Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition
Abstract
Simple Summary
Abstract
1. Introduction
2. The Role of EMT-TFs in Cancer
3. Targeting TFs of Basic Domain
3.1. bZIP Factors
3.1.1. Jun and FOS
3.1.2. BACH1
3.2. Basic Helix-Loop-Helix Factors (bHLH) Factors
3.2.1. E2A
3.2.2. Twist
4. Targeting TFs of Zinc-Coordination DNA-Binding Domain
4.1. Nuclear Receptors with C4 Zinc Fingers
4.1.1. Steroid Hormone Receptor (SHR)
4.1.2. Retinoic Acid Receptor (RAR)
4.2. C2H2 Zinc Finger Factors
Snail-like
5. Targeting TFs of Helix-Turn-Helix Domain
5.1. Homeo Domain Factors
5.1.1. ZEB
5.1.2. Intestine-Specific Homeobox (ISX)
5.2. Fork Head/Winged Helix Factors
FOX
6. Targeting TFs of Other All-Alpha-Helical DNA-Binding Domains
6.1. High-Mobility Group (HMG) Domain Factors
SOX
7. Targeting TFs of Immunoglobulin Fold
7.1. Rel Homology Region (RHR) Factors
NF-κB–Related
7.2. Signal Transducer and Activator of Transcription (STAT) Domain Factors
STAT
7.3. p53 Domain Factors
p53
7.4. Runt Domain Factors
RUNX1
8. Targeting TFs of Beta-Hairpin Exposed by an Alpha/Beta-Scaffold
8.1. SMAD/NF-1 DNA-Binding Domain Factors
SMAD
9. The Epigenetic Regulation Pathways of TFs Involved in EMT
9.1. SNAIL-Associated Regulation Pathway
9.2. Twist-Associated Regulation Pathway
9.3. ZEB-Associated Regulation Pathway
9.4. Intestine-Specific Homeobox (ISX) and P300/CBP-Associated Factor (PCAF)
10. Therapeutic Implications of Targeting EMT-TFs
11. Challenge of Targeting EMT-TFs in Cancer
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| hepatocellular carcinoma: | HCC |
| non-small cell lung carcinoma: | NCSLC |
| breast cancer: | BC |
| squamous cell carcinoma: | SCC |
| epithelial-to-mesenchymal transition: | EMT |
References
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 2014, 7, re8. [Google Scholar] [CrossRef] [PubMed]
- Fedele, M.; Sgarra, R.; Battista, S.; Cerchia, L.; Manfioletti, G. The Epithelial–Mesenchymal Transition at the Crossroads between Metabolism and Tumor Progression. Int. J. Mol. Sci. 2022, 23, 800. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial–mesenchymal transition and its transcription factors. Biosci. Rep. 2022, 42, BSR20211754. [Google Scholar] [CrossRef]
- Tarin, D.; Thompson, E.W.; Newgreen, D.F. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005, 65, 5996–6000, Discussion 6000–5991. [Google Scholar] [CrossRef]
- Jonckheere, S.; Adams, J.; De Groote, D.; Campbell, K.; Berx, G.; Goossens, S. Epithelial-Mesenchymal Transition (EMT) as a Therapeutic Target. Cells Tissues Organs 2022, 211, 157–182. [Google Scholar] [CrossRef]
- Mitra, P. Targeting transcription factors in cancer drug discovery. Explor. Target. Anti-Tumor Ther. 2020, 1, 401–412. [Google Scholar] [CrossRef]
- Yeh, J.E.; Toniolo, P.A.; Frank, D.A. Targeting transcription factors: Promising new strategies for cancer therapy. Curr. Opin. Oncol. 2013, 25, 652–658. [Google Scholar] [CrossRef]
- Wingender, E.; Schoeps, T.; Haubrock, M.; Krull, M.; Dönitz, J. TFClass: Expanding the classification of human transcription factors to their mammalian orthologs. Nucleic Acids Res. 2017, 46, D343–D347. [Google Scholar] [CrossRef]
- Wingender, E. Criteria for an updated classification of human transcription factor DNA-binding domains. J. Bioinform. Comput. Biol. 2013, 11, 1340007. [Google Scholar] [CrossRef]
- Wingender, E.; Schoeps, T.; Haubrock, M.; Dönitz, J. TFClass: A classification of human transcription factors and their rodent orthologs. Nucleic Acids Res. 2015, 43, D97–D102. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, A.; Dhungel, B.; Steel, J.C. Epithelial-to-mesenchymal plasticity of cancer stem cells: Therapeutic targets in hepatocellular carcinoma. J. Hematol. Oncol. 2016, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- López-Novoa, J.M.; Nieto, M.A. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 2009, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef]
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef]
- Meng, Q.; Xia, Y. c-Jun, at the crossroad of the signaling network. Protein Cell 2011, 2, 889–898. [Google Scholar] [CrossRef]
- Brennan, A.; Leech, J.T.; Kad, N.M.; Mason, J.M. Selective antagonism of cJun for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 184. [Google Scholar] [CrossRef]
- Mathas, S.; Hinz, M.; Anagnostopoulos, I.; Krappmann, D.; Lietz, A.; Jundt, F.; Bommert, K.; Mechta-Grigoriou, F.; Stein, H.; Dörken, B.; et al. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappaB. EMBO J. 2002, 21, 4104–4113. [Google Scholar] [CrossRef]
- Szabo, E.; Riffe, M.E.; Steinberg, S.M.; Birrer, M.J.; Linnoila, R.I. Altered cJUN expression: An early event in human lung carcinogenesis. Cancer Res. 1996, 56, 305–315. [Google Scholar] [PubMed]
- Vleugel, M.M.; Greijer, A.E.; Bos, R.; van der Wall, E.; van Diest, P.J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006, 37, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Birkenbach, M.; Hart, J. Expression of Jun family members in human colorectal adenocarcinoma. Carcinogenesis 2000, 21, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.-J.; Su, K.-Y.; Hsu, Y.-C.; Chang, G.-C.; Chen, J.-S.; Chen, H.-Y.; Hong, Q.-S.; Hsu, S.-C.; Kang, P.-H.; Hsu, C.-Y.; et al. SPANXA suppresses EMT by inhibiting c-JUN/SNAI2 signaling in lung adenocarcinoma. Oncotarget 2016, 7, 44417–44429. [Google Scholar] [CrossRef]
- Lin, G.; Yu, B.; Liang, Z.; Li, L.; Qu, S.; Chen, K.; Zhou, L.; Lu, Q.; Sun, Y.; Zhu, X. Silencing of c-jun decreases cell migration, invasion, and EMT in radioresistant human nasopharyngeal carcinoma cell line CNE-2R. OncoTargets Ther. 2018, 11, 3805–3815. [Google Scholar] [CrossRef]
- Bakiri, L.; Macho-Maschler, S.; Custic, I.; Niemiec, J.; Guío-Carrión, A.; Hasenfuss, S.C.; Wagner, E.F. Fra-1/AP-1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression. Cell Death Differ. 2015, 22, 336–350. [Google Scholar] [CrossRef]
- Luo, Y.-Z.; He, P.; Qiu, M.-X. FOSL1 enhances growth and metastasis of human prostate cancer cells through epithelial mesenchymal transition pathway. Eur. Rev. Med. Pharm. Sci. 2018, 22, 8609–8615. [Google Scholar]
- Feldker, N.; Ferrazzi, F.; Schuhwerk, H.; Widholz, S.A.; Guenther, K.; Frisch, I.; Jakob, K.; Kleemann, J.; Riegel, D.; Bönisch, U.; et al. Genome-wide cooperation of EMT transcription factor ZEB 1 with YAP and AP-1 in breast cancer. EMBO J. 2020, 39, e103209. [Google Scholar] [CrossRef]
