Cutaneous Side Effects of Modern Targeted Therapy and Immunotherapy in Patients with Dermatological Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
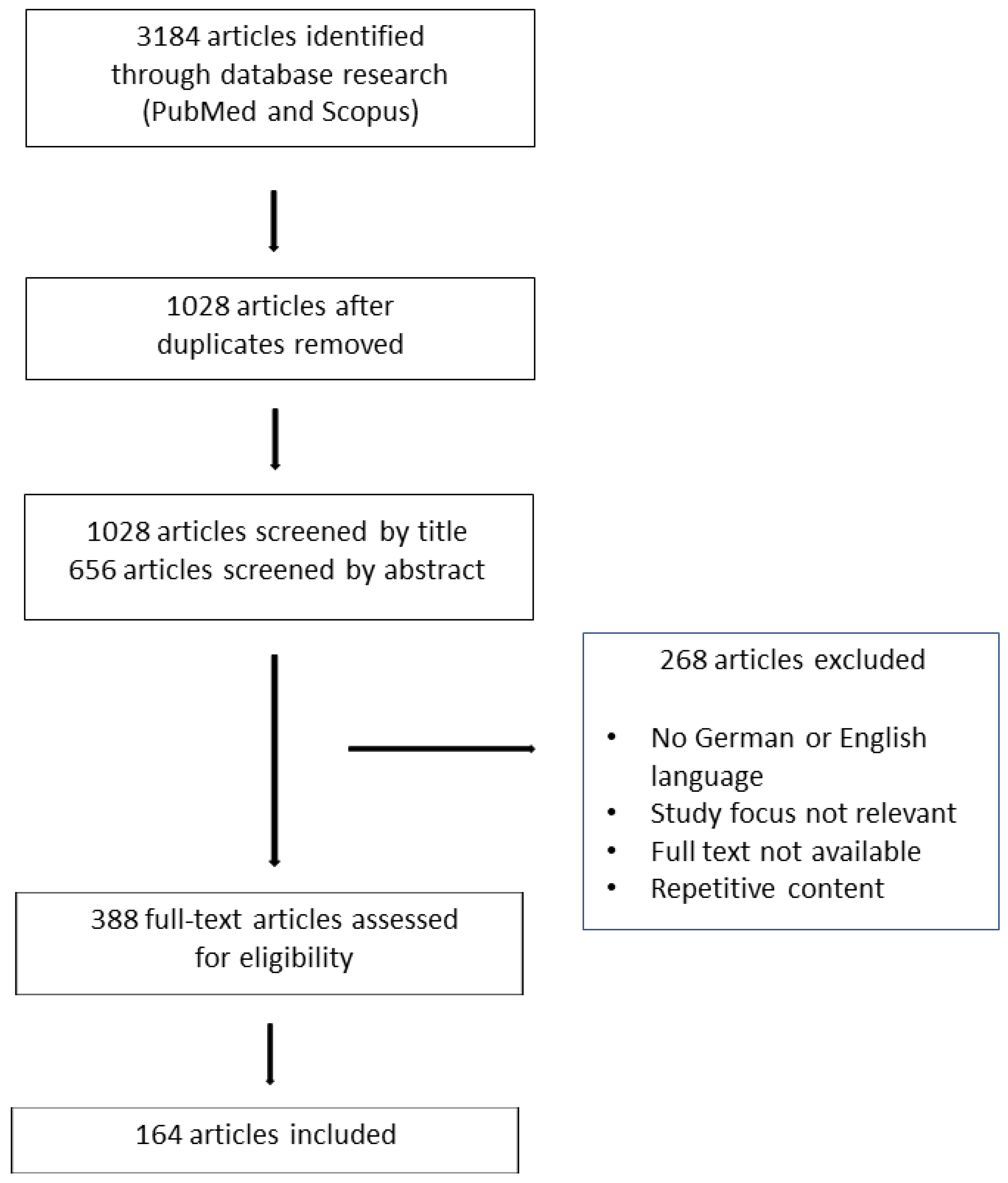
2. Materials and Methods
3. Results
3.1. ICIs
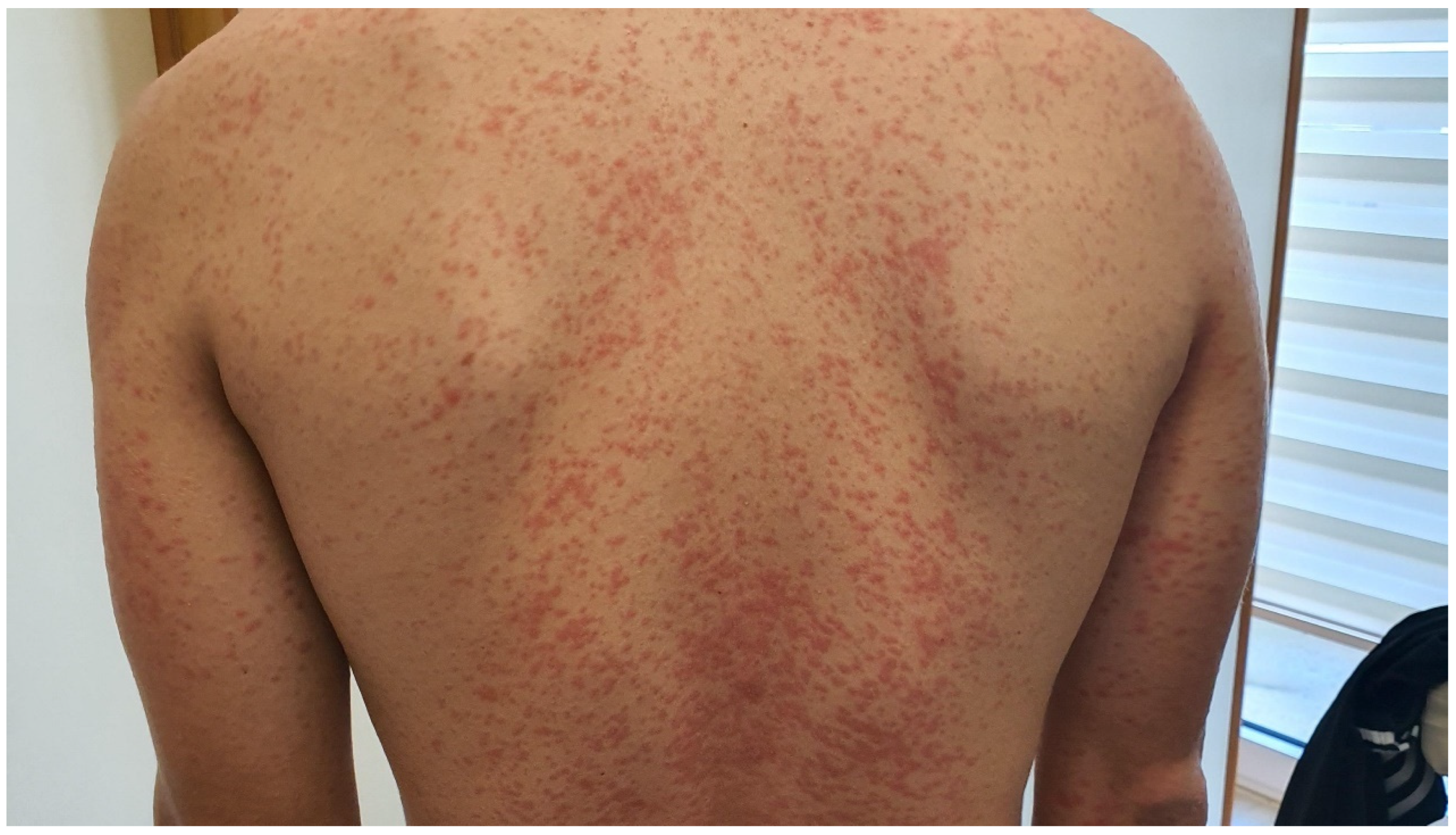
3.1.1. Maculopapular Rash
3.1.2. Lichenoid Rash
3.1.3. Psoriasis or Psoriasiform Rash
3.1.4. Bullous Eruptions
3.1.5. Pruritus
3.1.6. Vitiligo
3.1.7. Hair and Nail Toxicity
3.1.8. Mucosal Toxicity
3.1.9. Potentially Life-Threatening Adverse Reactions
3.1.10. Other Cutaneous Adverse Reactions
4. BRAF-Inhibitors
4.1. Photosensitivity
4.2. NMSC (Nonmelanoma Skin Cancer) and Benign Cutaneous Neoplasias
4.3. Benign and Malignant Melanocytic Lesions
4.4. Maculopapular Rash
4.5. Severe Cutaneous Adverse Reactions
4.6. Other Cutaneous Adverse Reactions
5. MEK-Inhibitors
5.1. Cutaneous Eruptions
5.2. Other Cutaneous Findings
6. Hedgehog-Inhibitors
6.1. Alopecia
6.2. Dysgeusia
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEP | Acute Generalized Exanthematous Pustulosis |
| BRAFi | BRAF-inhibitors |
| BSA | Body Surface Area |
| CTLA-4 | T lymphocyte-associated antigen-4 |
| DRESS | Drug Reaction with Eosinophilia and Systemic Symptoms |
| EGFR | Epidermal Growth Factor Receptor Inhibitors |
| ICIs | Immune Checkpoint-Inhibitors |
| IGF-1 | Insulin-like Growing Factor-1 |
| irAE | Immune-related Adverse Event |
| MAPK | Mitogen-activated Protein Kinase |
| MEKi | MEK-inhibitors |
| NMSC | Nonmelanoma Skin Cancer |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death Ligand 1 |
| PTCH1 | Patched Homologue 1 |
| SCC | Squamous Cell Carcinomas |
| SMO | Smoothened Homologue |
References
- Chen, C.-H.; Yu, H.-S.; Yu, S. Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article. Curr. Oncol. 2022, 29, 2871–2886. [Google Scholar] [CrossRef] [PubMed]
- Apalla, Z.; Papageorgiou, C.; Lallas, A.; Delli, F.; Fotiadou, C.; Kemanetzi, C.; Lazaridou, E. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Literature Review. Dermatol. Pract. Concept. 2021, 11, e2021155. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, J.B.; Macdonald, B.; Golitz, L.E.; LoRusso, P.; Sekulic, A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. J. Am. Acad. Dermatol. 2015, 72, 221–236. Available online: https://pubmed.ncbi.nlm.nih.gov/25592339 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Yokota, T.; Homma, A.; Kiyota, N.; Tahara, M.; Hanai, N.; Asakage, T.; Matsuura, K.; Ogawa, T.; Saito, Y.; Sano, D.; et al. Immunotherapy for squamous cell carcinoma of the head and neck. Jpn. J. Clin. Oncol. 2020, 50, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018, 19, 345–361. Available online: https://pubmed.ncbi.nlm.nih.gov/29256113 (accessed on 6 March 2023). [CrossRef]
- Luke, J.J.; Ott, P.A. PD-1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. Oncotarget 2015, 6, 3479–3492. Available online: https://pubmed.ncbi.nlm.nih.gov/25682878 (accessed on 6 March 2023). [CrossRef] [Green Version]
- Pennock, G.K.; Chow, L.Q.M. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015, 20, 812–822. Available online: https://pubmed.ncbi.nlm.nih.gov/26069281 (accessed on 6 March 2023). [CrossRef] [Green Version]
- Muntyanu, A.; Netchiporouk, E.; Gerstein, W.; Gniadecki, R.; Litvinov, I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2020, 25, 59–76. [Google Scholar] [CrossRef]
- Gençler, B.; Gönül, M. Cutaneous Side Effects of BRAF Inhibitors in Advanced Melanoma: Review of the Literature. Dermatol. Res. Pract. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Torres-Navarro, I.; de Unamuno-Bustos, B.; Botella-Estrada, R. Systematic review of BRAF/MEK inhibitors-induced Severe Cu-taneous Adverse Reactions (SCARs). J. Eur. Acad. Dermatol. Venereol. 2021, 35, 607–614. [Google Scholar] [CrossRef]
- Naqash, A.R.; File, D.M.; Ziemer, C.M.; Whang, Y.E.; Landman, P.; Googe, P.B.; Collichio, F.A. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. A single-institutional case-series. J. Immunother. Cancer 2019, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Lacouture, M.E.; Duvic, M.; Hauschild, A.; Prieto, V.G.; Robert, C.; Schadendorf, D.; Kim, C.C.; McCormack, C.J.; Myskowski, P.L.; Spleiss, O.; et al. Analysis of Dermatologic Events in Vemu-rafenib-Treated Patients with Melanoma. Oncologist 2013, 18, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Hertzman Johansson, C.; Egyhazi Brage, S. BRAF inhibitors in cancer therapy. Pharmacol. Ther. 2014, 142, 176–182. [Google Scholar] [CrossRef]
- De Golian, E.; Kwong, B.Y.; Swetter, S.M.; Pugliese, S.B. Cutaneous Complications of Targeted Melanoma Therapy. Curr. Treat. Options Oncol. 2016, 17, 57. Available online: https://pubmed.ncbi.nlm.nih.gov/27645330 (accessed on 11 March 2023). [CrossRef]
- Carlos, G.; Anforth, R.; Clements, A.; Menzies, A.M.; Carlino, M.S.; Chou, S.; Fernandez-Peñas, P. Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination with MEK Inhibitors for Metastatic Melanoma. JAMA Dermatol. 2015, 151, 1103–1109. Available online: https://pubmed.ncbi.nlm.nih.gov/26200476 (accessed on 13 March 2023). [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. Available online: https://pubmed.ncbi.nlm.nih.gov/21639808 (accessed on 12 March 2023). [CrossRef] [Green Version]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5278761 (accessed on 15 March 2023). [CrossRef] [Green Version]
- Oniangue-Ndza, C.; Gooden, K.M.; May, J.; Malcolm, B.; Du, E.X.; Yin, L.; Betts, K.A. PCN111 comparison of adverse event costs of nivolumab+ipilimumab versus sunitinib for previously untreated intermediate-/poor-risk ad-vanced renal cell carcinoma in Switzerland. Value Health 2019, 22, S457. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Mono-therapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.v.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. Available online: https://pubmed.ncbi.nlm.nih.gov/34986285 (accessed on 17 May 2023). [CrossRef] [PubMed]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Wolchok, J.D.; Yosipovitch, G.; Kähler, K.C.; Busam, K.J.; Hauschild, A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J. Am. Acad. Dermatol. 2014, 71, 161–169. [Google Scholar] [CrossRef]
