Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution
Abstract
:Simple Summary
Abstract
1. Introduction
2. When Should We Consider That an Oral Carcinoma Has Been Early Diagnosed?
3. Diagnosis of Oral Cancer: A Tortuous Path
4. Why Is Oral Cancer Diagnosis So Often Delayed? How to Fight It?
5. Final Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
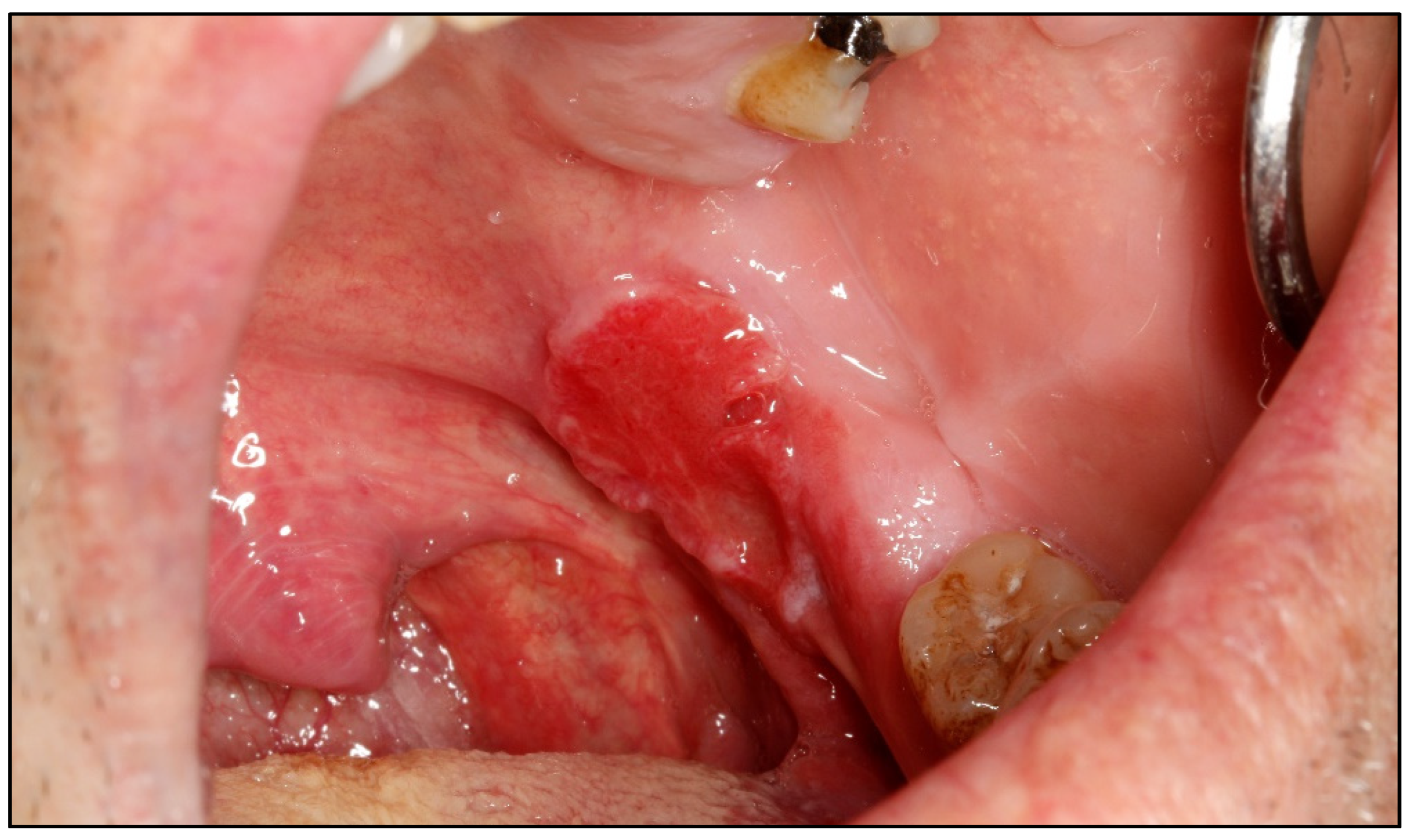
Appendix A. Clinical Presentation of OPMD
