Intraperitoneal BromAc® Does Not Interfere with the Healing of Colon Anastomosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Preparation
2.2. Study Ethics and Design
2.3. Colon-Anastomosis Surgery and Drug Treatment
2.4. Anastomotic Burst Pressure
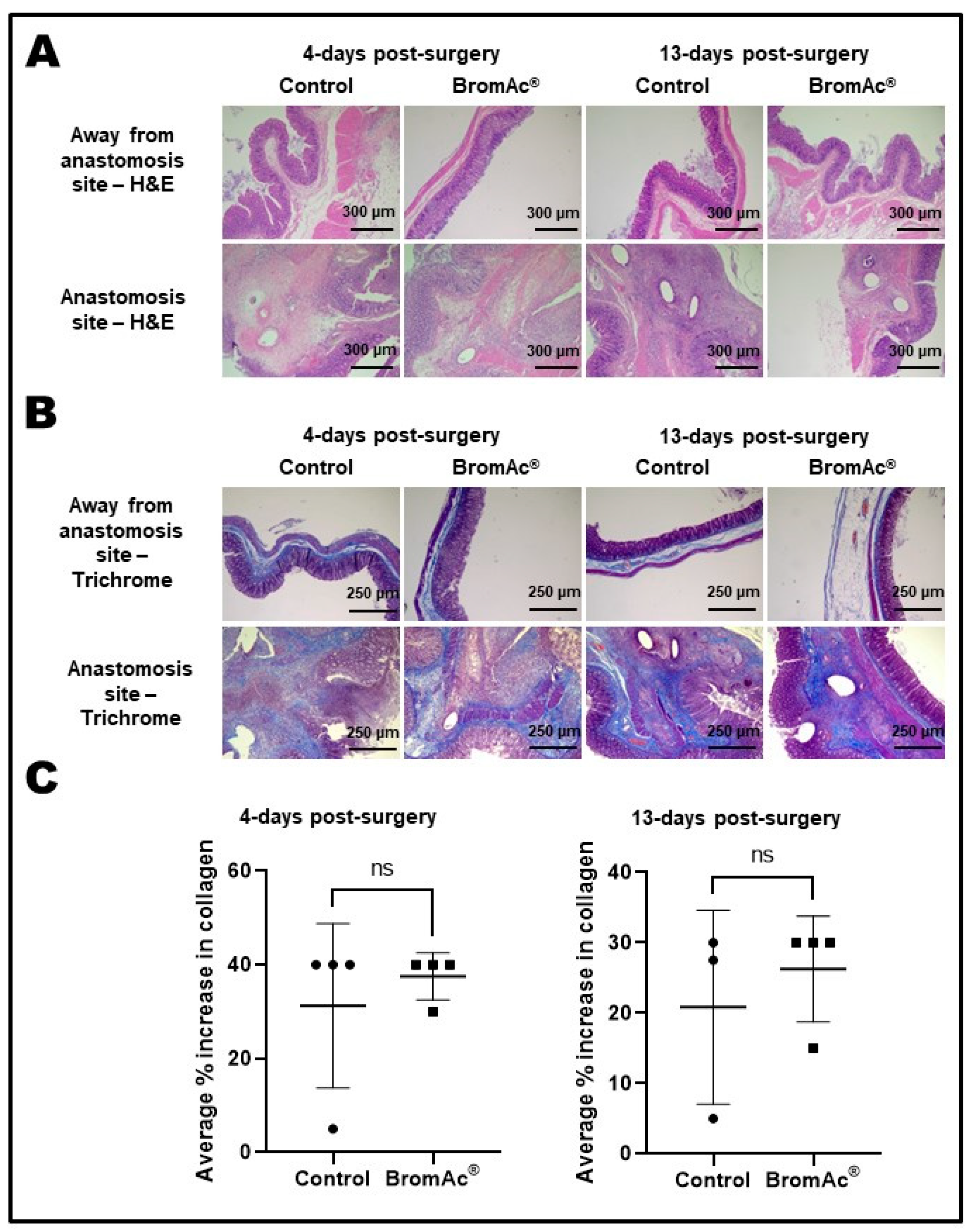
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gleeson, E.M.; Feldman, R.; Mapow, B.L.; Mackovick, L.T.; Ward, K.M.; Morano, W.F.; Rubin, R.R.; Bowne, W.B. Appendix-derived Pseudomyxoma Peritonei (PMP): Molecular Profiling Toward Treatment of a Rare Malignancy. Am. J. Clin. Oncol. 2017, 41, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Parvaiz, A.; Cecil, T.D.; Moran, B.J. Pseudomyxoma peritonei usually originates from the appendix: A review of the evidence. Eur. J. Gynaecol. Oncol. 2004, 25, 411–414. [Google Scholar]
- Noguchi, R.; Yano, H.; Gohda, Y.; Suda, R.; Igari, T.; Ohta, Y.; Yamashita, N.; Yamaguchi, K.; Terakado, Y.; Ikenoue, T.; et al. Molecular profiles of high-grade and low-grade pseudomyxoma peritonei. Cancer Med. 2015, 4, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.; Chandrakumaran, K.; Dayal, S.; Mohamed, F.; Cecil, T.D.; Moran, B.J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur. J. Surg. Oncol. 2016, 42, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.C.; Yan, T.D.; Smigielski, M.E.; Zhu, K.J.; Ng, K.M.; Zhao, J.; Morris, D.L. Long-term survival in patients with pseudomyxoma peritonei treated with cytoreductive surgery and perioperative intraperitoneal chemotherapy: 10 years of experience from a single institution. Ann. Surg. Oncol. 2009, 16, 1903–1911. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef] [Green Version]
- Dell, D.D.; Held-Warmkessel, J.; Jakubek, P.; O’Mara, T. Care of the open abdomen after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies. Oncol. Nurs. Forum 2014, 41, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.B.; Di Napoli, R. Hyperthermic Intraperitoneal Chemotherapy. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Brown, S.; Hunt, J.; Hill, R. Differential thermal sensitivity of tumour and normal tissue microvascular response during hyperthermia. Int. J. Hyperth. 1992, 8, 501–514. [Google Scholar] [CrossRef]
- Pillai, K.; Mekkawy, A.H.; Akhter, J.; Badar, S.; Dong, L.; Liu, A.I.; Morris, D.L. Enhancing the potency of chemotherapeutic agents by combination with bromelain and N-acetylcysteine—An in vitro study with pancreatic and hepatic cancer cells. Am. J. Transl. Res. 2020, 12, 7404–7419. [Google Scholar]
