Evaluation of Cardiac Substructures Exposure of DIBH-3DCRT, FB-HT, and FB-3DCRT in Hypofractionated Radiotherapy for Left-Sided Breast Cancer after Breast-Conserving Surgery: An In Silico Planning Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Cardiac Segmentation
2.3. DIBH-3DCRT and FB-3DCRT Treatment Planning
2.4. FB-HT Treatment Planning
2.5. Statistics
3. Results
3.1. DIBH-3DCRT vs. FB-3DCRT
3.1.1. Overall Outcome
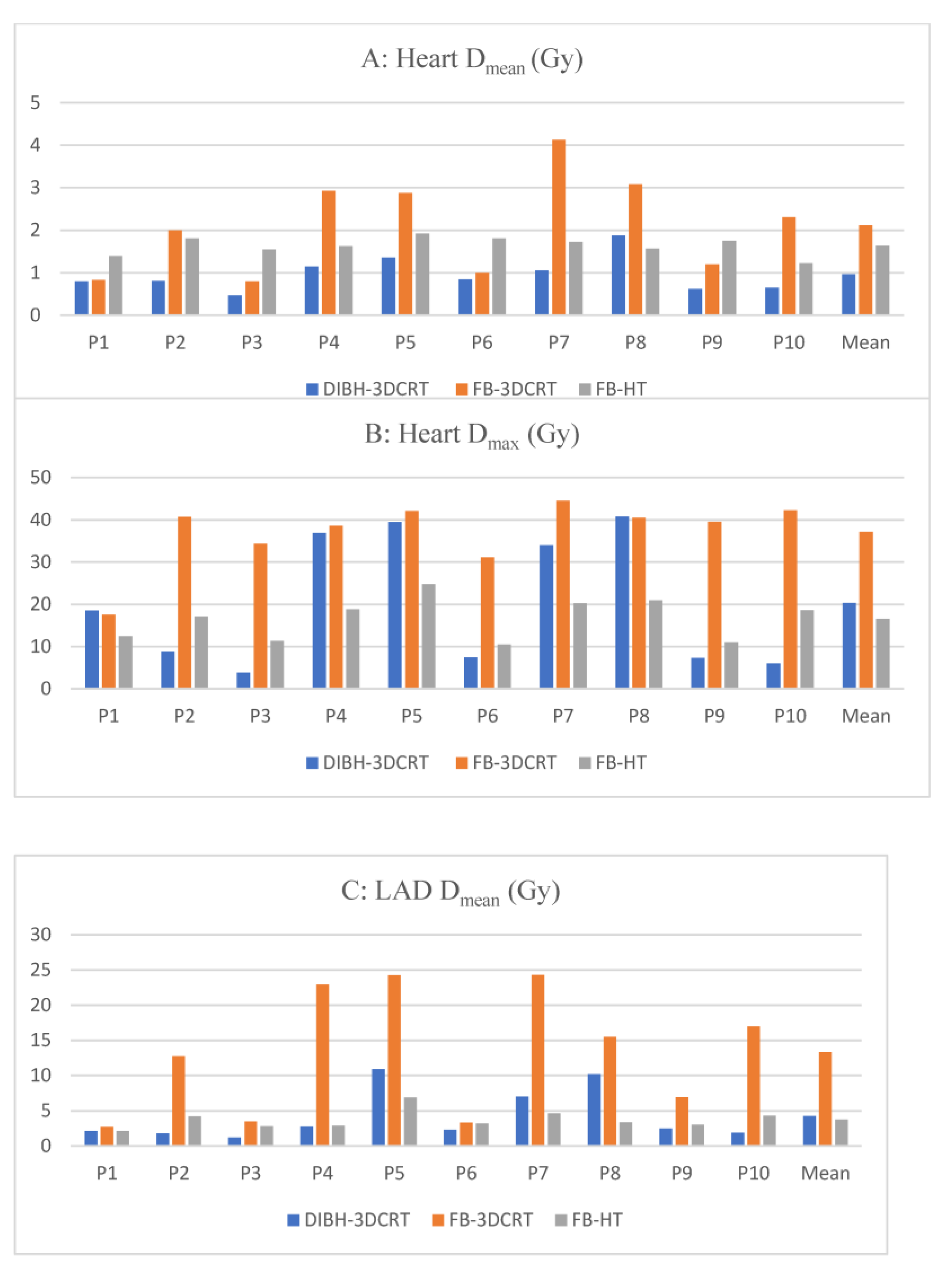
3.1.2. Heart Dosimetry
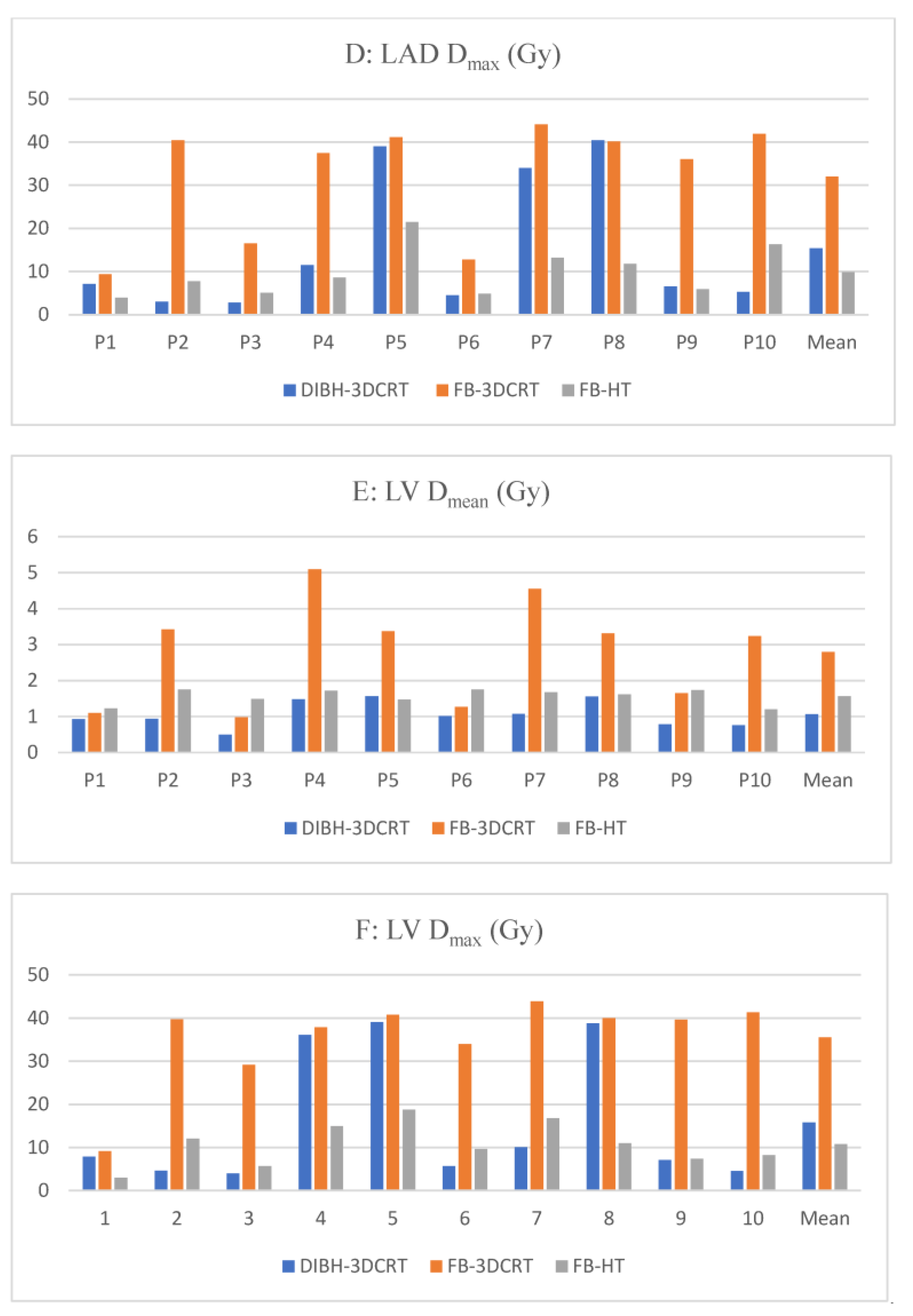
3.1.3. Cardiac Substructures Dosimetry
3.1.4. Contralateral Breast and Ipsilateral Lung Dosimetry
3.2. DIBH-3DCRT vs. FB-HT
3.2.1. Heart Dosimetry
3.2.2. Cardiac Substructures Dosimetry
3.2.3. Contralateral Breast and Ipsilateral Lung Dosimetry
3.3. FB-HT vs. FB-3DCRT
3.3.1. Heart Dosimetry
3.3.2. Cardiac Substructures Dosimetry
3.3.3. Contralateral Breast and Ipsilateral Lung Dosimetry
4. Discussion
4.1. DIBH Effectiveness
4.2. DIBH Limitations
4.3. FB-HT as an Alternative
4.4. Mean Heart Dose and Cardiac Substructures
4.5. FB-HT Limitations
4.6. What Technique Should Be Used?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGale, P.; Darby, S.C.; Hall, P.; Adolfsson, J.; Bengtsson, N.O.; Bennet, A.M.; Fornander, T.; Gigante, B.; Jensen, M.-B.; Peto, R.; et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother. Oncol. J. Eur. Soc. Radiol. Oncol. 2011, 100, 167–175. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, Y. The cardiac toxicity of radiotherapy—A review of characteristics, mechanisms, diagnosis, and prevention. Int. J. Radiat. Biol. 2021, 97, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.; Correa, C.; Duane, F.K.; Aznar, M.C.; Anderson, S.J.; Bergh, J.; Dodwell, D.; Ewertz, M.; Gray, R.; Jagsi, R.; et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence from Modern Radiation Doses to the Lungs and Heart and from Previous Randomized Trials. J. Clin. Oncol. 