- Shi, V.J.; Rodic, N.; Gettinger, S.; Leventhal, J.S.; Neckman, J.P.; Girardi, M.; Bosenberg, M.; Choi, J.N. Clinical and histologic features of lichenoid mucocu-taneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016, 152, 1128–1136. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.P.; Setser, A.; Anadkat, M.J.; Cotliar, J.; Olsen, E.A.; Garden, B.C.; Lacouture, M.E. Grading dermatologic adverse events of cancer treatments: The Common Terminology Criteria for Adverse Events Version 4.0. J. Am. Acad. Dermatol. 2012, 67, 1025–1039. Available online: https://pubmed.ncbi.nlm.nih.gov/22502948 (accessed on 17 May 2023). [CrossRef]
- Curry, J.L.; Tetzlaff, M.T.; Nagarajan, P.; Drucker, C.; Diab, A.; Hymes, S.R.; Duvic, M.; Hwu, W.-J.; Wargo, J.A.; Torres-Cabala, C.A.; et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J. Cutan. Pathol. 2016, 44, 158–176. Available online: https://pubmed.ncbi.nlm.nih.gov/27859479 (accessed on 7 March 2023). [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Gomes, N.; Sibaud, V.; Azevedo, F.; Magina, S. Cutaneous Toxicity of Immune Checkpoint Inhibitors: A Narrative Review. Acta Med. Port. 2020, 33, 335–343. Available online: https://pubmed.ncbi.nlm.nih.gov/32416756 (accessed on 7 March 2023). [CrossRef]
- Joseph, R.W.; Cappel, M.; Goedjen, B.; Gordon, M.; Kirsch, B.; Gilstrap, C.; Bagaria, S.; Jambusaria-Pahlajani, A. Lichenoid Dermatitis in Three Patients with Metastatic Melanoma Treated with Anti–PD-1 Therapy. Cancer Immunol. Res. 2015, 3, 18–22. Available online: https://pubmed.ncbi.nlm.nih.gov/25287118 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Schaberg, K.B.; Novoa, R.A.; Wakelee, H.A.; Kim, J.; Cheung, C.; Srinivas, S.; Kwong, B.Y. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. J. Cutan. Pathol. 2016, 43, 339–346. Available online: https://pubmed.ncbi.nlm.nih.gov/26762844 (accessed on 7 March 2023). [CrossRef]
- Guggina, L.M.; Yanes, D.A.; Choi, J.N. Inverse lichenoid drug eruption associated with nivolumab. JAAD Case Rep. 2016, 3, 7–9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5198731 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Tetzlaff, M.T.; Nagarajan, P.; Chon, S.; Huen, A.; Diab, A.; Omar, P.; Aung, P.P.; Torres-Cabala, C.A.; Mays, S.R.; Prieto, V.G.; et al. Lichenoid Dermatologic Toxicity From Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. Am. J. Dermatopathol. 2017, 39, 121–129. Available online: https://pubmed.ncbi.nlm.nih.gov/28134729 (accessed on 7 March 2023). [CrossRef]
- Sibaud, V.; Eid, C.; Belum, V.R.; Combemale, P.; Barres, B.; Lamant, L.; Mourey, L.; Gomez-Roca, C.; Estilo, C.L.; Motzer, R.; et al. Oral Lichenoid Reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e464. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645209 (accessed on 7 March 2023). [CrossRef]
- Mahil, S.K.; Capon, F.; Barker, J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. Semin. Immunopathol. 2015, 38, 11–27. Available online: https://pubmed.ncbi.nlm.nih.gov/26573299 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Chia, P.L.; John, T. Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non–Small Cell Lung Cancer (NSCLC). J. Immunother. 2016, 39, 202–204. Available online: https://pubmed.ncbi.nlm.nih.gov/27163740 (accessed on 7 March 2023). [CrossRef]
- Dulos, J.; Carven, G.J.; van Boxtel, S.J.; Evers, S.; Driessen-Engels, L.J.A.; Hobo, W.; Gorecka, M.A.; de Haan, A.F.J.; Mulders, P.; Punt, C.J.A.; et al. PD-1 Blockade Augments Th1 and Th17 and Suppresses Th2 Responses in Peripheral Blood from Patients with Prostate and Advanced Melanoma Cancer. J. Immunother. 2012, 35, 169–178. Available online: https://pubmed.ncbi.nlm.nih.gov/22306905 (accessed on 7 March 2023). [CrossRef]
- Matsumura, N.; Ohtsuka, M.; Kikuchi, N.; Yamamoto, T. Exacerbation of Psoriasis During Nivolumab Therapy for Metastatic Melanoma. Acta Derm. -Venereol. 2016, 96, 259–260. Available online: https://pubmed.ncbi.nlm.nih.gov/26270860 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Totonchy, M.B.; Ezaldein, H.H.; Ko, C.; Choi, J. Inverse Psoriasiform Eruption During Pembrolizumab Therapy for Metastatic Melanoma. JAMA Dermatol. 2016, 152, 590–592. Available online: https://pubmed.ncbi.nlm.nih.gov/26675815 (accessed on 7 March 2023). [CrossRef]
- Bonigen, J.; Raynaud-Donzel, C.; Hureaux, J.; Kramkimel, N.; Blom, A.; Jeudy, G.; Breton, A.-L.; Hubiche, T.; Bedane, C.; Legoupil, D.; et al. Anti-PD1-induced psoriasis: A study of 21 patients. J. Eur. Acad. Dermatol. Venereol. 2016, 31, e254–e257. Available online: https://pubmed.ncbi.nlm.nih.gov/27739129 (accessed on 7 March 2023). [CrossRef]
- Ruiz-Bañobre, J.; Abdulkader, I.; Anido, U.; León, L.; López-López, R.; García-González, J. Development of de novo psoriasis during nivolumab therapy for metastatic renal cell carcinoma: Immunohistochemical analyses and clinical outcome. Apmis 2017, 125, 259–263. Available online: https://pubmed.ncbi.nlm.nih.gov/28233446 (accessed on 7 March 2023). [CrossRef] [PubMed]
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor–related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. Available online: https://pubmed.ncbi.nlm.nih.gov/32454097 (accessed on 7 March 2023). [CrossRef] [PubMed]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Byth, K.; Kong, B.Y.; Chou, S.; Carlino, M.S.; Kefford, R.; Fernandez-Penas, P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J. Am. Acad. Dermatol. 2016, 74, 455–461.e1. Available online: https://pubmed.ncbi.nlm.nih.gov/26793994 (accessed on 7 March 2023). [CrossRef] [PubMed] [Green Version]
