Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Cell Culture
2.4. CRISPR
2.5. RNA Sequencing
2.6. Quantitative PCR
2.7. Flow Cytometry
2.8. Western Blot
2.9. Statistical Analysis
3. Results
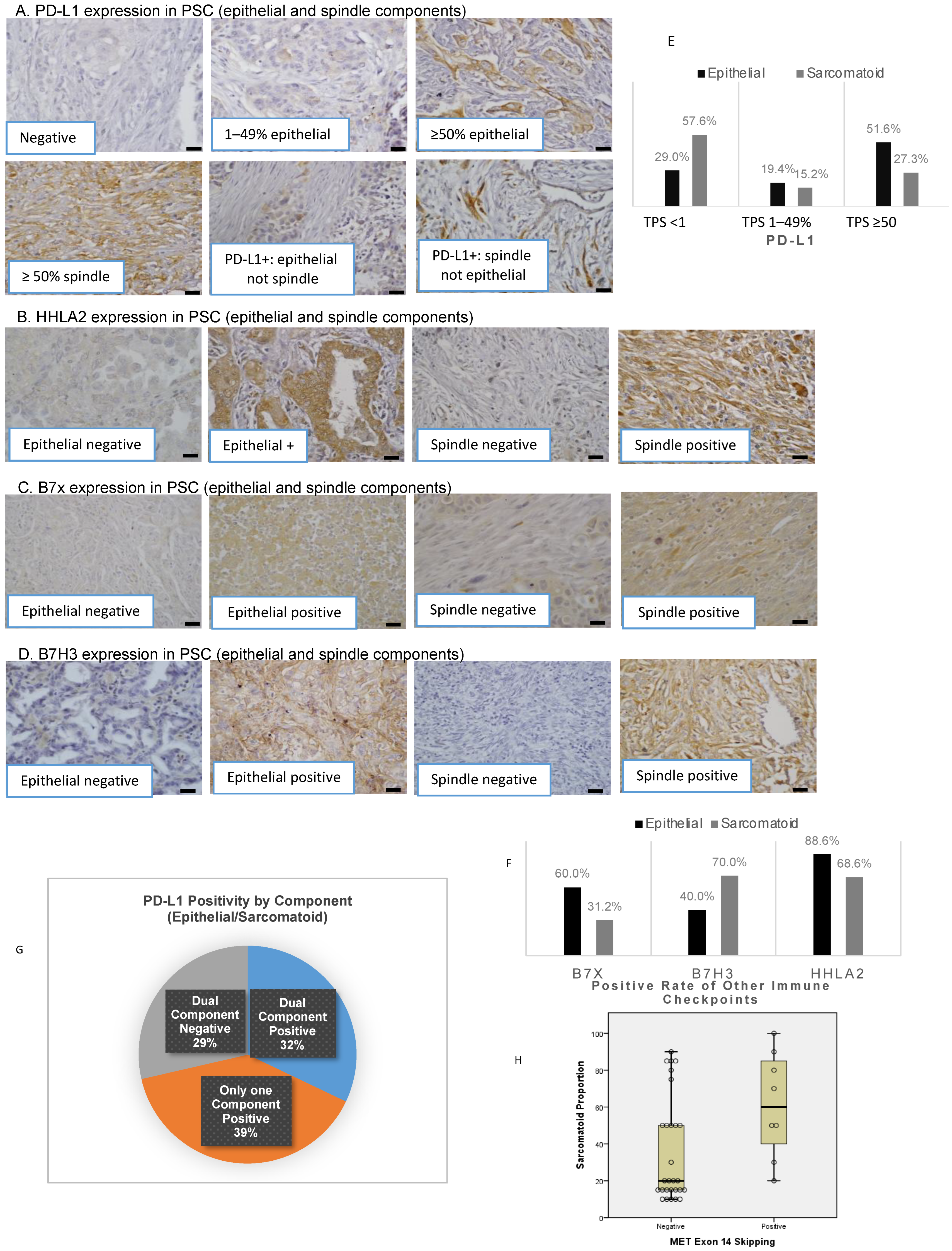
3.1. Clinicopathological Characteristics, Immune Checkpoint Expression, and Genetic Alterations in PSC Patients
3.2. Distinct Expression of Immune Checkpoints in Epithelial and Sarcomatoid Components
3.3. Association of Epithelial and Sarcomatoid Distribution in PSC Samples with Oncogenic Genomic Alterations and Immune Checkpoints
3.4. Genomic Alterations and Their Association with Immune Checkpoints and Tumor-Infiltrating Lymphocytes
3.5. Survival Analysis
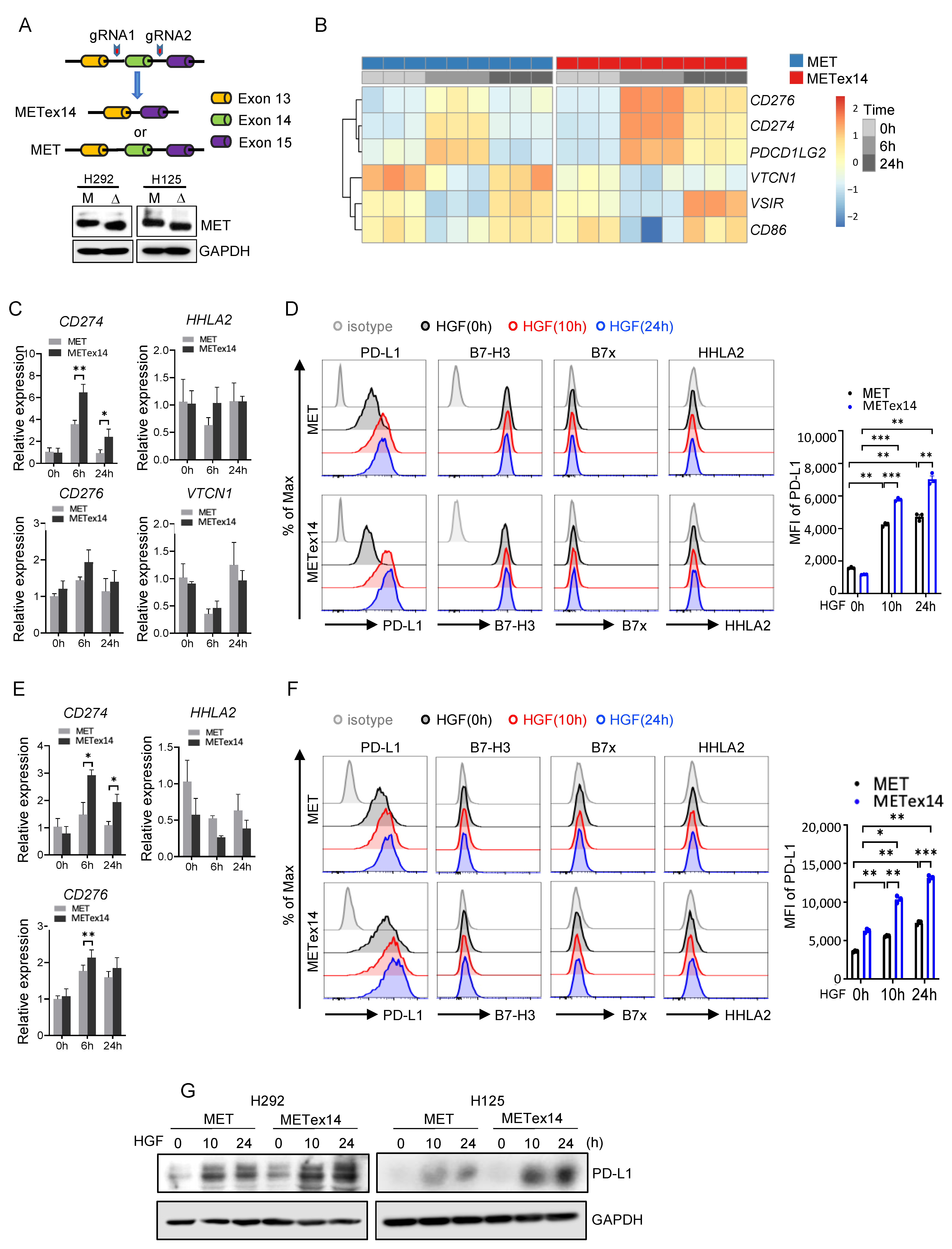
3.6. HGF/METex14 Signaling Specifically Induces PD-L1 Expression
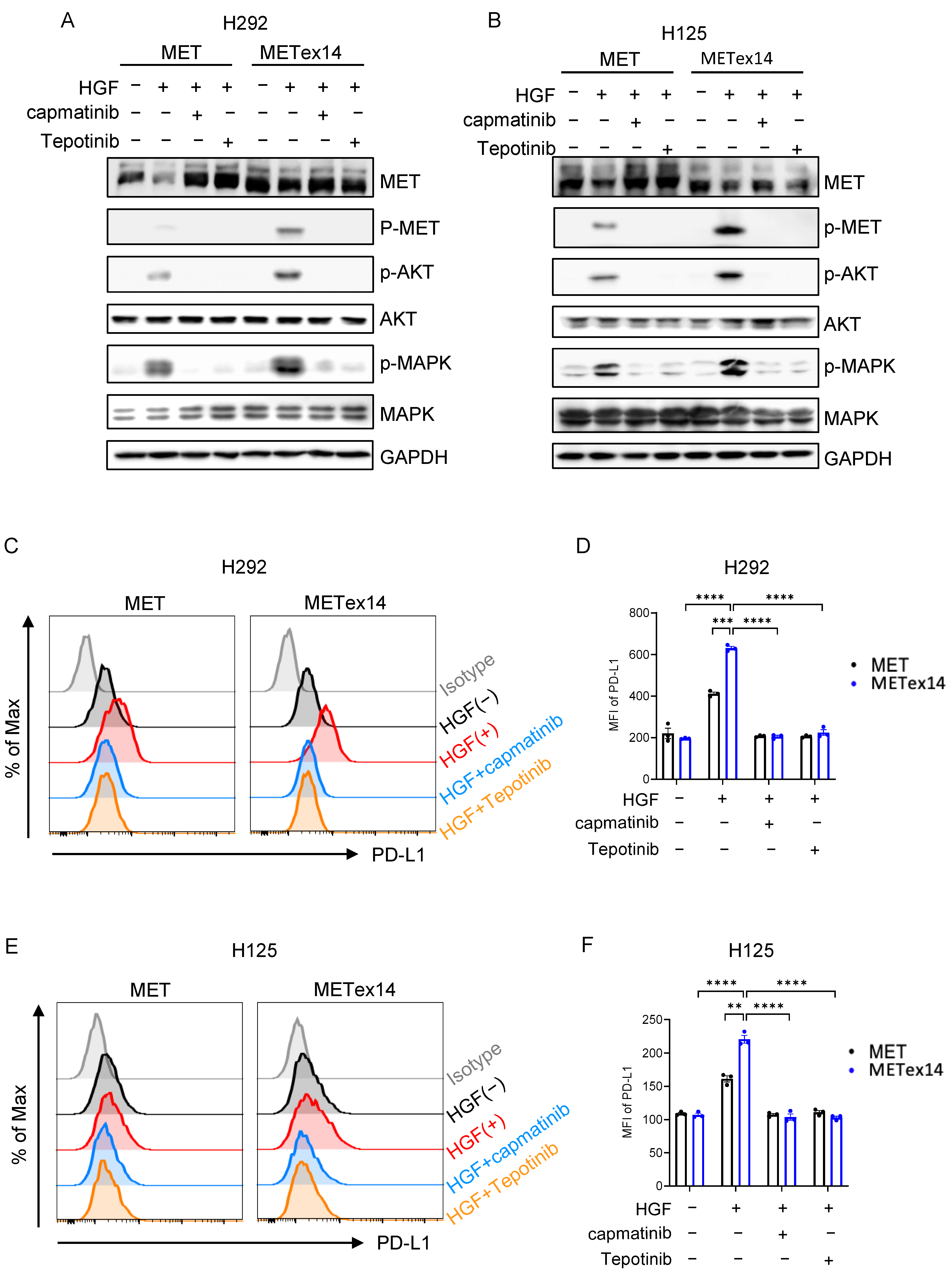
3.7. METex14 Drives PD-L1 Expression via PI3K/Akt and MAPK Signaling Cascades, and MET Inhibitors Block PD-L1 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahouma, M.; Kamel, M.; Narula, N.; Nasar, A.; Harrison, S.; Lee, B.; Stiles, B.; Altorki, N.K.; Port, J.L. Pulmonary Sarcomatoid Carcinoma: An Analysis of a Rare Cancer from the Surveillance, Epidemiology, and End Results Database. Eur. J. Cardiothorac. Surg. 2018, 53, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Maneenil, K.; Xue, Z.; Liu, M.; Boland, J.; Wu, F.; Stoddard, S.M.; Molina, J.; Yang, P. Sarcomatoid Carcinoma of the Lung: The Mayo Clinic Experience in 127 Patients. Clin. Lung Cancer 2018, 19, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef]
- Vieira, T.; Girard, N.; Ung, M.; Monnet, I.; Cazes, A.; Bonnette, P.; Duruisseaux, M.; Mazieres, J.; Antoine, M.; Cadranel, J.; et al. Efficacy of First-Line Chemotherapy in Patients with Advanced Lung Sarcomatoid Carcinoma. J. Thorac. Oncol. 2013, 8, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, D.; Liu, H.; Chen, J. Pulmonary Sarcomatoid Carcinoma: Progress, Treatment and Expectations. Ther. Adv. Med. Oncol. 2020, 12, 1758835920950207. [Google Scholar] [CrossRef] [PubMed]
- Shum, E.; Stuart, M.; Borczuk, A.; Wang, F.; Cheng, H.; Halmos, B. Recent Advances in the Management of Pulmonary Sarcomatoid Carcinoma. Expert Rev. Respir. Med. 2016, 10, 407–416. [Google Scholar] [CrossRef]
