Functional Imaging of Hypoxia: PET and MRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Pathophysiology of Hypoxia in Cancer
2.1. Hypoxia and the Cellular Response
2.2. Hypoxia and Acidosis
2.3. Hypoxia and Immune Function
2.4. Hypoxia and Treatment Resistance
3. Imaging Hypoxia in Cancer
3.1. Positron Emission Tomography (PET)
3.1.1. Techniques
3.1.2. Clinical Applications
3.2. Magnetic Resonance Imaging (MRI)
3.2.1. Techniques
3.2.2. Clinical Applications
3.3. Additional Techniques
3.4. Invasive Techniques
3.5. Hypoxia Imaging and Interventional Radiology
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kakkad, S.; Krishnamachary, B.; Jacob, D.; Pacheco-Torres, J.; Goggins, E.; Bharti, S.K.; Penet, M.-F.; Bhujwalla, Z.M. Molecular and functional imaging insights into the role of hypoxia in cancer aggression. Cancer Metastasis Rev. 2019, 38, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.; Li, Z.; Huang, C.; Yang, Y.; Lang, M.; Cao, J.; Jiang, W.; Xu, Y.; Dong, J.; et al. Hypoxia Inducible Factor 1 (HIF-1) Recruits Macrophage to Activate Pancreatic Stellate Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2016, 17, 799. [Google Scholar] [CrossRef]
- Kaelin, W.G.J.; Ratcliffe, P.J. Oxygen Sensing by Metazoans: The Central Role of the HIF Hydroxylase Pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Dor, Y.; Herbert, J.M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, F.; Bono, R.; Abramovitch, P.; Maxwell, P.; et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562. [Google Scholar] [CrossRef]
- Dewhirst, M.W. Relationships between Cycling Hypoxia, HIF-1, Angiogenesis and Oxidative Stress. Radiat. Res. 2009, 172, 653–665. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Cao, Y.; Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer 2008, 8, 425–437. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yasui, H.; Mitchell, J.B.; Krishna, M.C. Imaging Cycling Tumor Hypoxia. Cancer Res. 2010, 70, 10019–10023. [Google Scholar] [CrossRef]
- Masamune, A.; Kikuta, K.; Watanabe, T.; Satoh, K.; Hirota, M.; Shimosegawa, T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am. J. Physiol. Liver Physiol. 2008, 295, G709–G717. [Google Scholar] [CrossRef]
- Erkan, M.; Reiser-Erkan, C.; Michalski, C.W.; Deucker, S.; Sauliunaite, D.; Streit, S.; Esposito, I.; Friess, H.; Kleeff, J. Cancer-Stellate Cell Interactions Perpetuate the Hypoxia-Fibrosis Cycle in Pancreatic Ductal Adenocarcinoma. Neoplasia 2009, 11, 497–508. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, D.; Liu, Y.; Su, Z.; Zhang, L.; Chen, F.; Zhou, Y.; Wu, Y.; Yu, M.; Zhang, Z.; et al. Role of the Hypoxia-inducible factor-1 alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells. Cancer Cell Int. 2013, 13, 119. [Google Scholar] [CrossRef]
- Lindner, D.; Raghavan, D. Intra-tumoural extra-cellular pH: A useful parameter of response to chemotherapy in syngeneic tumour lines. Br. J. Cancer 2009, 100, 1287–1291. [Google Scholar] [CrossRef]
- Gillies, R.J.; Robey, I.; Gatenby, R.A. Causes and Consequences of Increased Glucose Metabolism of Cancers. J. Nucl. Med. 2008, 49, 24S–42S. [Google Scholar] [CrossRef]
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Chen, L.; Endler, A.; Shibasaki, F. Hypoxia and angiogenesis: Regulation of hypoxia-inducible factors via novel binding factors. Exp. Mol. Med. 2009, 41, 849–857. [Google Scholar] [CrossRef]
- Elia, A.R.; Cappello, P.; Puppo, M.; Fraone, T.; Vanni, C.; Eva, A.; Musso, T.; Novelli, F.; Varesio, L. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J. Leukoc. Biol. 2008, 84, 1472–1482. [Google Scholar] [CrossRef]
- Chen, J.L.-Y.; Lucas, J.E.; Schroeder, T.; Mori, S.; Wu, J.; Nevins, J.; Dewhirst, M.; West, M.; Chi, J.-T. The Genomic Analysis of Lactic Acidosis and Acidosis Response in Human Cancers. PLoS Genet. 2008, 4, e1000293. [Google Scholar] [CrossRef]
- Chun, Y.; Kim, J. AMPK-mTOR Signaling and Cellular Adaptations in Hypoxia. Int. J. Mol. Sci. 2021, 22, 9765. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Menegakis, A.; Klompmaker, R.; Vennin, C.; Arbusà, A.; Damen, M.; Broek, B.v.D.; Zips, D.; van Rheenen, J.; Krenning, L.; Medema, R.H. Resistance of Hypoxic Cells to Ionizing Radiation Is Mediated in Part via Hypoxia-Induced Quiescence. Cells 2021, 10, 610. [Google Scholar] [CrossRef]
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634. [Google Scholar] [CrossRef]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef]
- Goggins, E.; Kakkad, S.; Mironchik, Y.; Jacob, D.; Wildes, F.; Krishnamachary, B.; Bhujwalla, Z.M. Hypoxia Inducible Factors Modify Collagen I Fibers in MDA-MB-231 Triple Negative Breast Cancer Xenografts. Neoplasia 2018, 20, 131–139. [Google Scholar] [CrossRef]
- Kakkad, S.M.; Solaiyappan, M.; O’rourke, B.; Stasinopoulos, I.; Ackerstaff, E.; Raman, V.; Bhujwalla, Z.M.; Glunde, K. Hypoxic Tumor Microenvironments Reduce Collagen I Fiber Density. Neoplasia 2010, 12, 608–617. [Google Scholar] [CrossRef]
- Blagosklonny, M. Hypoxia-inducible factor: Achilles’ heel of antiangiogenic cancer therapy (Review). Int. J. Oncol. 2001, 19, 257–262. [Google Scholar] [CrossRef]
- Ni, J.; Wang, X.; Stojanovic, A.; Zhang, Q.; Wincher, M.; Bühler, L.; Arnold, A.; Correia, M.P.; Winkler, M.; Koch, P.-S.; et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1α Unleashes NK Cell Activity. Immunity 2020, 52, 1075–1087.e8. [Google Scholar] [CrossRef]
- Noman, M.Z.; Chouaib, S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 2014, 3, e954463. [Google Scholar] [CrossRef]
- Chiu, D.K.; Tse, A.; Xu, I.; Di Cui, J.; Lai, R.; Li, L.; Koh, H.; Tsang, F.; Wei, L.; Wong, C.; et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017, 8, 517. [Google Scholar] [CrossRef]
- Chiu, D.K.-C.; Xu, I.M.-J.; Lai, R.K.-H.; Tse, A.P.-W.; Wei, L.L.; Koh, H.-Y.; Li, L.L.; Lee, D.; Lo, R.C.-L.; Wong, C.-M.; et al. Hypoxia induces myeloid-derived suppressor cell recruitment to hepatocellular carcinoma through chemokine (C-C motif) ligand 26. Hepatology 2016, 64, 797–813. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Gao, Z.Q.; Ning, H.F.; Sun, Y.Q.; Cao, G.W. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008, 49, 523–529. [Google Scholar] [CrossRef]
- Li, X.; Feng, G.-S.; Zheng, C.-S.; Zhuo, C.-K.; Liu, X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J. Gastroenterol. 2004, 10, 2878–2882. [Google Scholar] [CrossRef]
- Virmani, S.; Rhee, T.K.; Ryu, R.K.; Sato, K.T.; Lewandowski, R.J.; Mulcahy, M.F.; Kulik, L.M.; Szolc-Kowalska, B.; Woloschak, G.E.; Yang, G.-Y.; et al. Comparison of Hypoxia-inducible Factor-1α Expression before and after Transcatheter Arterial Embolization in Rabbit VX2 Liver Tumors. J. Vasc. Interv. Radiol. 2008, 19, 1483–1489. [Google Scholar] [CrossRef]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- Ding, Z.; Yang, L.; Xie, X.; Xie, F.; Pan, F.; Li, J.; He, J. Expression and significance of hypoxia-inducible factor-1 alpha and MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J. Cancer Res. Clin. Oncol. 2010, 136, 1697–1707. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.P.; Luo, S.F.; Guan, J.; Zhang, W.G.; Zhang, B.X. Involvement of hypoxia-inducible factor-1-alpha in multidrug resistance induced by hypoxia in HepG2 cells. J. Exp. Clin. Cancer Res. 2005, 24, 565–574. [Google Scholar]