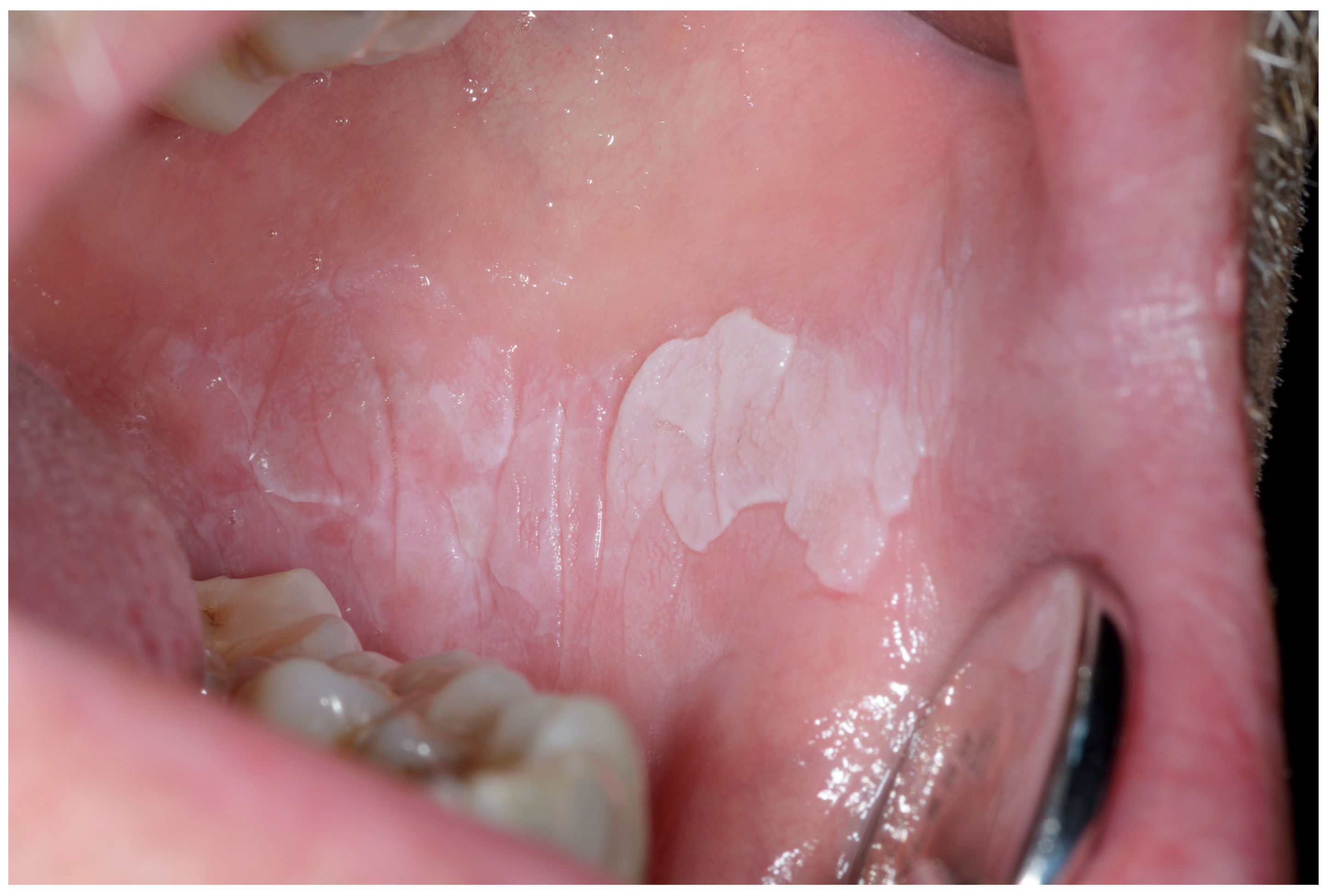
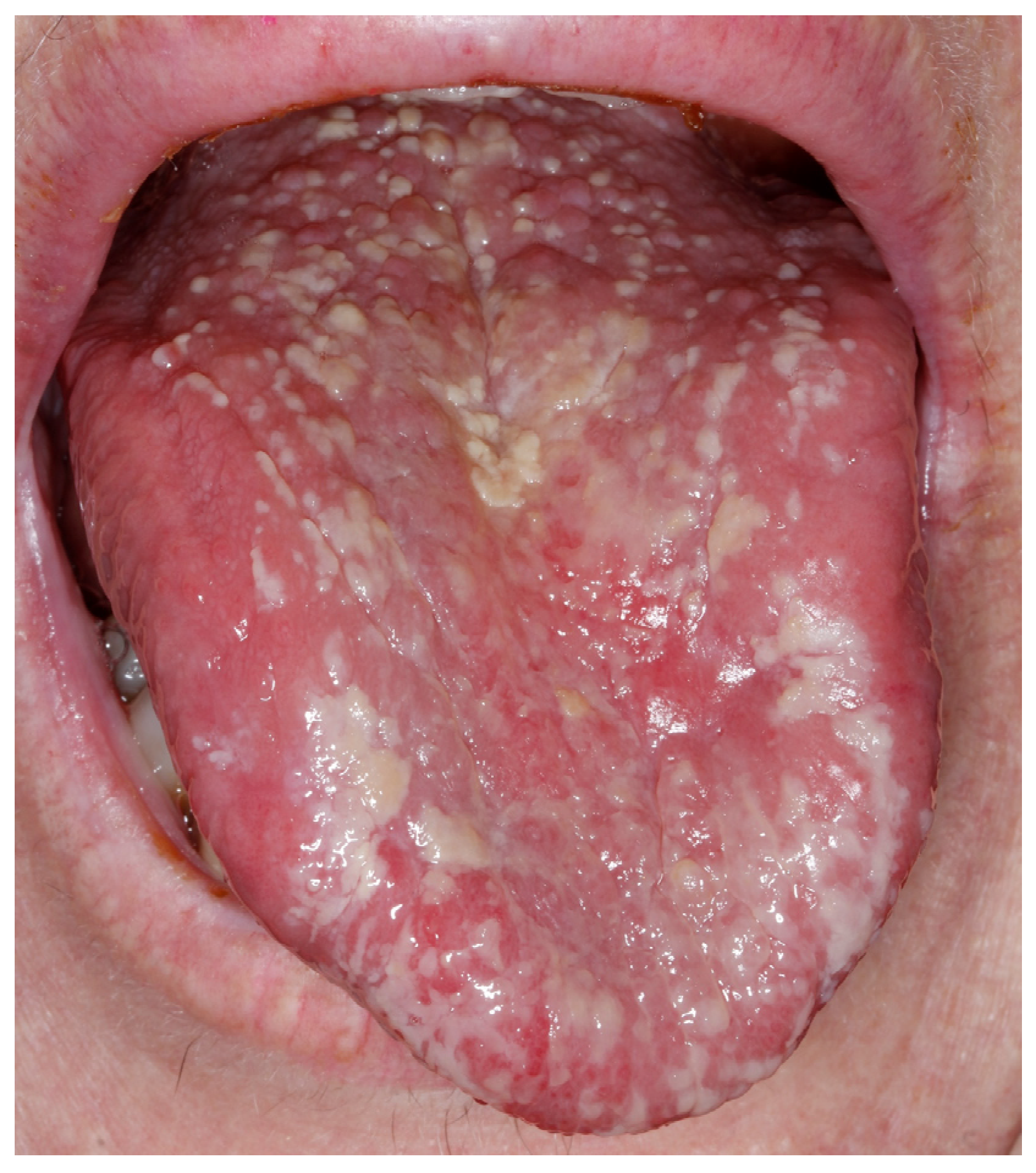
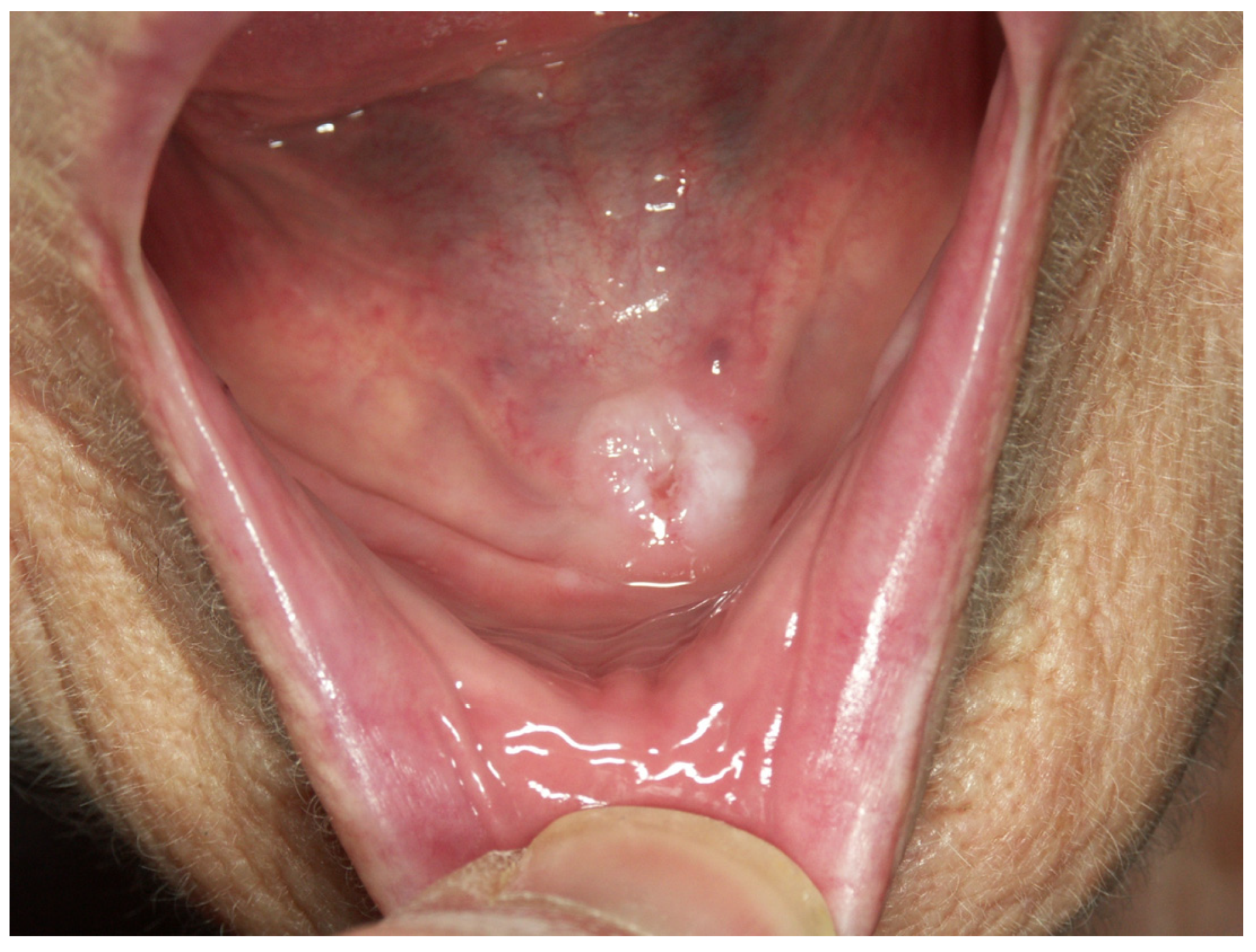

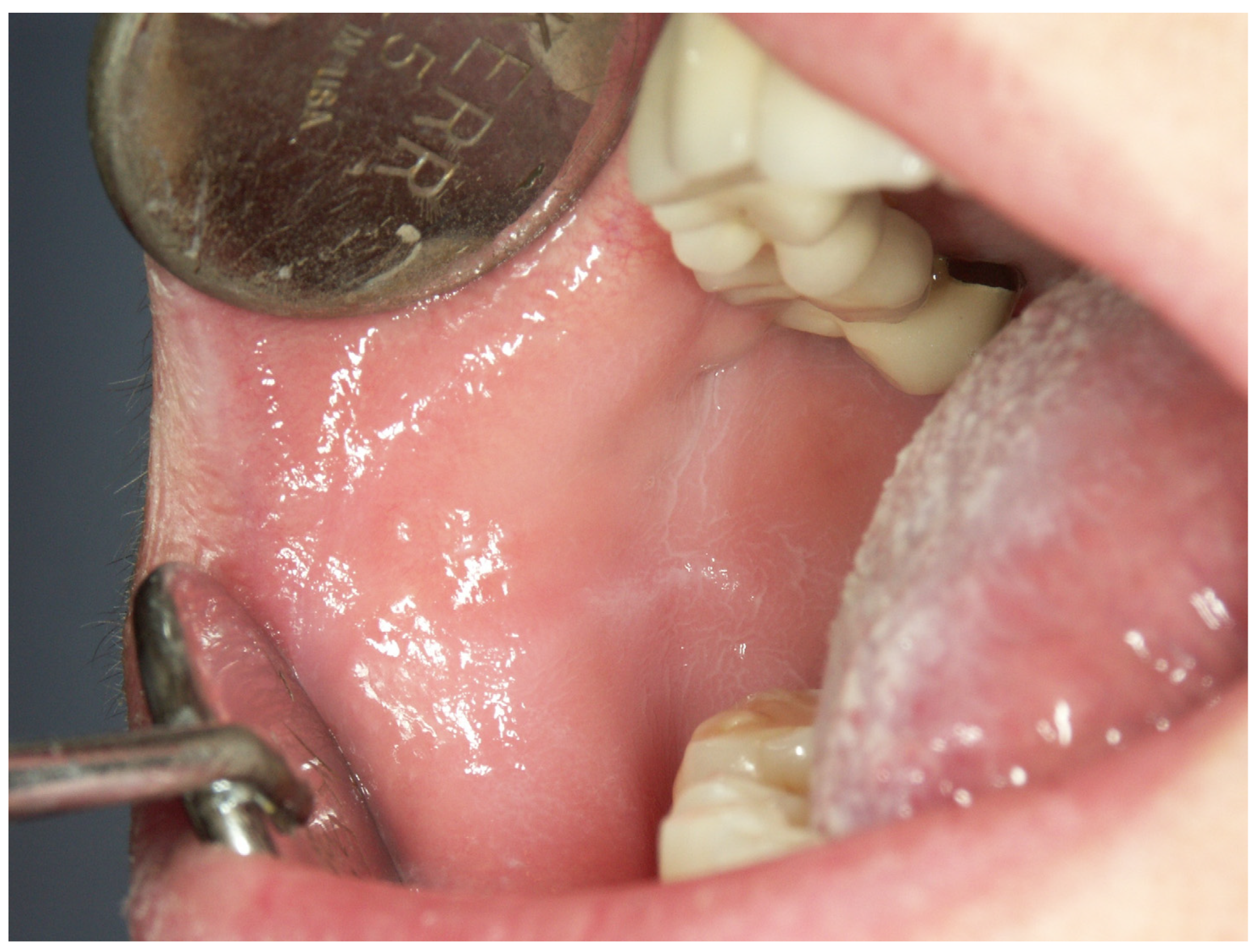



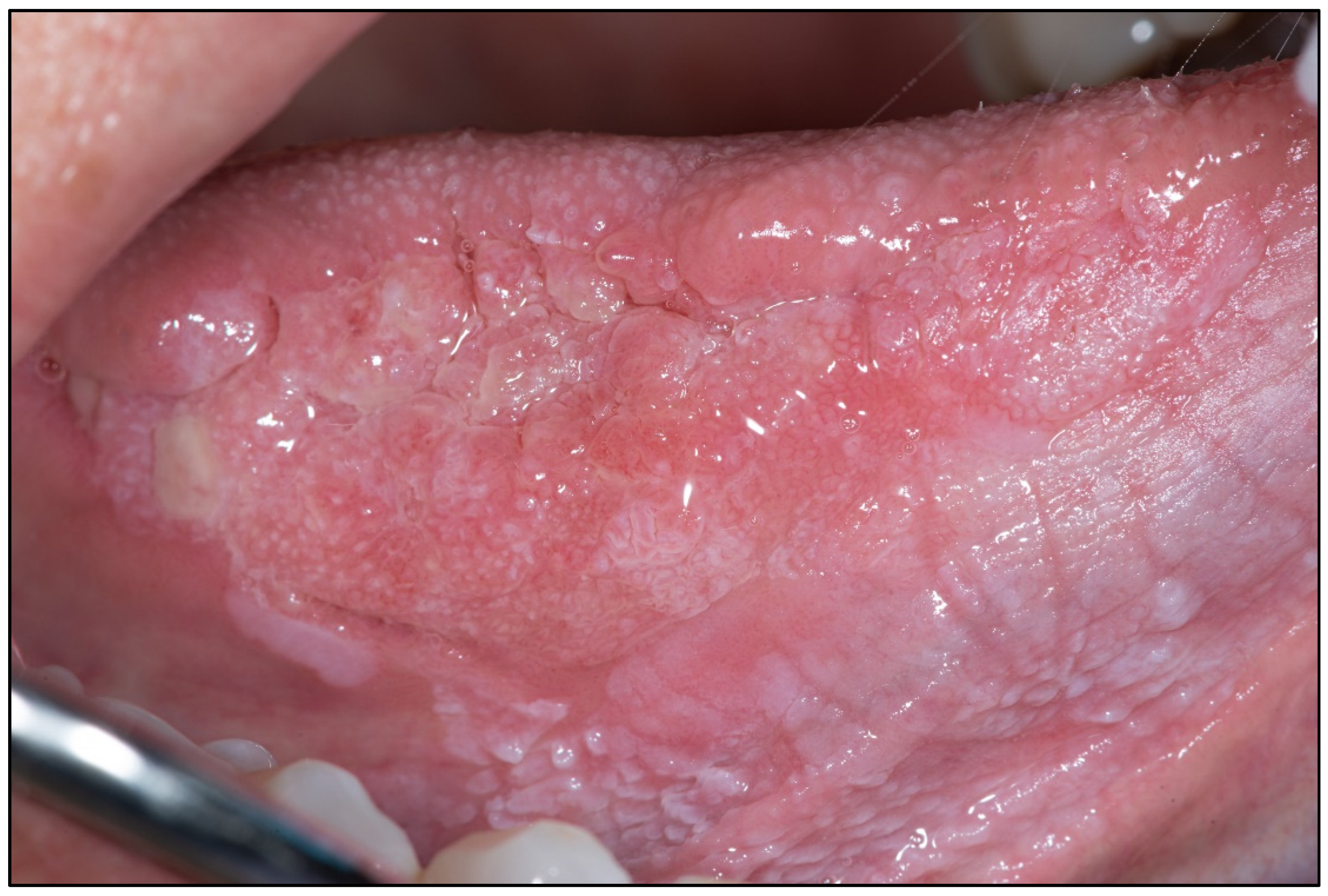