- Babacan, N.A.; Pina, I.B.; Signorelli, D.; Prelaj, A.; Garassino, M.C.; Tanvetyanon, T. Relationship Between Programmed Death Receptor-Ligand 1 Expression and Response to Checkpoint Inhibitor Immunotherapy in Pulmonary Sarcomatoid Carcinoma: A Pooled Analysis. Clin. Lung Cancer 2020, 21, e456–e463. [Google Scholar] [CrossRef]
- Domblides, C.; Leroy, K.; Monnet, I.; Mazières, J.; Barlesi, F.; Gounant, V.; Baldacci, S.; Mennecier, B.; Toffart, A.-C.; Audigier-Valette, C.; et al. Efficacy of Immune Checkpoint Inhibitors in Lung Sarcomatoid Carcinoma. J. Thorac. Oncol. 2020, 15, 860–866. [Google Scholar] [CrossRef]
- Sukrithan, V.; Sandler, J.; Gucalp, R.; Gralla, R.; Halmos, B. Immune Checkpoint Blockade Is Associated with Durable Responses in Pulmonary Sarcomatoid Carcinoma. Clin. Lung Cancer 2019, 20, e242–e246. [Google Scholar] [CrossRef]
- Janakiram, M.; Shah, U.A.; Liu, W.; Zhao, A.; Schoenberg, M.P.; Zang, X. The Third Group of the B7-CD28 Immune Checkpoint Family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol. Rev. 2017, 276, 26–39. [Google Scholar] [CrossRef]
- Suh, W.-K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 Family Member B7-H3 Preferentially down-Regulates T Helper Type 1–Mediated Immune Responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Picarda, E.; Galbo, P.M.; Zong, H.; Rajan, M.R.; Wallenius, V.; Zheng, D.; Börgeson, E.; Singh, R.; Pessin, J.; Zang, X. The Immune Checkpoint B7-H3 (CD276) Regulates Adipocyte Progenitor Metabolism and Obesity Development. Sci. Adv. 2022, 8, eabm7012. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Ren, X.; Galbo, P.M.; Moerdler, S.; Wang, H.; Sica, R.A.; Etemad-Gilbertson, B.; Shi, L.; Zhu, L.; Tang, X.; et al. KIR3DL3-HHLA2 Is a Human Immunosuppressive Pathway and a Therapeutic Target. Sci. Immunol. 2021, 6, eabf9792. [Google Scholar] [CrossRef]
- Cheng, H.; Borczuk, A.; Janakiram, M.; Ren, X.; Lin, J.; Assal, A.; Halmos, B.; Perez-Soler, R.; Zang, X. Wide Expression and Significance of Alternative Immune Checkpoint Molecules, B7x and HHLA2, in PD-L1 Negative Human Lung Cancers. Clin. Cancer Res. 2018, 24, 1954–1964. [Google Scholar] [CrossRef]
