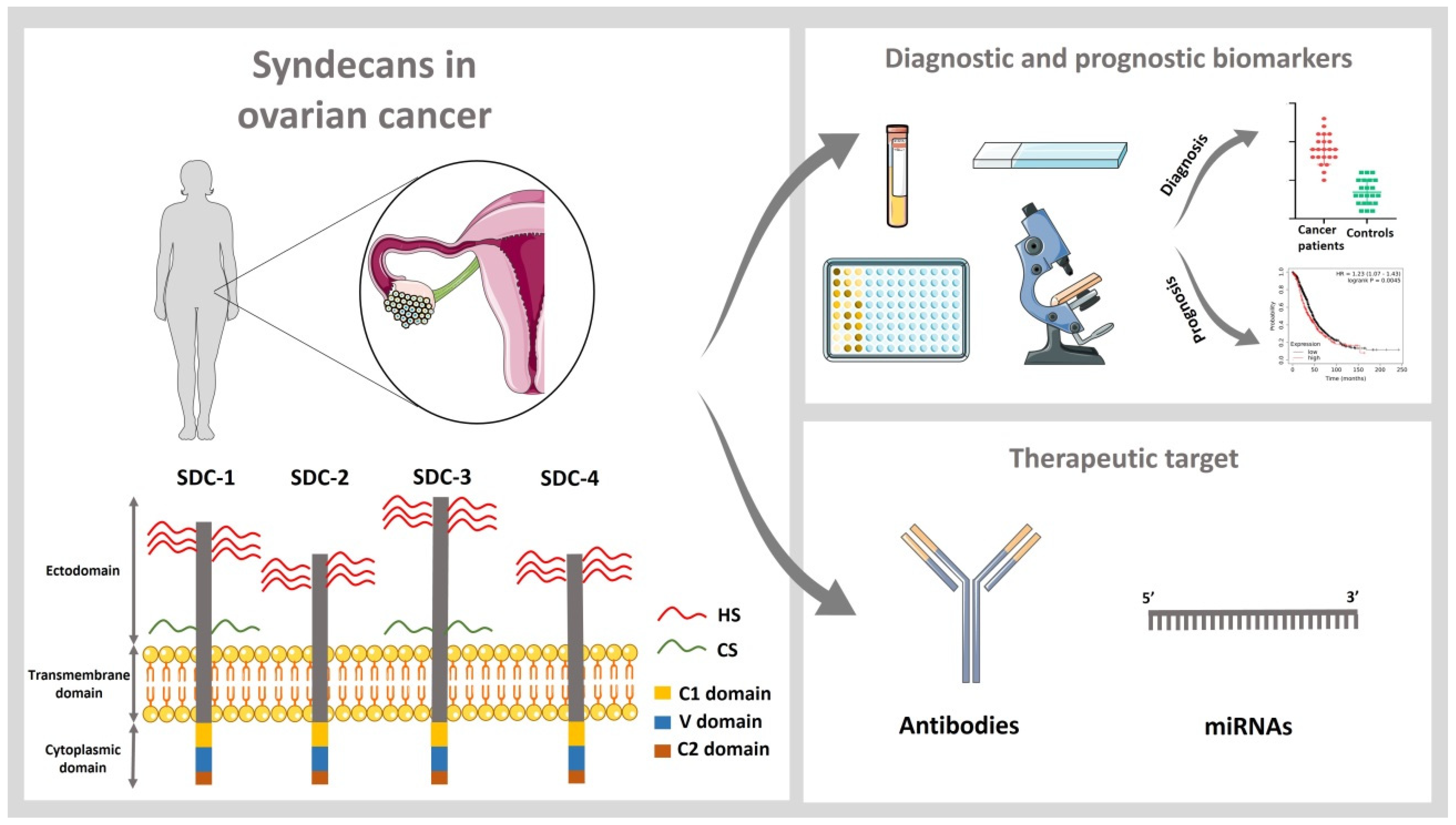
Role of Syndecans in Ovarian Cancer: New Diagnostic and Prognostic Biomarkers and Potential Therapeutic Targets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Syndecans as Diagnostic and Prognostic Biomarkers in Ovarian Cancer
3. Pathophysiological Role of Syndecans in Ovarian Cancer Tumorigenesis
4. Syndecans as Therapeutic Targets in Ovarian Cancer
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Atallah, G.A.; Abd Aziz, N.H.; Teik, C.K.; Shafiee, M.N.; Kampan, N.C. New Predictive Biomarkers for Ovarian Cancer. Diagnostics 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Passi, A.; Gotte, M.; Rousselle, P.; Vlodavsky, I. Extracellular matrix-based cancer targeting. Trends Mol. Med. 2021, 27, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat Res. Commun. 2021, 27, 100312. [Google Scholar] [CrossRef]
- Bernfield, M.; Gotte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brezillon, S.; Gotte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Onyeisi, J.O.S.; Lopes, C.C.; Gotte, M. Role of syndecan-4 in breast cancer pathophysiology. Am. J. Physiol. Cell Physiol. 2022, 323, C1345–C1354. [Google Scholar] [CrossRef] [PubMed]
- Yablecovitch, D.; Ben-Horin, S.; Picard, O.; Yavzori, M.; Fudim, E.; Nadler, M.; Levy, I.; Sakhnini, E.; Lang, A.; Engel, T.; et al. Serum Syndecan-1: A Novel Biomarker for Pancreatic Ductal Adenocarcinoma. Clin. Transl. Gastroenterol. 2022, 13, e00473. [Google Scholar] [CrossRef]
- Jechorek, D.; Haeusler-Pliske, I.; Meyer, F.; Roessner, A. Diagnostic value of syndecan-4 protein expression in colorectal cancer. Pathol. Res. Pract. 2021, 222, 153431. [Google Scholar] [CrossRef]
- Santos, N.J.; Barquilha, C.N.; Barbosa, I.C.; Macedo, R.T.; Lima, F.O.; Justulin, L.A.; Barbosa, G.O.; Carvalho, H.F.; Felisbino, S.L. Syndecan Family Gene and Protein Expression and Their Prognostic Values for Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 8669. [Google Scholar] [CrossRef]
- Hassan, N.; Greve, B.; Espinoza-Sanchez, N.A.; Gotte, M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell Signal 2021, 77, 109822. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.C.; Oegema, T.R., Jr.; Skubitz, K.M.; Pambuccian, S.E.; Grindle, S.M.; Skubitz, A.P. Cell membrane glycosylation mediates the adhesion, migration, and invasion of ovarian carcinoma cells. Clin. Exp. Metastasis 2003, 20, 143–152. [Google Scholar] [CrossRef]
- Davies, E.J.; Blackhall, F.H.; Shanks, J.H.; David, G.; McGown, A.T.; Swindell, R.; Slade, R.J.; Martin-Hirsch, P.; Gallagher, J.T.; Jayson, G.C. Distribution and clinical significance of heparan sulfate proteoglycans in ovarian cancer. Clin. Cancer Res. 2004, 10, 5178–5186. [Google Scholar] [CrossRef] [Green Version]