- Coleman, E.; Ko, C.; Dai, F.; Tomayko, M.M.; Kluger, H.; Leventhal, J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J. Am. Acad. Dermatol. 2019, 80, 990–997. Available online: https://pubmed.ncbi.nlm.nih.gov/30399387 (accessed on 7 March 2023). [CrossRef] [PubMed]
- Siegel, J.; Totonchy, M.; Damsky, W.; Berk-Krauss, J.; Castiglione, F.; Sznol, M.; Petrylak, D.P.; Fischbach, N.; Goldberg, S.B.; Decker, R.H.; et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J. Am. Acad. Dermatol. 2018, 79, 1081–1088. Available online: https://pubmed.ncbi.nlm.nih.gov/30025829 (accessed on 7 March 2023). [CrossRef]
- Beck, K.M.; Dong, J.; Geskin, L.J.; Beltrani, V.P.; Phelps, R.G.; Carvajal, R.D.; Schwartz, G.; Saenger, Y.M.; Gartrell, R.D. Disease stabilization with pembrolizumab for metastatic acral melanoma in the setting of autoimmune bullous pemphigoid. J. Immunother. Cancer 2016, 4, 20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4835882 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Damsky, W.; Kole, L.; Tomayko, M.M. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016, 2, 442–444. Available online: https://pubmed.ncbi.nlm.nih.gov/27981213 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Sowerby, L.; Dewan, A.K.; Granter, S.; Gandhi, L.; Leboeuf, N.R. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol. 2017, 153, 603–605. Available online: https://pubmed.ncbi.nlm.nih.gov/28355425 (accessed on 7 March 2023). [CrossRef]
- Naidoo, J.; Schindler, K.; Querfeld, C.; Busam, K.; Cunningham, J.; Page, D.B.; Postow, M.A.; Weinstein, A.; Lucas, A.S.; Ciccolini, K.T.; et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol. Res. 2016, 4, 383–389. Available online: https://pubmed.ncbi.nlm.nih.gov/26928461 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Garje, R.; Chau, J.J.; Chung, J.; Wanat, K.; Zakharia, Y. Acute Flare of Bullous Pemphigus with Pembrolizumab Used for Treatment of Metastatic Urothelial Cancer. J. Immunother. 2018, 41, 42–44. Available online: https://pubmed.ncbi.nlm.nih.gov/29111983 (accessed on 7 March 2023). [CrossRef]
- Clawson, R.C.; Tabata, M.M.; Chen, S.T. Pemphigus vulgaris flare in a patient treated with nivolumab. Dermatol. Ther. 2021, 34, e14871. Available online: https://pubmed.ncbi.nlm.nih.gov/33571394 (accessed on 7 March 2023). [CrossRef]
- Krammer, S.; Krammer, C.; Salzer, S.; Bağci, I.S.; French, L.E.; Hartmann, D. Recurrence of Pemphigus Vulgaris Under Nivolumab Therapy. Front. Med. 2019, 6, 262. Available online: https://pubmed.ncbi.nlm.nih.gov/31781569 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Yatim, A.; Bohelay, G.; Grootenboer-Mignot, S.; Prost-Squarcioni, C.; Alexandre, M.; Le Roux-Villet, C.; Martin, A.; Maubec, E.; Caux, F. Paraneoplastic Pemphigus Revealed by Anti-programmed Death-1 Pembrolizumab Therapy for Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa. Front. Med. 2019, 6, 249. Available online: https://pubmed.ncbi.nlm.nih.gov/31750309 (accessed on 7 March 2023). [CrossRef] [Green Version]
- McNally, M.A.; Vangipuram, R.; Campbell, M.T.; Nagarajan, P.; Patel, A.B.; Curry, J.L.; Heberton, M. Paraneoplastic pemphigus manifesting in a patient treated with pembrolizumab for urothelial carcinoma. JAAD Case Rep. 2021, 10, 82–84. Available online: https://pubmed.ncbi.nlm.nih.gov/33778141 (accessed on 7 March 2023). [CrossRef]
- Kwon, C.; Land, A.; Smoller, B.; Scott, G.; Beck, L.; Mercurio, M. Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e349–e350. Available online: https://pubmed.ncbi.nlm.nih.gov/28129461 (accessed on 7 March 2023). [CrossRef]
- Sibaud, V.; Meyer, N.; Lamant, L.; Vigarios, E.; Mazieres, J.; Delord, J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr. Opin. Oncol. 2016, 28, 254–263. Available online: https://pubmed.ncbi.nlm.nih.gov/27136138 (accessed on 7 March 2023). [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cispla-tin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. Available online: https://pubmed.ncbi.nlm.nih.gov/27939400 (accessed on 7 March 2023). [CrossRef] [Green Version]
- Peters, S.; Gettinger, S.; Johnson, M.L.; Jänne, P.A.; Garassino, M.C.; Christoph, D.; Toh, C.K.; Rizvi, N.A.; Chaft, J.E.; Costa, E.C.; et al. Phase II Trial of Atezolizumab as First-Line or Subsequent Therapy for Patients with Programmed Death-Ligand 1–Selected Advanced Non–Small-Cell Lung Cancer (BIRCH). J. Clin. Oncol. 2017, 35, 2781–2789. Available online: https://pubmed.ncbi.nlm.nih.gov/28609226 (accessed on 7 March 2023). [CrossRef]
- Sanlorenzo, M.; Vujic, I.; Daud, A.; Algazi, A.; Gubens, M.; Luna, S.A.; Lin, K.; Quaglino, P.; Rappersberger, K.; Ortiz-Urda, S. Pembrolizumab cutaneous adverse events and their asso-ciation with disease progression. JAMA Dermatol. 2015, 151, 1206–1212. [Google Scholar] [CrossRef] [Green Version]
- Yin, E.S.; Totonchy, M.B.; Leventhal, J.S. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: A novel finding. JAAD Case Rep. 2017, 3, 90–92. [Google Scholar] [CrossRef] [Green Version]
- Mathias, C.; Wakelee, H.I. 21 Checkpoint Inhibitor Combinations Among Themselves and with Chemotherapy. J. Thorac. Oncol. 2019, 14, S1166. [Google Scholar] [CrossRef]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.C.; Consuegra, G.; Chou, S.; Fernandez Peñas, P. Vitiligo-like depigmentation in oncology patients treated with immuno-therapies for nonmelanoma metastatic cancers. Clin. Exp. Dermatol. 2019, 44, 643–646. [Google Scholar] [CrossRef]
- Quaglino, P.; Marenco, F.; Osella-Abate, S.; Cappello, N.; Ortoncelli, M.; Salomone, B.; Fierro, M.T.; Savoia, P.; Bernengo, M.G. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: Results from a single-institution hospital-based observational cohort study. Ann. Oncol. 2009, 21, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Boussemart, L.; Mateus, C.; Routier, E.; Boutros, C.; Cazenave, H.; Viollet, R.; Thomas, M.; Roy, S.; Benannoune, N.; et al. Association of Vitiligo with Tumor Response in Patients with Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016, 152, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 76–83. [Google Scholar] [CrossRef]