- Cheng, H.; Janakiram, M.; Borczuk, A.; Lin, J.; Qiu, W.; Liu, H.; Chinai, J.M.; Halmos, B.; Perez-Soler, R.; Zang, X. HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status. Clin. Cancer Res. 2017, 23, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, W.; Chen, K.; Xie, Y.; Liu, Q.; Yao, M.; Duan, W.; Zhou, X.; Liang, R.; Tao, M. B7-H1 and B7-H3 Are Independent Predictors of Poor Prognosis in Patients with Non-Small Cell Lung Cancer. Oncotarget 2014, 6, 3452–3461. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zhao, J.; Gu, M.; Giscombe, R.; Lefvert, A.K.; Wang, X. B7-H3 and B7-H4 Expression in Non-Small-Cell Lung Cancer. Lung Cancer 2006, 53, 143–151. [Google Scholar] [CrossRef]
- John, P.; Wei, Y.; Liu, W.; Du, M.; Guan, F.; Zang, X. The B7x Immune Checkpoint Pathway: From Discovery to Clinical Trial. Trends Pharmacol. Sci. 2019, 40, 883–896. [Google Scholar] [CrossRef]
- Pang, A.; Carbini, M.; Moreira, A.L.; Maki, R.G. Carcinosarcomas and Related Cancers: Tumors Caught in the Act of Epithelial-Mesenchymal Transition. JCO 2018, 36, 210–216. [Google Scholar] [CrossRef]
- Nakagomi, T.; Goto, T.; Hirotsu, Y.; Shikata, D.; Yokoyama, Y.; Higuchi, R.; Amemiya, K.; Okimoto, K.; Oyama, T.; Mochizuki, H.; et al. New Therapeutic Targets for Pulmonary Sarcomatoid Carcinomas Based on Their Genomic and Phylogenetic Profiles. Oncotarget 2018, 9, 10635–10649. [Google Scholar] [CrossRef]
- Manzotti, G.; Torricelli, F.; Benedetta, D.; Lococo, F.; Sancisi, V.; Rossi, G.; Piana, S.; Ciarrocchi, A. An Epithelial-to-Mesenchymal Transcriptional Switch Triggers Evolution of Pulmonary Sarcomatoid Carcinoma (PSC) and Identifies Dasatinib as New Therapeutic Option. Clin. Cancer Res. 2019, 25, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Stoker, M.; Gherardi, E.; Perryman, M.; Gray, J. Scatter Factor Is a Fibroblast-Derived Modulator of Epithelial Cell Mobility. Nature 1987, 327, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–Mesenchymal Transitions in Tumour Progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Schrock, A.B.; Li, S.D.; Frampton, G.M.; Suh, J.; Braun, E.; Mehra, R.; Buck, S.C.; Bufill, J.A.; Peled, N.; Karim, N.A.; et al. Pulmonary Sarcomatoid Carcinomas Commonly Harbor Either Potentially Targetable Genomic Alterations or High Tumor Mutational Burden as Observed by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2017, 12, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Frampton, G.M.; Ali, S.M.; Rosenzweig, M.; Chmielecki, J.; Lu, X.; Bauer, T.M.; Akimov, M.; Bufill, J.A.; Lee, C.; Jentz, D.; et al. Activation of MET via Diverse Exon 14 Splicing Alterations Occurs in Multiple Tumor Types and Confers Clinical Sensitivity to MET Inhibitors. Cancer Discov. 