- Byers, S.A.; Schafer, B.; Sappal, D.S.; Brown, J.; Price, D.H. The antiproliferative agent MLN944 preferentially inhibits transcription. Mol. Cancer Ther. 2005, 4, 1260–1267. [Google Scholar] [CrossRef]
- Di Nicolantonio, F.; Knight, L.A.; Whitehouse, P.A.; Mercer, S.J.; Sharma, S.; Charlton, P.A.; Norris, D.; Cree, I.A. The ex vivo characterization of XR5944 (MLN944) against a panel of human clinical tumor samples. Mol. Cancer Ther. 2004, 3, 1631–1637. [Google Scholar] [CrossRef]
- Mishra, D.K.; Kim, M.P. SR 11302, an AP-1 Inhibitor, Reduces Metastatic Lesion Formation in Ex Vivo 4D Lung Cancer Model. Cancer Microenviron. 2017, 10, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Liu, K.; Li, H.; Wang, J.; Zhu, J.; Hao, P.; Zhu, L.; Zhang, S.; Shan, L.; Ma, W.; et al. Veratramine modulates AP-1-dependent gene transcription by directly binding to programmable DNA. Nucleic Acids Res. 2017, 46, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Kamide, D.; Yamashita, T.; Araki, K.; Tomifuji, M.; Tanaka, Y.; Tanaka, S.; Shiozawa, S.; Shiotani, A. Selective activator protein-1 inhibitor T-5224 prevents lymph node metastasis in an oral cancer model. Cancer Sci. 2016, 107, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Wu, B.; Li, J.; Wang, X.; Jiang, S.; Hu, F.; Dou, G.; Zhang, Y.; Sheng, C.; Zhao, G.; et al. T5224, RSPO2 and AZD5363 are novel drugs against functional pituitary adenoma. Aging 2019, 11, 9043–9059. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, K.; Kobatake, K.; Miura, K.; Fukushima, T.; Babasaki, T.; Miyamoto, S.; Sekino, Y.; Kitano, H.; Goto, K.; Ikeda, K.; et al. BACH1 promotes clear cell renal cell carcinoma progression by upregulating oxidative stress-related tumorigenicity. Cancer Sci. 2023, 114, 436–448. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Xie, X.; Nan, P.; Liu, F.; Sun, Y.; Zhao, X. BACH1 promotes the progression of esophageal squamous cell carcinoma by inducing the epithelial–mesenchymal transition and angiogenesis. Cancer Med. 2021, 10, 3413–3426. [Google Scholar] [CrossRef]
- Xie, M.; Sun, M.; Ji, X.; Li, D.; Chen, X.; Zhang, B.; Huang, W.; Zhang, T.; Wang, Y.; Tian, D.; et al. Overexpression of BACH1 mediated by IGF2 facilitates hepatocellular carcinoma growth and metastasis via IGF1R and PTK2. Theranostics 2022, 12, 1097–1116. [Google Scholar] [CrossRef]
- Sato, M.; Matsumoto, M.; Saiki, Y.; Alam, M.; Nishizawa, H.; Rokugo, M.; Brydun, A.; Yamada, S.; Kaneko, M.K.; Funayama, R.; et al. BACH1 Promotes Pancreatic Cancer Metastasis by Repressing Epithelial Genes and Enhancing Epithelial–Mesenchymal Transition. Cancer Res. 2020, 80, 1279–1292. [Google Scholar] [CrossRef]
- Igarashi, K.; Nishizawa, H.; Matsumoto, M. Iron in Cancer Progression: Does BACH1 Promote Metastasis by Altering Iron Homeostasis? Subcell Biochem. 2022, 100, 67–80. [Google Scholar]
- Padilla, J.; Lee, J. A Novel Therapeutic Target, BACH1, Regulates Cancer Metabolism. Cells 2021, 10, 634. [Google Scholar] [CrossRef]
- Igarashi, K.; Nishizawa, H.; Saiki, Y.; Matsumoto, M. The transcription factor BACH1 at the crossroads of cancer biology: From epithelial–mesenchymal transition to ferroptosis. J. Biol. Chem. 2021, 297, 101032. [Google Scholar] [CrossRef] [PubMed]
- López-Menéndez, C.; Vázquez-Naharro, A.; Santos, V.; Dubus, P.; Santamaría, P.G.; Martínez-Ramírez, Á.; Portillo, F.; Moreno-Bueno, G.; Faraldo, M.M.; Cano, A. E2A Modulates Stemness, Metastasis, and Therapeutic Resistance of Breast Cancer. Cancer Res. 2021, 81, 4529–4544. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chu, X.; Tian, H.; Liu, T.; Liu, H.; Gao, W.; Chen, S.; Hu, S.; Wu, D.; Xu, Y. The clinical outcomes and genomic landscapes of acute lymphoblastic leukemia patients with E2A-PBX1: A 10-year retrospective study. Am. J. Hematol. 2021, 96, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, H.; Nian, S.; Lv, C.; Ni, B.; Wang, D.; Tian, Z. Up-regulation of Transcription Factor 3 Is Correlated with Poor Prognosis in Cervical Carcinoma. Int. J. Gynecol. Cancer 2017, 27, 1422–1430. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Ito, K.; Pi, W.-C.; Lin, I.-H.; Chu, C.-S.; Malik, S.; Cheng, I.-H.; Chen, W.-Y.; Roeder, R.G. Mediator subunit MED1 is required for E2A-PBX1–mediated oncogenic transcription and leukemic cell growth. Proc. Natl. Acad. Sci. USA 2021, 118, e1922864118. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Li, H.; Zhang, D.; Liu, G. E2A attenuates tumor-initiating capacity of colorectal cancer cells via the Wnt/beta-catenin pathway. J. Exp. Clin. Cancer Res. 2019, 38, 276. [Google Scholar] [CrossRef]
- Vey, N.; Thomas, X.; Picard, C.; Kovascovicz, T.; Charin, C.; Cayuela, J.M.; Gabert, J. Allogeneic stem cell transplantation improves the outcome of adults with t(1;19)/E2A-PBX1 and t(4;11)/MLL-AF4 positive B-cell acute lymphoblastic leukemia: Results of the prospective multicenter LALA-94 study. Leukemia 2006, 20, 2155–2161. [Google Scholar] [CrossRef]
- Slattery, C.; McMorrow, T.; Ryan, M.P. Overexpression of E2A proteins induces epithelial-mesenchymal transition in human renal proximal tubular epithelial cells suggesting a potential role in renal fibrosis. FEBS Lett. 2006, 580, 4021–4030. [Google Scholar] [CrossRef]
- Pi, W.-C.; Wang, J.; Shimada, M.; Lin, J.-W.; Geng, H.; Lee, Y.-L.; Lu, R.; Li, D.; Wang, G.G.; Roeder, R.G.; et al. E2A-PBX1 functions as a coactivator for RUNX1 in acute lymphoblastic leukemia. Blood 2020, 136, 11–23. [Google Scholar] [CrossRef]
- Pei, H.; Li, Y.; Liu, M.; Chen, Y. Targeting Twist expression with small molecules. MedChemComm 2016, 8, 268–275. [Google Scholar] [CrossRef]
- Han, M.; Xu, W. EMP3 is induced by TWIST1/2 and regulates epithelial-to-mesenchymal transition of gastric cancer cells. Tumour Biol. 2017, 39, 1010428317718404. [Google Scholar] [CrossRef]
- Tan, Y.-E.; Xing, Y.; Ran, B.-L.; Zhang, C.; Pan, S.-W.; An, W.; Chen, Q.-C.; Xu, H.-M. LINC01235-TWIST2 feedback loop facilitates epithelial–mesenchymal transition in gastric cancer by inhibiting THBS2. Aging 2020, 12, 25060–25075. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Chen, H.-C.; Zhang, D.; Fu, J. Twist: A molecular target in cancer therapeutics. Tumor Biol. 2013, 34, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Scheller, K.; Sekeris, C.E. The Effects of Steroid Hormones on the Transcription of Genes Encoding Enzymes of Oxidative Phosphorylation. Exp. Physiol. 2003, 88, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Johnson, C.D.R. Growth Promotants for Beef Production: Anabolic Steroids: Performance Responses and Mode of Action. In Food Animal Practice, 5th ed.; David, E., Anderson, D.M.R., Eds.; W.B. Saunders: New York, NY, USA, 2009; pp. 643–651. [Google Scholar]