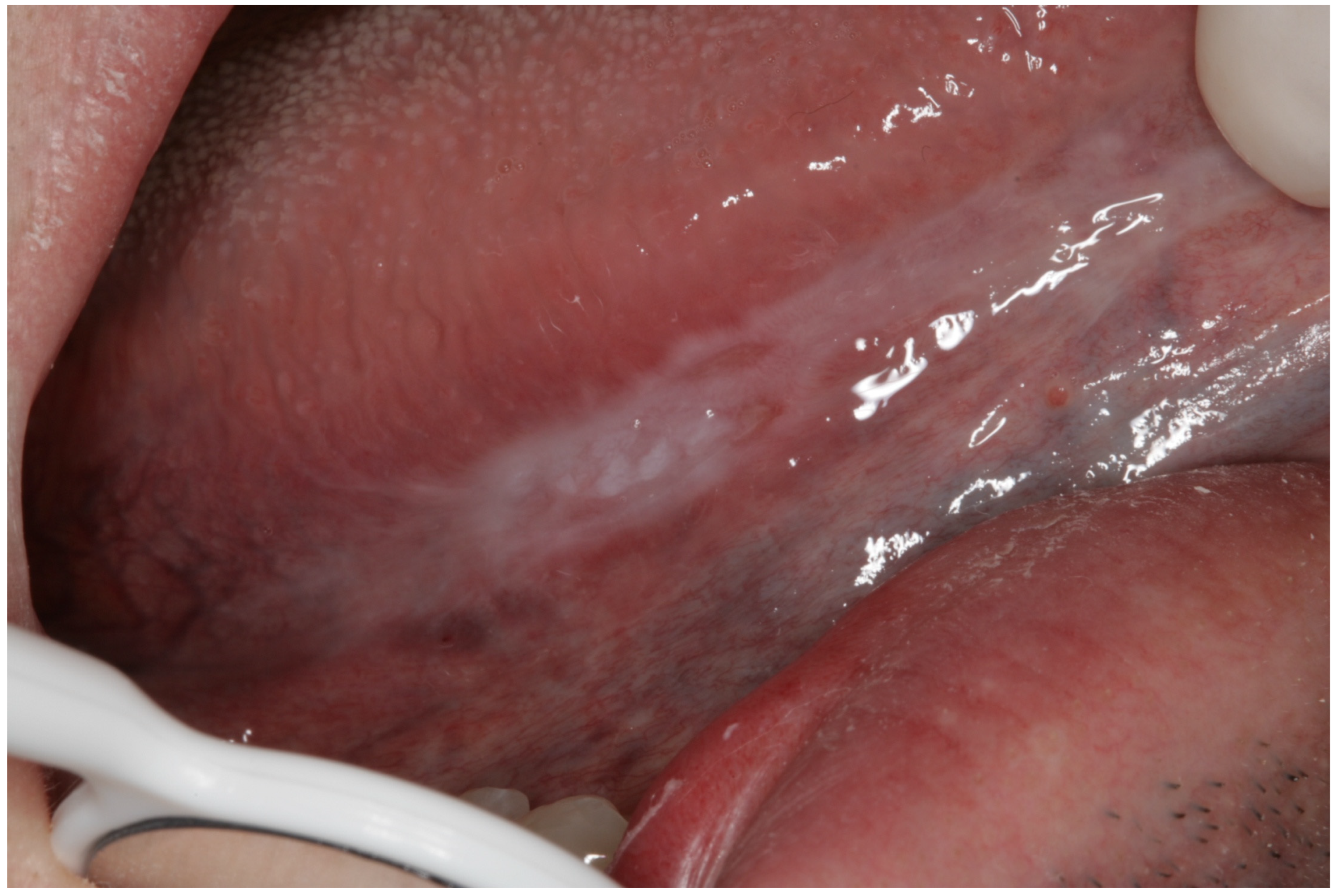

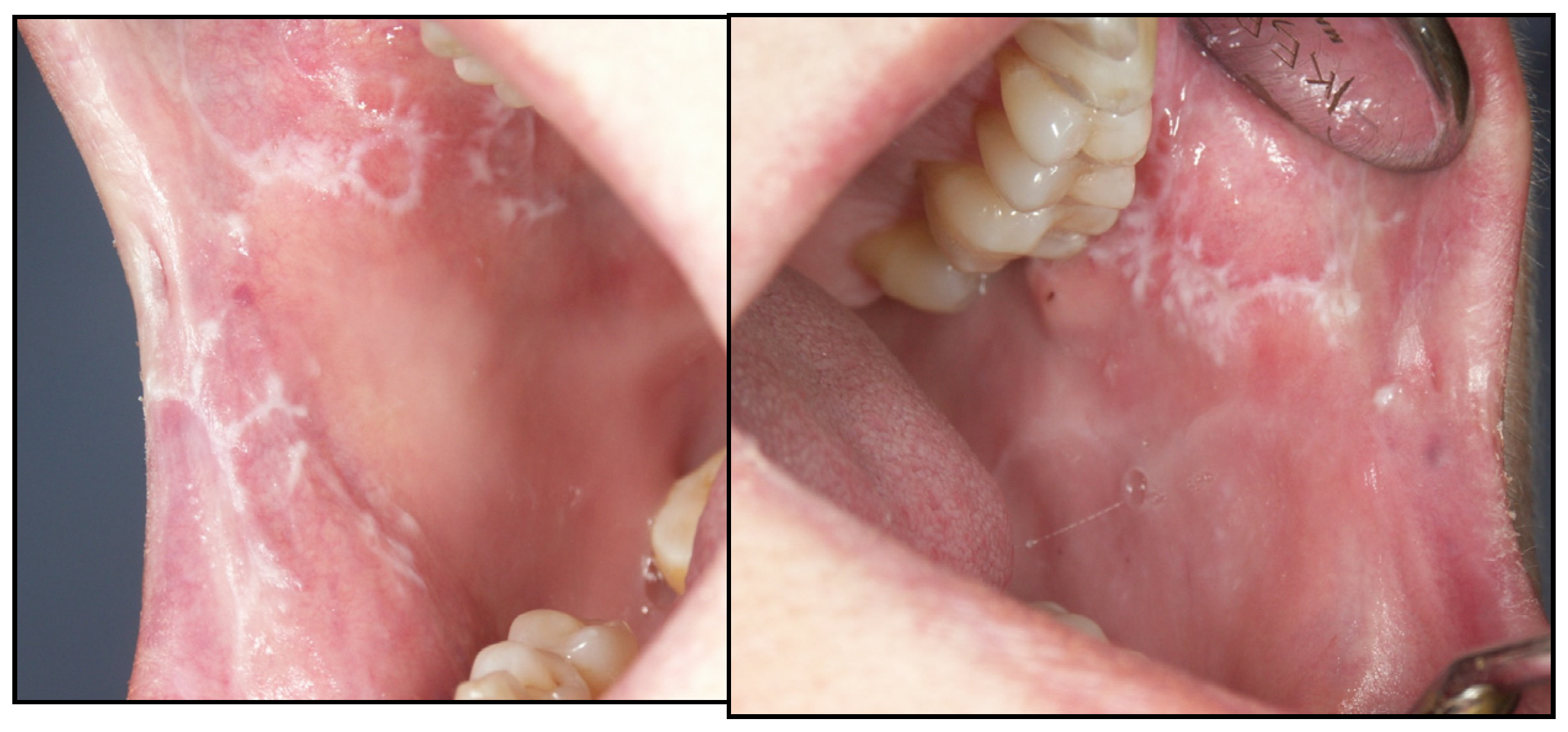
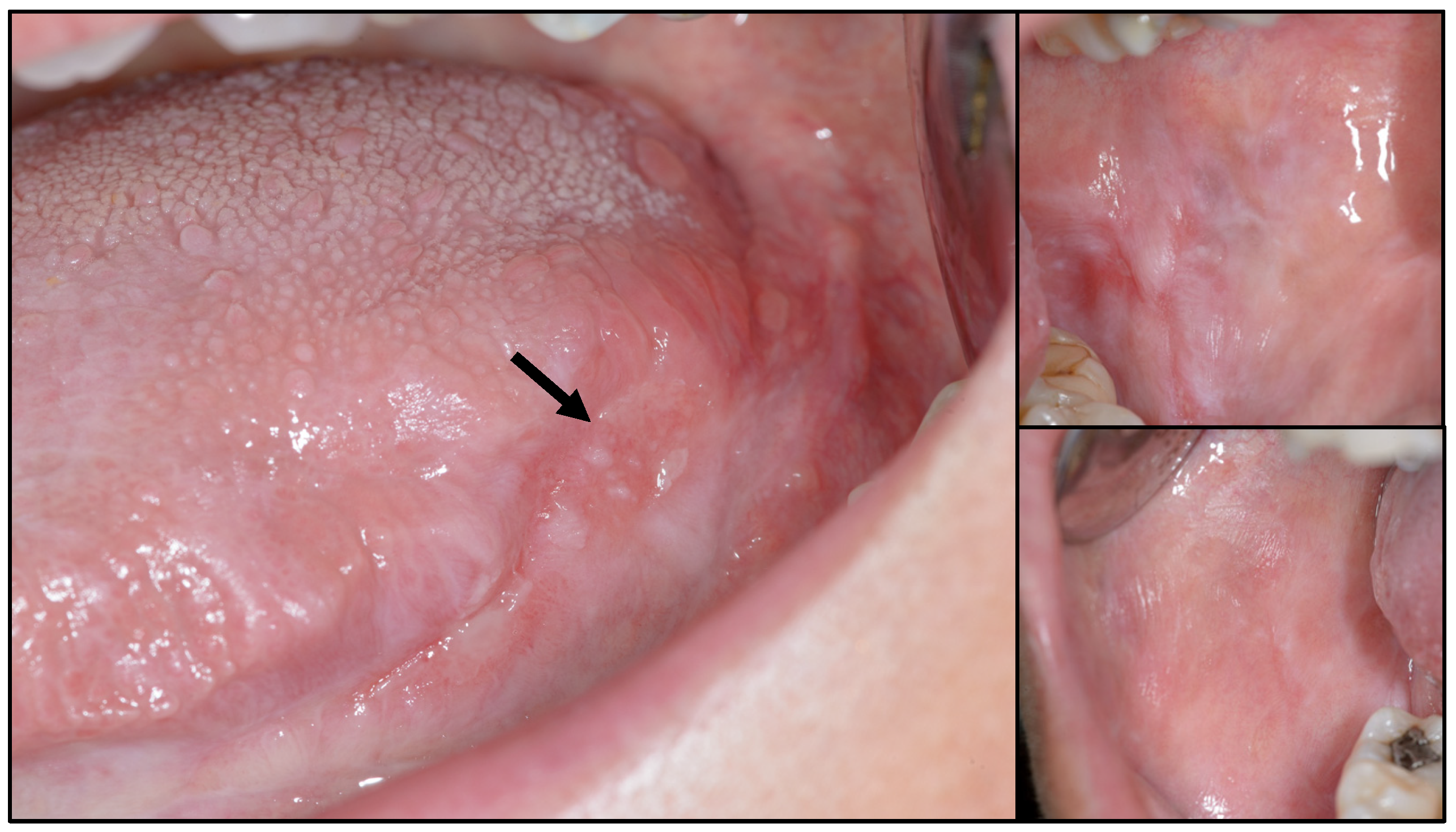
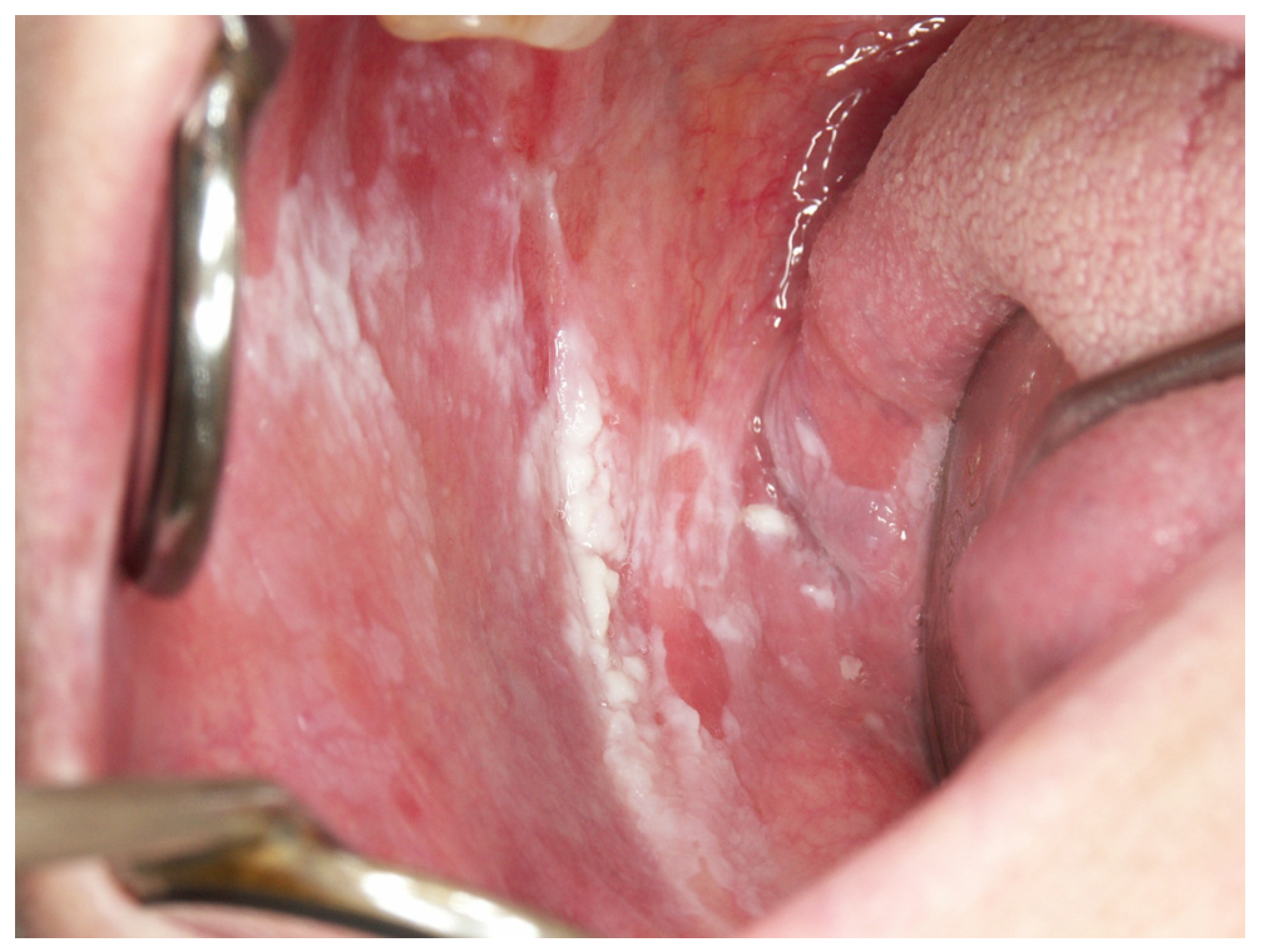
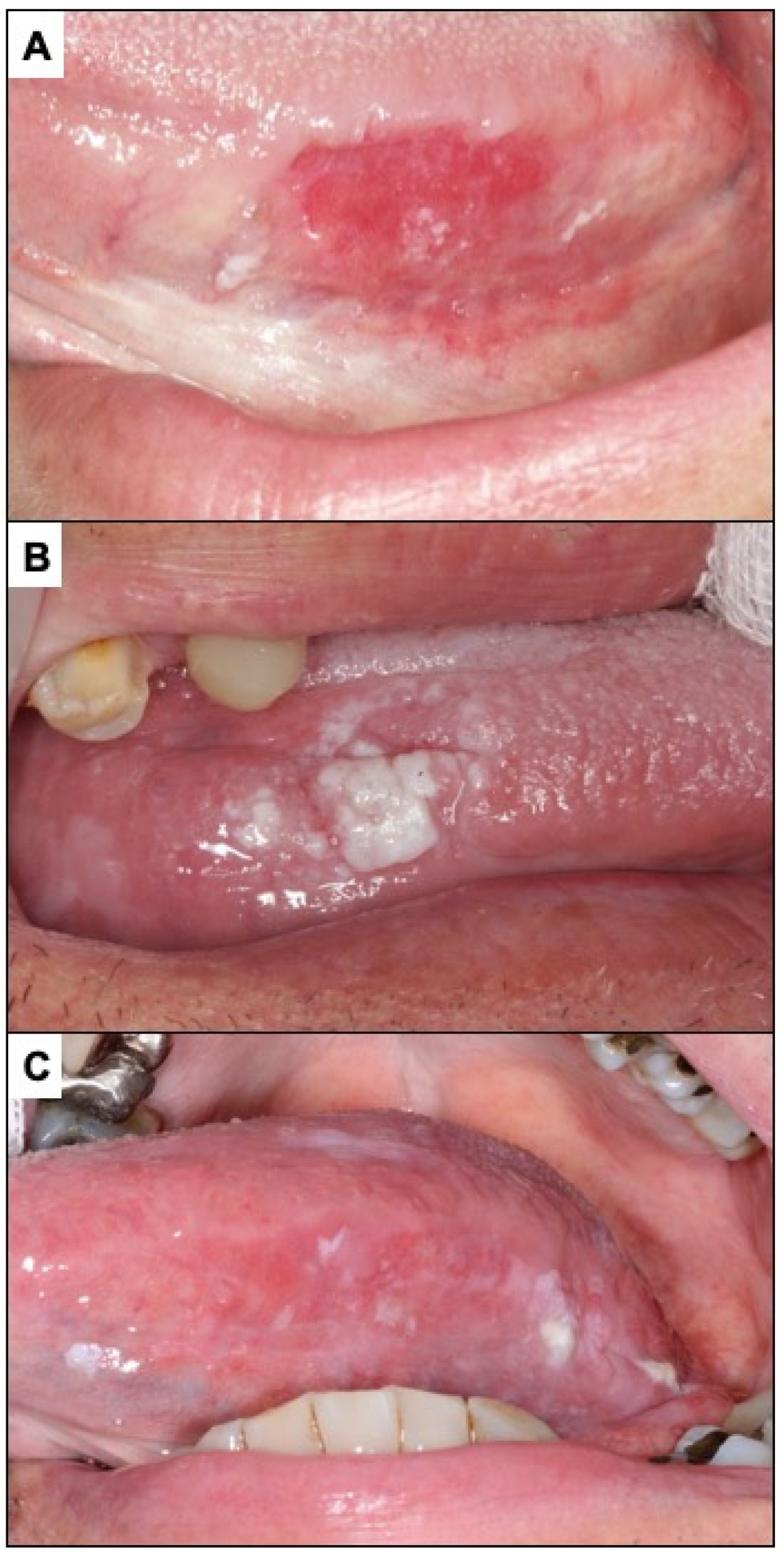

