Polyploid Giant Cancer Cells Are Frequently Found in the Urine of Prostate Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Urine Sample Collection and Stabilization
2.2. Human Cell Lines
2.3. Urine Processing
2.4. Cells Analysis—Giemsa Stain and Immunocytochemistry (ICC)
3. Results
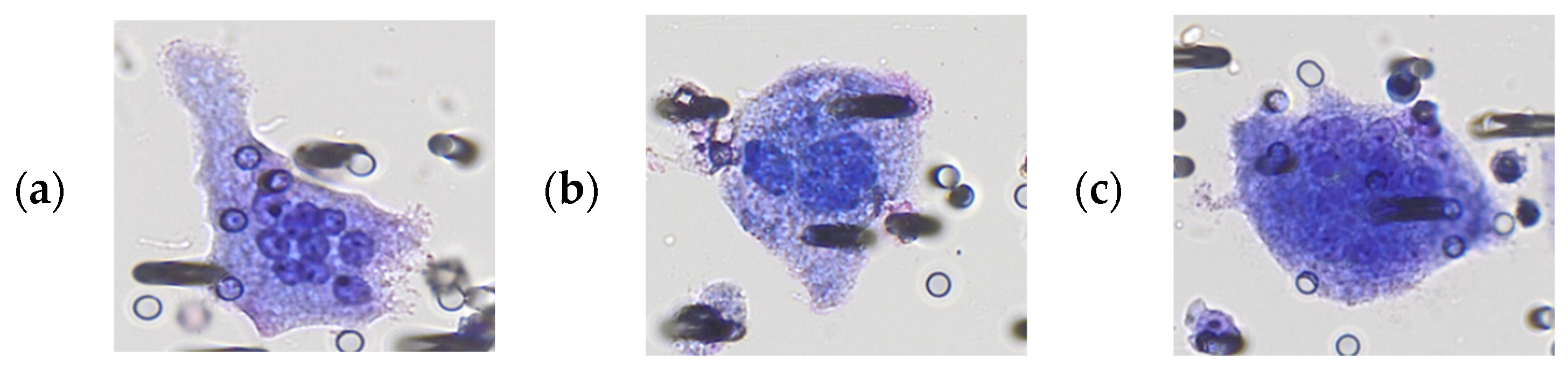
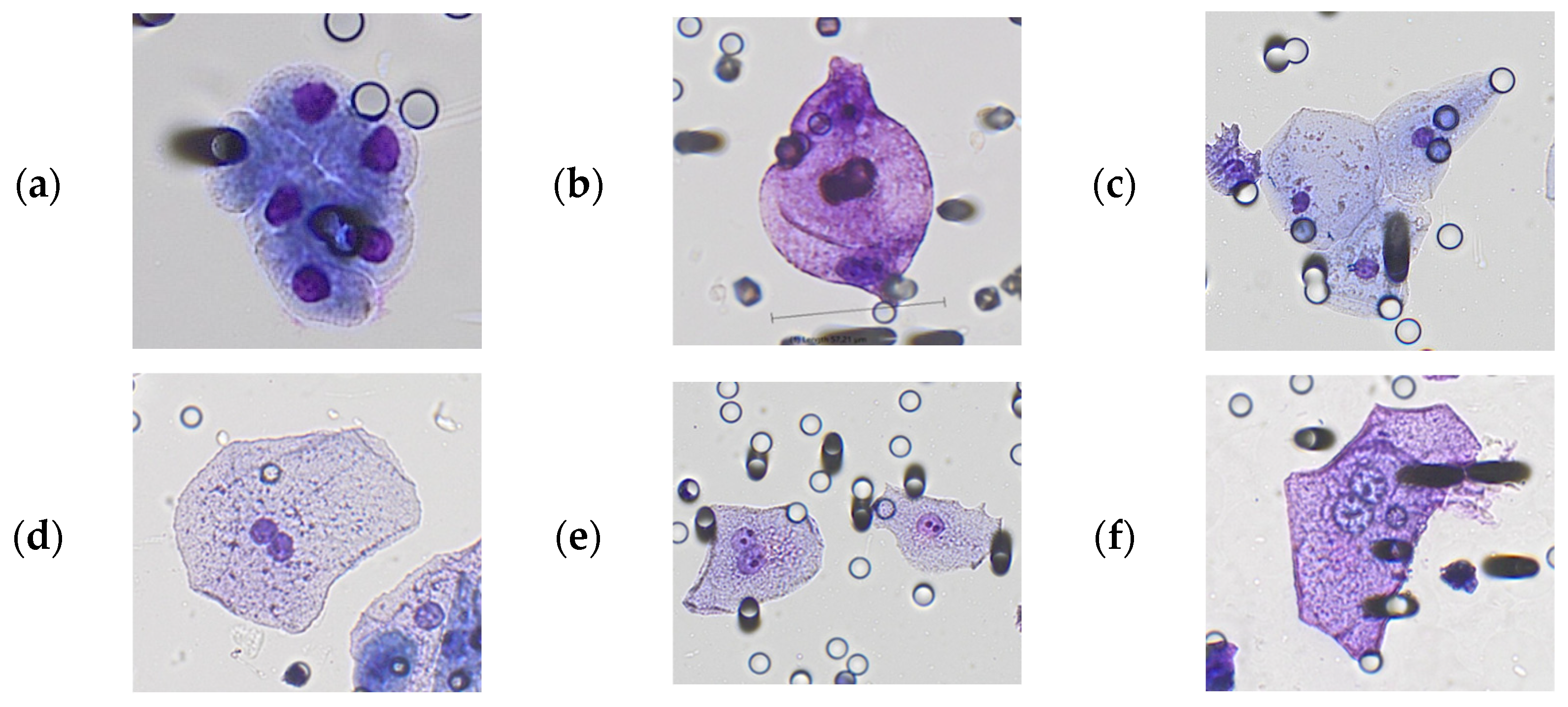
3.1. Morphological Characterization of Polyploid Giant Cancer Cells in Urine Samples
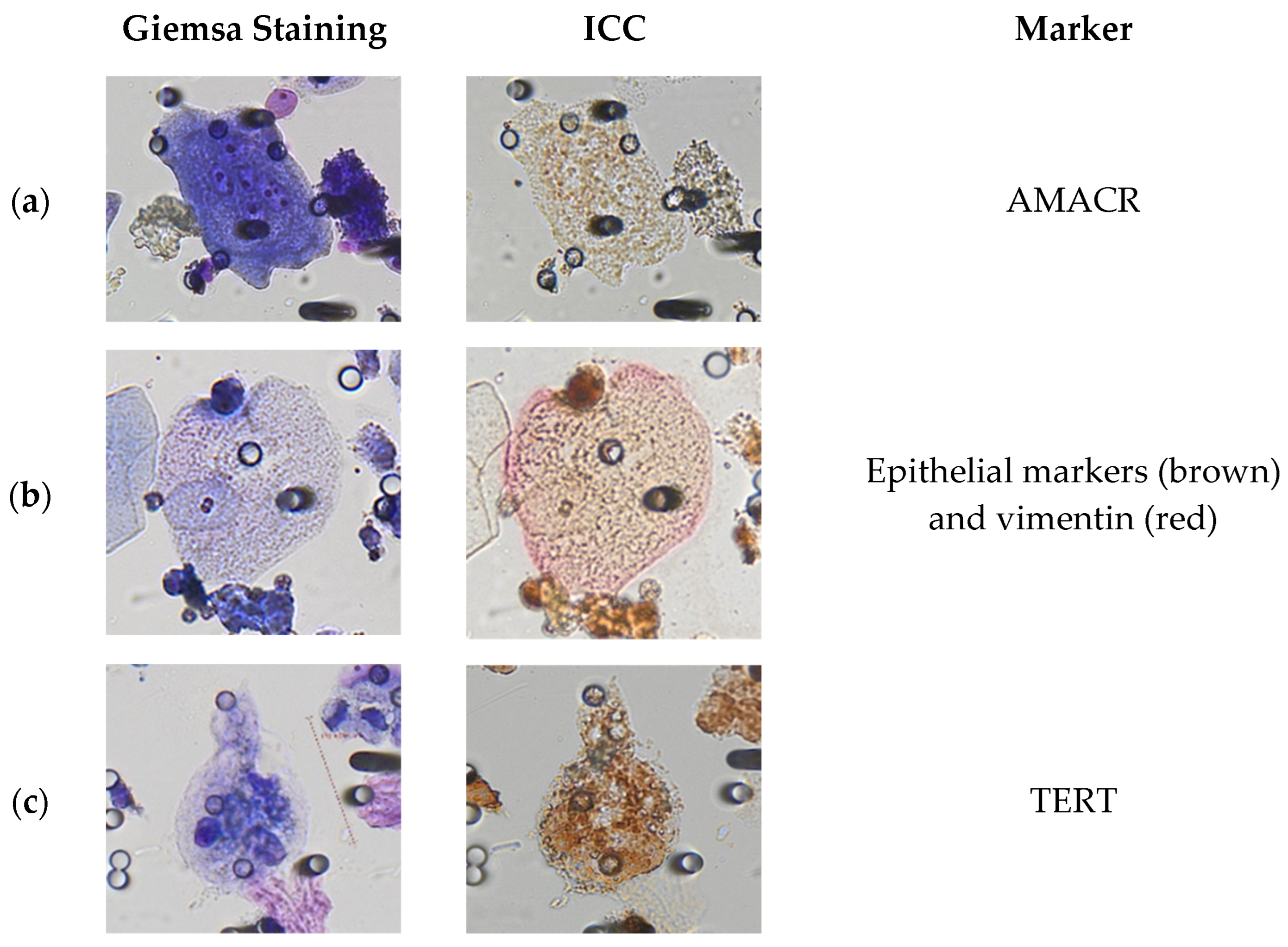
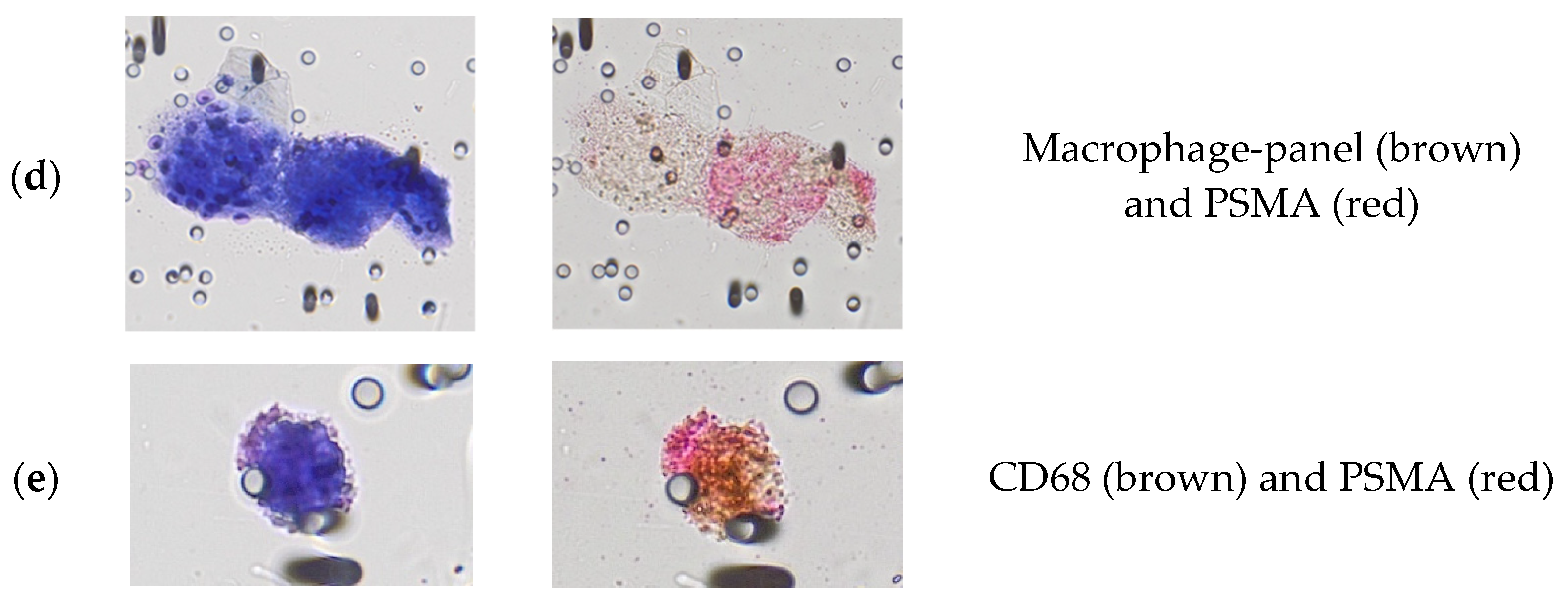
3.2. Immunocytological Characterization of Polyploid Giant Cancer Cells in Urine Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization International Agency for Research on Cancer (IARC). GLOBOCAN 2022: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: https://gco.iarc.fr/today/online-analysis (accessed on 1 June 2023).