- Saha, S.; Dey, S.; Nath, S. Steroid Hormone Receptors: Links with Cell Cycle Machinery and Breast Cancer Progression. Front. Oncol. 2021, 11, 620214. [Google Scholar] [CrossRef]
- Truong, T.H.; Lange, C.A. Deciphering Steroid Receptor Crosstalk in Hormone-Driven Cancers. Endocrinology 2018, 159, 3897–3907. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Epithelial-Mesenchymal Transition (EMT) and Regulation of EMT Factors by Steroid Nuclear Receptors in Breast Cancer: A Review and in Silico Investigation. J. Clin. Med. 2016, 5, 11. [Google Scholar] [CrossRef]
- Islam, S.; Afrin, S.; Jones, S.I.; Segars, J. Selective Progesterone Receptor Modulators—Mechanisms and Therapeutic Utility. Endocr. Rev. 2020, 41, bnaa012. [Google Scholar] [CrossRef]
- Lee, O.; Sullivan, M.E.; Xu, Y.; Rodgers, C.; Muzzio, M.; Helenowski, I.; Shidfar, A.; Zeng, Z.; Singhal, H.; Jovanovic, B.; et al. Selective Progesterone Receptor Modulators in Early-Stage Breast Cancer: A Randomized, Placebo-Controlled Phase II Window-of-Opportunity Trial Using Telapristone Acetate. Clin. Cancer Res. 2020, 26, 25–34. [Google Scholar] [CrossRef]
- Auricchio, F.; Ar, D.; Th, T.; Jh, O.; Ca, L. Faculty Opinions recommendation of 90 YEARS OF PROGESTERONE: Steroid receptors as MAPK signaling sensors in breast cancer: Let the fates decide. J. Mol. Endocrinol. 2020, 65, T35–T48. [Google Scholar] [CrossRef]
- Shi, W.; Wang, D.; Yuan, X.; Liu, Y.; Guo, X.; Li, J.; Song, J.-G. Glucocorticoid receptor–IRS-1 axis controls EMT and the metastasis of breast cancers. J. Mol. Cell Biol. 2019, 11, 1042–1055. [Google Scholar] [CrossRef] [PubMed]
- Bolt, M.J.; Stossi, F.; Newberg, J.Y.; Orjalo, A.; Johansson, H.E.; Mancini, M.A. Coactivators enable glucocorticoid receptor recruitment to fine-tune estrogen receptor transcriptional responses. Nucleic Acids Res. 2013, 41, 4036–4048. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jan, Y.; Kuo, L.; Wang, B.; Huo, C.; Jiang, S.S.; Chen, S.; Kuo, Y.; Chang, C.-R.; Chuu, C. Elevation of androgen receptor promotes prostate cancer metastasis by induction of epithelial-mesenchymal transition and reduction of KAT 5. Cancer Sci. 2018, 109, 3564–3574. [Google Scholar] [CrossRef]
- Ahram, M.; Bawadi, R.; Abdullah, M.S.; Alsafadi, D.B.; Abaza, H.; Abdallah, S.; Mustafa, E. Involvement of β-catenin in Androgen-induced Mesenchymal Transition of Breast MDA-MB-453 Cancer Cells. Endocr. Res. 2021, 46, 114–128. [Google Scholar] [CrossRef]
- Tripathi, A.; Gupta, S. Androgen receptor in bladder cancer: A promising therapeutic target. Asian J. Urol. 2020, 7, 284–290. [Google Scholar] [CrossRef]
- Jing, Y.; Cui, D.; Guo, W.; Jiang, J.; Jiang, B.; Lu, Y.; Zhao, W.; Wang, X.; Jiang, Q.; Han, B.; et al. Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition. Cancer Lett. 2014, 348, 135–145. [Google Scholar] [CrossRef]
- Williams, K.A.; Lee, M.; Winter, J.M.; Gildea, D.E.; Calagua, C.; Curry, N.L.; Lichtenberg, J.; Ye, H.; Crawford, N.P. Prostate cancer susceptibility gene HIST1H1A is a modulator of androgen receptor signaling and epithelial to mesenchymal transition. Oncotarget 2018, 9, 28532–28546. [Google Scholar] [CrossRef]
- Wang, G.-C.; Huang, T.-R.; Wang, K.-Y.; Wu, Z.-L.; Xie, J.-B.; Zhang, H.-L.; Yin, L.; Tang, W.-L.; Peng, B. Inflammation induced by lipopolysaccharide advanced androgen receptor expression and epithelial-mesenchymal transition progress in prostatitis and prostate cancer. Transl. Androl. Urol. 2021, 10, 4275–4287. [Google Scholar] [CrossRef]
- Culig, Z. Epithelial mesenchymal transition and resistance in endocrine-related cancers. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1368–1375. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. Targeting the Retinoic Acid Pathway to Eradicate Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 2373. [Google Scholar] [CrossRef] [PubMed]
- Di Masi, A.; Leboffe, L.; De Marinis, E.; Pagano, F.; Cicconi, L.; Rochette-Egly, C.; Lo-Coco, F.; Ascenzi, P.; Nervi, C. Retinoic acid receptors: From molecular mechanisms to cancer therapy. Mol. Asp. Med. 2015, 41, 1–115. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tokuda, K.; Yamashiro, C.; Higashijima, F.; Yoshimoto, T.; Ota, M.; Ogata, T.; Ashimori, A.; Hatano, M.; Kobayashi, M.; et al. Inhibition of epithelial–mesenchymal transition in retinal pigment epithelial cells by a retinoic acid receptor-α agonist. Sci. Rep. 2021, 11, 11842. [Google Scholar] [CrossRef]
- Gong, L.; Jiang, L.; Qin, Y.; Jiang, X.; Song, K.; Yu, X. Protective effect of retinoic acid receptor α on hypoxia-induced epithelial to mesenchymal transition of renal tubular epithelial cells associated with TGF-β/MMP-9 pathway. Cell Biol. Int. 2018, 42, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Giguère, V. Inactivation of RARβ Inhibits Wnt1-induced Mammary Tumorigenesis by Suppressing Epithelial-mesenchymal Transitions. Nucl. Recept. Signal. 2014, 12, e004. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.-L.; Song, W.; Zhou, P.; Fu, Q.-R.; Lin, C.-L.; Chen, Q.-X.; Shen, D.-Y. Oncogenic retinoic acid receptor γ knockdown reverses multi-drug resistance of human colorectal cancer via Wnt/β-catenin pathway. Cell Cycle 2017, 16, 685–692. [Google Scholar] [CrossRef]
- Doi, A.; Ishikawa, K.; Shibata, N.; Ito, E.; Fujimoto, J.; Yamamoto, M.; Shiga, H.; Mochizuki, H.; Kawamura, Y.; Goshima, N.; et al. Enhanced expression of retinoic acid receptor alpha (RARA) induces epithelial-to-mesenchymal transition and disruption of mammary acinar structures. Mol. Oncol. 2014, 9, 355–364. [Google Scholar] [CrossRef]