2015, 5, 850–859. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Jänne, P.A.; Verma, S.; et al. MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated with Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721–730. [Google Scholar] [CrossRef]
- Liu, X.; Jia, Y.; Stoopler, M.B.; Shen, Y.; Cheng, H.; Chen, J.; Mansukhani, M.; Koul, S.; Halmos, B.; Borczuk, A.C. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J. Clin. Oncol. 2016, 34, 794–802. [Google Scholar] [CrossRef]
- Sabari, J.K.; Leonardi, G.C.; Shu, C.A.; Umeton, R.; Montecalvo, J.; Ni, A.; Chen, R.; Dienstag, J.; Mrad, C.; Bergagnini, I.; et al. PD-L1 Expression, Tumor Mutational Burden, and Response to Immunotherapy in Patients with MET Exon 14 Altered Lung Cancers. Ann. Oncol. 2018, 29, 2085–2091. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yin, J.; Bohlman, S.; Walker, P.; Dacic, S.; Kim, C.; Khan, H.; Liu, S.V.; Ma, P.C.; Nagasaka, M.; et al. Characterization of MET Exon 14 Skipping Alterations (in NSCLC) and Identification of Potential Therapeutic Targets Using Whole Transcriptome Sequencing. JTO Clin. Res. Rep. 2022, 3, 100381. [Google Scholar] [CrossRef]
- Zhang, Z.; Kobayashi, S.; Borczuk, A.C.; Leidner, R.S.; LaFramboise, T.; Levine, A.D.; Halmos, B. Dual Specificity Phosphatase 6 (DUSP6) Is an ETS-Regulated Negative Feedback Mediator of Oncogenic ERK Signaling in Lung Cancer Cells. Carcinogenesis 2010, 31, 577–586. [Google Scholar] [CrossRef]
- Jeon, H.; Vigdorovich, V.; Garrett-Thomson, S.C.; Janakiram, M.; Ramagopal, U.A.; Abadi, Y.M.; Lee, J.S.; Scandiuzzi, L.; Ohaegbulam, K.C.; Chinai, J.M.; et al. Structure and Cancer Immunotherapy of the B7 Family Member B7x. Cell Rep. 2014, 9, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chinai, J.M.; Buhl, S.; Scandiuzzi, L.; Ray, A.; Jeon, H.; Ohaegbulam, K.C.; Ghosh, K.; Zhao, A.; Scharff, M.D.; et al. HHLA2 Is a Member of the B7 Family and Inhibits Human CD4 and CD8 T-Cell Function. Proc. Natl. Acad. Sci. USA 2013, 110, 9879–9884. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x Are Highly Expressed in Human Prostate Cancer and Associated with Disease Spread and Poor Outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Qiu, W.; Shum, E.; Feng, M.; Zhao, D.; Zheng, D.; Borczuk, A.; Cheng, H.; Halmos, B. Functional Analysis of MET Exon 14 Skipping Alteration in Cancer Invasion and Metastatic Dissemination. Cancer Res. 2022, 82, 1365–1379. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 8 December 2020).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.; Anders, S.; Huber, W. Differential Analysis of Count Data–the DESeq2 Package. Genome Biol. 2014, 15, 10–1186. [Google Scholar]
- Vieira, T.; Antoine, M.; Hamard, C.; Fallet, V.; Duruisseaux, M.; Rabbe, N.; Rodenas, A.; Cadranel, J.; Wislez, M. Sarcomatoid Lung Carcinomas Show High Levels of Programmed Death Ligand-1 (PD-L1) and Strong Immune-Cell Infiltration by TCD3 Cells and Macrophages. Lung Cancer 2016, 98, 51–58. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Y.; Li, Y.; Wang, Q.; Wang, H.; Jiang, L. 18F-FDG PET/CT Imaging in Pulmonary Sarcomatoid Carcinoma and Correlation with Clinical and Genetic Findings. Ann. Nucl. Med. 2019, 33, 647–656. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.-Y.; Koh, J.; Go, H.; Lee, D.S.; Jeon, Y.K.; Chung, D.H. Programmed Death-1 Ligand 1 and 2 Are Highly Expressed in Pleomorphic Carcinomas of the Lung: Comparison of Sarcomatous and Carcinomatous Areas. Eur. J. Cancer 2015, 51, 2698–2707. [Google Scholar] [CrossRef]
- Lamb, M.; Wei, Y.; Ren, X.; O’Connor, R.; Dulak, A.; Rausch, M.; Strand, J.; Etemad-Gilbertson, B.; Gilligan, R.; Chappel, S.; et al. 489 NPX267, a First-in-Class Monoclonal Antibody Targeting KIR3DL3, Blocks HHLA2-Mediated Immunosuppression and Potentiates T and NK Cell-Mediated Antitumor Immunity. J. Immunother. Cancer 2022, 10. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Descourt, R.; Babey, H.; Huchot, E.; Falchero, L.; Veillon, R.; Cortot, A.B.; Tissot, C.; Chouaid, C.; Decroisette, C. Brief Report: First-Line Pembrolizumab in Metastatic Non-Small Cell Lung Cancer Habouring MET Exon 14 Skipping Mutation and PD-L1 ≥50% (GFPC 01-20 Study). Clin. Lung Cancer 2022, 23, e545–e549. [Google Scholar] [CrossRef] [PubMed]

