A β-Arrestin 2-Biased Dopamine Receptor Type 2 (DRD2) Agonist Is More Efficacious Than Cabergoline in Reducing Cell Proliferation in PRL-Secreting but Not in Non-Functioning Pituitary Tumor Cells
Abstract
:Simple Summary
Abstract
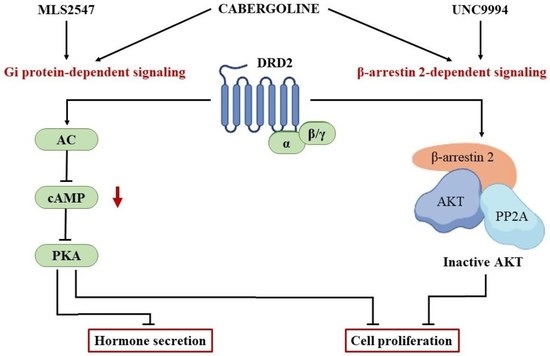
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Proliferation Assay
2.3. Western Blot Analysis
2.4. β-Arrestin 2 Silencing
2.5. Pertussis Toxin Pretreatment
2.6. PRL Secretion Assay
2.7. RT-PCR and Real-Time RT-PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. DRD2-Biased Agonists’ Effects on Cell Proliferation and PRL Secretion in MMQ Cells
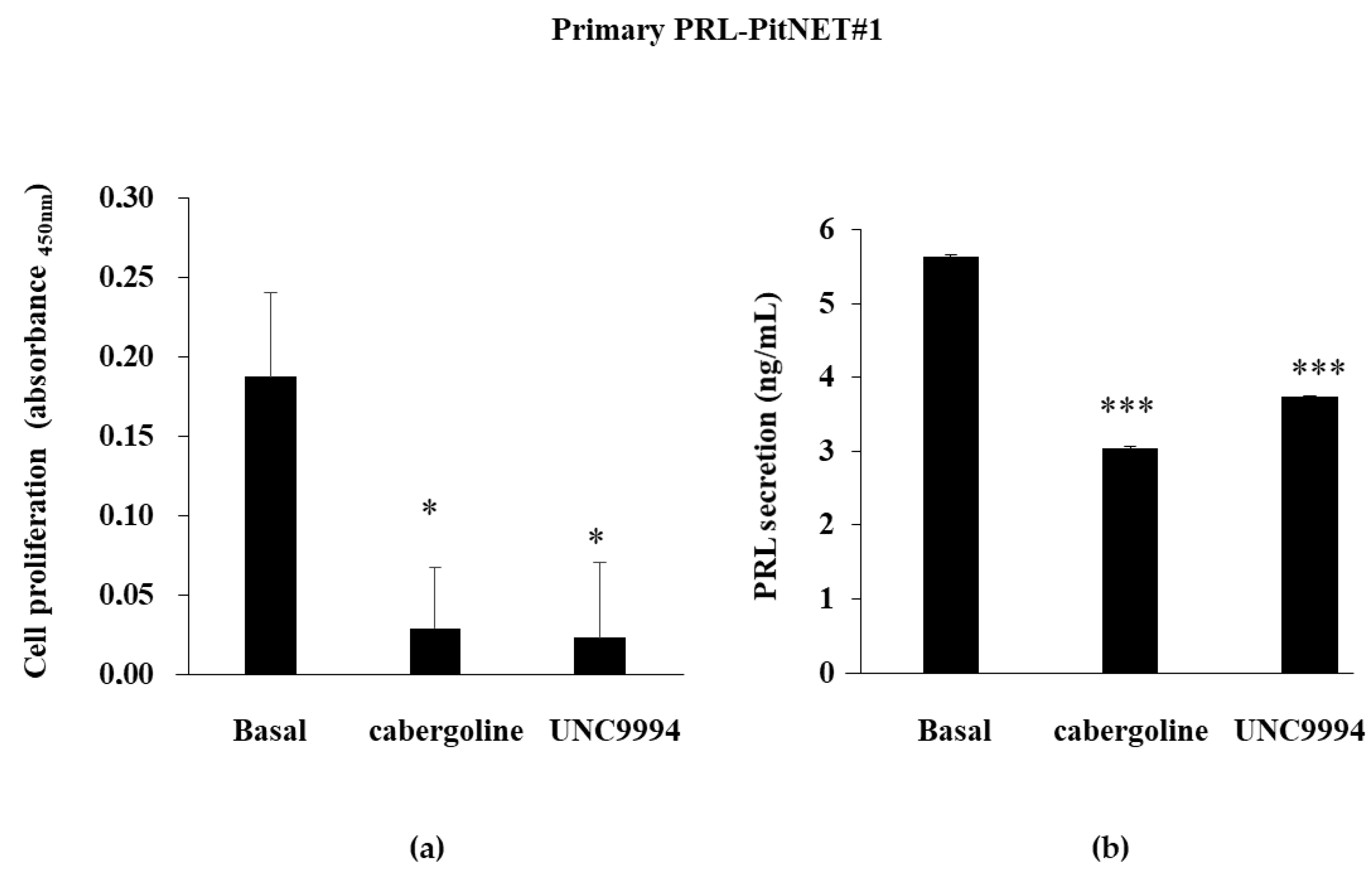
3.2. DRD2-Biased Agonists’ Effects on Cell Proliferation and PRL Secretion in Primary Cultured PRL-PitNET Cells
3.3. DRD2-Biased Agonists’ Effects on Cell Proliferation in Primary Cultured NF-PitNET Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Endocrine Society. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Greenman, Y.; Tordjman, K.; Osher, E.; Veshchev, I.; Shenkerman, G.; Reider-Groswasser, I.I.; Segev, Y.; Ouaknine, G.; Stern, N. Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth. Clin. Endocrinol. 2005, 63, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Greenman, Y.; Cooper, O.; Yaish, I.; Robenshtok, E.; Sagiv, N.; Jonas-Kimchi, T.; Yuan, X.; Gertych, A.; Shimon, I.; Ram, Z.; et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur. J. Endocrinol. 2016, 175, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colao, A.; Di Somma, C.; Pivonello, R.; Faggiano, A.; Lombardi, G.; Savastano, S. Medical therapy for clinically non-functioning pituitary adenomas. Endocr. Relat. Cancer 2008, 15, 905–915. [Google Scholar] [CrossRef]
- Cooper, O.; Greenman, Y. Dopamine Agonists for Pituitary Adenomas. Front. Endocrinol. 2018, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Even-Zohar, N.; Greenman, Y. Management of NFAs: Medical treatment. Pituitary 2018, 21, 168–175. [Google Scholar] [CrossRef]
- Peverelli, E.; Treppiedi, D.; Mangili, F.; Catalano, R.; Spada, A.; Mantovani, G. Drug resistance in pituitary tumours: From cell membrane to intracellular signalling. Nat. Rev. Endocrinol. 2021, 17, 560–571. [Google Scholar] [CrossRef]
- Sibley, D.R.; Monsma, F.J., Jr. Molecular biology of dopamine receptors. Trends Pharmacol. Sci. 1992, 13, 61–69. [Google Scholar] [CrossRef]