- Kimura, K.; Orita, T.; Liu, Y.; Yang, Y.; Tokuda, K.; Kurakazu, T.; Noda, T.; Yanai, R.; Morishige, N.; Takeda, A.; et al. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J. Mol. Med. 2015, 93, 749–758. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Kudo-Saito, C.; Shirako, H.; Takeuchi, T.; Kawakami, Y. Cancer Metastasis Is Accelerated through Immunosuppression during Snail-Induced EMT of Cancer Cells. Cancer Cell 2009, 15, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gross, K.M.; Kuperwasser, C. Molecular regulation of Snai2 in development and disease. J. Cell Sci. 2019, 132, jcs235127. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lei, H.; Zhang, S.; Peng, Y.; Fu, C.; Shu, G.; Yin, G. Non-canonical signaling pathway of SNAI2 induces EMT in ovarian cancer cells by suppressing miR-222-3p transcription and upregulating PDCD10. Theranostics 2020, 10, 5895–5913. [Google Scholar] [CrossRef]
- Tang, H.M.; Kuay, K.T.; Koh, P.F.; Asad, M.; Tan, T.Z.; Chung, V.Y.; Lee, S.C.; Thiery, J.P.; Huang, R.-J. An epithelial marker promoter induction screen identifies histone deacetylase inhibitors to restore epithelial differentiation and abolishes anchorage independence growth in cancers. Cell Death Discov. 2016, 2, 16041. [Google Scholar] [CrossRef] [PubMed]
- Mrkvicova, A.; Chmelarova, M.; Peterova, E.; Havelek, R.; Baranova, I.; Kazimirova, P.; Rudolf, E.; Rezacova, M. The effect of sodium butyrate and cisplatin on expression of EMT markers. PLoS ONE 2019, 14, e0210889. [Google Scholar] [CrossRef] [PubMed]
- Chalaskiewicz, K.; Karas, K.; Zaklos-Szyda, M.; Karwaciak, I.; Pastwinska, J.; Koziolkiewicz, M.; Ratajewski, M. Trichostatin A inhibits expression of the human SLC2A5 gene via SNAI1/SNAI2 transcription factors and sensitizes colon cancer cells to platinum compounds. Eur. J. Pharm. 2023, 949, 175728. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-M.; Bi, Y.-R.; Li, Y.; Fu, R.; Lv, W.-C.; Jiang, N.; Xu, Y.; Ren, B.-X.; Chen, Y.-D.; Xie, H.; et al. A potent CBP/p300-Snail interaction inhibitor suppresses tumor growth and metastasis in wild-type p53-expressing cancer. Sci. Adv. 2020, 6, eaaw8500. [Google Scholar] [CrossRef]
- Jing, C.; Li, X.; Zhou, M.; Zhang, S.; Lai, Q.; Liu, D.; Ye, B.; Li, L.; Wu, Y.; Li, H.; et al. The PSMD14 inhibitor Thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics 2021, 11, 5847–5862. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, Y.K.; Kim, H.; Lee, J.; Oh, M.J.; Kim, S.B.; Kim, H.S. Snail acetylation by autophagy-derived acetyl-coenzyme A promotes invasion and metastasis of KRAS-LKB1 co-mutated lung cancer cells. Cancer Commun. 2022, 42, 716–749. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, H.; Lee, C.H. Post-Translational Modification of ZEB Family Members in Cancer Progression. Int. J. Mol. Sci. 2022, 23, 15127. [Google Scholar] [CrossRef] [PubMed]
- Veloso, E.S.; Gonçalves, I.N.N.; Silveira, T.L.; Santo, J.T.E.; Figueiredo, L.V.; Varaschin, M.S.; Cassali, G.D.; Del Puerto, H.L.; Ferreira, E. ZEB and Snail expression indicates epithelial-mesenchymal transition in canine melanoma. Res. Veter. Sci. 2020, 131, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, L.; Zarrabi, A.; Hashemi, F.; Hashemi, F.; Zabolian, A.; Banihashemi, S.M.; Moghadam, S.S.; Hushmandi, K.; Samarghandian, S.; Ashrafizadeh, M.; et al. Role of ZEB family members in proliferation, metastasis and chemoresistance of prostate cancer cells: Revealing signaling networks. Curr. Cancer Drug Targets 2021, 21, 749–767. [Google Scholar] [CrossRef]
- Kalra, R.S.; Chaudhary, A.; Yoon, A.-R.; Bhargava, P.; Omar, A.; Garg, S.; Yun, C.-O.; Kaul, S.C.; Wadhwa, R. CARF enrichment promotes epithelial–mesenchymal transition via Wnt/β-catenin signaling: Its clinical relevance and potential as a therapeutic target. Oncogenesis 2018, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ang, H.L.; Moghadam, E.R.; Mohammadi, S.; Zarrin, V.; Hushmandi, K.; Samarghandian, S.; Zarrabi, A.; Najafi, M.; Mohammadinejad, R.; et al. MicroRNAs and Their Influence on the ZEB Family: Mechanistic Aspects and Therapeutic Applications in Cancer Therapy. Biomolecules 2020, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xu, J.; Ge, Y.; Shi, Y.; Wang, F.; Zhu, M. NR4A1 inhibits the epithelial-mesenchymal transition of hepatic stellate cells: Involvement of TGF-β-Smad2/3/4-ZEB signaling. Open Life Sci. 2022, 17, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sue, S.; Shibata, W.; Kameta, E.; Sato, T.; Ishii, Y.; Kaneko, H.; Miwa, H.; Sasaki, T.; Tamura, T.; Kondo, M.; et al. Intestine-specific homeobox (ISX) induces intestinal metaplasia and cell proliferation to contribute to gastric carcinogenesis. J. Gastroenterol. 2016, 51, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Wang, L.-T.; Lee, K.-T.; Chen, Y.-L.; Liu, K.-Y.; Suen, J.-L.; Chai, C.-Y.; Wang, S.-N. Proinflammatory Homeobox Gene, ISX, Regulates Tumor Growth and Survival in Hepatocellular Carcinoma. Cancer Res. 2013, 73, 508–518. [Google Scholar] [CrossRef]
- Wang, L.; Liu, K.; Jeng, W.; Chiang, C.; Chai, C.; Chiou, S.; Huang, M.; Yokoyama, K.K.; Wang, S.; Huang, S.; et al. PCAF -mediated acetylation of ISX recruits BRD 4 to promote epithelial-mesenchymal transition. EMBO Rep. 2020, 21, e48795. [Google Scholar] [CrossRef]
- Chuang, K.; Wang, S.; Hsu, S.; Wang, L. Impact of bromodomain-containing protein 4 (BRD4) and intestine-specific homeobox (ISX) expression on the prognosis of patients with hepatocellular carcinoma’ for better clarity. Cancer Med. 2021, 10, 5545–5556. [Google Scholar] [CrossRef]
- Wang, L.T.; Chiou, S.S.; Chai, C.Y.; His, E.; Yokoyama, K.K.; Wang, S.N.; Hsu, S.H. Intestine-Specific Homeobox Gene ISX Integrates IL6 Signaling, Tryptophan Catabolism, and Immune Suppression. Cancer Res. 2017, 77, 4065–4077. [Google Scholar] [CrossRef]
- Wang, S.-N.; Wang, L.-T.; Sun, D.-P.; Chai, C.-Y.; Hsi, E.; Kuo, H.-T.; Yokoyama, K.K.; Hsu, S.-H. Intestine-specific homeobox (ISX) upregulates E2F1 expression and related oncogenic activities in HCC. Oncotarget 2016, 7, 36924–36939. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Igarashi, M.; Fukuda, H.; Nakagama, H.; Katoh, M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013, 328, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liang, Z.; Lou, H. The Emerging Roles of Fox Family Transcription Factors in Chromosome Replication, Organization, and Genome Stability. Cells 2020, 9, 258. [Google Scholar] [CrossRef]
- Li, J.; Dai, S.; Chen, X.; Liang, X.; Qu, L.; Jiang, L.; Guo, M.; Zhou, Z.; Wei, H.; Zhang, H.; et al. Mechanism of forkhead transcription factors binding to a novel palindromic DNA site. Nucleic Acids Res. 2021, 49, 3573–3583. [Google Scholar] [CrossRef] [PubMed]