| Protein | Clonality | Clone | Company | Catalog No. | Dilution and Incubation Time |
|---|---|---|---|---|---|
| PD-L1 | Monoclonal | SP142 | Spring Biosciences | ab228462 | 1:100, incubate for 30 min at room temperature |
| B7x | Monoclonal | 1H3 | [31] | N/A | 1:500, incubate overnight at 4 °C |
| HHLA2 | Monoclonal | 566.1 | [15,32] | N/A | 1:500, incubate overnight at 4 °C |
| B7-H3 | Polyclonal | N/A | R&D sciences [33] | BAF1027 | 5 μg/mL, incubate overnight at 4 °C |
| Gene Name | Direction | Primer Sequence |
|---|---|---|
| CD274 | forward | 5′-TGGCATTTGCTGAACGCATTT-3′ |
| reverse | 5′-TGCAGCCAGGTCTAATTGTTTT-3′ | |
| CD276 | Forward | 5′-CTGGCTTTCGTGTGCTGGAGAA-3′ |
| reverse | 5′-GCTGTCAGAGTGTTTCAGAGGC-3′ | |
| VTCN1 | forward | 5′-TCTGGGCATCCCAAGTTGAC-3′ |
| reverse | 5′-TCCGCCTTTTGATCTCCGATT-3′ | |
| HHLA2 | forward | 5′-TACAAAGGCAGTGACCATTTGG-3′ |
| reverse | 5′-AGGTGTAAATTCCTTCGTCCAGA-3′ | |
| GAPDH | forward | 5′-GGAGCGAGATCCCTCCAAAAT-3′ |
| reverse | 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
| Protein | Company | Catalog No. | Source | Concentration |
|---|---|---|---|---|
| MET | Cell Signaling Technology | 8198 | Rabbit | 1:1000 |
| phospho-MET | Cell Signaling Technology | 3077 | Rabbit | 1:1000 |
| AKT | Cell Signaling Technology | 9272 | Rabbit | 1:1000 |
| phospho-AKT | Cell Signaling Technology | 9271 | Rabbit | 1:1000 |
| P42/44 MAPK | Cell Signaling Technology | 9102 | Rabbit | 1:1000 |
| phospho-p42/44 MAPK | Cell Signaling Technology | 9101 | Rabbit | 1:1000 |
| GAPDH | Cell Signaling Technology | 5174 | Rabbit | 1:1000 |
| PD-L1 | B7X | B7H3 | HHLA2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p | Negative | Positive | p |
| Age, year | 70 | 72 | 0.602 | 68 | 72 | 0.277 | 69.5 | 71.33 | 0.664 | 74.3 | 70.5 | 0.579 |
| Gender | 0.470 | 0.482 | 0.288 | 0.580 | ||||||||
| Female | 4 (44.4%) | 16 (59.3%) | 5 (41.7%) | 15 (60%) | 7 (70.0%) | 13 (48.1%) | 1 (33.3%) | 19 (55.9%) | ||||
| Male | 5 (55.6%) | 11 (40.7%) | 7 (58.3%) | 10 (40%) | 3 (30.0%) | 14 (51.9%) | 2 (66.7%) | 15 (44.1%) | ||||
| Smoking status | 0.921 | 0.207 | 0.282 | 0.536 | ||||||||
| Never smoker | 1 (12.5%) | 2 (8.0%) | 1 (9.1%) | 2 (8.7%) | 0 (0.0%) | 3 (11.5%) | 0 (0.0%) | 3 (9.7%) | ||||