- Kim, K.M.; Valenzano, K.J.; Robinson, S.R.; Yao, W.D.; Barak, L.S.; Caron, M.G. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J. Biol. Chem. 2001, 276, 37409–37414. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Kim, M.Y.; Lee, E.J.; Ahn, Y.S.; Baik, J.H. Distinct regulation of internalization and mitogen-activated protein kinase activation by two isoforms of the dopamine D2 receptor. Mol. Endocrinol. 2004, 18, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol. Sci. 2007, 28, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Mangili, F.; Giardino, E.; Treppiedi, D.; Barbieri, A.M.; Catalano, R.; Locatelli, M.; Lania, A.G.; Spada, A.; Arosio, M.; Mantovani, G.; et al. Beta-Arrestin 2 Is Required for Dopamine Receptor Type 2 Inhibitory Effects on AKT Phosphorylation and Cell Proliferation in Pituitary Tumors. Neuroendocrinology 2021, 111, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Sotnikova, T.D.; Marion, S.; Lefkowitz, R.J.; Gainetdinov, R.R.; Caron, M.G. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005, 122, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Vitali, E.; Peverelli, E.; Giardino, E.; Locatelli, M.; Lasio, G.B.; Beck-Peccoz, P.; Spada, A.; Lania, A.G.; Mantovani, G. Cyclic adenosine 3′-5′-monophosphate (cAMP) exerts proliferative and anti-proliferative effects in pituitary cells of different types by activating both cAMP-dependent protein kinase A (PKA) and exchange proteins directly activated by cAMP (Epac). Mol. Cell. Endocrinol. 2014, 383, 193–202. [Google Scholar] [CrossRef]
- Peverelli, E.; Mantovani, G.; Lania, A.G.; Spada, A. cAMP in the pituitary: An old messenger for multiple signals. J. Mol. Endocrinol. 2013, 52, R67–R77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, J.D.; Clarke, W.P.; von Zastrow, M.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.P.; Feng, B.; et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef] [Green Version]
- Free, R.B.; Chun, L.S.; Moritz, A.E.; Miller, B.N.; Doyle, T.B.; Conroy, J.L.; Padron, A.; Meade, J.A.; Xiao, J.; Hu, X.; et al. Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor. Mol. Pharmacol. 2014, 86, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Peverelli, E.; Giardino, E.; Treppiedi, D.; Meregalli, M.; Belicchi, M.; Vaira, V.; Corbetta, S.; Verdelli, C.; Verrua, E.; Serban, A.L.; et al. Dopamine receptor type 2 (DRD2) and somatostatin receptor type 2 (SSTR2) agonists are effective in inhibiting proliferation of progenitor/stem-like cells isolated from nonfunctioning pituitary tumors. Int. J. Cancer 2017, 140, 1870–1880. [Google Scholar] [CrossRef] [Green Version]
- Mangili, F.; Treppiedi, D.; Catalano, R.; Marra, G.; Di Muro, G.; Spada, A.; Arosio, M.; Peverelli, E.; Mantovani, G. A Novel Mechanism Regulating Dopamine Receptor Type 2 Signal Transduction in Pituitary Tumoral Cells: The Role of cAMP/PKA-Induced Filamin A Phosphorylation. Front. Endocrinol. 2021, 11, 611752. [Google Scholar] [CrossRef]