- Salani, R.; Neuberger, I.; Kurman, R.J.; Bristow, R.E.; Chang, H.W.; Wang, T.L.; Shih Ie, M. Expression of extracellular matrix proteins in ovarian serous tumors. Int. J. Gynecol. Pathol. 2007, 26, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Kusumoto, T.; Kodama, J.; Seki, N.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Clinical significance of syndecan-1 and versican expression in human epithelial ovarian cancer. Oncol. Rep. 2010, 23, 917–925. [Google Scholar]
- Guo, Q.; Yang, X.; Ma, Y.; Ma, L. Syndecan-1 serves as a marker for the progression of epithelial ovarian carcinoma. Eur. J. Gynaecol. Oncol. 2015, 36, 506–513. [Google Scholar]
- Hasby, E.A. Mammary serine protease inhibitor and CD138 immunohistochemical expression in ovarian serous and clear cell carcinomas. Tumour Biol. 2016, 37, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirstrom, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klaschik, S.; Gehlen, J.; Neumann, C.; Keyver-Paik, M.D.; Soehle, M.; Frede, S.; Velten, M.; Hoeft, A.; Hilbert, T. Network of Mediators for Vascular Inflammation and Leakage Is Dysbalanced during Cytoreductive Surgery for Late-Stage Ovarian Cancer. Mediators Inflamm. 2019, 2019, 5263717. [Google Scholar] [CrossRef] [Green Version]
- Kulbe, H.; Otto, R.; Darb-Esfahani, S.; Lammert, H.; Abobaker, S.; Welsch, G.; Chekerov, R.; Schafer, R.; Dragun, D.; Hummel, M.; et al. Discovery and Validation of Novel Biomarkers for Detection of Epithelial Ovarian Cancer. Cells 2019, 8, 713. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Han, Y.; Kim, S.I.; Lee, J.; Jo, H.; Wang, W.; Cho, U.; Park, W.Y.; Rando, T.A.; Dhanasekaran, D.N.; et al. Computational modeling of malignant ascites reveals CCL5-SDC4 interaction in the immune microenvironment of ovarian cancer. Mol. Carcinog. 2021, 60, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Wu, Z.; Zheng, J.; Ji, L. Integrated Bioinformatics Analysis for Identification of the Hub Genes Linked with Prognosis of Ovarian Cancer Patients. Comput. Math Methods Med. 2022, 2022, 5113447. [Google Scholar] [CrossRef]
- Ween, M.P.; Oehler, M.K.; Ricciardelli, C. Role of versican, hyaluronan and CD44 in ovarian cancer metastasis. Int. J. Mol. Sci. 2011, 12, 1009–1029. [Google Scholar] [CrossRef] [Green Version]
- Nash, M.A.; Loercher, A.E.; Freedman, R.S. In vitro growth inhibition of ovarian cancer cells by decorin: Synergism of action between decorin and carboplatin. Cancer Res. 1999, 59, 6192–6196. [Google Scholar]
- Lanczky, A.; Gyorffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef]
- Marques, C.; Reis, C.A.; Vivès, R.R.; Magalhães, A. Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression. Front Oncol. 2021, 11, 778752. [Google Scholar] [CrossRef]