2017, 35, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Degro, B.C.E.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; et al. Heart-sparing radiotherapy techniques in breast cancer patients: A recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther. Onkol. 2019, 195, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.M.; Knight, K.A.; Aarons, Y.K.; Wasiak, J. The cardiac dose-sparing benefits of deep inspiration breath-hold in left breast irradiation: A systematic review. J. Med. Radiat. Sci. 2015, 62, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, R.; Conroy, L.; Long, K.; Walrath, D.; Li, H.; Smith, W.; Hudson, A.; Phan, T. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat. Oncol. 2015, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Freislederer, P.; Kügele, M.; Öllers, M.; Swinnen, A.; Sauer, T.O.; Bert, C.; Giantsoudi, D.; Corradini, S.; Batista, V. Recent advanced in Surface Guided Radiation Therapy. Radiat. Oncol. 2020, 15, 187. [Google Scholar] [CrossRef]
- Nieder, C.; Schill, S.; Kneschaurek, P.; Molls, M. Influence of different treatment techniques on radiation dose to the LAD coronary artery. Radiat. Oncol. 2007, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Shah, C.; Badiyan, S.; Berry, S.; Khan, A.J.; Goyal, S.; Schulte, K.; Nanavati, A.; Lynch, M.; Vicini, F.A. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 112, 9–16. [Google Scholar] [CrossRef]
- Barnett, G.C.; Wilkinson, J.S.; Moody, A.M.; Wilson, C.B.; Twyman, N.; Wishart, G.C.; Burnet, N.G.; Coles, C.E. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: Interim results at 2 years. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 715–723. [Google Scholar] [CrossRef]