| Former smoker | 5 (62.5%) | 17 (68.0%) | 5 (45.5%) | 17 (73.9%) | 7 (87.5%) | 15 (57.7%) | 2 (66.7%) | 20 (64.5%) | ||||
| Active smoker | 2 (25.0%) | 6 (24.0%) | 5 (0.0%) | 4 (0.0%) | 1 (12.5%) | 8 (30.8%) | 1 (33.3%) | 8 (25.8%) | ||||
| Stage | 0.823 | 0.920 | 0.541 | 0.309 | ||||||||
| I | 4 (44.4%) | 8 (29.6%) | 4 (33.3%) | 8 (32.0%) | 5 (50.0%) | 7 (25.9%) | 0 (0.0%) | 12 (35.3%) | ||||
| II | 3 (33.3%) | 11 (40.7%) | 5 (41.7%) | 10 (40.0%) | 3 (30.0%) | 12 (44.4%) | 1 (33.3%) | 14 (41.2%) | ||||
| III | 2 (22.2%) | 7 (25.9%) | 3 (25.0%) | 6 (24.0%) | 2 (20.0%) | 7 (25.9%) | 2 (66.7%) | 7 (20.6%) | ||||
| IV | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 1 (4.0%) | 0 (0.0%) | 1 (3.7%) | 0 (0.0%) | 1 (2.9%) | ||||
| Lymph node | 0.427 | 0.478 | 1.000 | 1.000 | ||||||||
| Negative | 7 (77.8%) | 14 (56.0%) | 8 (72.7%) | 14 (58.3%) | 6 (66.7%) | 16 (61.5%) | 2 (66.7%) | 20 (62.5%) | ||||
| Positive | 2 (22.2%) | 11 (44.0%) | 3 (27.3%) | 10 (41.7%) | 3 (33.3%) | 10 (38.5%) | 1 (33.3%) | 12 (37.5%) | ||||
| Mutation status | ||||||||||||
| MET mutation | 0.160 | 1.000 | 1.000 | 1.000 | ||||||||
| Negative | 9 (100.0%) | 19 (70.4%) | 10 (83.3%) | 19 (17.0%) | 8 (80.0%) | 21 (77.8%) | 3 (100.0%) | 26 (76.5%) | ||||
| Positive | 0 (0.0%) | 8 (29.6%) | 2 (16.7%) | 6 (24.0%) | 2 (20.0%) | 6 (22.2%) | 0 (0.0%) | 8 (23.5%) | ||||
| KRAS mutation | 0.652 | 1.000 | 0.655 | 0.431 | ||||||||
| Negative | 8 (88.9%) | 21 (80.8%) | 10 (83.3%) | 20 (83.3%) | 9 (90.0%) | 21 (80.8%) | 2 (66.7%) | 28 (84.8%) | ||||
| Positive | 1 (11.1%) | 5 (19.2%) | 2 (16.7%) | 4 (16.7%) | 1 (10.0%) | 5 (19.2%) | 1 (33.3%) | 5 (15.2%) | ||||
| TIL score | 0.223 | 1.000 | 1.000 | 0.536 | ||||||||
| Low (<30%) | 8 (88.9%) | 17 (65.4%) | 8 (66.7%) | 17 (73.9%) | 7 (70.0%) | 18 (72.0%) | 2 (100.0%) | 23 (69.7%) | ||||
| High (≥30%) | 1 (11.1%) | 9 (34.6%) | 4 (33.3%) | 6 (26.1%) | 3 (30.0%) | 7 (28.0%) | 0 (0.0%) | 10 (30.3%) | ||||
| B7X | 1.000 | |||||||||||
| Negative | 3 (33.3%) | 9 (33.3%) | ||||||||||
| Positive | 6 (66.7%) | 18 (66.7%) | ||||||||||
| B7H3 | 0.226 | |||||||||||
| Negative | 4 (44.4%) | 6 (22.2%) | ||||||||||
| Positive | 5 (55.6%) | 21 (77.8%) | ||||||||||
| HHLA2 | 1.000 | |||||||||||
| Negative | 0 (0.0%) | 2 (7.4%) | ||||||||||
| Positive | 9 (100.0%) | 25 (92.6%) | ||||||||||