- Hassan, N.; Efing, J.; Kiesel, L.; Bendas, G.; Götte, M. The Tissue Factor Pathway in Cancer: Overview and Role of Heparan Sulfate Proteoglycans. Cancers 2023, 15, 1524. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Chen, H.J.; Chen, L.Y.; Chao, T.K.; Lin, W.Y.; Liew, P.L.; Su, P.H.; Weng, Y.C.; Wang, Y.C.; Liao, C.C.; et al. Epigenetic loss of heparan sulfate 3-O-sulfation sensitizes ovarian carcinoma to oncogenic signals and predicts prognosis. Int. J. Cancer 2018, 143, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.C.; Rushton, G.; Jayson, G.C.; Avizienyte, E. Ovarian cancer cell heparan sulfate 6-O-sulfotransferases regulate an angiogenic program induced by heparin-binding epidermal growth factor (EGF)-like growth factor/EGF receptor signaling. J. Biol. Chem. 2014, 289, 10488–10501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.H.; Huang, Y.-J.; Lu, K.H.; Liu, Z.; Mills, G.B.; Wei, Q.; Wang, L.-E. Polymorphisms in the SULF1 gene are associated with early age of onset and survival of ovarian cancer. J. Exp. Clin. Cancer Res. 2011, 30, 5. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.; Yang, J.; Wang, D.; Cao, L.; Tan, W.; Qian, H.; Sun, B.; Qian, Q.; Yin, Z.; Wu, M.; et al. hSulf-1 gene exhibits anticancer efficacy through negatively regulating VEGFR-2 signaling in human cancers. PLoS ONE 2011, 6, e23274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Chien, J.; Staub, J.; Avula, R.; Greene, E.L.; Matthews, T.A.; Smith, D.I.; Kaufmann, S.H.; Roberts, L.R.; Shridhar, V. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J. Biol. Chem. 2003, 278, 23107–23117. [Google Scholar] [CrossRef] [Green Version]
- Davidson, B.; Shafat, I.; Risberg, B.; Ilan, N.; Trope, C.G.; Vlodavsky, I.; Reich, R. Heparanase expression correlates with poor survival in metastatic ovarian carcinoma. Gynecol. Oncol. 2007, 104, 311–319. [Google Scholar] [CrossRef]
- Zheng, H.; Ruan, J.; Zhao, P.; Chen, S.; Pan, L.; Liu, J. Heparanase is involved in proliferation and invasion of ovarian cancer cells. Cancer Biomark. 2015, 15, 525–534. [Google Scholar] [CrossRef]
- Kokenyesi, R. Ovarian carcinoma cells synthesize both chondroitin sulfate and heparan sulfate cell surface proteoglycans that mediate cell adhesion to interstitial matrix. J. Cell. Biochem. 2001, 83, 259–270. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Kobayashi, H.; Yagyu, T.; Wakahara, K.; Kondo, T.; Kurita, N.; Sekino, H.; Inagaki, K.; Suzuki, M.; Kanayama, N.; et al. Reduced syndecan-1 expression stimulates heparin-binding growth factor-mediated invasion in ovarian cancer cells in a urokinase-independent mechanism. Oncol. Rep. 2005, 14, 449–457. [Google Scholar] [CrossRef]