- Dong, Z.; Wang, J.Z.; Yu, F.; Venkatachalam, M.A. Apoptosis-Resistance of Hypoxic Cells: Multiple Factors Involved and a Role for IAP-2. Am. J. Pathol. 2003, 163, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, Y.; Tomida, A.; Tsuruo, T. Nuclear localization of proteasomes participates in stress-inducible resistance of solid tumor cells to topoisomerase II-directed drugs. Cancer Res. 2002, 62, 5008–5012. [Google Scholar] [PubMed]
- Rharass, T.; Vigo, J.; Salmon, J.-M.; Ribou, A.-C. New method for the detection of reactive oxygen species in anti-tumoural activity of adriamycin: A comparison between hypoxic and normoxic cells. Free Radic. Res. 2008, 42, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Tomida, A.; Tsuruo, T. Drug resistance mediated by cellular stress response to the microenvironment of solid tumors. Anticancer Drug Des. 1999, 14, 169–177. [Google Scholar] [PubMed]
- Gewirtz, D. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Zhou, H.; Arias-Ramos, N.; López-Larrubia, P.; Mason, R.; Cerdán, S. Oxygenation Imaging by Nuclear Magnetic Resonance Methods. Methods Mol. Biol. 2018, 1718, 297–313. [Google Scholar]
- Krohn, K.A.; Link, J.M.; Mason, R.P. Molecular Imaging of Hypoxia. J. Nucl. Med. 2008, 49 (Suppl. S2), 129S–148S. [Google Scholar] [CrossRef]
- Bekaert, L.; Valable, S.; Lechapt-Zalcman, E.; Ponte, K.; Collet, S.; Constans, J.-M.; Levallet, G.; Bordji, K.; Petit, E.; Branger, P.; et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: Correlation with molecular markers of hypoxia and angiogenesis. Eur. J. Nucl. Med. 2017, 44, 1383–1392. [Google Scholar] [CrossRef]
- Cheng, J.; Lei, L.; Xu, J.; Sun, Y.; Zhang, Y.; Wang, X.; Pan, L.; Shao, Z.; Zhang, Y.; Liu, G. 18F-Fluoromisonidazole PET/CT: A Potential Tool for Predicting Primary Endocrine Therapy Resistance in Breast Cancer. J. Nucl. Med. 2013, 54, 333–340. [Google Scholar] [CrossRef]
- Löck, S.; Linge, A.; Seidlitz, A.; Bandurska-Luque, A.; Nowak, A.; Gudziol, V.; Buchholz, F.; Aust, D.E.; Baretton, G.B.; Zöphel, K.; et al. Repeat FMISO-PET imaging weakly correlates with hypoxia-associated gene expressions for locally advanced HNSCC treated by primary radiochemotherapy. Radiother. Oncol. 2019, 135, 43–50. [Google Scholar] [CrossRef]
- Thorwarth, D.; Welz, S.; Mönnich, D.; Pfannenberg, C.; Nikolaou, K.; Reimold, M.; La Fougère, C.; Reischl, G.; Mauz, P.-S.; Paulsen, F.; et al. Prospective Evaluation of a Tumor Control Probability Model Based on Dynamic 18F-FMISO PET for Head and Neck Cancer Radiotherapy. J. Nucl. Med. 2019, 60, 1698–1704. [Google Scholar] [CrossRef]
- Watanabe, S.; Inoue, T.; Okamoto, S.; Magota, K.; Takayanagi, A.; Sakakibara-Konishi, J.; Katoh, N.; Hirata, K.; Manabe, O.; Toyonaga, T.; et al. Combination of FDG-PET and FMISO-PET as a treatment strategy for patients undergoing early-stage NSCLC stereotactic radiotherapy. EJNMMI Res. 2019, 9, 104. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, J.; Li, Y.; Fu, S.; Chen, Y.; Wu, J. Imaging of Tumor Hypoxia with Radionuclide-Labeled Tracers for PET. Front. Oncol. 2021, 11, 731503. [Google Scholar] [CrossRef]
- Troost, E.G.; Laverman, P.; Philippens, M.; Lok, J.; van der Kogel, A.; Oyen, W.; Boerman, O.; Kaanders, J. Correlation of [18F]FMISO autoradiography and pimonidazole [corrected] immunohistochemistry in human head and neck carcinoma xenografts. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1803–1811. [Google Scholar] [CrossRef]
- Vāvere, A.L.; Lewis, J.S. Cu–ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007, 4893–4902. [Google Scholar] [CrossRef]
- Postema, E.J.; McEwan, A.J.B.; Riauka, T.A.; Kumar, P.; Richmond, D.A.; Abrams, D.N.; Wiebe, L. Initial results of hypoxia imaging using 1-α-d-(5-deoxy-5-[18F]-fluoroarabinofuranosyl)-2-nitroimidazole (18F-FAZA). Eur. J. Nucl. Med. 2009, 36, 1565–1573. [Google Scholar] [CrossRef]
- Trinkaus, M.E.; Blum, R.; Rischin, D.; Callahan, J.; Bressel, M.; Segard, T.; Roselt, P.; Eu, P.; Binns, D.; MacManus, M.P.; et al. Imaging of hypoxia with18F-FAZA PET in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 475–481. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Kerner, G.; Pruim, J.; Steenbakkers, R.; Wiegman, E.; Koole, M.; de Groot, E.; Willemsen, A.; Luurtsema, G.; Widder, J.; et al. PET imaging of tumor hypoxia using 18F-fluoroazomycin arabinoside in stage III-IV non-small cell lung cancer patients. J. Nucl. Med. 2013, 54, 1175–1180. [Google Scholar] [CrossRef]
- Graves, E.E.; Hicks, R.J.; Binns, D.; Bressel, M.; Le, Q.-T.; Peters, L.; Young, R.J.; Rischin, D. Quantitative and qualitative analysis of [18F]FDG and [18F]FAZA positron emission tomography of head and neck cancers and associations with HPV status and treatment outcome. Eur. J. Nucl. Med. 2016, 43, 617–625. [Google Scholar] [CrossRef]
- Mortensen, L.S.; Johansen, J.; Kallehauge, J.F.; Primdahl, H.; Busk, M.; Lassen, P.; Alsner, J.; Sørensen, B.S.; Toustrup, K.; Jakobsen, S.; et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother. Oncol. 2012, 105, 14–20. [Google Scholar] [CrossRef]
- Han, K.; Shek, T.; Vines, D.; Driscoll, B.; Fyles, A.; Jaffray, D.; Keller, H.; Metser, U.; Pintilie, M.; Xie, J.; et al. Measurement of Tumor Hypoxia in Patients with Locally Advanced Cervical Cancer Using Positron Emission Tomography with 18F-Fluoroazomyin Arabinoside. Int. J. Radiat. Oncol. 2018, 102, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, M.; Schmid, M.P.; Pötter, R.; Kommata, S.; Georg, D.; Lukic, D.; Dudczak, R.; Kletter, K.; Dimopoulos, J.; Karanikas, G.; et al. Evaluating repetitive18F-fluoroazomycin-arabinoside (18FAZA) PET in the setting of MRI guided adaptive radiotherapy in cervical cancer. Acta Oncol. 2010, 49, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.G.; Zegers, C.; Lieuwes, N.; van Elmpt, W.; Eriksson, J.; van Dongen, G.; Dubois, L. A comparative study of the hypoxia PET tracers [¹⁸F]HX4, [¹⁸F]FAZA, and [¹⁸F]FMISO in a preclinical tumor model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Havelund, B.M.; Holdgaard, P.C.; Rafaelsen, S.R.; Mortensen, L.S.; Theil, J.; Bender, D.; Pløen, J.; Spindler, K.-L.G.; Jakobsen, A. Tumour hypoxia imaging with 18F-fluoroazomycinarabinofuranoside PET/CT in patients with locally advanced rectal cancer. Nucl. Med. Commun. 2013, 34, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, Y.; Cabrera, A.R.; Sun, X.; Zhao, S.; Xie, P.; Zheng, J.; Ma, L.; Fu, Z. Measuring tumor hypoxia with 18F-FETNIM PET in esophageal squamous cell carcinoma: A pilot clinical study. Dis. Esophagus 2012, 25, 54–61. [Google Scholar] [CrossRef]