- Laissue, P. The forkhead-box family of transcription factors: Key molecular players in colorectal cancer pathogenesis. Mol. Cancer 2019, 18, 5. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, J.; Wu, H.; Mo, Z.; Lian, Y.; Wang, P.; Xie, C. Forkhead box (FOX) G1 promotes hepatocellular carcinoma epithelial-Mesenchymal transition by activating Wnt signal through forming T-cell factor-4/Beta-catenin/FOXG1 complex. J. Exp. Clin. Cancer Res. 2019, 38, 475. [Google Scholar] [CrossRef] [PubMed]
- Millour, J.; Constantinidou, D.; Stavropoulou, A.V.; Wilson, M.S.; Myatt, S.S.; Kwok, J.M.; Sivanandan, K.; Coombes, R.C.; Medema, R.H.; Hartman, J.; et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene 2010, 29, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.-H.; Long, N.P.; Luu, T.-T.; Anh, N.H.; Kwon, S.W.; Lee, S.K. The Dominant Role of Forkhead Box Proteins in Cancer. Int. J. Mol. Sci. 2018, 19, 3279. [Google Scholar] [CrossRef]
- She, Z.-Y.; Yang, W.-X. SOX family transcription factors involved in diverse cellular events during development. Eur. J. Cell Biol. 2015, 94, 547–563. [Google Scholar] [CrossRef]
- Grimm, D.; Bauer, J.; Wise, P.; Krüger, M.; Simonsen, U.; Wehland, M.; Infanger, M.; Corydon, T.J. The role of SOX family members in solid tumours and metastasis. Semin. Cancer Biol. 2020, 67, 122–153. [Google Scholar] [CrossRef] [PubMed]
- Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Role of SOX Protein Groups F and H in Lung Cancer Progression. Cancers 2020, 12, 3235. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Najafi, M.; Entezari, M.; Hushmandi, K.; Aref, A.R.; Khan, H.; Makvandi, P.; et al. The role of SOX family transcription factors in gastric cancer. Int. J. Biol. Macromol. 2021, 180, 608–624. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Mirzaei, S.; Gholami, M.H.; Zarrabi, A.; Zabolian, A.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Aref, A.R.; Samarghandian, S. Cervical cancer progression is regulated by SOX transcription factors: Revealing signaling networks and therapeutic strategies. Biomed. Pharmacother. 2021, 144, 112335. [Google Scholar] [CrossRef]
- Hanieh, H.; Ahmed, E.A.; Vishnubalaji, R.; Alajez, N.M. SOX4: Epigenetic regulation and role in tumorigenesis. Semin. Cancer Biol. 2020, 67, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Mandalos, N.; Rhinn, M.; Granchi, Z.; Karampelas, I.; Mitsiadis, T.; Economides, A.N.; Dollé, P.; Remboutsika, E. Sox2 acts as a rheostat of epithelial to mesenchymal transition during neural crest development. Front. Physiol. 2014, 5, 345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, W. SOX factors as cell-state regulators in the mammary gland and breast cancer. Semin. Cell Dev. Biol. 2021, 114, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Temme, A.; Senner, V.; Ebner, R.; Schwind, S.; Stevanovic, S.; Wehner, R.; Schackert, G.; Schackert, H.K.; Fussel, M.; et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br. J. Cancer 2007, 96, 1293–1301. [Google Scholar] [CrossRef]
- Wu, R.; Wang, C.; Li, Z.; Xiao, J.; Li, C.; Wang, X.; Kong, P.; Cao, J.; Huang, F.; Huang, Y.; et al. SOX2 promotes resistance of melanoma with PD-L1 high expression to T-cell-mediated cytotoxicity that can be reversed by SAHA. J. Immunother. Cancer 2020, 8, e001037. [Google Scholar] [CrossRef]
- Stolzenburg, S.; Rots, M.G.; Beltran, A.S.; Rivenbark, A.G.; Yuan, X.; Qian, H.; Strahl, B.D.; Blancafort, P. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012, 40, 6725–6740. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, L. Alternate Roles of Sox Transcription Factors beyond Transcription Initiation. Int. J. Mol. Sci. 2021, 22, 5949. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.A.; Mitchell, J.P.; Cook, S.J. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem. J. 2021, 478, 2619–2664. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zong, J.; Lin, W.; Wang, M.; Xu, Y.; Zhou, R.; Pan, J. EBV-miR-BART8-3p induces epithelial-mesenchymal transition and promotes metastasis of nasopharyngeal carcinoma cells through activating NF-κB and Erk1/2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 283. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Saghari, S.; Bassiri, F.; Raesi, R.; Zarrabi, A.; Hushmandi, K.; Tergaonkar, V. NF-κB as a regulator of cancer metastasis and therapy response: A focus on epithelial-mesenchymal transition. J. Cell Physiol. 2022, 237, 2770–2795. [Google Scholar] [CrossRef]
- Jiang, X.M.; Xu, Y.L.; Yuan, L.W.; Zhang, L.L.; Huang, M.Y.; Ye, Z.H.; Lu, J.J. TGFβ2-mediated epithelial-mesenchymal transition and NF-κB pathway activation contribute to osimertinib resistance. Acta Pharm. Sin. 2021, 42, 451–459. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Gaptulbarova, K.A.; Tsyganov, M.M.; Pevzner, A.M.; Ibragimova, M.K.; Litviakov, N.V. NF-kB as a potential prognostic marker and a candidate for targeted therapy of cancer. Exp. Oncol. 2020, 42, 263–269. [Google Scholar]
- Ramadass, V.; Vaiyapuri, T.; Tergaonkar, V. Small Molecule NF-κB Pathway Inhibitors in Clinic. Int. J. Mol. Sci. 2020, 21, 5164. [Google Scholar] [CrossRef]
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629. [Google Scholar] [CrossRef]
- Jin, W. Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells 2020, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.E.; Peluffo, G.; Qiu, X.; Temko, D.; Fassl, A.; Li, Z.; Trinh, A.; Seehawer, M.; Jovanović, B.; Alečković, M.; et al. JAK–STAT Signaling in Inflammatory Breast Cancer Enables Chemotherapy-Resistant Cell States. Cancer Res. 2023, 83, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Durham, G.; Williams, J.J.; Nasim, T.; Palmer, T.M. Targeting SOCS Proteins to Control JAK-STAT Signalling in Disease. Trends Pharm. Sci. 2019, 40, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Sarais, F.; Kummerow, S.; Montero, R.; Rebl, H.; Köllner, B.; Goldammer, T.; Rebl, A. PIAS Factors from Rainbow Trout Control NF-κB- and STAT-Dependent Gene Expression. Int. J. Mol. Sci. 2021, 22, 12815. [Google Scholar] [CrossRef] [PubMed]
- Sivaganesh, V.; Sivaganesh, V.; Scanlon, C.; Iskander, A.; Maher, S.; Lê, T.; Peethambaran, B. Protein Tyrosine Phosphatases: Mechanisms in Cancer. Int. J. Mol. Sci. 2021, 22, 12865. [Google Scholar] [CrossRef]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef]