- Nikolova, V.; Koo, C.Y.; Ibrahim, S.A.; Wang, Z.; Spillmann, D.; Dreier, R.; Kelsch, R.; Fischgräbe, J.; Smollich, M.; Rossi, L.H.; et al. Differential roles for membrane-bound and soluble syndecan-1 (CD138) in breast cancer progression. Carcinogenesis 2009, 30, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.A.; Yip, G.W.; Stock, C.; Pan, J.W.; Neubauer, C.; Poeter, M.; Pupjalis, D.; Koo, C.Y.; Kelsch, R.; Schüle, R.; et al. Targeting of syndecan-1 by microRNA miR-10b promotes breast cancer cell motility and invasiveness via a Rho-GTPase- and E-cadherin-dependent mechanism. Int. J. Cancer 2012, 131, E884–E896. [Google Scholar] [CrossRef]
- Whitworth, M.K.; Backen, A.C.; Clamp, A.R.; Wilson, G.; McVey, R.; Friedl, A.; Rapraeger, A.C.; David, G.; McGown, A.; Slade, R.J.; et al. Regulation of fibroblast growth factor-2 activity by human ovarian cancer tumor endothelium. Clin. Cancer Res. 2005, 11, 4282–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumbarello, D.A.; Temple, J.; Brenton, J.D. ss3 integrin modulates transforming growth factor beta induced (TGFBI) function and paclitaxel response in ovarian cancer cells. Mol. Cancer 2012, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.A.; Mills, A.D.; Ibrahim, A.E.; Temple, J.; Blenkiron, C.; Vias, M.; Massie, C.E.; Iyer, N.G.; McGeoch, A.; Crawford, R.; et al. The extracellular matrix protein TGFBI induces microtubule stabilization and sensitizes ovarian cancers to paclitaxel. Cancer Cell 2007, 12, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Kato, N.; Kurotaki, H.; Uchigasaki, S.; Fukase, M.; Kurose, A. Ovarian clear cell carcinoma with plasma cell-rich inflammatory stroma: A clear cell carcinoma subgroup with distinct clinicopathological features. Histopathology 2016, 68, 588–595. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, X.; Abasi, A.; Dai, Y.; Wu, R.; Zhang, T.; Li, K.; Yan, M.; Huang, X. LncRNA TRPM2-AS promotes ovarian cancer progression and cisplatin resistance by sponging miR-138-5p to release SDC3 mRNA. Aging 2021, 13, 6832–6848. [Google Scholar] [CrossRef]
- Espinoza-Sanchez, N.A.; Troschel, F.; Greve, B.; Götte, M. Proteoglycan Expression Studied by MicroRNAs. Methods Mol. Biol. 2023, 2619, 273–292. [Google Scholar]
- Hillemeyer, L.; Espinoza-Sanchez, N.A.; Greve, B.; Hassan, N.; Chelariu-Raicu, A.; Kiesel, L.; Gotte, M. The Cell Surface Heparan Sulfate Proteoglycan Syndecan-3 Promotes Ovarian Cancer Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5793. [Google Scholar] [CrossRef]