- Wolner, Z.J.; Marghoob, A.A.; Pulitzer, M.P.; Postow, M.A.; Marchetti, M.A. A case report of disappearing pigmented skin lesions as-sociated with pembrolizumab treatment for metastatic melanoma. Br. J. Dermatol. 2018, 178, 265–269. [Google Scholar] [CrossRef]
- Manson, G.; Marabelle, A.; Houot, R. Hair Repigmentation with Anti–PD-1 and Anti–PD-L1 Immunotherapy: A Novel Hypothesis. JAMA Dermatol. 2018, 154, 113. Available online: https://pubmed.ncbi.nlm.nih.gov/29167871 (accessed on 9 March 2023). [CrossRef]
- Rivera, N.; Boada, A.; Bielsa, M.I.; Fernández-Figueras, M.T.; Carcereny, E.; Moran, M.T.; Ferrándiz, C. Hair Repigmentation During Immuno-therapy Treatment with an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA Dermatol. 2017, 153, 1162–1165. Available online: https://pubmed.ncbi.nlm.nih.gov/28700789 (accessed on 9 March 2023). [CrossRef]
- Teulings, H.-E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-Like Depigmentation in Patients with Stage III-IV Melanoma Receiving Immunotherapy and Its Association with Survival: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Asami, Y.; Teramoto, Y.; Imamura, T.; Sato, S.; Maruyama, H.; Fujisawa, Y.; Matsuya, T.; Fujimoto, M.; et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 2016, 44, 117–122. [Google Scholar] [CrossRef]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Zarbo, A.; Belum, V.; Sibaud, V.; Oudard, S.; Postow, M.; Hsieh, J.; Motzer, R.; Busam, K.; Lacouture, M. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2016, 176, 1649–1652. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265, Erratum in Lancet 2017, 389, e5. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Dasanu, C.A.; Lippman, S.M.; Plaxe, S.C. Persistently curly hair phenotype with the use of nivolumab for squamous cell lung cancer. J. Oncol. Pharm. Pract. 2016, 23, 638–640. Available online: https://pubmed.ncbi.nlm.nih.gov/27824586 (accessed on 9 March 2023). [CrossRef]
- Jackson, L.K.; Johnson, D.B.; Sosman, J.A.; Murphy, B.A.; Epstein, J.B. Oral health in oncology: Impact of immunotherapy. Support. Care Cancer 2014, 23, 1–3. Available online: https://pubmed.ncbi.nlm.nih.gov/25216852 (accessed on 9 March 2023). [CrossRef]
- Vigarios, E.; Epstein, J.B.; Sibaud, V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint in-hibitors. Support Care Cancer 2017, 25, 1713–1739. Available online: https://pubmed.ncbi.nlm.nih.gov/28224235 (accessed on 9 March 2023). [CrossRef] [Green Version]
- McDermott, D.F.; Sosman, J.A.; Sznol, M.; Massard, C.; Gordon, M.S.; Hamid, O.; Powderly, J.D.; Infante, J.R.; Fassò, M.; Wang, Y.V.; et al. Atezolizumab, an Anti–Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates from a Phase Ia Study. J. Clin. Oncol. 2016, 34, 833–842. Available online: https://pubmed.ncbi.nlm.nih.gov/26755520 (accessed on 9 March 2023). [CrossRef]
- Collins, L.K.; Chapman, M.S.; Carter, J.B.; Samie, F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr. Probl. Cancer 2017, 41, 125–128. Available online: https://pubmed.ncbi.nlm.nih.gov/28190531 (accessed on 9 March 2023). [CrossRef]
- Inno, A.; Metro, G.; Bironzo, P.; Grimaldi, A.M.; Grego, E.; DI Nunno, V.; Picasso, V.; Massari, F.; Gori, S. Pathogenesis, Clinical Manifestations and Management of Immune Checkpoint Inhibitors Toxicity. Tumori J. 2017, 103, 405–421. Available online: https://pubmed.ncbi.nlm.nih.gov/28497847 (accessed on 10 March 2023). [CrossRef] [PubMed]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. Available online: https://pubmed.ncbi.nlm.nih.gov/24590637 (accessed on 10 March 2023). [CrossRef] [PubMed]
- Jour, G.; Glitza, I.C.; Ellis, R.M.; Torres-Cabala, C.A.; Tetzlaff, M.T.; Li, J.Y.; Nagarajan, P.; Huen, A.; Aung, P.P.; Ivan, D.; et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: A report on bullous skin eruptions. J. Cutan. Pathol. 2016, 43, 688–696. Available online: https://pubmed.ncbi.nlm.nih.gov/27086658 (accessed on 10 March 2023). [CrossRef] [PubMed]
- Voskens, C.J.; Goldinger, S.M.; Loquai, C.; Robert, C.; Kaehler, K.C.; Berking, C.; Bergmann, T.; Bockmeyer, C.L.; Eigentler, T.; Fluck, M.; et al. The Price of Tumor Control: An Analysis of Rare Side Effects of Anti-CTLA-4 Therapy in Metastatic Melanoma from the Ipilimumab Network. PLoS ONE 2013, 8, e53745. Available online: https://pubmed.ncbi.nlm.nih.gov/23341990 (accessed on 10 March 2023). [CrossRef] [Green Version]
- Hwang, S.J.E.; Carlos, G.; Wakade, D.; Sharma, R.; Fernandez-Penas, P. Ipilimumab-induced acute generalized exanthematous pus-tulosis in a patient with metastatic melanoma. Melanoma Res. 2016, 26, 417–420. Available online: https://pubmed.ncbi.nlm.nih.gov/27031538 (accessed on 10 March 2023). [CrossRef]
- Saw, S.; Lee, H.Y.; Ng, Q.S. Pembrolizumab-induced Stevens–Johnson syndrome in non-melanoma patients. Eur. J. Cancer 2017, 81, 237–239. Available online: https://pubmed.ncbi.nlm.nih.gov/28438440 (accessed on 10 March 2023). [CrossRef]
- Vivar, K.L.; Deschaine, M.; Messina, J.; Divine, J.M.; Rabionet, A.; Patel, N.; Harrington, M.A.; Seminario-Vidal, L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. J. Cutan. Pathol. 2017, 44, 381–384. Available online: https://pubmed.ncbi.nlm.nih.gov/28000240 (accessed on 10 March 2023). [CrossRef]
- Goldinger, S.M.; Stieger, P.; Meier, B.; Micaletto, S.; Contassot, E.; French, L.E.; Dummer, R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin. Cancer Res. 2016, 22, 4023–4029. Available online: https://pubmed.ncbi.nlm.nih.gov/26957557 (accessed on 10 March 2023). [CrossRef] [Green Version]