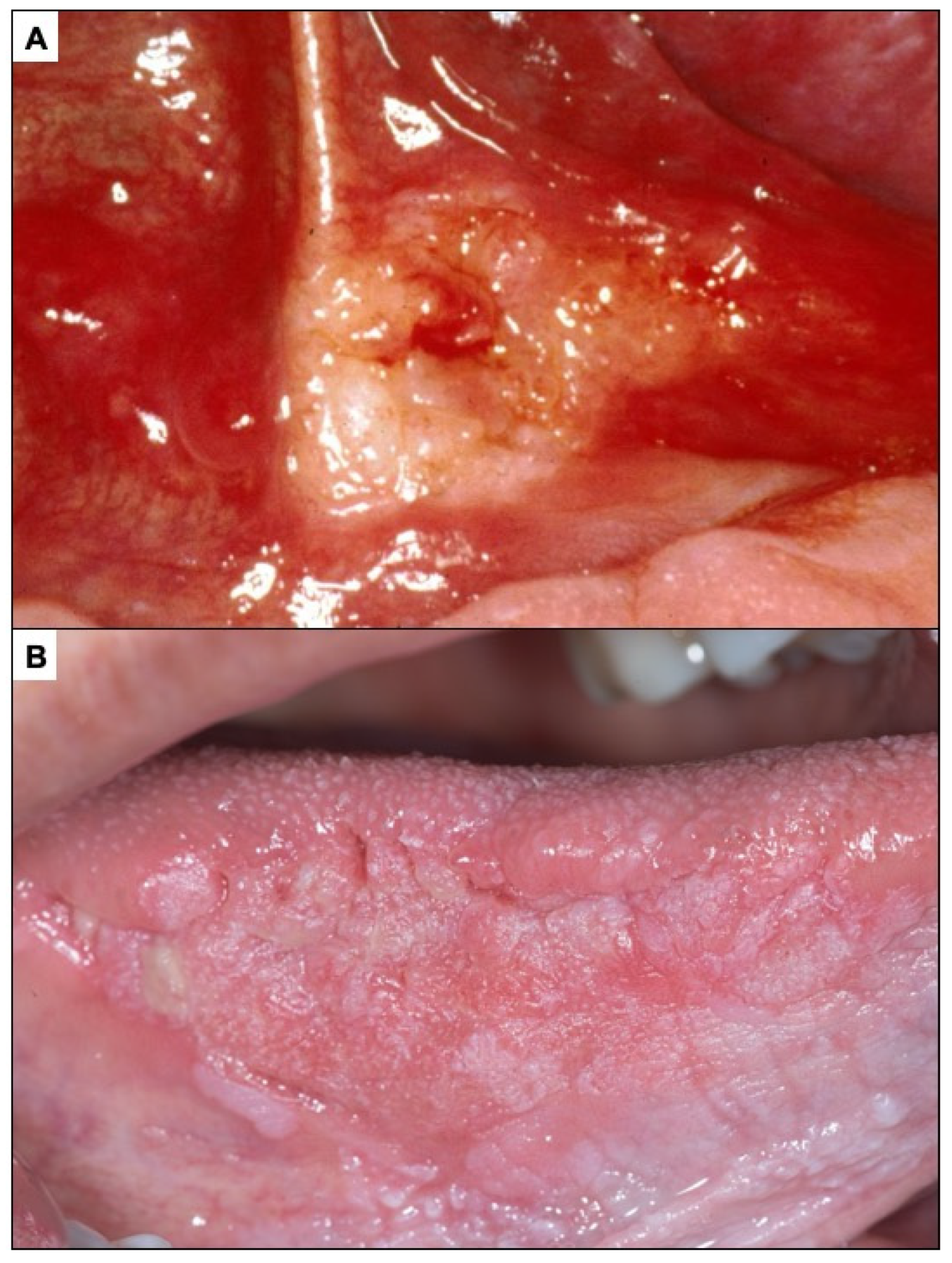
General Principles of Treatment of the Main OPMDs
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Available online: https://seer.cancer.gov (accessed on 4 May 2023).