- Chobotova, K.; Vernallis, A.B.; Majid, F.A. Bromelain’s activity and potential as an anti-cancer agent: Current evidence and perspectives. Cancer Lett. 2010, 290, 148–156. [Google Scholar] [CrossRef]
- Wang, S.L.; Lin, H.T.; Liang, T.W.; Chen, Y.J.; Yen, Y.H.; Guo, S.P. Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Bioresour. Technol. 2008, 99, 4386–4393. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Akhter, J.; Pillai, K.; Chua, T.C.; Alzarin, N.; Morris, D.L. Efficacy of a novel mucolytic agent on pseudomyxoma peritonei mucin, with potential for treatment through peritoneal catheters. Am. J. Cancer Res. 2014, 4, 495–507. [Google Scholar] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. A formulation for in situ lysis of mucin secreted in pseudomyxoma peritonei. Int. J. Cancer 2014, 134, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Liauw, W.; Morris, D.L. Potentiation of chemotherapeutics by bromelain and N-acetylcysteine: Sequential and combination therapy of gastrointestinal cancer cells. Am. J. Cancer Res. 2016, 6, 350–369. [Google Scholar] [PubMed]
- Erali, R.A.; Wajih, N.; Forsythe, S.; Shen, P.; Levine, E.; Soker, S.; Morris, D.L.V.K. Assessing the Mucolytic and Cytotoxic Activity of Bromelain in Appendiceal Cancer Organoids [abstract]. In Proceedings of the Society of Surgical Oncology SSO 2023—International Conference on Surgical Cancer Care. In Annals of Surgical Oncology, Boston, MA, USA, 22–25 March 2023. [Google Scholar]
- Mekkawy, A.H.; Pillai, K.; Badar, S.; Akhter, J.; Ke, K.; Valle, S.J.; Morris, D.L. Addition of bromelain and acetylcysteine to gemcitabine potentiates tumor inhibition in vivo in human colon cancer cell line LS174T. Am. J. Cancer Res. 2021, 11, 2252–2263. [Google Scholar] [PubMed]
- Mekkawy, A.H.; Pillai, K.; Suh, H.; Badar, S.; Akhter, J.; Képénékian, V.; Ke, K.; Valle, S.J.; Morris, D.L. Bromelain and Acetylcysteine (BromAc) alone and in combination with Gemcitabine inhibits subcutaneous deposits of pancreatic cancer after intraperitoneal injection. Am. J. Transl. Res. 2021, 13, 13524–13539. [Google Scholar]
- Dilly, A.K.; Honick, B.D.; Frederick, R.; Elapavaluru, A.; Velankar, S.; Makala, H.; Hitchens, T.K.; Foley, L.M.; Guo, J.; Beumer, J.H.; et al. Improved chemosensitivity following mucolytic therapy in patient-derived models of mucinous appendix cancer. Transl. Res. J. Lab. Clin. Med. 2021, 229, 100–114. [Google Scholar] [CrossRef]