- Lu, J.; Thuraisingam, T.; Chergui, M.; Nguyen, K. Nivolumab-associated DRESS syndrome: A case report. JAAD Case Rep. 2019, 5, 216–218. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6374958 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Ai, L.; Gao, J.; Zhao, S.; Li, Q.; Cui, Y.-H.; Liu, Q.; Wu, D.; Wang, Y.; Jin, X.; Ji, Y.; et al. Nivolumab-associated DRESS in a genetic susceptible individual. J. Immunother. Cancer 2021, 9, e002879. Available online: https://pubmed.ncbi.nlm.nih.gov/34599025 (accessed on 11 March 2023). [CrossRef]
- Chen, C.-B.; Wu, M.-Y.; Ng, C.Y.; Lu, C.-W.; Wu, J.; Kao, P.-H.; Yang, C.-K.; Peng, M.-T.; Huang, C.-Y.; Chang, W.-C.; et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag. Res. 2018, 10, 1259–1273. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5962313 (accessed on 10 March 2023). [CrossRef] [Green Version]
- Raschi, E.; Antonazzo, I.C.; La Placa, M.; Ardizzoni, A.; Poluzzi, E.; De Ponti, F. Serious Cutaneous Toxicities with Immune Checkpoint Inhibitors in the U.S. Food and Drug Administration Adverse Event Reporting System. Oncol. 2019, 24, e1228–e1231. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6853099 (accessed on 10 March 2023). [CrossRef] [Green Version]
- Bs, N.J.M.; Ravi, V.; Cheng, K.; Bach, D.Q.; Worswick, S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. Int. J. Dermatol. 2020, 59, e183–e188. Available online: https://pubmed.ncbi.nlm.nih.gov/32052409 (accessed on 10 March 2023).
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE 2016, 11, e0160221. Available online: https://pubmed.ncbi.nlm.nih.gov/27472273 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Estenaga, A.; Rodriguez-Garijo, N.; Tomás-Velázquez, A.; Antoñanzas-Pérez, J.; Alvarez-Gigli, M.L.; García-Tobar, L.; Espaa-Alonso, A.; Salido-Vallejo, R. Immuno-therapy-intensified paraneoplastic dermatomyositis. Indian J. Dermatol. Venereol. Leprol. 2021, 88, 93–96. Available online: https://pubmed.ncbi.nlm.nih.gov/34491672 (accessed on 11 March 2023). [CrossRef]
- Thomas, R.; Patel, H.; Scott, J. Dermatomyositis Flare with Immune Checkpoint Inhibitor Therapy for Melanoma. Cureus 2021, 13, e14387. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8106943 (accessed on 11 March 2023). [CrossRef]
- Coustal, C.; Du Thanh, A.; Roubille, F.; Assenat, E.; Maria, A.T. Rare cutaneous toxicity of immune checkpoint inhibitors: A case of durvalumab-induced dermatomyositis. Eur. J. Cancer 2021, 155, 25–27. Available online: https://pubmed.ncbi.nlm.nih.gov/34332401 (accessed on 11 March 2023). [CrossRef]
- Freites-Martinez, A.; Kwong, B.Y.; Rieger, K.E.; Coit, D.G.; Colevas, A.D.; Lacouture, M.E. Eruptive Keratoacanthomas Associated with Pembrolizumab Therapy. JAMA Dermatol. 2017, 153, 694–697. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523926 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Seban, R.-D.; Vermersch, C.; Champion, L.; Bonsang, B.; Roger, A.; Ghidaglia, J. Immune-Related Erythema Nodosum Mimicking in Transit Melanoma Metastasis on [18F]-FDG PET/CT. Diagnostics 2021, 11, 747. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8143543 (accessed on 11 March 2023). [CrossRef]
- Pach, J.; Moody, K.; Ring, N.; Panse, G.; Zhang, M.; Deverapalli, S.; Leventhal, J. Erythema nodosum-like panniculitis associated with immune checkpoint inhibitor therapy: Two cases reporting a rare cutaneous adverse event. JAAD Case Rep. 2021, 13, 118–120. Available online: https://pubmed.ncbi.nlm.nih.gov/34189226 (accessed on 11 March 2023). [CrossRef]
- Tetzlaff, M.T.; Jazaeri, A.A.; Torres-Cabala, C.A.; Korivi, B.R.; Landon, G.A.; Nagarajan, P.; Choksi, A.; Chen, L.; Uemura, M.; Aung, P.P.; et al. Erythema nodosum-like panniculitis mimicking disease recurrence: A novel toxicity from immune checkpoint blockade therapy-Report of 2 patients. J. Cutan. Pathol. 2017, 44, 1080–1086. Available online: https://pubmed.ncbi.nlm.nih.gov/28901560 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Munoz, J.; Guillot, B.; Girard, C.; Dereure, O.; Du-Thanh, A. First report of ipilimumab-induced Grover disease. Br. J. Dermatol. 2014, 171, 1236–1237. Available online: https://pubmed.ncbi.nlm.nih.gov/24749658 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Uemura, M.; Faisal, F.; Haymaker, C.; McQuail, N.; Sirmans, E.; Hudgens, C.W.; Barbara, L.; Bernatchez, C.; Curry, J.L.; Hwu, P.; et al. A case report of Grover’s disease from immu-notherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J. Immunother. Cancer 2016, 4, 55. Available online: https://pubmed.ncbi.nlm.nih.gov/27660709 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Koelzer, V.H.; Buser, T.; Willi, N.; Rothschild, S.I.; Wicki, A.; Schiller, P.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Grover’s-like drug eruption in a patient with metastatic melanoma under ipilimumab therapy. J. Immunother. Cancer 2016, 4, 47. Available online: https://pubmed.ncbi.nlm.nih.gov/27532022 (accessed on 11 March 2023). [CrossRef] [PubMed] [Green Version]
- Welborn, M.E.; Kubicki, S.L.; Patel, A.B. Pyoderma Gangrenosum Following Initiation of Immune Checkpoint Inhibitor Therapy. J. Immunother. Precis. Oncol. 2018, 1, 82–84. [Google Scholar] [CrossRef]
- Tsibris, H.; Lian, C.; Ho, A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell car-cinoma. Dermatol. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Lomax, A.J.; McGuire, H.M.; McNeil, C.; Choi, C.J.; Hersey, P.; Karikios, D.; Shannon, K.; van Hal, S.; Carr, U.; Crotty, A.; et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Int. J. Rheum. Dis. 2017, 20, 1277–1285. Available online: https://pubmed.ncbi.nlm.nih.gov/28480561 (accessed on 11 March 2023). [CrossRef]