- Vitale, D.; Kumar Katakam, S.; Greve, B.; Jang, B.; Oh, E.S.; Alaniz, L.; Götte, M. Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. FEBS J. 2019, 286, 2870–2882. [Google Scholar] [CrossRef] [Green Version]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef] [PubMed]
- Ponandai-Srinivasan, S.; Saare, M.; Boggavarapu, N.R.; Frisendahl, C.; Ehrstrom, S.; Riethmuller, C.; Garcia-Uribe, P.A.; Rettkowski, J.; Iyengar, A.; Salumets, A.; et al. Syndecan-1 modulates the invasive potential of endometrioma via TGF-beta signalling in a subgroup of women with endometriosis. Hum. Reprod. 2020, 35, 2280–2293. [Google Scholar] [CrossRef] [PubMed]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Bandari, S.K.; Purushothaman, A.; Ramani, V.C.; Brinkley, G.J.; Chandrashekar, D.S.; Varambally, S.; Mobley, J.A.; Zhang, Y.; Brown, E.E.; Vlodavsky, I.; et al. Chemotherapy induces secretion of exosomes loaded with heparanase that degrades extracellular matrix and impacts tumor and host cell behavior. Matrix Biol. 2018, 65, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, J.; Ruan, D.; Bian, C. The diagnostic and therapeutic prospects of exosomes in ovarian cancer. BJOG, 2023; early view. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Sawada, K.; Kimura, T. Pathophysiological Role and Potential Therapeutic Exploitation of Exosomes in Ovarian Cancer. Cells 2020, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Helpman, L.; Katz, B.Z.; Safra, T.; Schreiber, L.; Levine, Z.; Nemzer, S.; Kinar, Y.; Grisaru, D. Systematic antigenic profiling of hematopoietic antigens on ovarian carcinoma cells identifies membrane proteins for targeted therapy development. Am. J. Obstet. Gynecol. 2009, 201, 196.e1–196.e7. [Google Scholar] [CrossRef]
- Gashaw, I.; Ellinghaus, P.; Sommer, A.; Asadullah, K. What makes a good drug target? Drug Discov Today 2011, 16, 1037–1043. [Google Scholar] [CrossRef]
- Guo, T.; Yu, W.; Lv, S.; Zhang, C.; Tian, Y. MiR-302a inhibits the tumorigenicity of ovarian cancer cells by suppression of SDC1. Int. J. Clin. Exp. Pathol. 2015, 8, 4869–4880. [Google Scholar]
- Orecchia, P.; Conte, R.; Balza, E.; Petretto, A.; Mauri, P.; Mingari, M.C.; Carnemolla, B. A novel human anti-syndecan-1 antibody inhibits vascular maturation and tumour growth in melanoma. Eur. J. Cancer 2013, 49, 2022–2033. [Google Scholar] [CrossRef]
- Orecchia, P.; Conte, R.; Balza, E.; Pietra, G.; Mingari, M.C.; Carnemolla, B. Targeting Syndecan-1, a molecule implicated in the process of vasculogenic mimicry, enhances the therapeutic efficacy of the L19-IL2 immunocytokine in human melanoma xenografts. Oncotarget 2015, 6, 37426–37442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orecchia, P.; Balza, E.; Pietra, G.; Conte, R.; Bizzarri, N.; Ferrero, S.; Mingari, M.C.; Carnemolla, B. L19-IL2 Immunocytokine in Combination