- Hu, M.; Xie, P.; Lee, N.; Li, M.; Ho, F.; Lian, M.; Zhao, S.; Yang, G.; Fu, Z.; Zheng, J.; et al. Hypoxia with 18F-fluoroerythronitroimidazole integrated positron emission tomography and computed tomography (18F-FETNIM PET/CT) in locoregionally advanced head and neck cancer: Hypoxia changes during chemoradiotherapy and impact on clinical outcome. Medicine 2019, 98, e17067. [Google Scholar] [CrossRef]
- Vercellino, L.; Groheux, D.; Thoury, A.; Delord, M.; Schlageter, M.; Delpech, Y.; Barré, E.; Baruch-Hennequin, V.; Tylski, P.; Homyrda, L.; et al. Hypoxia imaging of uterine cervix carcinoma with (18)F-FETNIM PET/CT. Clin. Nucl. Med. 2012, 37, 1065–1068. [Google Scholar] [CrossRef]
- Lehtiö, K.; Oikonen, V.; Grönroos, T.; Eskola, O.; Kalliokoski, K.; Bergman, J.; Solin, O.; Grénman, R.; Nuutila, P.; Minn, H. Imaging of blood flow and hypoxia in head and neck cancer: Initial evaluation with [(15)O]H(2)O and [(18)F]fluoroerythronitroimidazole PET. J. Nucl. Med. 2001, 42, 1643–1652. [Google Scholar]
- Wei, Y.; Zhao, W.; Huang, Y.; Yu, Q.; Zhu, S.; Wang, S.; Zhao, S.; Hu, X.; Yu, J.; Yuan, S. A Comparative Study of Noninvasive Hypoxia Imaging with 18F-Fluoroerythronitroimidazole and 18F-Fluoromisonidazole PET/CT in Patients with Lung Cancer. PLoS ONE 2016, 11, e0157606. [Google Scholar] [CrossRef]
- Beppu, T.; Terasaki, K.; Sasaki, T.; Fujiwara, S.; Matsuura, H.; Ogasawara, K.; Sera, K.; Yamada, N.; Uesugi, N.; Sugai, T.; et al. Standardized Uptake Value in High Uptake Area on Positron Emission Tomography with 18F-FRP170 as a Hypoxic Cell Tracer Correlates with Intratumoral Oxygen Pressure in Glioblastoma. Mol. Imaging Biol. 2014, 16, 127–135. [Google Scholar] [CrossRef]
- Kaneta, T.; Takai, Y.; Iwata, R.; Hakamatsuka, T.; Yasuda, H.; Nakayama, K.; Ishikawa, Y.; Watanuki, S.; Furumoto, S.; Funaki, Y.; et al. Initial evaluation of dynamic human imaging using 18F-FRP170 as a new PET tracer for imaging hypoxia. Ann. Nucl. Med. 2007, 21, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Komar, G.; Seppänen, M.; Eskola, O.; Lindholm, P.; Grönroos, T.; Forsback, S.; Sipilä, H.; Evans, S.; Solin, O. 18F-EF5: A new PET tracer for imaging hypoxia in head and neck cancer. J. Nucl. Med. 2008, 49, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Narva, S.I.; Seppänen, M.P.M.; Raiko, J.R.M.; Forsback, S.J.M.; Orte, K.J.M.; Virtanen, J.M.M.; Hynninen, J.M.; Hietanen, S.M. Imaging of Tumor Hypoxia With 18F-EF5 PET/MRI in Cervical Cancer. Clin. Nucl. Med. 2021, 46, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J.; Scheuermann, J.S.; Divgi, C.; Judy, K.D.; Kachur, A.V.; Freifelder, R.; Reddin, J.S.; Karp, J.; Stubbs, J.B.; Hahn, S.M.; et al. Biodistribution and dosimetry of 18F-EF5 in cancer patients with preliminary comparison of 18F-EF5 uptake versus EF5 binding in human glioblastoma. Eur. J. Nucl. Med. 2010, 37, 2048–2059. [Google Scholar] [CrossRef]
- Zegers, C.M.; van Elmpt, W.; Reymen, B.; Even, A.; Troost, E.; Ollers, M.; Hoebers, F.; Houben, R.; Eriksson, J.; Windhorst, A.; et al. In vivo quantification of hypoxic and metabolic status of NSCLC tumors using [18F]HX4 and [18F]FDG-PET/CT imaging. Clin. Cancer Res. 2014, 20, 6389–6397. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Kolb, H.; Walsh, J.; Zhang, J. ¹⁸F-HX4 hypoxia imaging with PET/CT in head and neck cancer: A comparison with ¹⁸F-FMISO. Nucl. Med. Commun. 2012, 33, 1096–1102. [Google Scholar] [CrossRef]
- Zegers, C.M.L.; Hoebers, F.J.P.; Van Elmpt, W.; Bons, J.A.; Öllers, M.C.; Troost, E.G.C.; Eekers, D.; Balmaekers, L.; Arts-Pechtold, M.; Mottaghy, F.M.; et al. Evaluation of tumour hypoxia during radiotherapy using [18F]HX4 PET imaging and blood biomarkers in patients with head and neck cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2139–2146. [Google Scholar] [CrossRef]
- Klaassen, R.; Bennink, R.J.; van Tienhoven, G.; Bijlsma, M.F.; Besselink, M.G.; Henegouwen, M.I.v.B.; Wilmink, J.W.; Nederveen, A.J.; Windhorst, A.D.; Hulshof, M.C.; et al. Feasibility and repeatability of PET with the hypoxia tracer [18F]HX4 in oesophageal and pancreatic cancer. Radiother. Oncol. 2015, 116, 94–99. [Google Scholar] [CrossRef]
- Dubois, L.J.; Lieuwes, N.; Janssen, M.; Peeters, W.; Windhorst, A.; Walsh, J.; Kolb, H.; Ollers, M.; Bussink, J.; van Dongen, G.; et al. Preclinical evaluation and validation of [18F]HX4, a promising hypoxia marker for PET imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 14620–14625. [Google Scholar] [CrossRef]
- Minagawa, Y.; Shizukuishi, K.; Koike, I.; Horiuchi, C.; Watanuki, K.; Hata, M.; Omura, M.; Odagiri, K.; Tohnai, I.; Inoue, T.; et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: A pilot study. Ann. Nucl. Med. 2011, 25, 339–345. [Google Scholar] [CrossRef]
- Kinoshita, T.; Fujii, H.; Hayashi, Y.; Kamiyama, I.; Ohtsuka, T.; Asamura, H. Prognostic significance of hypoxic PET using 18 F-FAZA and 62 Cu-ATSM in non-small-cell lung cancer. Lung Cancer 2015, 91, 56–66. [Google Scholar] [CrossRef]
- Grigsby, P.W.; Malyapa, R.S.; Higashikubo, R.; Schwarz, J.K.; Welch, M.J.; Huettner, P.C.; Dehdashti, F. Comparison of Molecular Markers of Hypoxia and Imaging with 60Cu-ATSM in Cancer of the Uterine Cervix. Mol. Imaging Biol. 2007, 9, 278–283. [Google Scholar] [CrossRef]
- Dietz, D.W.; Dehdashti, F.; Grigsby, P.W.; Malyapa, R.S.; Myerson, R.J.; Picus, J.; Ritter, J.; Lewis, J.; Welch, M.J.; Siegel, B.A. Tumor Hypoxia Detected by Positron Emission Tomography with 60Cu-ATSM as a Predictor of Response and Survival in Patients Undergoing Neoadjuvant Chemoradiotherapy for Rectal Carcinoma: A Pilot Study. Dis. Colon Rectum 2008, 51, 1641–1648. [Google Scholar] [CrossRef]
- Tateishi, K.; Sato, M.; Yamanaka, S.; Kanno, H.; Murata, H.; Inoue, T.; Kawahara, N. Application of62Cu-Diacetyl-Bis (N4-Methylthiosemicarbazone) PET Imaging to Predict Highly Malignant Tumor Grades and Hypoxia-Inducible Factor-1α Expression in Patients with Glioma. Am. J. Neuroradiol. 2013, 34, 92–99. [Google Scholar] [CrossRef]
- Capasso, E.; Durzu, S.; Piras, S.; Zandieh, S.; Knoll, P.; Haug, A.; Hacker, M.; Meleddu, C.; Mirzaei, S. Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann. Nucl. Med. 2015, 29, 482–488. [Google Scholar] [CrossRef]
- Piccardo, A.; Paparo, F.; Puntoni, M.; Righi, S.; Bottoni, G.; Bacigalupo, L.; Zanardi, S.; DeCensi, A.; Ferrarazzo, G.; Gambaro, M.; et al. (64)CuCl(2) PET/CT in Prostate Cancer Relapse. J. Nucl. Med. 2018, 59, 444–451. [Google Scholar] [CrossRef]
- Lohith, T.G.; Kudo, T.; Demura, Y.; Umeda, Y.; Kiyono, Y.; Fujibayashi, Y. Pathophysiologic correlation between 62Cu-ATSM and 18F-FDG in lung cancer. J. Nucl. Med. 2009, 50, 1948–1953. [Google Scholar] [CrossRef]
- Kositwattanarerk, A.; Oh, M.; Kudo, T.; Kiyono, Y.; Mori, T.; Kimura, Y.; Maruyama, R.; Fujibayashi, Y.; Fujieda, S.; Okazawa, H. Different Distribution of 62Cu ATSM and 18F-FDG in Head and Neck Cancers. Clin. Nucl. Med. 2012, 37, 252–257. [Google Scholar] [CrossRef]
- Pfeiffer, D. Health Physics and Radiological Health, 4th Edition. Med. Phys. 2013, 40, 117301. [Google Scholar] [CrossRef]
- Dearling, J.L.J.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef]