| A. | ||||||||
| PD-L1 | B7x | B7H3 | HHLA2 | |||||
| Median (interquartile range) | p | Median (interquartile range) | p | Median (interquartile range) | p | Median (interquartile range) | p | |
| Percentage of tumor | 0.010 | 0.008 | 0.053 | 0.553 | ||||
| Epithelial | 50 (0–75) | 25 (0–75) | 0 (0–50) | 50 (75–100) | ||||
| Sarcomatoid | 0 (0–58.5) | 0 (0–23.75) | 50 (0–75) | 95 (57.5–95.0) | ||||
| H-Score | 0.014 | 0.013 | 0.176 | 0.9 | ||||
| Epithelial | 75 (0–150) | 25 (0–75) | 0 (0–100) | 100 (75–180) | ||||
| Sarcomatoid | 0 (0–100) | 0 (0–23.75) | 75 (0–150) | 100 (0–160) | ||||
| B. | ||||||||
| Any Other Immune Checkpoint Expression | ||||||||
| Epithelial | Sarcomatoid | |||||||
| PD-L1 Epithelial | Negative | Positive | PD-L1 Sarcomatoid | Negative | Positive | |||
| Negative | 2 (7.1%) | 13 (46.4%) | Negative | 0 (0.0%) | 8 (26.7%) | |||
| Positive | 0 (0.0%) | 13 (46.4%) | Positive | 1 (3.3%) | 21 (70.0%) | |||
| C. | ||||||||
| Epithelial | Sarcomatoid | |||||||
| B7x | 4 (50.0%) | 3 (33.3%) | ||||||
| B7H3 | 3 (37.5%) | 3 (33.3%) | ||||||
| HHLA2 | 8 (100%) | 6 (66.7%) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Cali Daylan, A.E.; Deng, L.; Yang, J.; Sharma, J.; Su, C.; Li, S.; Zang, X.; Halmos, B.; Borczuk, A.; et al. Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways. Cancers 2023, 15, 3372. https://doi.org/10.3390/cancers15133372
Wang F, Cali Daylan AE, Deng L, Yang J, Sharma J, Su C, Li S, Zang X, Halmos B, Borczuk A, et al. Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways. Cancers. 2023; 15(13):3372. https://doi.org/10.3390/cancers15133372
Chicago/Turabian StyleWang, Feng, Ayse Ece Cali Daylan, Lei Deng, Jihua Yang, Janaki Sharma, Christopher Su, Shenduo Li, Xingxing Zang, Balazs Halmos, Alain Borczuk, and et al. 2023. "Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways" Cancers 15, no. 13: 3372. https://doi.org/10.3390/cancers15133372
APA StyleWang, F., Cali Daylan, A. E., Deng, L., Yang, J., Sharma, J., Su, C., Li, S., Zang, X., Halmos, B., Borczuk, A., & Cheng, H. (2023). Heterogeneous Expression of PD-L1, B7x, B7-H3, and HHLA2 in Pulmonary Sarcomatoid Carcinoma and the Related Regulatory Signaling Pathways. Cancers, 15(13), 3372. https://doi.org/10.3390/cancers15133372