- Mukesh, M.B.; Barnett, G.C.; Wilkinson, J.S.; Moody, A.M.; Wilson, C.; Dorling, L.; Hak, C.C.W.; Qian, W.; Twyman, N.; Burnet, N.G.; et al. Randomized controlled trial of intensity-modulated radiotherapy for early breast cancer: 5-year results confirm superior overall cosmesis. J. Clin. Oncol. 2013, 31, 4488–4495. [Google Scholar] [CrossRef]
- Austin, A.M.; Douglass, M.J.J.; Nguyen, G.T.; Cunningham, L.; Le, H.; Hu, Y.; Penfold, S.N. Individualised selection of left-sided breast cancer patients for proton therapy based on cost-effectiveness. J. Med. Radiat. Sci. 2021, 68, 44–51. [Google Scholar] [CrossRef]
- Naimi, Z.; Moujahed, R.; Neji, H.; Yahyaoui, J.; Hamdoun, A.; Bohli, M.; Kochbati, L. Cardiac substructures exposure in left-sided breast cancer radiotherapy: Is the mean heart dose a reliable predictor of cardiac toxicity? Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2021, 25, 229–236. [Google Scholar] [CrossRef]
- Loap, P.; Vu-Bezin, J.; Monceau, V.; Jacob, S.; Fourquet, A.; Kirova, Y. Dosimetric evaluation of the benefit of deep inspiration breath hold (DIBH) for locoregional irradiation of right breast cancer with volumetric modulated arctherapy (VMAT). Acta Oncol. 2023, 62, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Biete Sola, A.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Moran, J.M.; Koelling, T.; Chughtai, A.; Chan, J.L.; Freedman, L.; Hayman, J.A.; Jagsi, R.; Jolly, S.; Larouere, J.; et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, N.; Hussain, K.; Shlofmitz, E. Pericardial Calcification; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK538342/ (accessed on 14 June 2023).