- Hueting, R.; Kersemans, V.; Cornelissen, B.; Tredwell, M.; Hussien, K.; Christlieb, M.; Gee, A.; Passchier, J.; Smart, S.; Dilworth, J.; et al. A comparison of the behavior of (64)Cu-acetate and (64)Cu-ATSM in vitro and in vivo. J. Nucl. Med. 2014, 55, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Gaustad, J.-V.; Hauge, A.; Wegner, C.S.; Simonsen, T.G.; Lund, K.V.; Hansem, L.M.K.; Rofstad, E.K. DCE-MRI of Tumor Hypoxia and Hypoxia-Associated Aggressiveness. Cancers 2020, 12, 1979. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Bozo, J.C.; Manavaki, R.; Woitek, R.; Torheim, T.; Baxter, G.; Caracò, C.; Provenzano, E.; Graves, M.; Fryer, T.; Patterson, A.; et al. Hypoxia and perfusion in breast cancer: Simultaneous assessment using PET/MR imaging. Eur. Radiol. 2021, 31, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hu, L.; Zeng, Q.; Peng, D.; Chen, Z.; Huang, C.; Liu, Z.; Wen, Q.; Zou, F.; Yan, L. Dynamic contrast-enhanced MRI of nasopharyngeal carcinoma: Correlation of quantitative dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) parameters with hypoxia-inducible factor 1α expression and tumor grade/stage. Ann. Palliat. Med. 2021, 10, 2238–2253. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, I. Insights into Hypoxia: Non-invasive Assessment through Imaging Modalities and Its Application in Breast Cancer. J. Breast Cancer 2019, 22, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torres, J.; López-Larrubia, P.; Ballesteros, P. Imaging tumor hypoxia by magnetic resonance methods. NMR Biomed. 2011, 24, 1–16. [Google Scholar] [CrossRef]
- Dubec, M.J.; Buckley, D.L.; Berks, M.; Clough, A.; Gaffney, J.; Datta, A.; McHugh, D.J.; Porta, N.; Little, R.A.; Cheung, S.; et al. First-in-human technique translation of oxygen-enhanced MRI to an MR Linac system in patients with head and neck cancer. Radiother. Oncol. 2023, 183, 109592. [Google Scholar] [CrossRef]
- Little, R.A.; Datta, A.; Featherstone, A.; Watson, Y.; Cheung, S.; Buckley, L.; Saunders, M.; Parker, G. OE-MRI, DCE-MRI and DWI provide complementary response evaluation in patients with rectal cancer treated with chemoradiotherapy. In Proceedings of the ISMRM 27th Annual Meeting & Exhibition, Montréal, QC, Canada, 11–16 May 2019. [Google Scholar]
- Datta, A.; Dubec, M.; Buckley, D.; McHugh, D.; Salah, A.; Little, R.; Berks, M.; Cheung, S.; West, C.; Choudhury, A. Quantifying and mapping hypoxia modification in patients with uterine cervical cancer using oxygen-enhanced MRI. In Proceedings of the Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting, London, UK, 7–12 May 2022. [Google Scholar]
- Arnold, J.F.; Kotas, M.; Fidler, F.; Pracht, E.D.; Flentje, M.; Jakob, P. Quantitative regional oxygen transfer imaging of the human lung. J. Magn. Reson. Imaging 2007, 26, 637–645. [Google Scholar] [CrossRef]
- Remmele, S.; Sprinkart, A.; Müller, A.; Träber, F.; von Lehe, M.; Gieseke, J.; Flacke, S.; Willinek, W.; Schild, H.; Sénégas, J.; et al. Dynamic and simultaneous MR measurement of R1 and R2* changes during respiratory challenges for the assessment of blood and tissue oxygenation. Magn. Reason. Med. 2013, 70, 136–146. [Google Scholar] [CrossRef]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef]
- Rijpkema, M.; Kaanders, J.H.; Joosten, F.B.; van der Kogel, A.J.; Heerschap, A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int. J. Radiat. Oncol. 2002, 53, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Foltz, W.D.; Milosevic, M.F.; Toi, A.; Bristow, R.G.; Ménard, C.; Haider, M.A. Comparing oxygen-sensitive MRI (BOLD R2*) with oxygen electrode measurements: A pilot study in men with prostate cancer. Int. J. Radiat. Biol. 2009, 85, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Weatherall, P.T.; McColl, R.W.; Tripathy, D.; Mason, R. Blood oxygenation level-dependent (BOLD) contrast magnetic resonance imaging (MRI) for prediction of breast cancer chemotherapy response: A pilot study. J. Magn. Reson. Imaging 2013, 37, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Engineer, R.; Chopra, S.; Mahanshetty, U.; Juvekar, S.; Shrivastava, S.; Desekar, N.; Thakur, M. Role of 3T multiparametric-MRI with BOLD hypoxia imaging for diagnosis and post therapy response evaluation of postoperative recurrent cervical cancers. Eur. J. Radiol. Open 2016, 3, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Maralani, P.J.; Das, S.; Mainprize, T.; Phan, N.; Bharatha, A.; Keith, J.; Munoz, D.G.; Sahgal, A.; Symons, S.; Ironside, S.; et al. Hypoxia Detection in Infiltrative Astrocytoma: Ferumoxytol-based Quantitative BOLD MRI with Intraoperative and Histologic Validation. Radiology 2018, 288, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, J.H.; Kim, E.; Choi, S. Introducing a New Biomarker Named R2 *- BOLD-MRI Parameter to Assess Treatment Response in Osteosarcoma. J. Magn. Reson. Imaging 2022, 56, 538–546. [Google Scholar] [CrossRef]
- Christen, T.; Lemasson, B.; Pannetier, N.; Farion, R.; Remy, C.; Zaharchuk, G.; Barbier, E.L.; Maralani, P.J.; Das, S.; Mainprize, T.; et al. Is T2* Enough to Assess Oxygenation? Quantitative Blood Oxygen Level–Dependent Analysis in Brain Tumor. Radiology 2012, 262, 495–502. [Google Scholar] [CrossRef]
- Zhang, C.; Moonshi, S.S.; Wang, W.; Ta, H.T.; Han, Y.; Han, F.Y.; Peng, H.; Král, P.; Rolfe, B.E.; Gooding, J.J.; et al. High F-Content Perfluoropolyether-Based Nanoparticles for Targeted Detection of Breast Cancer by 19F Magnetic Resonance and Optical Imaging. ACS Nano 2018, 12, 9162–9176. [Google Scholar] [CrossRef]
- Mishima, H.; Kobayashi, T.; Shimizu, M.; Tamaki, Y.; Baba, M.; Shimano, T.; Itoh, S.; Yamazaki, M.; Iriguchi, N.; Takahashi, M.; et al. In vivo F-19 chemical shift imaging with FTPA and antibody-coupled FMIQ. J. Magn. Reson. Imaging 1991, 1, 705–709. [Google Scholar] [CrossRef]
- Kadayakkara, D.K.K.; Janjic, J.M.; Pusateri, L.K.; Young, W.-B.; Ahrens, E.T. In vivo observation of intracellular oximetry in perfluorocarbon-labeled glioma cells and chemotherapeutic response in the CNS using fluorine-19 MRI. Magn. Reson. Med. 2010, 64, 1252–1259. [Google Scholar] [CrossRef]
- Chapelin, F.; Leach, B.I.; Chen, R.; Lister, D.; Messer, K.; Okada, H.; Ahrens, E.T. Assessing Oximetry Response to Chimeric Antigen Receptor T-cell Therapy against Glioma with 19F MRI in a Murine Model. Radiol. Imaging Cancer 2021, 3, e200062. [Google Scholar] [CrossRef]
- Zhao, D.; Ran, S.; Constantinescu, A.; Hahn, E.W.; Mason, R.P. Tumor Oxygen Dynamics: Correlation of In Vivo MRI with Histological Findings. Neoplasia 2003, 5, 308–318. [Google Scholar] [CrossRef]
- Schmieder, A.H.; Wang, K.; Zhang, H.; Senpan, A.; Pan, D.; Keupp, J.; Caruthers, S.D.; Wickline, S.A.; Shen, B.; Wagner, E.M.; et al. Characterization of early neovascular response to acute lung ischemia using simultaneous 19F/1H MR molecular imaging. Angiogenesis 2014, 17, 51–60. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, L.; Hahn, E. Comparison of 1H blood oxygen level-dependent (BOLD) and 19F MRI to investigate tumor oxygenation. Magn. Reson. Med. 2009, 62, 357–364. [Google Scholar] [CrossRef]