- Valle, S.J.; Akhter, J.; Mekkawy, A.H.; Lodh, S.; Pillai, K.; Badar, S.; Glenn, D.; Power, M.; Liauw, W.; Morris, D.L. A novel treatment of bromelain and acetylcysteine (BromAc) in patients with peritoneal mucinous tumours: A phase I first in man study. Eur. J. Surg. Oncol. 2021, 47, 115–122. [Google Scholar] [CrossRef]
- dos Reis, J.G.A.C.; Ferreira, G.M.; Lourenço, A.A.; Ribeiro, A.Á.L.; de Melo Oliveira, P.; de Almeida Marques, D.P.; Ferreira, L.L.; Clarindo, F.A.; da Silva, M.F.; Póvoas Filho, H.P. Ex-vivo mucolytic and anti-inflammatory activity of BromAc in tracheal aspirates from COVID-19. Biomed. Pharmacother. 2022, 148, 112753. [Google Scholar] [CrossRef]
- Rosendorf, J.; Klicova, M.; Herrmann, I.; Anthis, A.; Cervenkova, L.; Palek, R.; Treska, V.; Liska, V. Intestinal Anastomotic Healing: What do We Know About Processes Behind Anastomotic Complications. Front. Surg. 2022, 9, 904810. [Google Scholar] [CrossRef] [PubMed]
- Isabela Avila-Rodriguez, M.; Melendez-Martinez, D.; Licona-Cassani, C.; Manuel Aguilar-Yanez, J.; Benavides, J.; Lorena Sanchez, M. Practical context of enzymatic treatment for wound healing: A secreted protease approach (Review). Biomed. Rep. 2020, 13, 3–14. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain a potential bioactive compound: A comprehensive overview from a pharmacological perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Kreken Almeland, S.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef]
- AlMatar, M.; Batool, T.; Makky, E.A. Therapeutic Potential of N-Acetylcysteine for Wound Healing, Acute Bronchiolitis, and Congenital Heart Defects. Curr. Drug Metab. 2016, 17, 156–167. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Xu, F.; Kang, X.; Zhou, J.; Yao, Q.; Lin, Y.; Zhang, W. The antioxidant N-acetylcysteine promotes immune response and inhibits epithelial-mesenchymal transition to alleviate pulmonary fibrosis in chronic obstructive pulmonary disease by suppressing the VWF/p38 MAPK axis. Mol. Med. 2021, 27, 97. [Google Scholar] [CrossRef]
- Echeverri-Ruiz, N.; Haynes, T.; Landers, J.; Woods, J.; Gemma, M.J.; Hughes, M.; Del Rio-Tsonis, K. A biochemical basis for induction of retina regeneration by antioxidants. Dev. Biol. 2018, 433, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Oguz, A.; Uslukaya, O.; Alabalık, U.; Turkoglu, A.; Kapan, M.; Bozdag, Z. Topical N-acetylcysteine improves wound healing comparable to dexpanthenol: An experimental study. Int. Surg. 