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Gómez, I.; Warnakulasuriya, S.; Varela-Centelles, P.I.; López-Jornet, P.; Suárez-Cunqueiro, M.; Diz-Dios, P.; Seoane, J. Is early diagnosis of oral cancer a feasible objective? Who is to blame for diagnostic delay? Oral Dis. 2010, 16, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; Takkouche, B.; Varela-Centelles, P.; Tomás, I.; Seoane-Romero, J.M. Impact of delay in diagnosis on survival to head and neck carcinomas: A systematic review with meta-analysis. Clin. Otolaryngol. 2012, 37, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; Alvarez-Novoa, P.; Gomez, I.; Takkouche, B.; Diz, P.; Warnakulasiruya, S.; Seoane-Romero, J.M.; Varela-Centelles, P. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck 2016, 38 (Suppl. 1), E2182–E2189. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, J.L.; Thornton-Evans, G. Total diagnostic delay in oral cancer may be related to advanced disease stage at diagnosis. J. Evid. Based Dent. Pract. 2012, 12, 84–86. [Google Scholar] [CrossRef] [Green Version]
- Kantola, S.; Jokinen, K.; Hyrynkangas, K.; Mäntyselkä, P.; Alho, O.P. Detection of tongue cancer in primary care. Br. J. Gen. Pract. 2001, 51, 106–111. [Google Scholar]
- Facione, N.C. Delay versus help seeking for breast cancer symptoms: A critical review of the literature on patient and provider delay. Soc. Sci. Med. 1993, 36, 1521–1534. [Google Scholar] [CrossRef]
- Porta, M.; Gallen, M.; Malats, N.; Planas, J. Influence of “diagnostic delay” upon cancer survival: An analysis of five tumour sites. J. Epidemiol. Community Health 1991, 45, 225–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erwenne, C.M.; Franco, E.L. Age and lateness of referral as determinants of extra-ocular retinoblastoma. Ophthalmic Genet. 1989, 10, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.E.; Grunfeld, E.A.; McGurk, M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005, 41, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Moeckelmann, N.; Ebrahimi, A.; Tou, Y.K.; Gupta, R.; Low, T.H.; Ashford, B.; Ch’ng, S.; Palme, C.E.; Clark, J.R. Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) staging system in oral cavity squamous cell carcinoma. Oral Oncol. 2018, 85, 82–86. [Google Scholar] [CrossRef]
- Gómez, I.; Seoane, J.; Varela-Centelles, P.; Diz, P.; Takkouche, B. Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 2009, 117, 541–546. [Google Scholar] [CrossRef]
- Bruun, J.P. Time lapse by diagnosis of oral cancer. Oral Surg. Oral Med. Oral Pathol. 1976, 42, 139–149. [Google Scholar] [CrossRef]
- Shafer, W.G. Initial mismanagement and delay in diagnosis of oral cancer. J. Am. Dent. Assoc. 1975, 90, 1262–1264. [Google Scholar] [CrossRef]
- Allison, P.; Franco, E.; Feine, J. Predictors of professional diagnostic delays for upper aerodigestive tract carcinoma. Oral Oncol. 1998, 34, 127–132. [Google Scholar] [CrossRef]
- Brouha, X.D.R.; Tromp, D.M.; Hordijk, G.J.; Winnubst, J.A.M.; De Leeuw, J.R.J. Oral and pharyngeal cancer: Analysis of patient delay at different tumor stages. Head Neck 2005, 27, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Morelatto, R.A.; Herrera, M.C.; Fernández, E.N.; Corball, A.G.; López De Blanc, S.A. Diagnostic delay of oral squamous cell carcinoma in two diagnosis centers in Córdoba Argentina. J. Oral Pathol. Med. 2007, 36, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Kerdpon, D.; Sriplung, H. Factors related to delay in diagnosis of oral squamous cell carcinoma in southern Thailand. Oral Oncol. 2001, 37, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Brouha, X.; Tromp, D.; Hordijk, G.J.; Winnubst, J.; De Leeuw, R. Role of alcohol and smoking in diagnostic delay of head and neck cancer patients. Acta Otolaryngol. 2005, 125, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Tromp, D.M.; Brouha, X.D.; Hordijk, G.J.; Winnubst, J.A.; de Leeuw, R.J. Patient and tumour factors associated with advanced carcinomas of the head and neck. Br. Dent. J. 2005, 199, 151. [Google Scholar] [CrossRef] [PubMed]
- Pitiphat, W.; Diehl, S.R.; Laskaris, G.; Cartsos, V.; Douglass, C.W.; Zavras, A.I. Factors associated with delay in the diagnosis of oral cancer. J. Dent. Res. 2002, 81, 192–197. [Google Scholar] [CrossRef]
- Onizawa, K.; Nishihara, K.; Yamagata, K.; Yusa, H.; Yanagawa, T.; Yoshida, H. Factors associated with diagnostic delay of oral squamous cell carcinoma. Oral Oncol. 2003, 39, 781–788. [Google Scholar] [CrossRef]
- McGurk, M.; Chan, C.; Jones, J.; O’regan, E.; Sherriff, M. Delay in diagnosis and its effect on outcome in head and neck cancer. Br. Dent. J. 2005, 199, 721. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Pintos, J.; Schlecht, N.F.; Oliveira, B.V.; Fava, A.S.; Curado, M.P.; Kowalski, L.P.; Franco, E.L. Predictive factors for diagnosis of advanced-stage squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 313–318. [Google Scholar] [CrossRef] [Green Version]
- Weller, D.; Vedsted, P.; Rubin, G.; Walter, F.M.; Emery, J.; Scott, S.; Campbell, C.; Andersen, R.S.; Hamilton, W.; Olesen, F.; et al. The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. Br. J. Cancer 2012, 106, 1262–1267. [Google Scholar] [CrossRef]
- Seoane-Romero, J.M.; Vázquez-Mahía, I.; Seoane, J.; Varela-Centelles, P.; Tomás, I.; López-Cedrún, J.L. Factors related to late stage diagnosis of oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e35–e40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teppo, H.; Alho, O.P. Relative importance of diagnostic delays in different head and neck cancers. Clin. Otolaryngol. 2008, 33, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulis, G.; Reade, P.; Wiesenfeld, D. Referral patterns of patients with oral squamous cell carcinoma, Australia. Eur. J. Cancer Part B Oral Oncol. 1992, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Diz Dios, P.; Padron Gonzalez, N.; Seoane Leston, J.; Tomas Carmona, I.; Limeres Posse, J.; Varela-Centelles, P. “Scheduling delay” in oral cancer diagnosis: A new protagonist. Oral Oncol. 2005, 41, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Peacock, Z.S.; Pogrel, M.A.; Schmidt, B.L. Exploring the reasons for delay in treatment of oral cancer. J. Am. Dent. Assoc. 2008, 139, 1346–1352. [Google Scholar] [CrossRef]