- Mangili, F.; Esposito, E.; Treppiedi, D.; Catalano, R.; Marra, G.; Di Muro, G.; Barbieri, A.M.; Locatelli, M.; Lania, A.G.; Mangone, A.; et al. DRD2 Agonist Cabergoline Abolished the Escape Mechanism Induced by mTOR Inhibitor Everolimus in Tumoral Pituitary Cells. Front. Endocrinol. 2022, 13, 867822. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Tan, Z.; Lei, Z.; Yan, Z.; Ji, X.; Chang, X.; Cai, Z.; Lu, L.; Qi, Y.; Yin, X.; Han, X.; et al. Exploiting D2 receptor β-arrestin2-biased signalling to suppress tumour growth of pituitary adenomas. Br. J. Pharmacol. 2021, 178, 3570–3586. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, A.E.; Murano, T.; Li, S.; Tomić, M.; Stojilkovic, S.S. Dopamine inhibits basal prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology 2008, 149, 1470–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burris, T.P.; Nguyen, D.N.; Smith, S.G.; Freeman, M.E. The stimulatory and inhibitory effects of dopamine on prolactin secretion involve different G-proteins. Endocrinology 1992, 130, 926–932. [Google Scholar] [PubMed]
- Enjalbert, A.; Bockaert, J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol. Pharmacol. 1983, 23, 576–584. [Google Scholar] [PubMed]
- Cronin, M.J.; Myers, G.A.; MacLeod, R.M.; Hewlett, E.L. Pertussis toxin uncouples dopamine agonist inhibition of prolactin release. Am. J. Physiol. 1983, 244, E499–E504. [Google Scholar] [CrossRef]
- Ågren, R.; Århem, P.; Nilsson, J.; Sahlholm, K. The Beta-Arrestin-Biased Dopamine D2 Receptor Ligand, UNC9994, Is a Partial Agonist at G-Protein-Mediated Potassium Channel Activation. Int. J. Neuropsychopharmacol. 2018, 21, 1102–1108. [Google Scholar] [CrossRef] [Green Version]
- Melkersson, K.; Hulting, A.L. Prolactin-secreting pituitary adenoma in neuroleptic treated patients with psychotic disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 6–10. [Google Scholar] [CrossRef]
- Broekhof, R.; Gosselink, M.J.; Pijl, H.; Giltay, E.J. The effect of aripiprazole and quinagolide, a dopamine agonist, in a patient with symptomatic pituitary prolactinoma and chronic psychosis. Gen. Hosp. Psychiatry 2012, 34, 209.e1–209.e3. [Google Scholar] [CrossRef]