2015, 100, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessler, B.; Eriksson, O.; Angenete, E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int. J. Color. Dis. 2017, 32, 549–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Snellman, A.; Tuomisto, A.; Koski, A.; Latvanlehto, A.; Pihlajaniemi, T. The role of disulfide bonds and α-helical coiled-coils in the biosynthesis of type XIII collagen and other collagenous transmembrane proteins. J. Biol. Chem. 2007, 282, 14898–14905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayne, K. Assessing Pain and Distress: A Veterinary Behaviorist’s Perspective. In Definition of Pain and Distress and Reporting Requirements for Laboratory Animals: Proceedings of the Workshop Held June 22, 2000; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Despoudi, K.; Mantzoros, I.; Ioannidis, O.; Loutzidou, L.; Christidis, P.; Chatzakis, C.; Gkasdaris, G.; Raptis, D.; Pramateftakis, M.G.; Angelopoulos, S. Healing of colonic anastomosis in rats under obstructive ileus conditions. Discoveries 2021, 9, e129. [Google Scholar] [CrossRef]
- Ghiselli, R.; Lucarini, G.; Ortenzi, M.; Salvolini, E.; Saccomanno, S.; Orlando, F.; Provinciali, M.; Casciani, F.; Guerrieri, M. Anastomotic healing in a rat model of peritonitis after non-steroidal anti-inflammatory drug administration. Eur. J. Histochem. 2020, 64, 3085. [Google Scholar] [CrossRef]
- Kosmidis, C.; Efthimiadis, C.; Anthimidis, G.; Basdanis, G.; Apostolidis, S.; Hytiroglou, P.; Vasiliadou, K.; Prousalidis, J.; Fahantidis, E. Myofibroblasts and colonic anastomosis healing in Wistar rats. BMC Surg. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czeiger, D.; Osyntsov, A.; Osyntsov, L.; Ball, C.G.; Gigi, R.; Shaked, G. Examining the safety of colon anastomosis on a rat model of ischemia-reperfusion injury. World J. Emerg. Surg. 2013, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Mantzoros, I.; Kanellos, I.; Demetriades, H.; Christoforidis, E.; Kanellos, D.; Pramateftakis, M.-G.; Zaraboukas, T.; Betsis, D. Effects of steroid on the healing of colonic anastomoses in the rat. Tech. Coloproctology 2004, 8, s180–s183. [Google Scholar] [CrossRef]