- Suozzi, K.C.; Stahl, M.; Ko, C.J.; Chiang, A.; Gettinger, S.N.; Siegel, M.D.; Bunick, C.G. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep. 2016, 2, 264–268. Available online: https://pubmed.ncbi.nlm.nih.gov/27486590 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Cotliar, J.; Raja, N.; Raz, D.; Boswell, W.J.; Chen, R.; Querfeld, C. Pembrolizumab-associated sarcoidosis. JAAD Case Rep. 2016, 2, 290–293. Available online: https://pubmed.ncbi.nlm.nih.gov/27504482 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Tetzlaff, M.T.; Nelson, K.C.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/sarcoid-like lesions asso-ciated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J. Immunother. Cancer 2018, 6, 14. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5810034 (accessed on 11 March 2023). [CrossRef]
- Gkiozos, I.; Kopitopoulou, A.; Kalkanis, A.; Vamvakaris, I.N.; Judson, M.A.; Syrigos, K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. J. Thorac. Oncol. 2018, 13, 1076–1082. Available online: https://pubmed.ncbi.nlm.nih.gov/29763666 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Cappelli, L.C.; Gutierrez, A.K.; Baer, A.N.; Albayda, J.; Manno, R.L.; Haque, U.; Lipson, E.J.; Bleich, K.B.; Shah, A.A.; Naidoo, J.; et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis. 2016, 76, 43–50. Available online: https://pubmed.ncbi.nlm.nih.gov/27307501 (accessed on 11 March 2023). [CrossRef]
- Yaşar, H.A.; Akkus, E.; Heper, A.O.; Akay, B.N.; Urun, Y.; Utkan, G. Sweet’s syndrome under ipilimumab therapy and a brief comparison of the cases in literature. J. Oncol. Pharm. Pract. 2020, 26, 1762–1764. Available online: https://pubmed.ncbi.nlm.nih.gov/32089071 (accessed on 11 March 2023). [CrossRef]
- Pintova, S.; Sidhu, H.; Friedlander, P.A.; Holcombe, R.F. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. Melanoma Res. 2013, 23, 498–501. Available online: https://pubmed.ncbi.nlm.nih.gov/24113862 (accessed on 11 March 2023). [CrossRef]
- Bousquet, E.; Zarbo, A.; Tournier, E.; Chevreau, C.; Mazieres, J.; Lacouture, M.; Sibaud, V. Development of Papulopustular Rosacea during Nivolumab Therapy for Metastatic Cancer. Acta Derm.-Venereol. 2017, 97, 539–540. Available online: https://pubmed.ncbi.nlm.nih.gov/27826614 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Gambichler, T.; Strutzmann, S.; Tannapfel, A.; Susok, L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. BMC Cancer 2017, 17, 327. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3313-6 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Le Burel, S.; Champiat, S.; Routier, E.; Aspeslagh, S.; Albiges, L.; Szwebel, T.A.; Michot, J.-M.; Chretien, P.; Mariette, X.; Voisin, A.-L.; et al. Onset of connective tissue disease following an-ti-PD1/PD-L1 cancer immunotherapy. Ann. Rheum. Dis. 2018, 77, 468–470. Available online: https://pubmed.ncbi.nlm.nih.gov/28242618 (accessed on 11 March 2023). [CrossRef]
- Sanlorenzo, M.; Choudhry, A.; Vujic, I.; Posch, C.; Chong, K.; Johnston, K.; Meier, M.; Osella-Abate, S.; Quaglino, P.; Daud, A.; et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. J. Am. Acad. Dermatol. 2014, 71, 1102–1109.e1. Available online: https://pubmed.ncbi.nlm.nih.gov/25440439 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Dummer, R.; Rinderknecht, J.; Goldinger, S.M. Ultraviolet A and Photosensitivity during Vemurafenib Therapy. N. Engl. J. Med. 2012, 366, 480–481. Available online: https://www.nejm.org/doi/full/10.1056/nejmc1113752 (accessed on 11 March 2023). [CrossRef]
- Boussemart, L.; Routier, E.; Mateus, C.; Opletalova, K.; Sebille, G.; Kamsu-Kom, N.; Thomas, M.; Vagner, S.; Favre, M.; Tomasic, G.; et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: A study of 42 patients. Ann. Oncol. 2013, 24, 1691–1697. Available online: http://www.annalsofoncology.org/article/S0923753419373016/fulltext (accessed on 11 March 2023). [CrossRef]
- Reyes-Habito, C.M.; Roh, E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. J. Am. Acad. Dermatol. 2014, 71, 217.e1–217.e11. Available online: https://pubmed.ncbi.nlm.nih.gov/25037801 (accessed on 11 March 2023).
- Anforth, R.; Fernandez-Penas, P. BRAF Inhibitor Induced Verrucal Keratosis. Am. J. Dermatopathol. 2014, 36, 192. Available online: https://pubmed.ncbi.nlm.nih.gov/23435364 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Anforth, R.; Carlos, G.; Clements, A.; Kefford, R.; Fernandez-Peñas, P. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks. Br. J. Dermatol. 2014, 172, 239–243. Available online: https://pubmed.ncbi.nlm.nih.gov/25040674 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Mattei, P.L.; Alora-Palli, M.B.; Kraft, S.; Lawrence, D.P.; Flaherty, K.T.; Kimball, A.B. Cutaneous effects of BRAF inhibitor therapy: A case series. Ann. Oncol. 2013, 24, 530–537. Available online: https://pubmed.ncbi.nlm.nih.gov/23035153 (accessed on 11 March 2023). [CrossRef] [PubMed]
- Anforth, R.; Tembe, V.; Blumetti, T.; Fernandez-Peñas, P. Mutational analysis of cutaneous squamous cell carcinomas and verrucal keratosis in patients taking BRAF inhibitors. Pigment. Cell Melanoma Res. 2012, 25, 569–572. Available online: https://pubmed.ncbi.nlm.nih.gov/22726224 (accessed on 11 March 2023). [CrossRef]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib. N. Engl. J. Med. 2012, 366, 707–714. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa1112302 (accessed on 11 March 2023). [CrossRef] [Green Version]
- Anforth, R.; Blumetti, T.; Kefford, R.; Sharma, R.; Scolyer, R.; Kossard, S.; Long, G.; Fernandez-Peñas, P. Cutaneous manifestations of dabrafenib (GSK2118436): A selective inhibitor of mutant BRAF in patients with metastatic melanoma. Br. J. Dermatol. 2012, 167, 1153–1160. Available online: https://pubmed.ncbi.nlm.nih.gov/22804352 (accessed on 11 March 2023). [CrossRef]
- Cohen, P.R.; Bedikian, A.Y.; Kim, K.B. Appearance of New Vemurafenib-associated Melanocytic Nevi on Normal-appearing Skin: Case Series and a Review of Changing or New Pigmented Lesions in Patients with Metastatic Malignant Melanoma After Ini-tiating Treatment with Vemurafenib. J. Clin. Aesthet. Dermatol. 2013, 6, 27. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3662681 (accessed on 12 March 2023).