- Panzarella, V.; Pizzo, G.; Calvino, F.; Compilato, D.; Colella, G.; Campisi, G. Diagnostic delay in oral squamous cell carcinoma: The role of cognitive and psychological variables. Int. J. Oral Sci. 2014, 6, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Andersen, B.L.; Cacioppo, J.T. Delay in seeking a cancer diagnosis: Delay stages and psychophysiological comparison processes. Br. J. Soc. Psychol. 1995, 34 Pt 1, 33–52. [Google Scholar] [CrossRef]
- Morse, D.E.; Kerr, A.R. Disparities in oral and pharyngeal cancer incidence, mortality and survival among black and white Americans. J. Am. Dent. Assoc. 2006, 137, 203–212. [Google Scholar] [CrossRef] [Green Version]
- McLean, A.; LeMay, W.; Vila, P.; Wegner, M.; Remington, P. Disparities in oral and pharyngeal cancer incidence and mortality among Wisconsin residents, 1999–2002. Wis. Med. J. 2006, 105, 32–35. [Google Scholar]
- Cruz, G.D.; Salazar, C.R.; Morse, D.E. Oral and pharyngeal cancer incidence and mortality among Hispanics, 1996-2002: The need for ethnoregional studies in cancer research. Am. J. Public Health 2006, 96, 2194–2200. [Google Scholar] [CrossRef]
- Andersen, R.S.; Vedsted, P.; Olesen, F.; Bro, F.; Søndergaard, J. Patient delay in cancer studies: A discussion of methods and measures. BMC Health Serv. Res. 2009, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Carter, L.M.; Ogden, G.R. Oral cancer awareness of general medical and general dental practitioners. Br. Dent. J. 2007, 203, E10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenheimer, J.; Verbin, R.S.; Johnson, J.T.; Horkowitz, C.A.; Myers, E.N. Factors delaying the diagnosis of oral and oropharyngeal carcinomas. Cancer 1989, 64, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Holland, J. Psychologic aspects of cancer. Cancer Med. 1982, 53, 1175–1177. [Google Scholar]
- McAndrew, P.G. Oral cancer biopsy in general practice. Br. Dent. J. 1998, 185, 428. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, N.; Duxbury, A.J.; Ariyaratnam, S.; Macfarlane, T.V. Attitudes to biopsy procedures in general dental practice. Br. Dent. J. 2002, 192, 588–592. [Google Scholar] [CrossRef] [Green Version]
- Wan, A.; Savage, N.W. Biopsy and diagnostic histopathology in dental practice in Brisbane: Usage patterns and perceptions of usefulness. Aust. Dent. J. 2010, 55, 162–169. [Google Scholar] [CrossRef]
- López Jornet, P.; Velandrino Nicolás, A.; Martínez Beneyto, Y.; Fernández Soria, M. Attitude towards oral biopsy among general dentists in Murcia. Med. Oral Patol. Oral Cir. Bucal 2007, 12, 78–83. [Google Scholar]
- Warnakulasuriya, K.A.A.S.; Johnson, N.W. Dentists and oral cancer prevention in the UK: Opinions, attitudes and practices to screening for mucosal lesions and to counselling patients on tobacco and alcohol use: Baseline data from 1991. Oral Dis. 1999, 5, 10–14. [Google Scholar] [CrossRef]
- Cowan, C.G.; Gregg, T.A.; Kee, F. Prevention and detection of oral cancer: The views of primary care dentists in Northern Ireland. Br. Dent. J. 1995, 179, 338–342. [Google Scholar] [CrossRef]
- Ergun, S.; Özel, S.; Koray, M.; Kürklü, E.; Ak, G.; Tanyeri, H. Dentists’ knowledge and opinions about oral mucosal lesions. Int. J. Oral Maxillofac. Surg. 2009, 38, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Moles, D.R.; Downer, M.C.; Speight, P.M. Opportunistic screening for oral cancer and precancer in general dental practice: Results of a demonstration study. Br. Dent. J. 2003, 194, 497–502. [Google Scholar] [CrossRef]
- Moles, D.R.; Downer, M.C.; Speight, P.M. Meta-analysis of measures of performance reported in oral cancer and precancer screening studies. Br. Dent. J. 2002, 192, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Brocklehurst, P.; Kujan, O.; O’Malley, L.A.; Ogden, G.; Shepherd, S.; Glenny, A.-M. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst. Rev. 2013, CD004150. [Google Scholar] [CrossRef] [Green Version]
- Kujan, O.; Glenny, A.M.; Oliver, R.J.; Thakker, N.; Sloan, P. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst. Rev. 2006, CD004150. [Google Scholar] [CrossRef] [Green Version]
- Garrote, L.F.; Sankaranarayanan, R.; Anta, J.J.L.; Salvá, A.R.; Parkin, D.M. An evaluation of the oral cancer control program in Cuba. Epidemiology 1995, 6, 428–431. [Google Scholar] [CrossRef]
- Santana, J.C.; Delgado, L.; Miranda, J.; Sánchez, M. Oral cancer case finding program (OCCFP). Eur. J. Cancer Part B Oral Oncol. 1997, 33, 10–12. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Effect of screening on oral cancer mortality in Kerala, India: A cluster-randomised controlled trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef]
- Rengaswamy, S.; Kunnambath, R.; Somanathan, T.; Muwonge, R.; Gigi, T.; Gopan, A.; Babu, M. Long term effect of visual screening on oral cancer incidence and mortality in a randomized trial in Kerala, India. Oral Oncol. 2013, 49, 314–321. [Google Scholar]
- Sankaranarayanan, R.; Mathew, B.; Jacob, B.; Thomas, G.; Somanathan, T.; Pisani, P.; Pandey, M.; Ramadas, K.; Najeeb, K.; Abraham, E. Early findings from a community-based, cluster-randomized, controlled oral cancer screening trial in Kerala, India. The Trivandrum Oral Cancer Screening Study Group. Cancer 2020, 88, 664–673. [Google Scholar] [CrossRef]
- Aguirre-Urizar, J.M.; Lafuente-Ibáñez de Mendoza, I.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and meta-analysis of the last 5 years. Oral Dis. 2021, 27, 1881–1895. [Google Scholar] [CrossRef] [PubMed]