- Kanellos, I.; Mantzoros, I.; Demetriades, H.; Kalfadis, S.; Sakkas, L.; Kelpis, T.; Betsis, D. Sutureless colonic anastomosis in the rat: A randomized controlled study. Tech. Coloproctology 2002, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Jansen-Winkeln, B.; Tagkalos, E.; Heimann, A.; Gaiser, T.; Hirsch, D.; Gockel, I.; Mittler, J.; Lang, H.; Heinrich, S. Pringle maneuver increases the risk of anastomotic leakage after colonic resection in rats. HPB 2018, 20, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Maimónides Biomedical Research Institute of Córdoba (Spain). Intratumoral Bromelain + Acetylcysteine in Relapsed and Unresectable Pseudomyxoma Peritonei. Identifier NCT04982146. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04982146 (accessed on 6 January 2023).

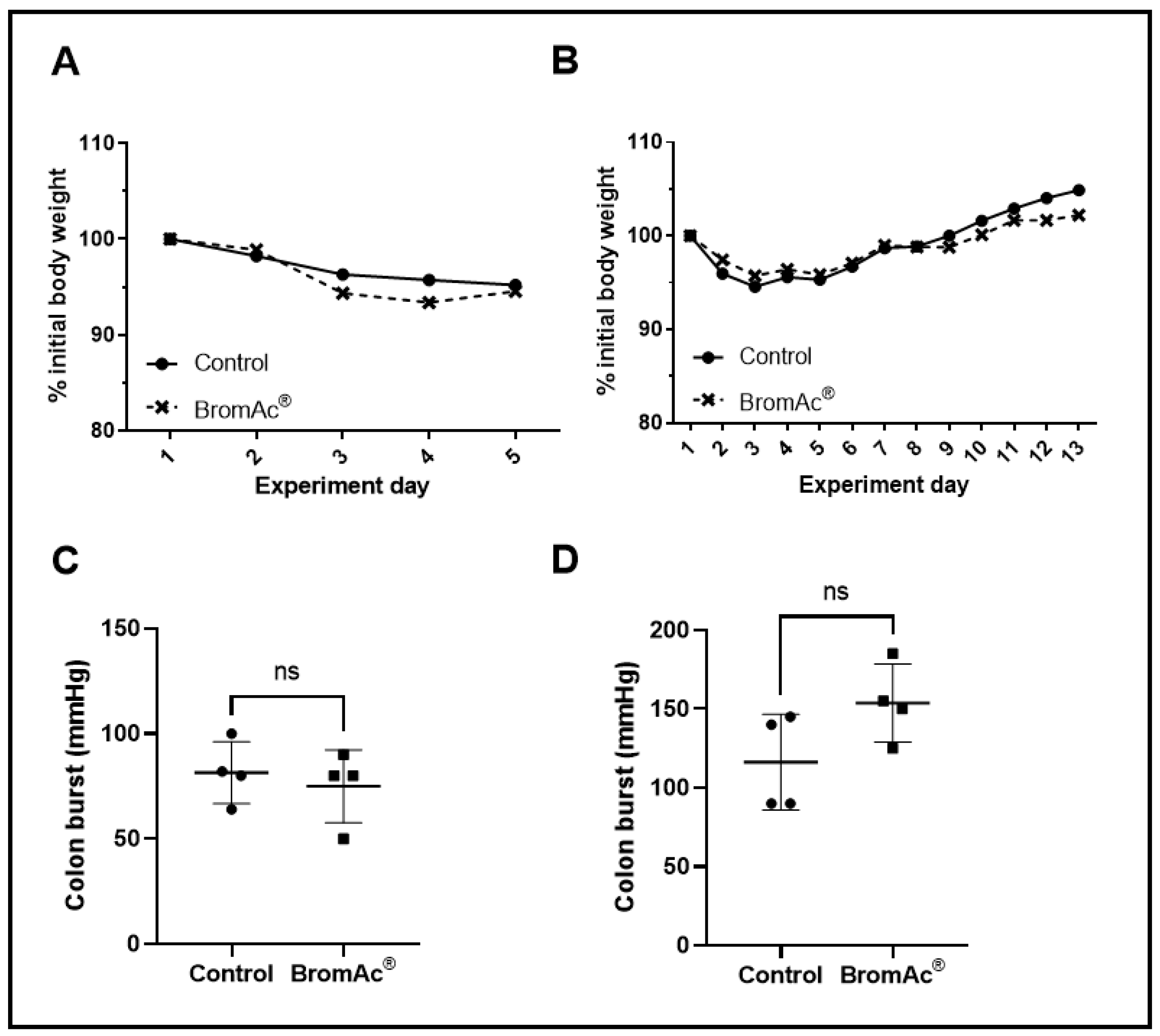

| 4 Days Post-Surgery Range (Mean); mmHg | 13 Days Post-Surgery Range (Mean); mmHg | ||||
|---|---|---|---|---|---|
| Control | BromAc® | p-Value | Control | BromAc® | p-Value |
| 64–100 (81.50) | 50–90 (75.00) | 0.59 | 90–145 (116.3) | 125–185 (153.8) | 0.10 |
| Histopathological Findings | 4 Days Post-Surgery | 13 Days Post-Surgery | ||
|---|---|---|---|---|
| Control ^ | BromAc® | Control | BromAc® | |
| Colon, away from surgical site–lamina propria | ||||
| Diffuse lymphocytic infiltration | 3/3 | 2/4 | 0/4 | 0/4 |
| Mixed inflammatory infiltration | 0/3 | 2/4 | 4/4 | 4/4 |
| Colon, surgical site–lamina propria | ||||
| Diffuse colitis, or mixed inflammation | 4/4 | 3/4 | 4/4 | 4/4 |
| Multifocal necrosis | 4/4 | 4/4 | 1/4 | 1/4 |
| Multifocal granulomata with multinucleated giant cells | 0/4 | 0/4 | 3/4 | 4/4 |
| Foreign-body pyogranuloma | 0/4 | 1/4 | 2/4 | 1/4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekkawy, A.H.; Breakeit, M.; Pillai, K.; Badar, S.; Akhter, J.; Valle, S.J.; Morris, D.L. Intraperitoneal BromAc® Does Not Interfere with the Healing of Colon Anastomosis. Cancers 2023, 15, 3321. https://doi.org/10.3390/cancers15133321
Mekkawy AH, Breakeit M, Pillai K, Badar S, Akhter J, Valle SJ, Morris DL. Intraperitoneal BromAc® Does Not Interfere with the Healing of Colon Anastomosis. Cancers. 2023; 15(13):3321. https://doi.org/10.3390/cancers15133321
Chicago/Turabian StyleMekkawy, Ahmed H., Mohammad Breakeit, Krishna Pillai, Samina Badar, Javed Akhter, Sarah J. Valle, and David L. Morris. 2023. "Intraperitoneal BromAc® Does Not Interfere with the Healing of Colon Anastomosis" Cancers 15, no. 13: 3321. https://doi.org/10.3390/cancers15133321
APA StyleMekkawy, A. H., Breakeit, M., Pillai, K., Badar, S., Akhter, J., Valle, S. J., & Morris, D. L. (2023). Intraperitoneal BromAc® Does Not Interfere with the Healing of Colon Anastomosis. Cancers, 15(13), 3321. https://doi.org/10.3390/cancers15133321