- Mignion, L.; Magat, J.; Schakman, O.; Marbaix, E.; Gallez, B.; Jordan, B.F. Hexafluorobenzene in comparison with perfluoro-15-crown-5-ether for repeated monitoring of oxygenation using 19F MRI in a mouse model. Magn. Reson. Med. 2013, 69, 248–254. [Google Scholar] [CrossRef]
- Ratai, E.-M.; Zhang, Z.; Fink, J.; Muzi, M.; Hanna, L.; Greco, E.; Richards, T.; Kim, D.; Andronesi, O.C.; Mintz, A.; et al. ACRIN 6684: Multicenter, phase II assessment of tumor hypoxia in newly diagnosed glioblastoma using magnetic resonance spectroscopy. PLoS ONE 2018, 13, e0198548. [Google Scholar] [CrossRef]
- Pinker, K.; Helbich, T.H.; Morris, E.A. The potential of multiparametric MRI of the breast. Br. J. Radiol. 2017, 90, 20160715. [Google Scholar] [CrossRef]
- O’Flynn, E.A.; DeSouza, N. Functional magnetic resonance: Biomarkers of response in breast cancer. Breast Cancer Res. 2011, 13, 204. [Google Scholar] [CrossRef]
- Maldonado, X.; Alonso, J.; Giralt, J.; Cucurella, M.; del Campo, J.; Rovira, A.; Felip, E.; Capellades, J.; Grivé, E.; Rubio, D.; et al. 31Phosphorus magnetic resonance spectroscopy in the assessment of head and neck tumors. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 309–312. [Google Scholar] [CrossRef]
- Moestue, S.; Sitter, B.; Bathen, T.F.; Tessem, M.-B.; Gribbestad, I.S. HR MAS MR spectroscopy in metabolic characterization of cancer. Curr. Top. Med. Chem. 2011, 11, 2–26. [Google Scholar] [CrossRef]
- Schult, T.A.; Lauer, M.J.; Berker, Y.; Cardoso, M.R.; Vandergrift, L.A.; Habbel, P.; Nowak, J.; Taupitz, M.; Aryee, M.; Mino-Kenudson, M.A.; et al. Screening human lung cancer with predictive models of serum magnetic resonance spectroscopy metabolomics. Proc. Natl. Acad. Sci. USA 2021, 118, e2110633118. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Brender, J.R.; Chandramouli, G.V.R.; Saida, Y.; Yamamoto, K.; Mitchell, J.B.; Krishna, M.C. Hypoxia-Activated Prodrug Evofosfamide Treatment in Pancreatic Ductal Adenocarcinoma Xenografts Alters the Tumor Redox Status to Potentiate Radiotherapy. Antioxid. Redox Signal 2021, 35, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Kawai, T.; Matsumoto, S.; Saito, K.; Devasahayam, N.; Mitchell, J.B.; Camphausen, K.; Inanami, O.; Krishna, M.C. Quantitative imaging of pO2 in orthotopic murine gliomas: Hypoxia correlates with resistance to radiation. Free Radic Res. 2017, 51, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Barth, E.D.; Lee, T.-H.; Chen, C.-T.; Epel, B.; Halpern, H.J.; Lo, L.-W. Highly sensitive electron paramagnetic resonance nanoradicals for quantitative intracellular tumor oxymetric images. Int. J. Nanomed. 2019, 14, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Yasui, H.; Batra, S.; Kinoshita, Y.; Bernardo, M.; Munasinghe, J.P.; Utsumi, H.; Choudhuri, R.; Devasahayam, N.; Subramanian, S.; et al. Simultaneous imaging of tumor oxygenation and microvascular permeability using Overhauser enhanced MRI. Proc. Natl. Acad. Sci. USA 2009, 106, 17898–17903. [Google Scholar] [CrossRef]
- Ahn, K.-H.; Scott, G.; Stang, P.; Conolly, S.; Hristov, D. Multiparametric imaging of tumor oxygenation, redox status, and anatomical structure using overhauser-enhanced MRI-prepolarized MRI system. Magn. Reson. Med. 2011, 65, 1416–1422. [Google Scholar] [CrossRef]
- Samouilov, A.; Efimova, O.V.; Bobko, A.A.; Sun, Z.; Petryakov, S.; Eubank, T.D.; Trofimov, D.G.; Kirilyuk, I.A.; Grigor’ev, I.A.; Takahashi, W.; et al. In Vivo Proton–Electron Double-Resonance Imaging of Extracellular Tumor pH Using an Advanced Nitroxide Probe. Anal. Chem. 2014, 86, 1045–1052. [Google Scholar] [CrossRef]
- Gorodetskii, A.A.; Eubank, T.D.; Driesschaert, B.; Poncelet, M.; Ellis, E.; Khramtsov, V.V.; Bobko, A.A. Development of multifunctional Overhauser-enhanced magnetic resonance imaging for concurrent in vivo mapping of tumor interstitial oxygenation, acidosis and inorganic phosphate concentration. Sci. Rep. 2019, 9, 12093. [Google Scholar] [CrossRef]
- Colliez, F.; Gallez, B.; Jordan, B.F. Assessing Tumor Oxygenation for Predicting Outcome in Radiation Oncology: A Review of Studies Correlating Tumor Hypoxic Status and Outcome in the Preclinical and Clinical Settings. Front. Oncol. 2017, 7, 10. [Google Scholar] [CrossRef]
- Sun, X.; Niu, G.; Chan, N.; Shen, B.; Chen, X. Tumor Hypoxia Imaging. Mol. Imaging Biol. 2011, 13, 399–410. [Google Scholar] [CrossRef]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET radiopharmaceuticals for imaging of tumor hypoxia: A review of the evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365–384. [Google Scholar]
- Delso, G.; ter Voert, E.; Veit-Haibach, P. How does PET/MR work? Basic physics for physicians. Abdom. Imaging 2015, 40, 1352–1357. [Google Scholar] [CrossRef]
- Chapman, J.D. Hypoxic Sensitizers — Implications for Radiation Therapy. N. Engl. J. Med. 1979, 301, 1429–1432. [Google Scholar] [CrossRef]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Imaging of Tumor Characteristics and Molecular Pathways with PET: Developments Over the Last Decade Toward Personalized Cancer Therapy. Int. J. Radiat. Oncol. 2018, 102, 1165–1182. [Google Scholar] [CrossRef]
- Obata, A.; Yoshimi, E.; Waki, A.; Lewis, J.S.; Oyama, N.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann. Nucl. Med. 2001, 15, 499–504. [Google Scholar] [CrossRef]
- Dehdashti, F.; Mintun, M.A.; Lewis, J.S.; Bradley, J.; Govindan, R.; Laforest, R.; Welch, M.J.; Siegel, B.A. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur. J. Nucl. Med. 2003, 30, 844–850. [Google Scholar] [CrossRef]
- Dehdashti, F.; Grigsby, P.W.; Mintun, M.A.; Lewis, J.S.; Siegel, B.A.; Welch, M.J. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: Relationship to therapeutic response—A preliminary report. Int. J. Radiat. Oncol. 2003, 55, 1233–1238. [Google Scholar] [CrossRef]
- Takahashi, N.; Fujibayashi, Y.; Yonekura, Y.; Welch, M.J.; Waki, A.; Tsuchida, T.; Sadato, N.; Sugimoto, K.; Itoh, H. Evaluation of 62Cu labeled diacetyl-bis(N 4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer. Ann. Nucl. Med. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Lewis, J.S.; McCarthy, D.W.; McCarthy, T.J.; Fujibayashi, Y.; Welch, M.J. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J. Nucl. Med. 1999, 40. [Google Scholar]
- Holland, J.P.; Lewis, J.S.; Dehdashti, F. Assessing tumor hypoxia by positron emission tomography with Cu-ATSM. Q. J. Nucl. Med. Mol. Imaging 2009, 53, 193–200. [Google Scholar]
- Fujibayashi, Y.; Taniuchi, H.; Yonekura, Y.; Ohtani, H.; Konishi, J.; Yokoyama, A. Copper-62-ATSM: A new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997, 38. [Google Scholar]
- Seelam, S.R.; Lee, J.Y.; Lee, Y.-S.; Hong, M.K.; Kim, Y.J.; Banka, V.K.; Lee, D.S.; Chung, J.-K.; Jeong, J.M. Development of 68Ga-labeled multivalent nitroimidazole derivatives for hypoxia imaging. Bioorganic Med. Chem. 2015, 23, 7743–7750. [Google Scholar] [CrossRef] [PubMed]