with the Anti-Syndecan-1 46F2SIP Antibody Format: A New Targeted Treatment Approach in an Ovarian Carcinoma Model. Cancers 2019, 11, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, R.F.; do Canto, L.M.; Abildgaard, C.; Aagaard, M.M.; Tronhjem, M.S.; Waldstrom, M.; Jensen, L.H.; Steffensen, K.D.; Rogatto, S.R. Single-cell and bulk RNA sequencing reveal ligands and receptors associated with worse overall survival in serous ovarian cancer. Cell Commun. Signal. 2022, 20, 176. [Google Scholar] [CrossRef]
- Connor, J.P.; Felder, M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor 3 (DcR3) and is associated with platinum resistance. Gynecol. Oncol. 2008, 111, 330–335. [Google Scholar] [CrossRef]
- Connor, J.P.; Felder, M.; Kapur, A.; Onujiogu, N. DcR3 binds to ovarian cancer via heparan sulfate proteoglycans and modulates tumor cells response to platinum with corresponding alteration in the expression of BRCA1. BMC Cancer 2012, 12, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| N | Type of Sample | Detection | Relevant Finding | Reference |
|---|---|---|---|---|
| 50 control ovaries 20 primary OCs 17 omental metastases 7 normal omenta | Frozen tissue | Gene expression | SDC1 upregulated in ovarian carcinoma tumor vs. normal ovary and in secondary omental metastases vs. normal omenta. | Casey RC et al., 2003 [16] |
| 115 EOC patients 10 benign epithelial ovarian tumor patients 12 controls | FFPE tissue | IHC | SDC2, -3, and -4 were expressed in both normal ovary and benign and malignant ovarian tumors. Negative expression of SDC1 in controls, positive in tumors. Presence of stromal SDC1 and its intensity were associated with poor OS and PFS. | Davies EJ et al., 2004 [17] |
| 138 EOC patients 17 atypical proliferative serous tumors 22 ovarian serous cystadenomas 12 controls | FFPE tissue | RT-qPCR IHC | SCD1 expression (mRNA and protein) upregulated in OC samples. | Salani R. et al., 2007 [18] |
| 111 patients | FFPE tissue | IHC | Epithelial SDC1: Lower expression in patients with advanced disease. Higher PFS in patients with negative expression. Stromal SDC1: Higher expression in patients with advanced disease. Lower PFS and OS in patients with high expression compared to patients with low expression. | Kusumoto T et al., 2010 [19] |
| 26 EOC patients 5 borderline 27 benign 2 controls | FFPE tissue | RT-qPCR IHC | Negative expression of SDC1 in controls, positive in tumors, and in borderline samples. Expression of both syndecan-1 and its mRNA detected at the original site of the tumor and in the metastatic foci. | Guo Q et al., 2015 [20] |
| 41 EOC patients | FFPE tissue | IHC | SDC1 expression higher in HGSC and clear cell carcinoma compared to LGSC. SDC1 expression correlated significantly to FIGO stage. | Hasby E.A. 2016 [21] |