- Piroth, M.D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; et al. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther. Onkol. 2019, 195, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loap, P.; Kirova, Y. The challenge of cardiac dose constraint adaptation to hypofractionated breast radiotherapy in clinical practice. Strahlenther. Onkol. 2021, 197, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.; Cresswell, K.; Robertson, A.; Huby, G.; Avery, A.; Sheikh, A. The case study approach. BMC Med. Res. Methodol. 2011, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Carlson, L.E.; Watt, G.P.; Tonorezos, E.S.; Chow, E.J.; Yu, A.F.; Woods, M.; Lynch, C.F.; John, E.M.; Mellemkjӕr, L.; Brooks, J.D.; et al. Coronary Artery Disease in Young Women After Radiation Therapy for Breast Cancer. JACC Cardio Oncol. 2021, 3, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, V.A.B.; Ta, B.D.P.; van der Schaaf, A.; Bouma, A.B.; Middag, A.M.H.; Bantema-Joppe, E.J.; Van Dijk, L.V.; Van Dijk-Peters, F.B.; Marteijn, L.A.; De Bock, G.H.; et al. Validation and Modification of a Prediction Model for Acute Cardiac Events in Patients with Breast Cancer Treated with Radiotherapy Based on Three-Dimensional Dose Distributions to Cardiac Substructures. J. Clin. Oncol. 2017, 35, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ishikawa, Y.; Ito, K.; Yamamoto, T.; Umezawa, R.; Jingu, K. Evaluation of DIBH and VMAT in Hypofractionated Radiotherapy for Left-Sided Breast Cancers After Breast-Conserving Surgery: A Planning Study. Technol. Cancer Res. Treat. 2021, 20, 153303382110487. [Google Scholar] [CrossRef]
- Swanson, T.; Grills, I.S.; Ye, H.; Entwistle, A.; Teahan, M.; Letts, N.; Yan, D.; Duquette, J.; Vicini, F.A. Six-year Experience Routinely Using Moderate Deep Inspiration Breath-hold for the Reduction of Cardiac Dose in Left-sided Breast Irradiation for Patients with Early-stage or Locally Advanced Breast Cancer. Am. J. Clin. Oncol. 2013, 36, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Rochet, N.; Drake, J.I.; Harrington, K.; Wolfgang, J.A.; Napolitano, B.; Sadek, B.T.; Shenouda, M.N.; Keruakous, A.; Niemierko, A.; Taghian, A.G. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: Evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract. Radiat. Oncol. 2015, 5, e127–e134. [Google Scholar] [CrossRef] [PubMed]
- Ferini, G.; Molino, L.; Tripoli, A.; Valenti, V.; Illari, S.I.; Marchese, V.A.; Cravagno, I.R.; Borzi, G.R. Anatomical Predictors of Dosimetric Advantages for Deep-inspiration-breath-hold 3D-conformal Radiotherapy Among Women with Left Breast Cancer. Anticancer Res. 2021, 41, 1529–1538. [Google Scholar] [CrossRef]
- Cao, N.; Kalet, A.M.; Young, L.A.; Fang, L.C.; Kim, J.N.; Mayr, N.A.; Meyer, J. Predictors of cardiac and lung dose sparing in DIBH for left breast treatment. Phys. Med. 2019, 67, 27–33. [Google Scholar] [CrossRef]
- Corradini, S.; Ballhausen, H.; Weingandt, H.; Freislederer, P.; Schönecker, S.; Niyazi, M.; Simonetto, C.; Eidemuller, M.; Ganswindt, U.; Belka, C. Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease: Effects of modern radiotherapy techniques. Strahlenther. Onkol. 2018, 194, 196–205. [Google Scholar] [CrossRef]