- Jonker, D.J.; Nott, L.; Yoshino, T.; Gill, S.; Shapiro, J.; Ohtsu, A.; Zalcberg, J.; Vickers, M.M.; Wei, A.C.; Gao, Y.; et al. Napabucasin versus placebo in refractory advanced colorectal cancer: A randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 263–270. [Google Scholar] [CrossRef]
- Yang, M.-Y.; Lee, H.-T.; Chen, C.-M.; Shen, C.-C.; Ma, H.-I. Celecoxib Suppresses the Phosphorylation of STAT3 Protein and Can Enhance the Radiosensitivity of Medulloblastoma-Derived Cancer Stem-Like Cells. Int. J. Mol. Sci. 2014, 15, 11013–11029. [Google Scholar] [CrossRef]
- Heppler, L.N.; Attarha, S.; Persaud, R.; Brown, J.I.; Wang, P.; Petrova, B.; Tošić, I.; Burton, F.B.; Flamand, Y.; Walker, S.R.; et al. The antimicrobial drug pyrimethamine inhibits STAT3 transcriptional activity by targeting the enzyme dihydrofolate reductase. J. Biol. Chem. 2022, 298, 101531. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Capaci, V.; Bascetta, L.; Fantuz, M.; Beznoussenko, G.V.; Sommaggio, R.; Cancila, V.; Bisso, A.; Campaner, E.; Mironov, A.A.; Wiśniewski, J.R.; et al. Mutant p53 induces Golgi tubulo-vesiculation driving a prometastatic secretome. Nat. Commun. 2020, 11, 3945. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, M.; Guo, W.; Zhao, X.; Tu, X.; Huang, S.; Zou, X.; Peng, X. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR-145. Int. J. Oncol. 2013, 42, 1473–1481. [Google Scholar] [CrossRef]
- Jiang, F.-Z.; He, Y.-Y.; Wang, H.-H.; Zhang, H.-L.; Zhang, J.; Yan, X.-F.; Wang, X.-J.; Che, Q.; Ke, J.-Q.; Chen, Z.; et al. Mutant p53 induces EZH2 expression and promotes epithelial-mesenchymal transition by disrupting p68-Drosha complex assembly and attenuating miR-26a processing. Oncotarget 2015, 6, 44660–44674. [Google Scholar] [CrossRef]
- Babaei, G.; Aliarab, A.; Asghari Vostakolaei, M.; Hotelchi, M.; Neisari, R.; Gholizadeh-Ghaleh Aziz, S.; Bazl, M.R. Crosslink between p53 and metastasis: Focus on epithelial-mesenchymal transition, cancer stem cell, angiogenesis, autophagy, and anoikis. Mol. Biol. Rep. 2021, 48, 7545–7557. [Google Scholar] [CrossRef]
- Semenov, O.; Daks, A.; Fedorova, O.; Shuvalov, O.; Barlev, N.A. Opposing Roles of Wild-type and Mutant p53 in the Process of Epithelial to Mesenchymal Transition. Front. Mol. Biosci. 2022, 9, 928399. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Topatana, W.; Juengpanich, S.; Li, S.; Bin Zhang, B.; Shen, J.; Cai, L.; Cai, X.; Chen, M. Targeting mutant p53 for cancer therapy: Direct and indirect strategies. J. Hematol. Oncol. 2021, 14, 157. [Google Scholar] [CrossRef]
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef]
- Marine, J.-C.; Francoz, S.; Maetens, M.; Wahl, G.M.; Toledo, F.; Lozano, G. Keeping p53 in check: Essential and synergistic functions of Mdm2 and Mdm4. Cell Death Differ. 2006, 13, 927–934. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Cai, L.; Zhang, X.-Q.; Lei, Z.; Yi, C.-S.; Liu, X.-D.; Yang, J.-G. Dysregulated RUNX1 Predicts Poor Prognosis by Mediating Epithelialmesenchymal Transition in Cervical Cancer. Curr. Med. Sci. 2022, 42, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lai, Q.; He, C.; Fang, Y.; Yan, Q.; Zhang, Y.; Wang, X.; Gu, C.; Wang, Y.; Ye, L.; et al. RUNX1 promotes tumour metastasis by activating the Wnt/β-catenin signalling pathway and EMT in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 334. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Luo, M.; Cai, W.; Zhou, S.; Feng, D.; Xu, C.; Wang, H. Runt-Related Transcription Factor 1 (RUNX1) Promotes TGF-β-Induced Renal Tubular Epithelial-to-Mesenchymal Transition (EMT) and Renal Fibrosis through the PI3K Subunit p110δ. Ebiomedicine 2018, 31, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Tuo, Z.; Zhang, Y.; Wang, X.; Dai, S.; Liu, K.; Xia, D.; Wang, J.; Bi, L. RUNX1 is a promising prognostic biomarker and related to immune infiltrates of cancer-associated fibroblasts in human cancers. BMC Cancer 2022, 22, 523. [Google Scholar] [CrossRef]
- Lin, T.C. RUNX1 and cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188715. [Google Scholar] [CrossRef]
- Sood, R.; Kamikubo, Y.; Liu, P. Role of RUNX1 in hematological malignancies. Blood 2017, 129, 2070–2082. [Google Scholar] [CrossRef]
- Ariffin, N.S. RUNX1 as a Novel Molecular Target for Breast Cancer. Clin. Breast Cancer 2022, 22, 499–506. [Google Scholar] [CrossRef]
- Mill, C.P.; Fiskus, W.; DiNardo, C.D.; Qian, Y.; Raina, K.; Rajapakshe, K.; Perera, D.; Coarfa, C.; Kadia, T.M.; Khoury, J.D.; et al. RUNX1-targeted therapy for AML expressing somatic or germline mutation in RUNX1. Blood 2019, 134, 59–73. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Dijke, P.T. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Syed, V. TGF-β Signaling in Cancer. J. Cell Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, H.; Dong, Z.; Shen, Y.; Wang, Z.; Wei, W.; Yi, J.; Wang, R.; Wu, N.; Jin, S. NRP1 regulates radiation-induced EMT via TGF-β/Smad signaling in lung adenocarcinoma cells. Int. J. Radiat. Biol. 2020, 96, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-J.; Jiao, Y.-L.; Chen, P.; Kong, J.-Y.; Gu, B.-L.; Liu, K.; Feng, D.-D.; Zhu, Y.-F.; Ruan, H.-J.; Lan, Z.-J.; et al. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFβ-dependent Smad/YAP/TAZ signaling. PLoS Biol. 2020, 18, e3000825. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Gu, J.; Peng, Q.; Wang, X.; Liu, L.; Shu, X.; He, Q.; Tan, Y. Berberine Suppresses EMT in Liver and Gastric Carcinoma Cells through Combination with TGFβR Regulating TGF-β/Smad Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 2337818. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Mehrotra, S.; Sadaria, M.R.; Kumar, S.; Shortle, N.H.; Roman, Y.; Sheridan, C.; Campbell, R.A.; Murry, D.J.; Badve, S.; et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol. Cancer Ther. 2005, 4, 1004–1012. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’shea, J.J. Mechanisms of Jak/STAT Signaling in Immunity and Disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Walerych, D.; Lisek, K.; Sommaggio, R.; Piazza, S.; Ciani, Y.; Dalla, E.; Rajkowska, K.; Gaweda-Walerych, K.; Ingallina, E.; Tonelli, C.; et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat. Cell Biol. 2016, 18, 897–909. [Google Scholar] [CrossRef]