- Reichart, P.A.; Samaranayake, L.P.; Philipsen, H.P. Pathology and clinical correlates in oral candidiasis and its variants: A review. Oral Dis. 2000, 6, 85–91. [Google Scholar] [CrossRef]
- Atkinson, J.C.; Harvey, K.E.; Domingo, D.L.; Trujillo, M.I.; Guadagnini, J.P.; Gollins, S.; Giri, N.; Hart, T.C.; Alter, B.P. Oral and dental phenotype of dyskeratosis congenita. Oral Dis. 2008, 14, 419–427. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Aguilar-Ruiz, M.; Ramos-García, P. Challenges in the Early Diagnosis of Oral Cancer, Evidence Gaps and Strategies for Improvement: A Scoping Review of Systematic Reviews. Cancers 2022, 14, 4967. [Google Scholar] [CrossRef]
- Odell, E.; Kujan, O.; Warnakulasuriya, S.; Sloan, P. Oral epithelial dysplasia: Recognition, grading and clinical significance. Oral Dis. 2021, 27, 1947–1976. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Worldwide prevalence of oral lichen planus: A systematic review and meta-analysis. Oral Dis. 2021, 27, 813–828. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ruiz-Ávila, I.; González-Ruiz, L.; Ayén, Á.; Gil-Montoya, J.A.; Ramos-García, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Ramos-García, P.; González-Moles, M.Á.; Warnakulasuriya, S. Oral cancer development in lichen planus and related conditions-3.0 evidence level: A systematic review of systematic reviews. Oral Dis. 2021, 27, 1919–1935. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. An appraisal of highest quality studies reporting malignant transformation of oral lichen planus based on a systematic review. Oral Dis. 2021, 27, 1908–1918. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P. Oral lichen planus and related lesions. What should we accept based on the available evidence? Oral Dis. 2022. [Google Scholar] [CrossRef]
- Andreasen, J.O. Oral lichen planus. 1. A clinical evaluation of 115 cases. Oral Surg. Oral Med. Oral Pathol. 1968, 25, 31–42. [Google Scholar] [CrossRef]
- Migliari, D. Will there be a critical review on the malignant transformation of oral lichen planus? Clinics 2023, 78, 100146. [Google Scholar] [CrossRef] [PubMed]
- van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Scully, C.; Gil-Montoya, J.A. Oral lichen planus: Controversies surrounding malignant transformation. Oral Dis. 2008, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.-Á.; Warnakulasuriya, S.; González-Ruiz, I.; Ayén, Á.; González-Ruiz, L.; Ruiz-Ávila, I.; Ramos-García, P. Dysplasia in oral lichen planus: Relevance, controversies and challenges. A position paper. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e541–e548. [Google Scholar] [CrossRef] [PubMed]
- Ramos-García, P.; González-Moles, M.Á.; Mello, F.W.; Bagan, J.V.; Warnakulasuriya, S. Malignant transformation of oral proliferative verrucous leukoplakia: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1896–1907. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; Ramos-García, P. Prognosis Parameters of Oral Carcinomas Developed in Proliferative Verrucous Leukoplakia: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4843. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Ramos-García, P.; Warnakulasuriya, S. A scoping review on gaps in the diagnostic criteria for proliferative verrucous leukoplakia: A conceptual proposal and diagnostic evidence-based criteria. Cancers 2021, 13, 3669. [Google Scholar] [CrossRef]
- González-Moles, M.Á.; Warnakulasuriya, S.; González-Ruiz, I.; González-Ruiz, L.; Ayén, Á.; Lenouvel, D.; Ruiz-Ávila, I.; Ramos-García, P. Clinicopathological and prognostic characteristics of oral squamous cell carcinomas arising in patients with oral lichen planus: A systematic review and a comprehensive meta-analysis. Oral Oncol. 2020, 106, 104688. [Google Scholar] [CrossRef]
- van der Waal, I.; Schepman, K.P.; van der Meij, E.H.; Smeele, L.E. Oral leukoplakia: A Clinicopathological review. Oral Oncol. 1997, 33, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Reibel, J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit. Rev. Oral Biol. Med. 2003, 14, 47–62. [Google Scholar] [CrossRef]
- Reichart, P.A.; Philipsen, H.P. Oral erythroplakia—A review. Oral Oncol. 2005, 41, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.; Bravo, M.; Gonzalez-Ruiz, L.; Ramos, P.; Gil-Montoya, J. Outcomes of oral lichen planus and oral lichenoid lesions treated with topical corticosteroid. Oral Dis. 2018, 24, 573–579. [Google Scholar] [CrossRef] [PubMed]


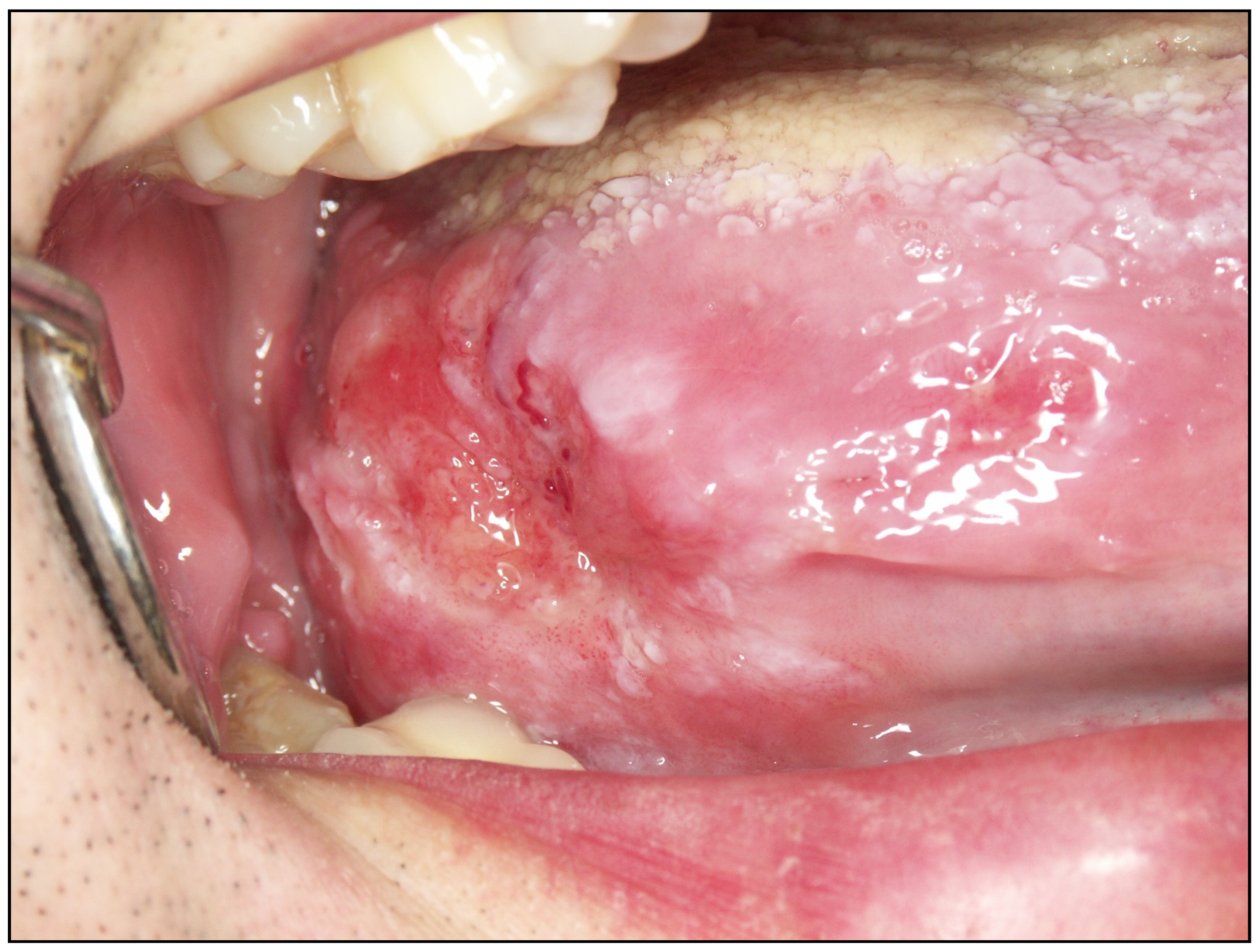
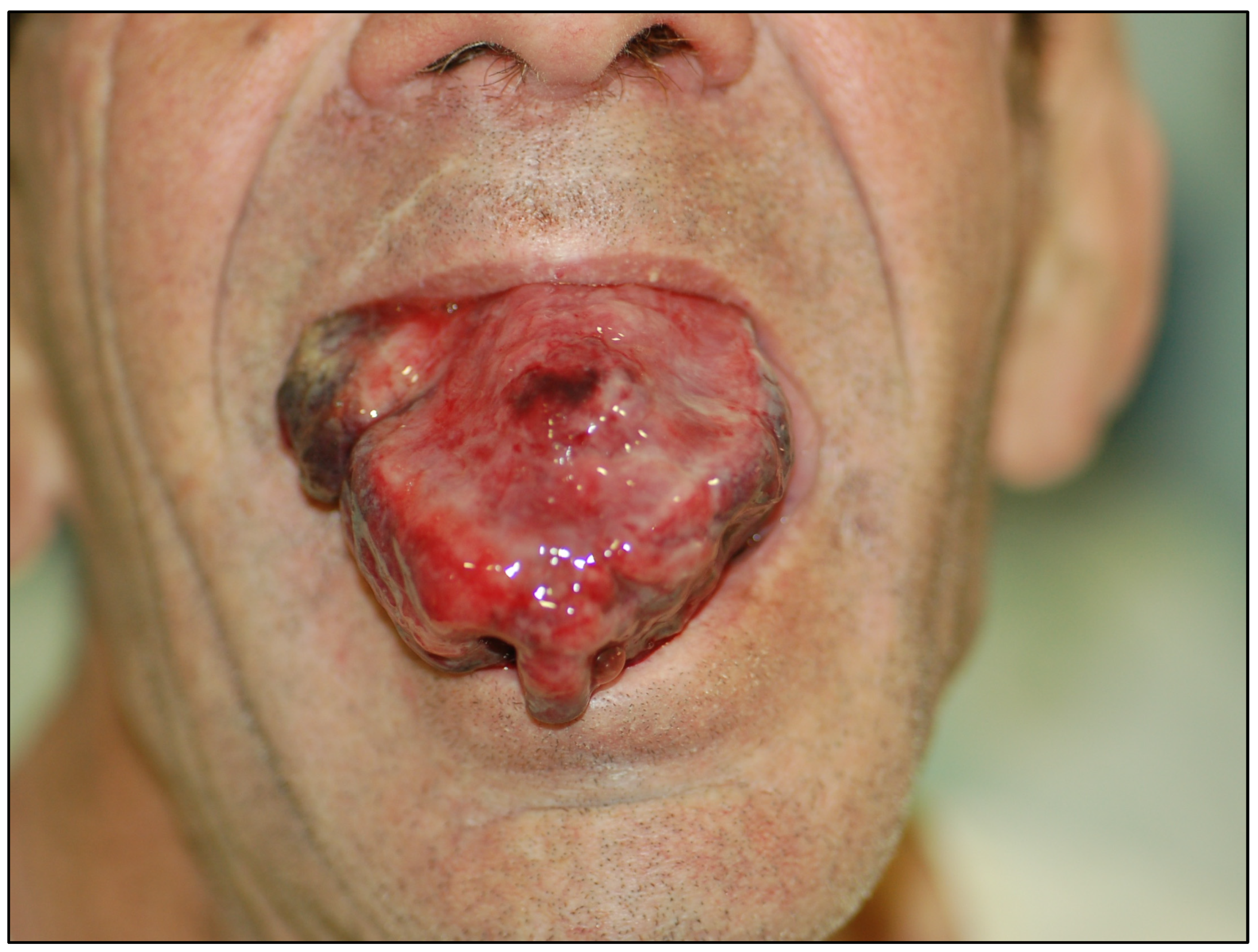

| Oral Potentially Malignant Disorders | Sample Size (Primary-Level Studies) | Number of Patients | Malignant Transformation * |
|---|---|---|---|
| Oral Leukoplakia | n = 24 ** | 16,192 | PP = 9.8% (95% CI: 7.9–11.7) |
| Oral Lichen Planus | n = 10 *** | 3206 | PP = 2.28% (95% CI = 1.49–3.20) |
| Oral Lichenoid Lesions | n = 3 | 197 | PP = 2.11% (95% CI = 0.01–6.33) |
| Proliferative Verrucous Leukoplakia | n = 17 | 474 | PP = 43.87% (95% CI = 31.93–56.13) |
| Oral Submucous Fibrosis | n = 9 | 6337 | PP = 4.2% (95% CI: 2.7%–5.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ruiz, I.; Ramos-García, P.; Ruiz-Ávila, I.; González-Moles, M.Á. Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution. Cancers 2023, 15, 3270. https://doi.org/10.3390/cancers15133270
González-Ruiz I, Ramos-García P, Ruiz-Ávila I, González-Moles MÁ. Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution. Cancers. 2023; 15(13):3270. https://doi.org/10.3390/cancers15133270
Chicago/Turabian StyleGonzález-Ruiz, Isabel, Pablo Ramos-García, Isabel Ruiz-Ávila, and Miguel Ángel González-Moles. 2023. "Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution" Cancers 15, no. 13: 3270. https://doi.org/10.3390/cancers15133270
APA StyleGonzález-Ruiz, I., Ramos-García, P., Ruiz-Ávila, I., & González-Moles, M. Á. (2023). Early Diagnosis of Oral Cancer: A Complex Polyhedral Problem with a Difficult Solution. Cancers, 15(13), 3270. https://doi.org/10.3390/cancers15133270