- Riedl, C.C.; Brader, P.; Zanzonico, P.B.; Chun, Y.S.; Woo, Y.; Singh, P.; Carlin, S.; Wen, B.; Ling, C.C.; Hricak, H.; et al. Imaging Hypoxia in Orthotopic Rat Liver Tumors with Iodine 124–labeled Iodoazomycin Galactopyranoside PET. Radiology 2008, 248, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Kudo, T.; Mutou, Y.; Umeda, I.O.; Miyano, A.; Ogawa, K.; Ono, M.; Fujii, H.; Kizaka-Kondoh, S.; Hiraoka, M.; et al. Evaluation of [125I]IPOS as a molecular imaging probe for hypoxia-inducible factor-1-active regions in a tumor: Comparison among single-photon emission computed tomography/X-ray computed tomography imaging, autoradiography, and immunohistochemistry. Cancer Sci. 2011, 102, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Sun, X.; Ma, Y.; Suehiro, M.; Zhang, M.; Russell, J.; Humm, J.L.; Ling, C.C.; O’donoghue, J.A. Detection of hypoxia in microscopic tumors using 131I-labeled iodo-azomycin galactopyranoside (131I-IAZGP) digital autoradiography. Eur. J. Nucl. Med. 2010, 37, 339–348. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, H.; Li, Z.; Wang, X. Effect of a second nitroimidazole redox centre on the accumulation of a hypoxia marker: Synthesis and in vitro evaluation of 99mTc-labeled bisnitroimidazole propylene amine oxime complexes. Bioorg. Med. Chem. Lett. 2012, 22, 172–177. [Google Scholar] [CrossRef]
- Bonnitcha, P.; Grieve, S.; Figtree, G. Clinical imaging of hypoxia: Current status and future directions. Free Radic Biol. Med. 2018, 126, 296–312. [Google Scholar] [CrossRef]
- Minn, I.; Koo, S.M.; Lee, H.S.; Brummet, M.; Rowe, S.P.; Gorin, M.A.; Sysa-Shah, P.; Lewis, W.D.; Ahn, H.-H.; Wang, Y.; et al. [64Cu]XYIMSR-06: A dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget 2016, 7, 56471–56479. [Google Scholar] [CrossRef]
- Hoeben, B.A.; Kaanders, J.; Franssen, G.; Troost, E.; Rijken, P.; Oosterwijk, E.; van Dongen, G.; Oyen, W.; Boerman, O. PET of hypoxia with 89Zr-labeled cG250-F(ab’)2 in head and neck tumors. J. Nucl. Med. 2010, 51, 1076–1083. [Google Scholar] [CrossRef]
- Sato, J.; Kitagawa, Y.; Watanabe, S.; Asaka, T.; Ohga, N.; Hirata, K.; Shiga, T.; Satoh, A.; Tamaki, N. Hypoxic volume evaluated by 18 F-fluoromisonidazole positron emission tomography (FMISO-PET) may be a prognostic factor in patients with oral squamous cell carcinoma: Preliminary analyses. Int. J. Oral Maxillofac. Surg. 2018, 47, 553–560. [Google Scholar] [CrossRef]
- Yamane, T.; Aikawa, M.; Yasuda, M.; Fukushima, K.; Seto, A.; Okamoto, K.; Koyama, I.; Kuji, I. [18F]FMISO PET/CT as a preoperative prognostic factor in patients with pancreatic cancer. EJNMMI Res. 2019, 9, 39. [Google Scholar] [CrossRef]
- Zschaeck, S.; Zöphel, K.; Seidlitz, A.; Zips, D.; Kotzerke, J.; Baumann, M.; Troost, E.G.; Löck, S.; Krause, M. Generation of biological hypotheses by functional imaging links tumor hypoxia to radiation induced tissue inflammation/glucose uptake in head and neck cancer. Radiother. Oncol. 2021, 155, 204–211. [Google Scholar] [CrossRef]
- Saga, T.; Inubushi, M.; Koizumi, M.; Yoshikawa, K.; Zhang, M.-R.; Obata, T.; Tanimoto, K.; Harada, R.; Uno, T.; Fujibayashi, Y. Prognostic value of PET/CT with 18F-fluoroazomycin arabinoside for patients with head and neck squamous cell carcinomas receiving chemoradiotherapy. Ann. Nucl. Med. 2016, 30, 217–224. [Google Scholar] [CrossRef]
- Saga, T.; Inubushi, M.; Koizumi, M.; Yoshikawa, K.; Zhang, M.; Tanimoto, K.; Horiike, A.; Yanagitani, N.; Ohyanagi, F. Prognostic value of (18) F-fluoroazomycin arabinoside PET/CT in patients with advanced non-small-cell lung cancer. Cancer Sci. 2015, 106, 1554–1560. [Google Scholar] [CrossRef]
- Saksø, M.; Mortensen, L.S.; Primdahl, H.; Johansen, J.; Kallehauge, J.; Hansen, C.R.; Overgaard, J. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial (NCT01017224). Radiother. Oncol. 2020, 151, 126–133. [Google Scholar] [CrossRef]
- Lehtiö, K.; Eskola, O.; Viljanen, T.; Oikonen, V.; Grönroos, T.; Sillanmäki, L.; Grénman, R.; Minn, H. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int. J. Radiat. Oncol. 2004, 59, 971–982. [Google Scholar] [CrossRef]
- Beppu, T.; Sasaki, T.; Terasaki, K.; Saura, H.; Mtsuura, H.; Ogasawara, K.; Sasaki, M.; Ehara, S.; Iwata, R.; Takai, Y. High-uptake areas on positron emission tomography with the hypoxic radiotracer 18F-FRP170 in glioblastomas include regions retaining proliferative activity under hypoxia. Ann. Nucl. Med. 2015, 29, 336–341. [Google Scholar] [CrossRef]
- Ali, R.; Apte, S.; Vilalta, M.; Subbarayan, M.; Miao, Z.; Chin, F.T.; Graves, E.E. 18F-EF5 PET Is Predictive of Response to Fractionated Radiotherapy in Preclinical Tumor Models. PLoS ONE 2015, 10, e0139425. [Google Scholar] [CrossRef]
- Carlin, S.; Zhang, H.; Reese, M.; Ramos, N.N.; Chen, Q.; Ricketts, S.-A. A Comparison of the Imaging Characteristics and Microregional Distribution of 4 Hypoxia PET Tracers. J. Nucl. Med. 2014, 55, 515–521. [Google Scholar] [CrossRef]
- Asano, A.; Ueda, S.; Kuji, I.; Yamane, T.; Takeuchi, H.; Hirokawa, E.; Sugitani, I.; Shimada, H.; Hasebe, T.; Osaki, A.; et al. Intracellular hypoxia measured by 18F-fluoromisonidazole positron emission tomography has prognostic impact in patients with estrogen receptor-positive breast cancer. Breast Cancer Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Smith, R.C.; McCarthy, S. Physics of magnetic resonance. J. Reprod. Med. 1992, 37, 19–26. [Google Scholar] [PubMed]
- Yan, Y.; Sun, X.; Shen, B. Contrast agents in dynamic contrast-enhanced magnetic resonance imaging. Oncotarget 2017, 8, 43491–43505. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Jackson, A.; Buonaccorsi, G.A.; Buckley, D.L.; Roberts, C.; Watson, Y.; Cheung, S.; McGrath, D.M.; Naish, J.H.; Rose, C.J.; et al. Organ-specific effects of oxygen and carbogen gas inhalation on tissue longitudinal relaxation times. Magn. Reson. Med. 2007, 58, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pacheco-Torres, J.; Hallac, R.; White, D.; Peschke, P.; Cerdan, S. Dynamic oxygen challenge evaluated by NMR T1 and T2*—Insights into tumor oxygenation. NMR Biomed. 2015, 28, 937–947. [Google Scholar] [CrossRef]
- Ma, M.; Liang, J.; Zhang, D.; Xu, X.; Cheng, Q.; Xiao, Z.; Shi, C.; Luo, L. Monitoring Treatment Efficacy of Antiangiogenic Therapy Combined With Hypoxia-Activated Prodrugs Online Using Functional MRI. Front. Oncol. 2021, 11, 672047. [Google Scholar] [CrossRef]
- Thulborn, K.R.; Waterton, J.C.; Matthews, P.M.; Radda, G.K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Biophys. Acta Gen. Subj. 1982, 714, 265–270. [Google Scholar] [CrossRef]
- Chen, J.; Lanza, G.M.; Wickline, S.A. Quantitative magnetic resonance fluorine imaging: Today and tomorrow. WIREs Nanomed. Nanobiotechnol. 2010, 2, 431–440. [Google Scholar] [CrossRef]
- Spiess, B.D. Perfluorocarbon emulsions as a promising technology: A review of tissue and vascular gas dynamics. J. Appl. Physiol. 2009, 106, 1444–1452. [Google Scholar] [CrossRef]
- Thomas, S.R.; Clark, L.C.J.; Ackerman, J.L.; Pratt, R.G.; Hoffmann, R.E.; Busse, L.J.; Kinsey, R.A.; Samaratunga, R.C. MR Imaging of the Lung Using Liquid Perfluorocarbons. J. Comput. Assist. Tomogr. 1986, 10, 1–9. [Google Scholar] [CrossRef]