- Tyler, K.L.; Selvaggi, S.M. Morphologic features of prostatic adenocarcinoma on ThinPrep® urinary cytology. Diagn. Cytopathol. 2011, 39, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Niu, N.; Li, X.; Zhang, X.; Sood, A.K. The life cycle of polyploid giant cancer cells and dormancy in cancer: Opportunities for novel therapeutic interventions. Semin. Cancer Biol. 2022, 81, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Martin, S.S.; Alpaugh, R.K.; Charpentier, M.; Tsai, S.; Bergan, R.C.; Ogden, I.M.; Catalona, W.; Chumsri, S.; Tang, C.-M.; et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 3514–3519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasabwala, D.M.; Bergan, R.C.; Gardner, K.P.; Lapidus, R.; Tsai, S.; Aldakkak, M.; Adams, D.L. Micronuclei in Circulating Tumor Associated Macrophages Predicts Progression in Advanced Colorectal Cancer. Biomedicines 2022, 10, 2898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mercado-Uribe, I.; Xing, Z.; Sun, B.; Kuang, J.; Liu, J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014, 33, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Patel, R.K.; Anderson, A.N.; Bowden, S.G.; Whalen, R.; Giske, N.R.; Wong, M.H. Circulating Cells with Macrophage-like Characteristics in Cancer: The Importance of Circulating Neoplastic-Immune Hybrid Cells in Cancer. Cancers 2022, 14, 3871. [Google Scholar] [CrossRef]
- Amend, S.R.; Torga, G.; Lin, K.; Kostecka, L.G.; de Marzo, A.; Austin, R.H.; Pienta, K.J. Polyploid giant cancer cells: Unrecognized actuators of tumorigenesis, metastasis, and resistance. Prostate 2019, 79, 1489–1497. [Google Scholar] [CrossRef]
- Pienta, K.J.; Hammarlund, E.U.; Brown, J.S.; Amend, S.R.; Axelrod, R.M. Cancer recurrence and lethality are enabled by enhanced survival and reversible cell cycle arrest of polyaneuploid cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2020838118. [Google Scholar] [CrossRef]
- Niu, N.; Mercado-Uribe, I.; Liu, J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene 2017, 36, 4887–4900. [Google Scholar] [CrossRef] [Green Version]
- Niwa, A.; Tomita, H.; Watanabe, N.; Kiriyama, S.; Hara, A.; Tanaka, T. Case Report: A Case of Gallbladder Carcinosarcoma With Osteoclast-like Multinucleated Giant Cells that Was Associated With RANK-RANKL Signaling. Pathol. Oncol. Res. 2022, 28, 1610134. [Google Scholar] [CrossRef]
- Manjunath, Y.; Mitchem, J.B.; Suvilesh, K.N.; Avella, D.M.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Deroche, C.B.; Pantel, K.; Li, G.; Kaifi, J.T. Circulating Giant Tumor-Macrophage Fusion Cells Are Independent Prognosticators in Patients With NSCLC. J. Thorac. Oncol. 2020, 15, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Ruano, A.P.C.; Guimarães, A.P.G.; Braun, A.C.; Flores, B.C.T.C.P.; Tariki, M.S.; Abdallah, E.A.; Torres, J.A.; Nunes, D.N.; Tirapelli, B.; de Lima, V.C.C.; et al. Fusion Cell Markers in Circulating Tumor Cells from Patients with High-Grade Ovarian Serous Carcinoma. Int. J. Mol. Sci. 2022, 23, 14687. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, C.; Markmann, B.; Konczalla, L.; Kropidlowski, J.; Pereira-Veiga, T.; Scognamiglio, P.; Schönrock, M.; Sinn, M.; Tölle, M.; Izbicki, J.; et al. Circulating Cancer Associated Macrophage-like Cells as a Potential New Prognostic Marker in Pancreatic Ductal Adenocarcinoma. Biomedicines 2022, 10, 2955. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.L.; Adams, D.K.; Alpaugh, R.K.; Cristofanilli, M.; Martin, S.S.; Chumsri, S.; Tang, C.-M.; Marks, J.R. Circulating Cancer-Associated Macrophage-Like Cells Differentiate Malignant Breast Cancer and Benign Breast Conditions. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1037–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzatoğlu, K.; Durak, H.; Canberk, Ş; Aydın, Ö; Huq, G.; Oznur, M.; Özyalvaçlı, G.; Yıldız, P. Giant cell tumor-like lesion of the urinary bladder: A report of two cases and literature review; giant cell tumor or undifferentiated carcinoma? Diagn. Pathol. 2009, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, H.D.; Malalasekera, A. Giant Cell Urothelial Carcinoma of Bladder. Case Rep. Urol. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Ikenberg, H.; Bergeron, C.; Schmidt, D.; Griesser, H.; Alameda, F.; Angeloni, C.; Bogers, J.; Dachez, R.; Denton, K.; Hariri, J.; et al. Screening for Cervical Cancer Precursors With p16/Ki-67 Dual-Stained Cytology: Results of the PALMS Study. J. Natl. Cancer Inst. 2013, 105, 1550–1557. [Google Scholar] [CrossRef]
- Khandelwal, P.; Abraham, S.N.; Apodaca, G. Cell biology and physiology of the uroepithelium. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1477–F1501. [Google Scholar] [CrossRef] [Green Version]
- Wiggins, R.; Horner, P.J.; Whittington, K.; Holmes, C.H. Quantitative analysis of epithelial cells in urine from men with and without urethritis: Implications for studying epithelial: Pathogen interactions in vivo. BMC Res. Notes 2009, 2, 139. [Google Scholar] [CrossRef] [Green Version]
- Becker, G.J.; Garigali, G.; Fogazzi, G.B. Advances in Urine Microscopy. Am. J. Kidney Dis. 2016, 67, 954–964. [Google Scholar] [CrossRef]
- Fogazzi, G.B.; Pallotti, F.; Garigali, G. Atypical/malignant urothelial cells in routine urinary sediment: Worth knowing and reporting. Clin. Chim. Acta 2015, 439, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, A.A.; Gould, E.W. High-Grade Urothelial Carcinoma on Urine Cytology Resembling Umbrella Cells. Acta Cytol. 2018, 62, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Broncy, L.; Ben Njima, B.; Méjean, A.; Béroud, C.; Ben Romdhane, K.; Ilie, M.; Hofman, V.; Muret, J.; Hofman, P.; Bouhamed, H.C.; et al. Single-cell genetic analysis validates cytopathological identification of circulating cancer cells in patients with clear cell renal cell carcinoma. Oncotarget 2018, 9, 20058–20074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, L.; Zhuang, S. New Insights Into the Role and Mechanism of Partial Epithelial-Mesenchymal Transition in Kidney Fibrosis. Front. Physiol. 2020, 11, 569322. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Ali, A.M.; Murty, V.V.; Raza, A. Mutation in SF3B1 gene promotes formation of polyploid giant cells in Leukemia cells. Med. Oncol. 2022, 39, 65. [Google Scholar] [CrossRef]
- Kim, C.-J.; Gonye, A.L.; Truskowski, K.; Lee, C.-F.; Cho, Y.-K.; Austin, R.H.; Pienta, K.J.; Amend, S.R. Nuclear morphology predicts cell survival to cisplatin chemotherapy. Neoplasia 2023, 42, 100906. [Google Scholar] [CrossRef]