- Haenssle, H.A.; Kraus, S.L.; Brehmer, F.; Kretschmer, L.; Völker, B.; Asper, H.; Kapp, A.; Gutzmer, R. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: Importance of sequential dermoscopy. Arch. Dermatol. 2012, 148, 1183–1185. Available online: https://pubmed.ncbi.nlm.nih.gov/22911096 (accessed on 12 March 2023). [CrossRef]
- Meneguzzo, A.; Lazzarotto, A.; Alaibac, M. Eruptive Melanocytic Nevi Secondary to Encorafenib for BRAF Mutant Metastatic Colorectal Cancer. Vivo 2019, 34, 441–445. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6984112 (accessed on 12 March 2023). [CrossRef] [Green Version]
- Anforth, R.M.; Carlos, G.R.; Scolyer, R.A.; Chou, S.; Fernandez-Peñas, P. Eruptive naevi in a patient treated with LGX818 for BRAF mutant metastatic melanoma. Melanoma Res. 2015, 25, 91–94. Available online: https://pubmed.ncbi.nlm.nih.gov/25380183 (accessed on 12 March 2023). [CrossRef]
- Zimmer, L.; Hillen, U.; Livingstone, E.; Lacouture, M.E.; Busam, K.; Carvajal, R.D.; Egberts, F.; Hauschild, A.; Kashani-Sabet, M.; Goldinger, S.M.; et al. Atypical Melanocytic Proliferations and New Primary Melanomas in Patients with Advanced Melanoma Undergoing Selective BRAF Inhibition. J. Clin. Oncol. 2012, 30, 2375–2383. Available online: https://pubmed.ncbi.nlm.nih.gov/22614973 (accessed on 12 March 2023). [CrossRef] [Green Version]
- Morita, H.; Nagai, R. Vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 365, 1448, author reply 1450. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21995398 (accessed on 12 March 2023).
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. Available online: https://pubmed.ncbi.nlm.nih.gov/25399551 (accessed on 12 March 2023). [CrossRef] [Green Version]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. Available online: https://pubmed.ncbi.nlm.nih.gov/30219628 (accessed on 12 March 2023). [CrossRef]
- Piraccini, B.M.; Patrizi, A.; Fanti, P.A.; Starace, M.; Bruni, F.; Melotti, B.; Misciali, C.; Dika, E. RASopathic alopecia: Hair changes associated with vemu-rafenib therapy. J. Am. Acad. Dermatol. 2015, 72, 738–741. Available online: http://www.jaad.org/article/S019096221500050X/fulltext (accessed on 12 March 2023). [CrossRef]
- Cavalieri, S.; Di Guardo, L.; Cossa, M.; Cimminiello, C.; Del Vecchio, M. Unusual Skin Carcinomas Induced by BRAF Inhibitor for Metastatic Melanoma: A Case Report. J. Clin. Diagn. Res. 2017, 11, XD06–XD08. Available online: https://pubmed.ncbi.nlm.nih.gov/28893027 (accessed on 12 March 2023). [CrossRef]
- Hui Ong, E.L.; Sinha, R.; Jmor, S.; Fearfield, L. BRAF Inhibitor-Associated Granulomatous Dermatitis: A Report of 3 Cases. Am. J. Dermatopathol. 2019, 41, 214–217. [Google Scholar] [CrossRef]
- Garrido, M.C.; Gutierrez, C.; Riveiro-Falkenbach, E.; Ortiz, P.; Rodriguez-Peralto, J.L. BRAF Inhibitor-Induced Antitumoral Granu-lomatous Dermatitis Eruption in Advanced Melanoma. Am. J. Dermatopathol. 2015, 37, 795–798. Available online: https://pubmed.ncbi.nlm.nih.gov/26381028 (accessed on 13 March 2023). [CrossRef]
- Park, J.J.; Hawryluk, E.B.; Tahan, S.R.; Flaherty, K.; Kim, C.C. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014, 150, 307–311. Available online: https://pubmed.ncbi.nlm.nih.gov/24352115 (accessed on 13 March 2023). [CrossRef] [Green Version]
- Chu, E.Y.; Wanat, K.A.; Miller, C.J.; Amaravadi, R.K.; Fecher, L.A.; Brose, M.S.; McGettigan, S.; Giles, L.R.; Schuchter, L.M.; Seykora, J.T.; et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: A clinicopathologic study. J. Am. Acad. Dermatol. 2012, 67, 1265–1272. Available online: https://pubmed.ncbi.nlm.nih.gov/22609219 (accessed on 13 March 2023). [CrossRef] [PubMed] [Green Version]
- Lilly, E.; Burke, M.; Kluger, H.; Choi, J. Pregabalin for the Treatment of Painful Hand-Foot Skin Reaction Associated with Dabrafenib. JAMA Dermatol. 2015, 151, 102. Available online: https://jamanetwork.com/journals/jamadermatology/fullarticle/1910311 (accessed on 12 March 2023). [CrossRef] [PubMed] [Green Version]
- Vázquez-Osorio, I.; Sánchez-Aguilar, M.D.; García-Rodiño, S.; Suárez-Peñaranda, J.M.; Aliste, C.; Vázquez-Veiga, H. Vemuraf-enib-induced neutrophilic panniculitis: A new case and review of the literature. Am. J. Dermatopathol. 2016, 38, e93–e96. [Google Scholar] [CrossRef] [PubMed]
- Monfort, J.-B.; Pagès, C.; Schneider, P.; Neyns, B.; Comte, C.; Bagot, M.; Vignon-Pennamen, M.-D.; Viguier, M.; Lebbé, C. Vemurafenib-induced neutrophilic panniculitis. Melanoma Res. 2012, 22, 399–401. Available online: https://pubmed.ncbi.nlm.nih.gov/22828248 (accessed on 13 March 2023). [CrossRef]
- Alonso-Castro, L.; Ríos-Buceta, L.; Vano-Galvan, S.; Moreno, C.; Soria-Rivas, A.; Jaén, P. Vitiligo in 2 patients receiving vemurafenib for metastatic melanoma. J. Am. Acad. Dermatol. 2013, 69, e28–e29. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23768302 (accessed on 13 March 2023). [CrossRef]
- Nasca, M.R.; Lacarrubba, F.; Ferraù, F.; Micali, G. Vitiligo of the Face in a Patient Treated with Vemurafenib for Metastatic Melanoma. J. Drugs Dermatol. 2016, 15, 766–768. Available online: https://pubmed.ncbi.nlm.nih.gov/27272087 (accessed on 13 March 2023).