- Mattrey, R.F.; Long, D.C. Potential role of PFOB in diagnostic imaging. Investig. Radiol. 1988, 23 (Suppl. S1), S298–S301. [Google Scholar] [CrossRef]
- Zhang, W.; Ito, Y.; Berlin, E.; Roberts, R.; Berkowitz, B.A. Role of Hypoxia during Normal Retinal Vessel Development and in Experimental Retinopathy of Prematurity. Investig. Opthalmol. Vis. Sci. 2003, 44, 3119–3123. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, L.; Mason, R.P. Measuring Changes in Tumor Oxygenation. Methods Enzym. 2004, 386, 378–418. [Google Scholar]
- Zhao, D.; Constantinescu, A.; Jiang, L.; Hahn, E.W.; Mason, R. Prognostic Radiology: Quantitative Assessment of Tumor Oxygen Dynamics by MRI. Am. J. Clin. Oncol. 2001, 24, 462–466. [Google Scholar] [CrossRef]
- Xie, D.; Kim, S.; Kohli, V.; Banerjee, A.; Yu, M.; Enriquez, J.S.; Luci, J.J.; Que, E.L. Hypoxia-Responsive 19F MRI Probes with Improved Redox Properties and Biocompatibility. Inorg. Chem. 2017, 56, 6429–6437. [Google Scholar] [CrossRef]
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.; Grover, V.P.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic Resonance Spectroscopy: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [Google Scholar] [CrossRef]
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular mechanisms of hypoxia in cancer. Clin. Transl. Imaging 2017, 5, 225–253. [Google Scholar] [CrossRef] [PubMed]
- Iversen, A.B.; Ringgaard, S.; Laustsen, C.; Stødkilde-Jørgensen, H.; Bentzen, L.; Busk, M.; Horsman, M.R. Hyperpolarized magnetic resonance spectroscopy for assessing tumor hypoxia. Acta Oncol. 2015, 54, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Matsumoto, S.; Yasui, H.; Saito, K.; Devasahayam, N.; Subramanian, S. Electron paramagnetic resonance imaging of tumor pO2. Radiat. Res. 2012, 177, 376–386. [Google Scholar] [CrossRef]
- Beeman, S.C.; Shui, Y.-B.; Perez-Torres, C.J.; Engelbach, J.A.; Ackerman, J.J.H.; Garbow, J.R. O2-sensitive MRI distinguishes brain tumor versus radiation necrosis in murine models. Magn. Reson. Med. 2016, 75, 2442–2447. [Google Scholar] [CrossRef]
- White, D.A.; Zhang, Z.; Li, L.; Gerberich, J.; Stojadinovic, S.; Peschke, P.; Mason, R.P. Developing oxygen-enhanced magnetic resonance imaging as a prognostic biomarker of radiation response. Cancer Lett. 2016, 380, 69–77. [Google Scholar] [CrossRef]
- Cao-Pham, T.-T.; Tran, L.-B.; Colliez, F.; Joudiou, N.; El Bachiri, S.; Grégoire, V.; Levêque, P.; Gallez, B.; Jordan, B.F. Monitoring Tumor Response to Carbogen Breathing by Oxygen-Sensitive Magnetic Resonance Parameters to Predict the Outcome of Radiation Therapy: A Preclinical Study. Int. J. Radiat. Oncol. 2016, 96, 149–160. [Google Scholar] [CrossRef]
- Little, R.A.; Tessyman, V.; Babur, M.; Cheung, S.; Watson, Y.; Gieling, R.; Finegan, K.; Ashton, T.; Parker, G.; Mckenna, W.; et al. In vivo OE-MRI quantification and mapping of response to hypoxia modifying drugs Banoxantrone and Atovaquone in Calu6 xenografts. Curr. Med. Imaging 2017, 25, 2919. [Google Scholar]
- Qian, J.; Yu, X.; Li, B.; Fei, Z.; Huang, X.; Luo, P.; Zhang, L.; Zhang, Z.; Lou, J.; Wang, H. In vivo Monitoring of Oxygen Levels in Human Brain Tumor Between Fractionated Radiotherapy Using Oxygen-enhanced MR Imaging. Curr. Med. Imaging Former. Curr. Med. Imaging Rev. 2020, 16, 427–432. [Google Scholar] [CrossRef]
- Bluemke, E.; Bertrand, A.; Chu, K.-Y.; Syed, N.; Murchison, A.G.; Cooke, R.; Greenhalgh, T.; Burns, B.; Craig, M.; Taylor, N.; et al. Oxygen-enhanced MRI and radiotherapy in patients with oropharyngeal squamous cell carcinoma. Clin. Transl. Radiat. Oncol. 2023, 39, 100563. [Google Scholar] [CrossRef]
- Salem, A.; Little, R.; Latif, A.; Featherstone, A.; Babur, M.; Peset, I.; Cheung, S.; Watson, Y.; Tessyman, V.; Mistry, H.; et al. Oxygen-enhanced MRI Is Feasible, Repeatable, and Detects Radiotherapy-induced Change in Hypoxia in Xenograft Models and in Patients with Non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 3818–3829. [Google Scholar] [CrossRef]
- Little, R.A.; Jamin, Y.; Boult, J.K.R.; Naish, J.H.; Watson, Y.; Cheung, S.; Holliday, K.F.; Lu, H.; McHugh, D.J.; Irlam, J.; et al. Mapping Hypoxia in Renal Carcinoma with Oxygen-enhanced MRI: Comparison with Intrinsic Susceptibility MRI and Pathology. Radiology 2018, 288, 739–747. [Google Scholar] [CrossRef]
- Linnik, I.V.; Scott, M.L.J.; Holliday, K.F.; Woodhouse, N.; Waterton, J.C.; O’Connor, J.P.B.; Barjat, H.; Liess, C.; Ulloa, J.; Young, H.; et al. Noninvasive tumor hypoxia measurement using magnetic resonance imaging in murine U87 glioma xenografts and in patients with glioblastoma. Magn. Reson. Med. 2014, 71, 1854–1862. [Google Scholar] [CrossRef]
- Hectors, S.J.; Wagner, M.; Bane, O.; Besa, C.; Lewis, S.; Remark, R.; Chen, N.; Fiel, M.I.; Zhu, H.; Gnjatic, S.; et al. Quantification of hepatocellular carcinoma heterogeneity with multiparametric magnetic resonance imaging. Sci. Rep. 2017, 7, 2452. [Google Scholar] [CrossRef]
- Napier, T.S.; Lynch, S.E.; Lu, Y.; Song, P.N.; Burns, A.C.; Sorace, A.G. Molecular Imaging of Oxygenation Changes during Immunotherapy in Combination with Paclitaxel in Triple Negative Breast Cancer. Biomedicines 2023, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cheng, Q.; Huang, J.; Ma, M.; Zhang, D.; Lei, X.; Xiao, Z.; Zhang, D.; Shi, C.; Luo, L. Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis 2019, 22, 457–470. [Google Scholar] [CrossRef] [PubMed]
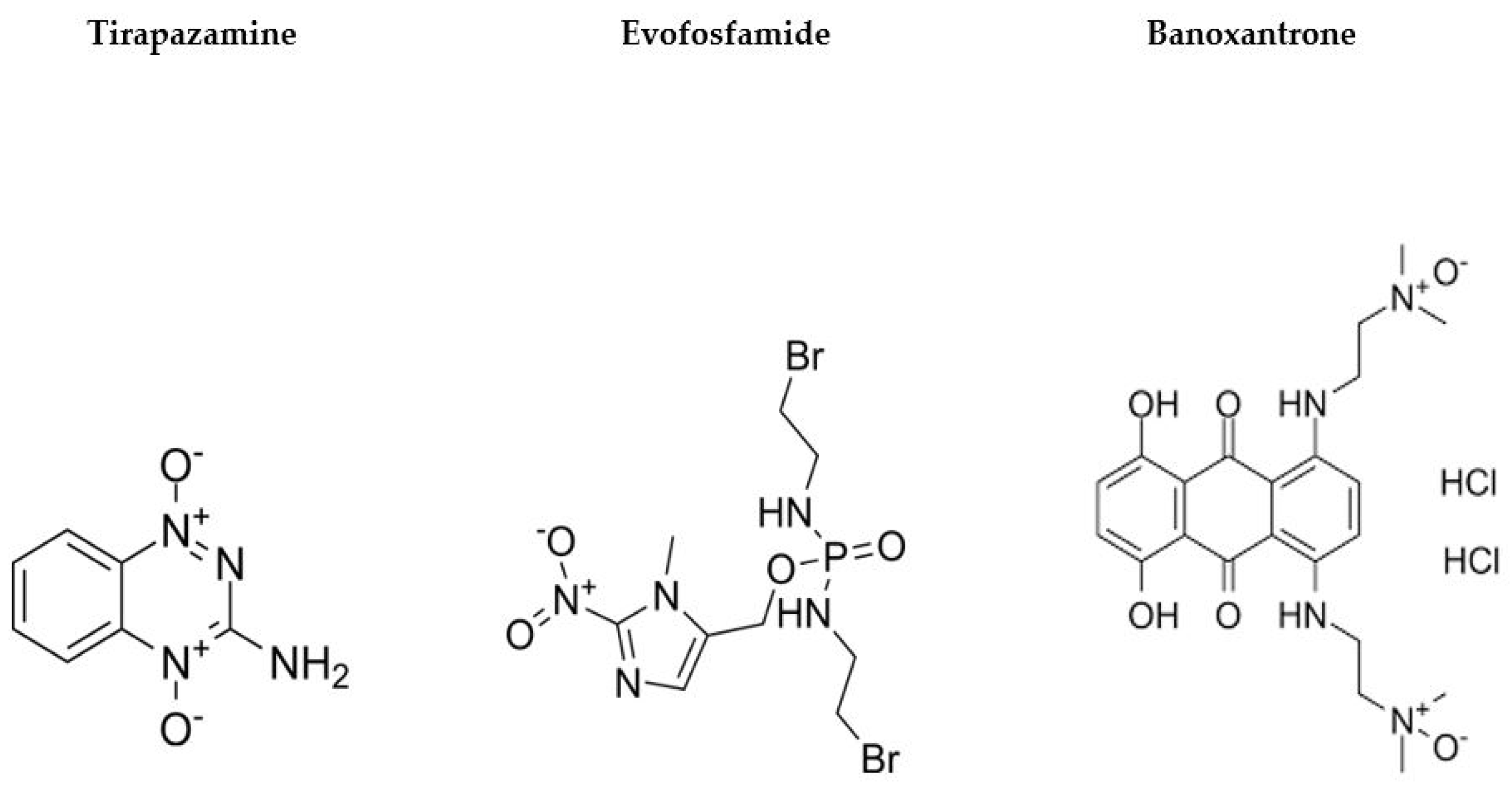
- Reeves, K.M.; Song, P.N.; Angermeier, A.; Della Manna, D.; Li, Y.; Wang, J.; Yang, E.S.; Sorace, A.G.; Larimer, B.M. 18F-FMISO PET Imaging Identifies Hypoxia and Immunosuppressive Tumor Microenvironments and Guides Targeted Evofosfamide Therapy in Tumors Refractory to PD-1 and CTLA-4 Inhibition. Clin. Cancer Res. 2021, 28, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Kang, S.Y.; Lee, K.-H.; Cheon, G.J.; Oh, D.-Y. Targeting Hypoxia Using Evofosfamide and Companion Hypoxia Imaging of FMISO-PET in Advanced Biliary Tract Cancer. Cancer Res. Treat 2021, 53, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Abi-Jaoudeh, N.; Dayyani, F.; Chen, P.J.; Fernando, D.; Fidelman, N.; Javan, H.; Liang, P.-C.; Hwang, J.-I.; Imagawa, D.K. Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.P.; Laeseke, P.F.; Shin, L.K.; Chin, F.T.; Kothary, N.; Segall, G.M. Limitations of Fluorine 18 Fluoromisonidazole in Assessing Treatment-induced Tissue Hypoxia after Transcatheter Arterial Embolization of Hepatocellular Carcinoma: A Prospective Pilot Study. Radiol. Imaging Cancer 2022, 4, e210094. [Google Scholar] [CrossRef]
- Gordon, A.C.; White, S.B.; Gates, V.L.; Procissi, D.; Harris, K.R.; Yang, Y.; Zhang, Z.; Li, W.; Lyu, T.; Huang, X.; et al. Yttrium-90 Radioembolization and Tumor Hypoxia: Gas-challenge BOLD Imaging in the VX2 Rabbit Model of Hepatocellular Carcinoma. Acad. Radiol. 2021, 28, 849–858. [Google Scholar] [CrossRef]
- Al-Hallaq, H.A.; Zamora, M.; Fish, B.L.; Farrell, A.; Moulder, J.E.; Karczmar, G.S. Mri measurements correctly predict the relative effects of tumor oxygenating agents on hypoxic fraction in rodent BA1112 tumors. Int. J. Radiat. Oncol. 2000, 47, 481–488. [Google Scholar] [CrossRef]