- Röpke, M.; Hald, J.; Guldberg, P.; Zeuthen, J.; Nørgaard, L.; Fugger, L.; Svejgaard, A.; Van Der Burg, S.; Nijman, H.W.; Melief, C.J.M.; et al. Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. Proc. Natl. Acad. Sci. USA 1996, 93, 14704–14707. [Google Scholar] [CrossRef]
- Ma, S.; Ba, Y.; Ji, H.; Wang, F.; Du, J.; Hu, S. Recognition of Tumor-Associated Antigens and Immune Subtypes in Glioma for mRNA Vaccine Development. Front. Immunol. 2021, 12, 738435. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Ziros, P.G.; Georgakopoulos-Soares, I.; Smith, A.A.T.; Marques, A.C.; Ibberson, M.; Kopp, P.A.; Habeos, I.; Trougakos, I.P.; Khoo, N.K.H.; et al. The Transcriptomic Response of the Murine Thyroid Gland to Iodide Overload and the Role of the Nrf2 Antioxidant System. Antioxidants 2020, 9, 884. [Google Scholar] [CrossRef]
- Seino, Y.; Miki, T.; Kiyonari, H.; Abe, T.; Fujimoto, W.; Kimura, K.; Seino, S. Isx Participates in the Maintenance of Vitamin A Metabolism by Regulation of {beta}-Carotene 15,15′-Monooxygenase (Bcmo1) Expression. J. Biol. Chem. 2008, 283, 4905–4911. [Google Scholar] [CrossRef] [PubMed]
- Gallinari, P.; Di Marco, S.; Jones, P.; Pallaoro, M.; Steinkühler, C. HDACs, histone deacetylation and gene transcription: From molecular biology to cancer therapeutics. Cell Res. 2007, 17, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Stemmler, M.P. PCAF, ISX, and BRD4: A maleficent alliance serving lung cancer malignancy. EMBO Rep. 2020, 21, e49766. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Shim, J.S. Targeting Epithelial–Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Sharma, G.; Shah, S.; Rath, H.; Senapati, S.N.; Mishra, E. Effectiveness of curcumin mouthwash on radiation-induced oral mucositis among head and neck cancer patients: A triple-blind, pilot randomised controlled trial. Indian J. Dent. Res. 2020, 31, 718–727. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Parulekar, W.R. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Soucek, L. Blocking Myc to Treat Cancer: Reflecting on Two Decades of Omomyc. Cells 2020, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Drugging "undruggable" genes for cancer treatment: Are we making progress? Int. J. Cancer 2021, 148, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Svetlovska, D.; Angelis, V.D.; Kalavska, K.; Lesko, P.; Makovník, M.; Obertova, J.; Orszaghova, Z.; Palacka, P.; Rečková, M.; et al. Phase II study of Disulfiram and Cisplatin in Refractory Germ Cell Tumors. The GCT-SK-006 phase II trial. Investig. New Drugs 2022, 40, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Shorstova, T.; Foulkes, W.D.; Witcher, M. Achieving clinical success with BET inhibitors as anti-cancer agents. Br. J. Cancer 2021, 124, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, K.; Scaravilli, M.; Ilvesmäki, A.; Staff, S.; Tolonen, T.; Mäenpää, J.U.; Visakorpi, T.; Auranen, A. Expression of the miR-200 family in tumor tissue, plasma and urine of epithelial ovarian cancer patients in comparison to benign counterparts. BMC Res. Notes 2020, 13, 311. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Plets, M.; Melissa, P.; Higano, C.S.; Tangen, C.M.; Agarwal, N.; Vogelzang, N.J.; Hussain, M.; Thompson, I.M., Jr.; Tewari, M.; et al. Circulating microRNAs and treatment response in the Phase II SWOG S0925 study for patients with new metastatic hormone-sensitive prostate cancer. Prostate 2017, 78, 121–127. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, B.; Zhang, K. miR-186 Suppresses the Progression of Cholangiocarcinoma Cells through Inhibition of Twist1. Oncol. Res. 2019, 27, 1061–1068. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, Y. microRNA-342-3p targets FOXQ1 to suppress the aggressive phenotype of nasopharyngeal carcinoma cells. BMC Cancer 2019, 19, 104. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Biagioni, A.; Arunkumar, G.; Shapiro, R.; Chang, K.-C.; Sedeeq, M.; Taiyab, A.; Hashemabadi, M.; Pardakhty, A.; Mandegary, A.; et al. EMT signaling: Potential contribution of CRISPR/Cas gene editing. Cell Mol. Life Sci. 2020, 77, 2701–2722. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Madden, S.K.; de Araujo, A.D.; Gerhardt, M.; Fairlie, D.P.; Mason, J.M. Taking the Myc out of cancer: Toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 2021, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef] [PubMed]
| Domain | Domain Character | Class | Class Character | Representation | References |
|---|---|---|---|---|---|
Basic Domain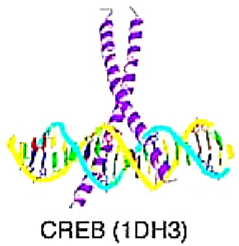 | TFs that are part of this superfamily interact with DNA by means of a basic region, which is disordered in solution and folds into an alpha-helix when binding to DNA. | basic motif leucine zipper (bZIP) factors | Characterized by a leucine zipper region that mediates dimerization with another bZIP domain and a basic region that binds to DNA. | JUN, FOS, BACH1 | [9,10,11] |
| bHLH factors | DNA-binding basic region followed by a motif of two potential amphipathic alpha-helices connected by a loop, possibly an omega loop. | E2A, TWIST | |||
Zinc-coordination DNA-binding domain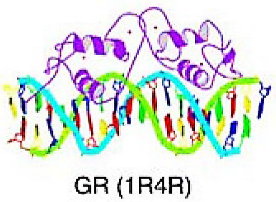 | The zinc-coordinating domains are characterized by the presence of a zinc ion that is coordinated by two or more conserved cysteine or histidine residues in the protein structure. | Nuclear receptors with C4 zinc fingers | C4 zinc finger motif consists of four cysteine residues coordinating one zinc ion and binds to DNA through the recognition of specific DNA sequences known as hormone response elements. In each molecule of the nuclear receptor, there are two DNA-binding motifs that are different in size, composition, and function. The first zinc finger binds to DNA through the major groove, and the second zinc finger mediates dimerization upon DNA binding, with an alpha-helix conformation. | AR, RAR | |
| C2H2 Zinc finger factors | Feature of zinc finger motif of TFIIIA/Krueppel type, consisting of two cysteine and two histidine residues coordinating a zinc ion, with some cases replacing a histidine with another cysteine. This zinc ion is crucial for DNA binding. Typically, the first half of the finger sequence is arranged in two antiparallel beta-strands, while the second half is organized as an alpha-helix and partially as a 310-helix. The conserved phenylalanine and leucine residues create hydrophobic contacts between the beta-strands and the alpha-helix, which binds to DNA via the major groove. | Snail-like | |||