- Trabzonlu, L.; Pienta, K.J.; Trock, B.J.; De Marzo, A.M.; Amend, S.R. Presence of cells in the polyaneuploid cancer cell (PACC) state predicts the risk of recurrence in prostate cancer. Prostate 2023, 83, 277–285. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, Q.; Xu, H.; Zhou, R.; Liu, X. Human cell polyploidization: The good and the evil. Semin. Cancer Biol. 2022, 81, 54–63. [Google Scholar] [CrossRef]
- Glybochko, P.; Zezerov, E.; Glukhov, A.; Alyaev, Y.; Severin, S.; Polyakovsky, K.; Varshavsky, V.; Severin, E.; Vinarov, A. Telomerase as a tumor marker in diagnosis of prostatic intraepithelial neoplasia and prostate cancer: Telomerase-A Tumor Marker. Prostate 2014, 74, 1043–1051. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, H.; Hu, L.; Kong, D.; Li, G.; Zhao, C.; Feng, L.; Cheng, S.; Shou, J.; Zhang, W.; et al. Improving the diagnosis of prostate cancer by telomerase-positive circulating tumor cells: A prospective pilot study. EClinicalMedicine 2022, 43, 101161. [Google Scholar] [CrossRef]
- Moon, J.H.; Nikas, I.P.; Moon, K.C.; Kim, B.; Ryu, H.S. Clinical application of the anti-human telomerase reverse transcriptase (h TERT ) antibody (SCD-A7) immunocytochemistry in liquid-based urine cytology: A prospective, single institute study. Cancer Med. 2023, 12, 10363–10370. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, W.; Li, C.; Yang, Y.; Shang, Y.-K.; Chen, C.; Zhang, J.; Yao, R.; Wang, P.; Wen, W.; et al. Promoter mutations and cellular distribution of telomerase in non-clear cell and clear cell hepatocellular carcinoma. Oncotarget 2017, 8, 26288–26297. [Google Scholar] [CrossRef] [Green Version]
- Nishi, Y.; Aoki, T.; Shimizu, T.; Sato, S.; Matsumoto, T.; Shiraki, T.; Sakuraoka, Y.; Mori, S.; Iso, Y.; Ishizuka, M.; et al. Significance of cytoplasmic expression of telomerase reverse transcriptase in patients with hepatocellular carcinoma undergoing liver resection. Mol. Clin. Oncol. 2021, 15, 244. [Google Scholar] [CrossRef]
- Moreno-Acosta, P.; Molano, M.; Morales, N.; Acosta, J.; González-Prieto, C.; Mayorga, D.; Buitrago, L.; Gamboa, O.; Mejía, J.C.; Castro, J.; et al. hTERT Protein Expression in Cytoplasm and Nucleus and its Association With HPV Infection in Patients With Cervical Cancer. Cancer Genom. Proteom. 2020, 17, 615–625. [Google Scholar] [CrossRef]
- Gasinska, A.; Luczynska, E.; Wilk, W.; Cichocka, A. Differences in the expression of telomerase and prostate-specific membrane antigen in non-advanced prostatic cancer. Folia Histochem. Cytobiol. 2013, 51, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson, A.; Carlsson, J.; Lundholm, M.; Fält, A.; Andersson, S.-O.; Andrén, O.; Davidsson, S. M2 macrophages and regulatory T cells in lethal prostate cancer. Prostate 2019, 79, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Deng, Y.; Xu, W.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. The Roles of Tumor-Associated Macrophages in Prostate Cancer. J. Oncol. 2022, 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.-C.; Zhang, M.; Meng, Y.-Y.; Liang, Y.-F.; Wang, Y.-Y.; Liu, X.-Q.; Cai, W.-Q.; Zhou, Y.; Wang, X.-W.; Ma, Z.-W.; et al. Cell-cell fusion as an important mechanism of tumor metastasis (Review). Oncol. Rep. 2021, 46, 145. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Foss, C.A.; Horhota, A.; Pullambhatla, M.; McDonnell, K.; Zale, S.; Pomper, M.G. 111 In- and IRDye800CW-Labeled PLA–PEG Nanoparticle for Imaging Prostate-Specific Membrane Antigen-Expressing Tissues. Biomacromolecules 2017, 18, 201–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland, B.T.; Shallal, H.; Shen, C.; Pienta, K.J.; Foss, C.A.; Pomper, M.G. Imaging and Characterization of Macrophage Distribution in Mouse Models of Human Prostate Cancer. Mol. Imaging Biol. 2019, 21, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- El-Zaatari, Z.M.; Thomas, J.S.; Divatia, M.K.; Shen, S.S.; Ayala, A.G.; Monroig-Bosque, P.; Shehabeldin, A.; Ro, J.Y. Pleomorphic giant cell carcinoma of prostate: Rare tumor with unique clinicopathological, immunohistochemical, and molecular features. Ann. Diagn. Pathol. 2021, 52, 151719. [Google Scholar] [CrossRef]
- Alharbi, A.M.; De Marzo, A.M.; Hicks, J.L.; Lotan, T.L.; Epstein, J.I. Prostatic Adenocarcinoma With Focal Pleomorphic Giant Cell Features: A Series of 30 Cases. Am. J. Surg. Pathol. 2018, 42, 1286–1296. [Google Scholar] [CrossRef] [PubMed]

| Clinical Parameter | Number (%) or Median (Range) |
|---|---|
| Age * average (range) | 73 (55–94) |
| Serum PSA (ng/mL) | |
| Average | 39.0 |
| Median | 12.0 |
| Range | 3.34–275 |
| Unknown | 2 patients |
| Gleason score | |
| 6 | 1 (2.2%) |
| 7 | 18 (40.0%) |
| ≥8 | 26 (57.8%) |
| Metastasis | 13 (28.9%) |
| Antibody | Type | Control Positive Cell Line | Staining Pattern | Supplier |
|---|---|---|---|---|
| PSMA (3E6) | Mouse monoclonal | VCaP, LNCaP | Membranous/cytoplasmic | Agilent (Dako, Santa Clara, United States), M3620 |
| AMACR (13H4) | Rabbit monoclonal | LNCaP | Cytoplasmic granular | Agilent (Dako, Santa Clara, United States), M3616 |
| Panel–Macrophage markers (CD11b, CD68, CD163) | Rabbit monoclonal | HeLa | Cytoplasmic | Abcam (Cambridge, UK), ab254013 |
| Vimentin (SP20) | Rabbit monoclonal | A375 | Cytoplasmic | ThermoFisher Scientific (Waltham, MA, USA), MA5-14564 |
| Panel–Epithelial markers (AE1/AE3, KL-1, EpCAM, E-cadherin) | Mouse monoclonal | A375, DU-145 | Cytoplasmic and membranous | Agilent, M3515 Santa Cruz Biotechnology (Dallas, TX, USA), SC-58825, SC-25308, SC-8425 |
| TERT (2D8) | Mouse monoclonal | LNCaP, HeLa | Nuclear/cytoplasmic | Thermo Fisher, MA5-16033 |
| PSA (EP1588Y) | Rabbit monoclonal | PC-3, VCaP | Cytoplasmic/ membranous | Invitrogen, (Waltham, MA, USA), MA5-14470 |
| Number | With Urinary Giant Cells | With Urinary PGCC | |
|---|---|---|---|
| Prostate cancer patients | 45 | 32 (175) | 22 (50) |
| Healthy donors | 43 | 11 (23) | 1 (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido Castillo, L.N.; Anract, J.; Delongchamps, N.B.; Huillard, O.; BenMohamed, F.; Decina, A.; Lebret, T.; Dachez, R.; Paterlini-Bréchot, P. Polyploid Giant Cancer Cells Are Frequently Found in the Urine of Prostate Cancer Patients. Cancers 2023, 15, 3366. https://doi.org/10.3390/cancers15133366
Garrido Castillo LN, Anract J, Delongchamps NB, Huillard O, BenMohamed F, Decina A, Lebret T, Dachez R, Paterlini-Bréchot P. Polyploid Giant Cancer Cells Are Frequently Found in the Urine of Prostate Cancer Patients. Cancers. 2023; 15(13):3366. https://doi.org/10.3390/cancers15133366
Chicago/Turabian StyleGarrido Castillo, Laura Nalleli, Julien Anract, Nicolas Barry Delongchamps, Olivier Huillard, Fatima BenMohamed, Alessandra Decina, Thierry Lebret, Roger Dachez, and Patrizia Paterlini-Bréchot. 2023. "Polyploid Giant Cancer Cells Are Frequently Found in the Urine of Prostate Cancer Patients" Cancers 15, no. 13: 3366. https://doi.org/10.3390/cancers15133366