- Baudelet, C.; Gallez, B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn. Reson. Med. 2002, 48, 980–986. [Google Scholar] [CrossRef]
- Kishimoto, S.; Matsumoto, K.-I.; Saito, K.; Enomoto, A.; Matsumoto, S.; Mitchell, J.B.; Devasahayam, N.; Krishna, M.C. Pulsed Electron Paramagnetic Resonance Imaging: Applications in the Studies of Tumor Physiology. Antioxid. Redox Signal. 2018, 28, 1378–1393. [Google Scholar] [CrossRef]
- Gertsenshteyn, I.; Giurcanu, M.; Vaupel, P.; Halpern, H. Biological validation of electron paramagnetic resonance (EPR) image oxygen thresholds in tissue. J. Physiol. 2021, 599, 1759–1767. [Google Scholar] [CrossRef]
- Yasui, H.; Matsumoto, S.; Devasahayam, N.; Munasinghe, J.; Choudhuri, R.; Saito, K.; Subramanian, S.; Mitchell, J. Low-field magnetic resonance imaging to visualize chronic and cycling hypoxia in tumor-bearing mice. Cancer Res. 2010, 70, 6427–6436. [Google Scholar] [CrossRef]
- Gerling, M.; Zhao, Y.; Nania, S.; Norberg, K.J.; Verbeke, C.S.; Englert, B.; Kuiper, R.V.; Bergström, A.; Hassan, M.; Neesse, A.; et al. Real-Time Assessment of Tissue Hypoxia In Vivo with Combined Photoacoustics and High-Frequency Ultrasound. Theranostics 2014, 4, 604–613. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Shao, Q.; Morgounova, E.; Jiang, C.; Choi, J.; Bischof, J. In vivo photoacoustic lifetime imaging of tumor hypoxia in small animals. J. Biomed. Opt. 2013, 18, 076019. [Google Scholar] [CrossRef]
- Knox, H.J.; Kim, T.W.; Zhu, Z.; Chan, J. Photophysical Tuning of N-Oxide-Based Probes Enables Ratiometric Photoacoustic Imaging of Tumor Hypoxia. ACS Chem. Biol. 2018, 13, 1838–1843. [Google Scholar] [CrossRef]
- Umehara, Y.; Kageyama, T.; Son, A.; Kimura, Y.; Kondo, T.; Tanabe, K. Biological reduction of nitroimidazole-functionalized gold nanorods for photoacoustic imaging of tumor hypoxia. RSC Adv. 2019, 9, 16863–16868. [Google Scholar] [CrossRef]
- Nyayapathi, N.; Xia, J. Photoacoustic imaging of breast cancer: A mini review of system design and image features. J. Biomed. Opt. 2019, 24, 121911–121913. [Google Scholar] [CrossRef]
- Tank, A.; Peterson, H.M.; Pera, V.; Tabassum, S.; Leproux, A.; O’Sullivan, T.; Jones, E.; Cabral, H.; Ko, N.; Mehta, R.S.; et al. Diffuse optical spectroscopic imaging reveals distinct early breast tumor hemodynamic responses to metronomic and maximum tolerated dose regimens. Breast Cancer Res. 2020, 22, 29. [Google Scholar] [CrossRef]
- Cerussi, A.E.; Shah, N.S.; Hsiang, D.; Durkin, A.; Butler, J.A.; Tromberg, B.J. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J. Biomed. Opt. 2006, 11, 044005. [Google Scholar] [CrossRef]
- de Boer, L.L.; Kho, E.; Van de Vijver, K.K.; Peeters, M.-J.T.F.D.V.; van Duijnhoven, F.; Hendriks, B.H.W.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Optical tissue measurements of invasive carcinoma and ductal carcinoma in situ for surgical guidance. Breast Cancer Res. 2021, 23, 59. [Google Scholar] [CrossRef]
- Sandhu, S.; Kydd, L.; Jaworski, J. Luminescent Probe Based Techniques for Hypoxia Imaging. J. Nanomed. Res. 2017, 6, 00160. [Google Scholar] [PubMed]
- Ciarrocchi, E.; Belcari, N. Cerenkov luminescence imaging: Physics principles and potential applications in biomedical sciences. EJNMMI Phys. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, E.; Courteau, A.; Bellaye, P.-S.; Guillemin, M.; Drouet, C.; Walker, P.; Collin, B.; Decréau, R.A. Cherenkov luminescence imaging is a fast and relevant preclinical tool to assess tumour hypoxia in vivo. EJNMMI Res. 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The Clinical Importance of Assessing Tumor Hypoxia: Relationship of Tumor Hypoxia to Prognosis and Therapeutic Opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and Characterization of Tumor Hypoxia Using pO2 Histography. Antioxid. Redox Signal 2007, 9, 1221–1236. [Google Scholar] [CrossRef]
- Dent, J.G.; Netter, K.J. Errors in oxygen tension measurements caused by halothane. Br. J. Anaesth. 1976, 48, 195–197. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef]
- Verburg, F.A.; Wiessmann, M.; Neuloh, G.; Mottaghy, F.M.; Brockmann, M.-A. Intraindividual comparison of selective intraarterial versus systemic intravenous 68Ga-DOTATATE PET/CT in patients with inoperable meningioma. Nuklearmedizin 2019, 58, 23–27. [Google Scholar] [CrossRef]
| Imaging | Cancers Studied | Advantages | Disadvantages |
|---|---|---|---|
| PET Tracers | |||
| PET—18F-FMISO | Most commonly used PET tracer for clinical and research applications [53] | Slow uptake and washout kinetics [53,54] Low uptake [55] 5–7 mm resolution [54] | |
| PET—18F-FAZA | Favorable vascular clearance and improved hypoxia–normoxia contrast when compared with 18F-FMISO [53] | ||
| PET—FETNIM | Rapid renal clearance and low liver absorption [68] | Low tumor/non-tumor uptake ratio [69] | |
| PET—18F-RP-170 | Favorable time interval before scanning and hypoxia contrast [71] | ||
| PET—18F-EF5 | High plasma half life [74] | ||
| PET—18F-HX4 | High maximum tumor-to-blood ratio [63] 3-h half-life [63] | ||
| PET—Cu-ATSM | Favorable pharmacokinetic profile, signal-to-noise ratio, and is not taken up by the bladder [85,86,87,88,89] | Unclear mechanism of hypoxia selectivity [90,91] | |
| Magnetic Resonance Techniques | |||
| DCE MRI—most commonly used with gadolinium-based contrast agents | Over-time assessment [95] | Dependent on perfusion [92] Limited resolution [96] | |
| TOLD MRI—hyperoxic inhalation | Maps oxygen delivery in tissues [102] | Motion artifact susceptibility [95] | |
| BOLD MRI—optional hyperoxic inhalation | qBOLD quantitative O2 mapping [109] High resolution [95] | Dependent on perfusion [109] | |
| MRI—Fluorine—19F probes | Quantitative PO2 measurement [116] | Probe toxicity [117] Low availability | |
| MRS—endogenous lactate | Provides quantitative measurement of metabolic byproducts of hypoxia | Time consuming | |
| EPRI/ESR—paramagnetic probe | Measuring cycling hypoxia [9] | Limited sensitivity compared with OMRI | |
| OMRI—hyperpolarized paramagnetic contrast | High image resolution [130] Rapid image acquisition [130] | Limited equipment Undesired heating of sample [131] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, R.C.; Kim, D.; Maxwell, A.W.P.; Camacho, J.C. Functional Imaging of Hypoxia: PET and MRI. Cancers 2023, 15, 3336. https://doi.org/10.3390/cancers15133336
Perez RC, Kim D, Maxwell AWP, Camacho JC. Functional Imaging of Hypoxia: PET and MRI. Cancers. 2023; 15(13):3336. https://doi.org/10.3390/cancers15133336
Chicago/Turabian StylePerez, Ryan C., DaeHee Kim, Aaron W. P. Maxwell, and Juan C. Camacho. 2023. "Functional Imaging of Hypoxia: PET and MRI" Cancers 15, no. 13: 3336. https://doi.org/10.3390/cancers15133336
APA StylePerez, R. C., Kim, D., Maxwell, A. W. P., & Camacho, J. C. (2023). Functional Imaging of Hypoxia: PET and MRI. Cancers, 15(13), 3336. https://doi.org/10.3390/cancers15133336