- Chang, S.C.; Chen, C.H.; Lu, M.L. Cabergoline-induced psychotic exacerbation in schizophrenic patients. Gen. Hosp. Psychiatry 2008, 30, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Urs, N.M.; Gee, S.M.; Pack, T.F.; McCorvy, J.D.; Evron, T.; Snyder, J.C.; Yang, X.; Rodriguiz, R.M.; Borrelli, E.; Wetsel, W.C.; et al. Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc. Natl. Acad. Sci. USA 2016, 113, E8178–E8186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pack, T.F.; Orlen, M.I.; Ray, C.; Peterson, S.M.; Caron, M.G. The dopamine D2 receptor can directly recruit and activate GRK2 without G protein activation. J. Biol. Chem. 2018, 293, 6161–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatto, F.; Feelders, R.; van der Pas, R.; Kros, J.M.; Dogan, F.; van Koetsveld, P.M.; van der Lelij, A.J.; Neggers, S.J.C.M.M.; Minuto, F.; de Herder, W.; et al. Hofland. β-Arrestin 1 and 2 and G Protein-Coupled Receptor Kinase 2 Expression in Pituitary Adenomas: Role in the Regulation of Response to Somatostatin Analogue Treatment in Patients with Acromegaly. Endocrinology 2013, 154, 4715–4725. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muro, G.; Mangili, F.; Esposito, E.; Barbieri, A.M.; Catalano, R.; Treppiedi, D.; Marra, G.; Nozza, E.; Lania, A.G.A.; Ferrante, E.; et al. A β-Arrestin 2-Biased Dopamine Receptor Type 2 (DRD2) Agonist Is More Efficacious Than Cabergoline in Reducing Cell Proliferation in PRL-Secreting but Not in Non-Functioning Pituitary Tumor Cells. Cancers 2023, 15, 3218. https://doi.org/10.3390/cancers15123218
Di Muro G, Mangili F, Esposito E, Barbieri AM, Catalano R, Treppiedi D, Marra G, Nozza E, Lania AGA, Ferrante E, et al. A β-Arrestin 2-Biased Dopamine Receptor Type 2 (DRD2) Agonist Is More Efficacious Than Cabergoline in Reducing Cell Proliferation in PRL-Secreting but Not in Non-Functioning Pituitary Tumor Cells. Cancers. 2023; 15(12):3218. https://doi.org/10.3390/cancers15123218
Chicago/Turabian StyleDi Muro, Genesio, Federica Mangili, Emanuela Esposito, Anna Maria Barbieri, Rosa Catalano, Donatella Treppiedi, Giusy Marra, Emma Nozza, Andrea G. A. Lania, Emanuele Ferrante, and et al. 2023. "A β-Arrestin 2-Biased Dopamine Receptor Type 2 (DRD2) Agonist Is More Efficacious Than Cabergoline in Reducing Cell Proliferation in PRL-Secreting but Not in Non-Functioning Pituitary Tumor Cells" Cancers 15, no. 12: 3218. https://doi.org/10.3390/cancers15123218
APA StyleDi Muro, G., Mangili, F., Esposito, E., Barbieri, A. M., Catalano, R., Treppiedi, D., Marra, G., Nozza, E., Lania, A. G. A., Ferrante, E., Locatelli, M., Arosio, M., Peverelli, E., & Mantovani, G. (2023). A β-Arrestin 2-Biased Dopamine Receptor Type 2 (DRD2) Agonist Is More Efficacious Than Cabergoline in Reducing Cell Proliferation in PRL-Secreting but Not in Non-Functioning Pituitary Tumor Cells. Cancers, 15(12), 3218. https://doi.org/10.3390/cancers15123218