- Jänne, P.A.; Shaw, A.T.; Pereira, J.R.; Jeannin, G.; Vansteenkiste, J.; Barrios, C.; Franke, F.A.; Grinsted, L.; Zazulina, V.; Smith, P.; et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013, 14, 38–47. Available online: https://pubmed.ncbi.nlm.nih.gov/23200175 (accessed on 13 March 2023). [CrossRef]
- Balagula, Y.; Huston, K.B.; Busam, K.J.; Lacouture, M.E.; Chapman, P.B.; Myskowski, P.L. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886). Investig. New Drugs 2011, 29, 1114. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5691597 (accessed on 13 March 2023). [CrossRef] [Green Version]
- Volontè, M.; Isoletta, E.; Gordon, S.; Foiadelli, T.; Bassanese, F.; Rossi, A.; Marseglia, G.L.; Savasta, S.; Brazzelli, V. Acneiform rash as a side effect of selumetinib in a child with neurofibromatosis type 1 treated for inoperable plexiform neurofibromas: Good results with doxycycline. Dermatol. Ther. 2022, 35, e15607. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9541585 (accessed on 13 March 2023). [CrossRef]
- Smith, T.M.; Gilliland, K.; Clawson, G.A.; Thiboutot, D. IGF-1 Induces SREBP-1 Expression and Lipogenesis in SEB-1 Sebocytes via Activation of the Phosphoinositide 3-Kinase/Akt Pathway. J. Investig. Dermatol. 2008, 128, 1286–1293. [Google Scholar] [CrossRef] [Green Version]
- Patel, U.; Cornelius, L.; Anadkat, M.J. MEK inhibitor-induced dusky erythema: Characteristic drug hypersensitivity manifestation in 3 patients. JAMA Dermatol. 2015, 151, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Carlberg, V.M.; Davies, O.M.T.; Brandling-Bennett, H.A.; Leary, S.E.S.; Huang, J.T.; Coughlin, C.C.; Gupta, D. Cutaneous reactions to pediatric cancer treatment part II: Targeted therapy. Pediatr. Dermatol. 2020, 38, 18–30. Available online: https://pubmed.ncbi.nlm.nih.gov/33378085 (accessed on 13 March 2023). [CrossRef]
- Hwang, S.J.E.; Anforth, R.; Carlos, G.; Fernandez-Peñas, P. Cutaneous Adverse Events of New Anti-melanoma Therapies: Classification and Management. Actas Dermosifiliogr. 2017, 108, 6–16. Available online: https://pubmed.ncbi.nlm.nih.gov/27642030 (accessed on 14 March 2023). [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012, 367, 107–114. Available online: https://pubmed.ncbi.nlm.nih.gov/22663011 (accessed on 14 March 2023). [CrossRef] [Green Version]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. Available online: https://pubmed.ncbi.nlm.nih.gov/28029918 (accessed on 14 March 2023). [CrossRef]
- Song, H.; Zhong, C.S.; Kieran, M.W.; Chi, S.N.; Wright, K.D.; Huang, J.T. Cutaneous reactions to targeted therapies in children with CNS tumors: A cross-sectional study. Pediatr. Blood Cancer 2019, 66, e27682. Available online: https://pubmed.ncbi.nlm.nih.gov/30821092 (accessed on 14 March 2023). [CrossRef]
- Alkeraye, S.; Maire, C.; Desmedt, E.; Templier, C.; Mortier, L. Persistent alopecia induced by vismodegib. Br. J. Dermatol. 2015, 172, 1671–1672. [Google Scholar] [CrossRef]
- Puig, S.; Serra-Guillén, C.; Pérez-Pastor, G.; Martínez-Domenech, Á.; Cabrera, R.F.-D. Experience with sonidegib in patients with advanced basal cell carcinoma: Case reports. Drugs Context 2022, 11, 1–8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9132536 (accessed on 17 May 2023). [CrossRef]
- Fife, K.; Herd, R.; Lalondrelle, S.; Plummer, R.; Strong, A.; Jones, S.; Lear, J.T. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Futur. Oncol. 2017, 13, 175–184. [Google Scholar] [CrossRef]
- Reversible Cutaneous Side Effects of Vismodegib Treatment|MDedge Dermatology. Available online: https://www.mdedge.com/dermatology/article/133890/hair-nails/reversible-cutaneous-side-effects-vismodegib-treatment (accessed on 15 March 2023).
- Le Moigne, M.; Saint-Jean, M.; Jirka, A.; Quéreux, G.; Peuvrel, L.; Brocard, A.; Gaultier, A.; Khammari, A.; Darmaun, M.; Dréno, B. Dysgeusia and weight loss under treatment with vismodegib: Benefit of nutritional management. Support. Care Cancer 2015, 24, 1689–1695. [Google Scholar] [CrossRef]
- Yang, H.; Cong, W.-N.; Yoon, J.S.; Egan, J.M. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. Cancer Med. 2015, 4, 245–252. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4329008 (accessed on 18 March 2023). [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Carrillo-Gijon, R.; Sendagorta, E.; Rios-Buceta, L. Acute generalized exanthematous pustulosis simulating Stevens-Johnson syndrome/toxic epidermal necrolysis associated with the use of vismodegib. JAAD Case Rep. 2018, 4, 123–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplash, G.; Curragh, D.S.; Halliday, L.; Huilgol, S.C.; Selva, D. Report of cutaneous side effects of vismodegib treatment. Clin. Exp. Ophthalmol. 2019, 48, 123–125. Available online: https://pubmed.ncbi.nlm.nih.gov/31569297 (accessed on 18 March 2023). [CrossRef] [PubMed]
- Aasi, S.; Silkiss, R.; Tang, J.Y.; Wysong, A.; Liu, A.; Epstein, E.; Oro, A.E.; Chang, A.L.S. New Onset of Keratoacanthomas After Vismodegib Treatment for Locally Advanced Basal Cell Carcinomas: A Report of 2 Cases. JAMA Dermatol. 2013, 149, 242–243. Available online: https://pubmed.ncbi.nlm.nih.gov/23426496 (accessed on 18 March 2023). [CrossRef] [Green Version]



| Adverse Reaction | Grace 1 | Grade 2 | Grade 3 | Life-Threatening Reactions |
|---|---|---|---|---|
| Maculopapular rash | Macules/papules covering\10% BSA with or without symptoms (e.g., pruritus, burning, tightness) | Macules/papules covering 10–30% BSA with or without symptoms (e.g., pruritus, burning, tightness) | Macules/papules covering 30% BSA with or without associated symptoms; limiting self-care activities | - |
| Pruritus | Mild or localized; topical intervention indicated | Intense or widespread; intermittent; skin changes from scratching (e.g., edema, papulation, excoriations, lichenification, oozing/crusts); oral intervention indicated | Intense or widespread; constant; limiting self-care activities or sleep; oral corticosteroid or immunosuppressive therapy indicated |
| Less Frequently Encountered ICI-Associated irAEs |
|---|
| Acneiform rash/ papulopustular folliculitis [5,43,94] |
| Dermatomyositis [95,96,97] |
| Eruptive keratoacanthomas [98] |
| Erythema nodosum [99,100,101] |
| Grover’s disease [102,103,104] |
| Photosensitivity [5] |
| Pyoderma gangrenosum [105,106] |
| Sarcoidosis [107,108,109,110,111] |
| Sjogren’s syndrome [112] |
| Sweet syndrome [113,114] |
| Rosacea [115] |
| Urticaria [5] |
| Vasculitis [116,117] |
| Less Frequently Encountered BRAFi-Associated Cutaneous Adverse Events |
|---|
| Acneiform eruption [123,124] |
| Alopecia (telogen effluvium) [136] |
| Basal cell carcinoma [137] |
| Cheilitis [9] |
| Granulomatous eruption [138,139,140] |
| Grover’s disease [141] |
| Hand-foot skin reaction [142] |
| Milia [9] |
| Panniculitis [143,144] |
| Pruritus [10,14] |
| Vitiligo [145,146] |
| Xerosis [9] |
| Less Frequently Encountered MEKi-Associated Cutaneous Adverse Events |
|---|
| Angular cheilitis [14] |
| Cellulitis [153,155,156] |
| DRESS syndrome [153,155,156] |
| Hair disorders [152] |
| Mucositis [14] |
| Paronychia and periungual fissuring [14] |
| Psoriasiform scalp dermatitis |
| Pruritus [153] |
| Teleangiectasias [14] |
| Urticaria [153,155,156] |
| Xerosis [152] |
| Less Frequently Encountered Cutaneous Adverse Reactions under Treatment with Hedgehog Inhibitors |
|---|
| AGEP [163] |
| Cutaneous eruptions (maculopapular, papulopustular) [164] |
| Folliculitis [160] |
| Grover’s disease [164] |
| Hypersensitivity reactions [160] |
| Keratoacanthomas [165] |
| Stevens-Johnson syndrome/Toxic epidermal necrolysis [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachouri, K.-M.; Florou, V.; Georgiou, V.; Georgiou, S. Cutaneous Side Effects of Modern Targeted Therapy and Immunotherapy in Patients with Dermatological Malignancies. Cancers 2023, 15, 3126. https://doi.org/10.3390/cancers15123126
Plachouri K-M, Florou V, Georgiou V, Georgiou S. Cutaneous Side Effects of Modern Targeted Therapy and Immunotherapy in Patients with Dermatological Malignancies. Cancers. 2023; 15(12):3126. https://doi.org/10.3390/cancers15123126
Chicago/Turabian StylePlachouri, Kerasia-Maria, Vaia Florou, Vasileios Georgiou, and Sophia Georgiou. 2023. "Cutaneous Side Effects of Modern Targeted Therapy and Immunotherapy in Patients with Dermatological Malignancies" Cancers 15, no. 12: 3126. https://doi.org/10.3390/cancers15123126
APA StylePlachouri, K.-M., Florou, V., Georgiou, V., & Georgiou, S. (2023). Cutaneous Side Effects of Modern Targeted Therapy and Immunotherapy in Patients with Dermatological Malignancies. Cancers, 15(12), 3126. https://doi.org/10.3390/cancers15123126