| 154 EOC patients 38 benign 33 omental metastases | FFPE tissue (TMAs) | IHC | High CD20 and SDC1 expression correlated significantly with high tumor grade. High SDC1 expression correlates with a poor OS and with poor ovarian cancer-specific survival. | Lundgren et al., 2016 [22] |
| 26 late-stage ovarian cancer patients (pre- and post-cytoreductive surgery) | Serum | Multiplex array | SDC1 was significantly increased in the post-surgery samples compared to baseline conditions. | Klaschik S et al., 2019 [23] |
| Screening: 3 datasets from GEO database (GSE29156, GSE40595, and GSE14407) Validation: 10 EOC patients 10 benign | Tissue Blood | Screening: In silico analysis Validation: Gene expression analysis (custom designed mRNA set containing 48 genes) | SDC3 was confirmed as potential biomarker. | Kulbe H et al., 2019 [24] |
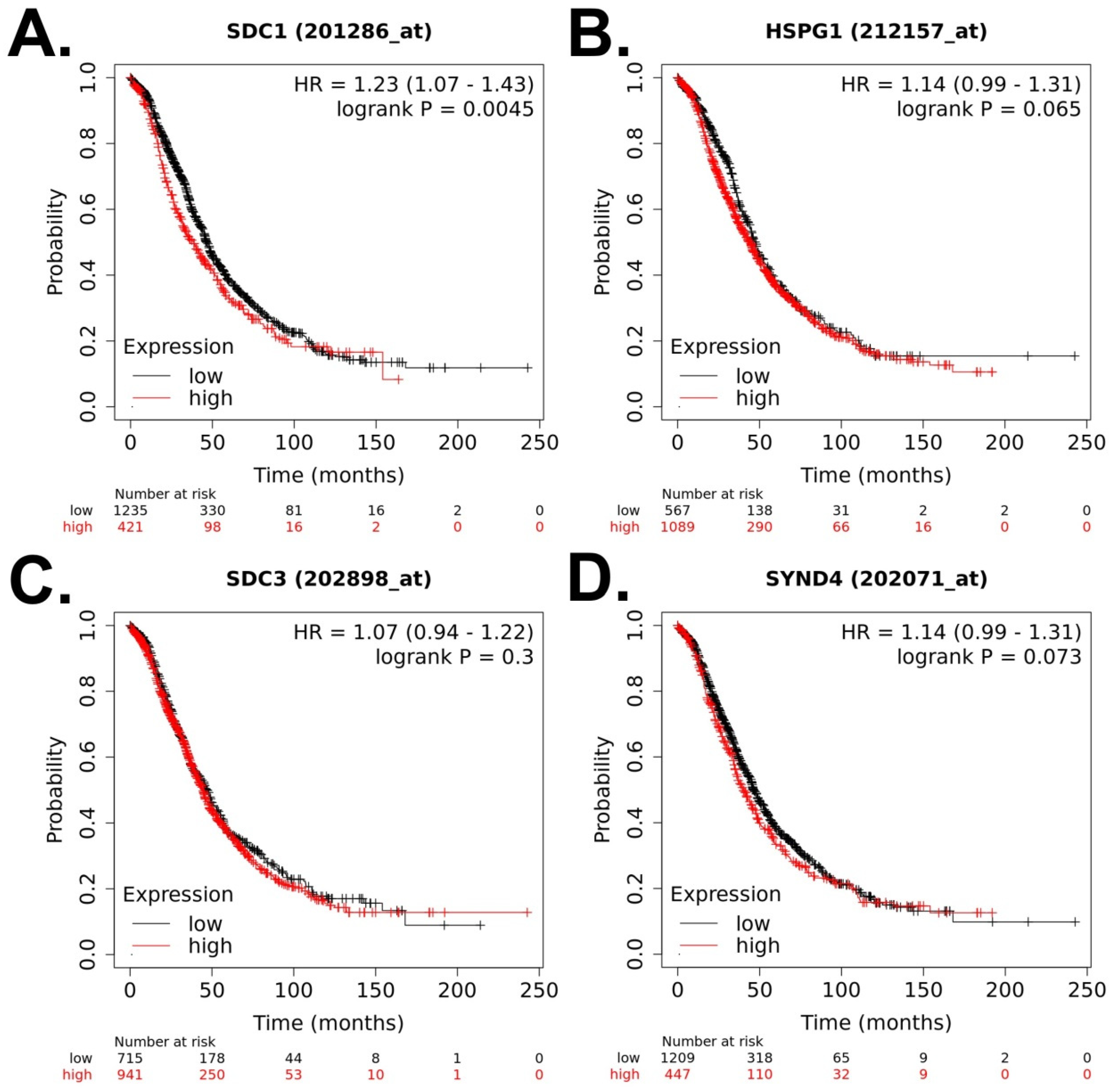
| Datasets from TCGA and GEO repositories (GSE9891, GSE3149, GSE26193, and GSE63885) | Tissue | In silico analysis | Higher expression of SDC4 is correlated with poor OS. SDC4 expression increased across the tumor stage. | Kim S et al., 2021 [25] |
| 3 datasets from GEO database (GSE38666, GSE40595, and GSE66957) | Tissue | In silico analysis (expression profiling, bioinformatic analysis) | Higher expression of SDC1 is correlated with poor OS. | Li X et al., 2022 [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oto, J.; Le, Q.-K.; Schäfer, S.D.; Kiesel, L.; Marí-Alexandre, J.; Gilabert-Estellés, J.; Medina, P.; Götte, M. Role of Syndecans in Ovarian Cancer: New Diagnostic and Prognostic Biomarkers and Potential Therapeutic Targets. Cancers 2023, 15, 3125. https://doi.org/10.3390/cancers15123125
Oto J, Le Q-K, Schäfer SD, Kiesel L, Marí-Alexandre J, Gilabert-Estellés J, Medina P, Götte M. Role of Syndecans in Ovarian Cancer: New Diagnostic and Prognostic Biomarkers and Potential Therapeutic Targets. Cancers. 2023; 15(12):3125. https://doi.org/10.3390/cancers15123125
Chicago/Turabian StyleOto, Julia, Quang-Khoi Le, Sebastian D. Schäfer, Ludwig Kiesel, Josep Marí-Alexandre, Juan Gilabert-Estellés, Pilar Medina, and Martin Götte. 2023. "Role of Syndecans in Ovarian Cancer: New Diagnostic and Prognostic Biomarkers and Potential Therapeutic Targets" Cancers 15, no. 12: 3125. https://doi.org/10.3390/cancers15123125
APA StyleOto, J., Le, Q.-K., Schäfer, S. D., Kiesel, L., Marí-Alexandre, J., Gilabert-Estellés, J., Medina, P., & Götte, M. (2023). Role of Syndecans in Ovarian Cancer: New Diagnostic and Prognostic Biomarkers and Potential Therapeutic Targets. Cancers, 15(12), 3125. https://doi.org/10.3390/cancers15123125