Helix-turn-helix domain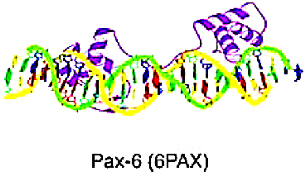 | The helix-turn-helix domain composed of two alpha helices connected by a short beta turn, forming a “V” shape. The first helix is often referred to as the recognition helix, as it makes specific contacts with the DNA. The second helix helps stabilize the structure of the domain and interacts with other proteins in some cases. | Homeo Domain factors | Made up of a series of three alpha-helices in a row, where the third helix predominantly interacts with the major groove of the DNA, and some interactions with the minor groove can also be seen. The homeodomain binds to DNA as a monomer, recognizing short DNA sequences typically 5–8 base pairs in length, and often functions in transcriptional regulation. | ZEB, ISX | |
| Fork head/winged helix factors | The DBD is about 110 amino acids long. It has three closely packed alpha-helices, where the third alpha-helix is exposed towards the major groove of the DNA. The domain also makes minor groove contacts. When it binds to DNA, it causes a bend of 13 degrees. | FOX | |||
Alpha-helical DNA-binding domains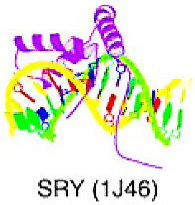 | The superclass includes DBDs that exhibits alpha-helically structured interfaces which interacting with the DNA. | HMG domain factors | The proteins with a HMG domain shared an identical structure, the HMG box. This domain shows a typical L-shaped conformation composed of three alpha-helices and an extended N-terminal extension of the first helix. | SOX | |
Beta-core (Immunoglobulin fold) | The DNA-binding domains in this superclass possess an immunoglobulin-like structure, consisting of a beta-core with a beta-sandwich architecture. The DNA-contact interface is primarily composed of loops, but may also include other elements of secondary structure, with DNA-binding residues extending from this interface. | Rel homology region factors | The structure of the Rel-type protein shows a bipartite subdomain structure, with each subdomain comprises a beta-barrel with five loops that form an extensive contact surface to the DNA’s major groove. | NF-κB | |
| STAT domain factors | The DNA binding motif of STAT proteins involves a dimeric organization with an eight-stranded beta-barrel and a four-helix bundle at the N-terminus, followed by an alpha-helical connector region at the C-terminus. | STAT | |||
| p53 domain factors | The p53 domain subtype is identified as a beta sandwich composed of a scaffold in addition to several loops and a loop-sheet-helix motif. One of the loops forms a contact of an arginine residue in the minor groove of the DNA, and side chains of the loop-sheet-helix motif in the major one. | P53 | |||
| Runt domain factors | The Runt domain is composed of 12 beta strands, with seven forming an immunoglobulin-like beta sandwich fold (S-type Ig fold), and is preceded by an alpha helix at the N-terminus. | RUNX1 | |||
Beta-sheet binding to DNA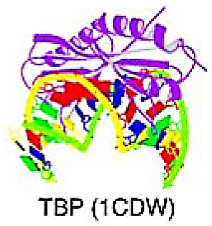 | The DBD of this superclass attach to DNA using either individual elongated strands or beta-sheets | TATA-binding proteins (TBP) | The structure of TBP involves a 10-stranded beta-sheet that forms a symmetrical saddle shape, with four alpha-helices on the convex side and hydrophobic interactions on the concave side, which bind to the minor groove of the TATA-box. This interaction causes a noticeable bend in the DNA helix. | TBP | |
Beta-hairpin exposed by an alpha/beta-scaffold | The alpha/beta-structured scaffold of the DNA-binding domains in this superclass reveals a beta-hairpin, which acts as the primary DNA-binding element by inserting into the major groove of the DNA. | SMAD/NF-1 DNA-binding domain factors | The alpha/beta-structured scaffold of the DBDs in this superclass exposes a beta-hairpin, which serves as the primary DNA-contacting element and inserts into the major groove of the DNA. | SMAD |
| Domain | Class | Representation | Consensus Binding Sequence | Oncogenic Effects and Current Findings | References |
|---|---|---|---|---|---|
| Basic domain | bZIP | JUN | TGAGTC |
| [9,19,29,30,31,32,33,34] |
| FOS | TGAGTC | ||||
| BACH1 | GCTGAG |
| [2,35,36,37,38,39,40,41] | ||
| bHLH | E2A | CAGGTG |
| [9,42,43,44,45,46,47,49] | |
| TWIST * | CGTCTG |
| [50,51,52,53] | ||
| Zinc-coordination DNA-binding domain | Nuclear receptors with C4 zinc fingers | AR | AGAACA |
| [65,66,67,69,70,71] |
| RAR | TGACCT |
| [74,75,76,79] | ||
| C2H2 Zinc finger factors | Snail-like * | CACCTGA |
| [81,82,83,84,85,89,90,91] | |
| Helix-turn-helix domain | Homeo Domain factors | ZEB * | CACCTG |
| [92,93,94,95,96,97] |
| ISX | CTAATT |
| [9,98,99,100,101,102,103] | ||
| Fork head/winged helix factors | FOX | TGTTT(A/G) |
| [4,9,104,105,106,107,108,109,110] | |
| alpha-helical DNA-binding domains | HMG domain factors | SOX | AACAAT |
| [9,111,112,113,114,115,116,117,118,119,120,121,122] |
| Beta-core (Immunoglobulin fold) | Rel homology region factors | NF-κB | NF-κB p50-like: GGGAATNF-κB p65-like: GAAAAT |
| [9,123,124,125,126,127,128,129,130] |
| STAT domain factors | STAT | TTC(N2-4)GAA |
| [9,131,132,133,134,135,136,137,138,139,140,141,142] | |
| p53 domain factors | p53 | RRRCWWGYYY-NNN-RRRCWWGYYY(R = A or G, W = A or T, Y = C or T, and N = any nucleotide.) |
| [9,143,144,145,146,147,148,149,150,151] | |
| Runt domain factors | RUNX1 | TGTGGTTAAC |
| [9,152,153,154,155,156,157,158,159] | |
| beta-hairpin exposed by an alpha/beta –scaffold | SMAD/NF-1 DNA-binding domain factors | SMAD | GTCTAGAC |
| [160,161,162,163,164,165,166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, K.-T.; Chiou, S.-S.; Hsu, S.-H. Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition. Cancers 2023, 15, 3338. https://doi.org/10.3390/cancers15133338
Chuang K-T, Chiou S-S, Hsu S-H. Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition. Cancers. 2023; 15(13):3338. https://doi.org/10.3390/cancers15133338
Chicago/Turabian StyleChuang, Kai-Ting, Shyh-Shin Chiou, and Shih-Hsien Hsu. 2023. "Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition" Cancers 15, no. 13: 3338. https://doi.org/10.3390/cancers15133338
APA StyleChuang, K.-T., Chiou, S.-S., & Hsu, S.-H. (2023). Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition. Cancers, 15(13), 3338. https://doi.org/10.3390/cancers15133338