- Ferini, G.; Valenti, V.; Viola, A.; Umana, G.E.; Martorana, E. A Critical Overview of Predictors of Heart Sparing by Deep-Inspiration-Breath-Hold Irradiation in Left-Sided Breast Cancer Patients. Cancers 2022, 14, 3477. [Google Scholar] [CrossRef]
- Mathieu, D.; Bedwani, S.; Mascolo-Fortin, J.; Côté, N.; Bernard, A.A.; Roberge, D.; Yassa, M.; Bahig, H.; Vu, T. Cardiac Sparing with Personalized Treatment Planning for Early-stage Left Breast Cancer. Cureus 2020, 12, e7247. [Google Scholar] [CrossRef] [Green Version]
- Wennstig, A.K.; Garmo, H.; Isacsson, U.; Gagliardi, G.; Rintelä, N.; Lagerqvist, B.; Holmberg, L.; Blomqvist, C.; Sund, M.; Nilsson, G. The relationship between radiation doses to coronary arteries and location of coronary stenosis requiring intervention in breast cancer survivors. Radiat. Oncol. 2019, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.; McGale, P.; Brønnum, D.; Correa, C.; Cutter, D.; Duane, F.K.; Gigante, B.; Jensen, M.-B.; Lorenzen, E.L.; Rahimi, K.; et al. Cardiac Structure Injury After Radiotherapy for Breast Cancer: Cross-Sectional Study with Individual Patient Data. J. Clin. Oncol. 2018, 36, 2288–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikäheimo, M.J.; Niemelä, K.O.; Linnaluoto, M.M.; Jakobsson, M.J.; Takkunen, J.T.; Taskinen, P.J. Early cardiac changes related to radiation therapy. Am. J. Cardiol. 1985, 56, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, J.L.; Darcourt, J.; Benoliel, J.; Bensadoun, R.J.; Migneco, O. Acute cardiac effects of mediastinal irradiation: Assessment by radionuclide angiography. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Jingu, K.; Kaneta, T.; Nemoto, K.; Ichinose, A.; Oikawa, M.; Takai, Y.; Ogawa, Y.; Nakata, E.; Sakayauchi, T.; Takai, K.; et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 845–851. [Google Scholar] [CrossRef]
- Eber, J.; Leroy-Freschini, B.; Antoni, D.; Noël, G.; Pflumio, C. Increased cardiac uptake of (18F)-fluorodeoxyglucose incidentally detected on positron emission tomography after left breast irradiation: How to interpret? Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2022, 26, 724–729. [Google Scholar] [CrossRef]
- Pak, S.; Hawash, A.A.; Linares, J.; Valencia, D.; Kilgore, A.; Valencia, V.; Markovic, J.-P. Myocardial damage on SPECT imaging among patients treated with radiotherapy for left-sided breast cancer: Systematic review with meta-analysis and narrative synthesis. J. BUON 2018, 23, 910–918. [Google Scholar]
- Evans, J.D.; Gomez, D.R.; Chang, J.Y.; Gladish, G.W.; Erasmus, J.J.; Rebueno, N.; Banchs, J.; Komaki, R.; Welsh, J.W. Cardiac 18F-fluorodeoxyglucose uptake on positron emission tomography after thoracic stereotactic body radiation therapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 109, 82–88. [Google Scholar] [CrossRef]
- Konski, A.; Li, T.; Christensen, M.; Cheng, J.D.; Yu, J.Q.; Crawford, K.; Haluszka, O.; Tokar, J.; Scott, W.; Meropol, N.J.; et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother. Oncol. 2012, 104, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Vinogradskiy, Y.; Diot, Q.; Jones, B.; Castillo, R.; Castillo, E.; Kwak, J.; Bowles, D.; Grills, I.; Myziuk, N.; Guerrero, T.; et al. Evaluating Positron Emission Tomography-Based Functional Imaging Changes in the Heart after Chemo-Radiation for Patients With Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1063–1070. [Google Scholar] [CrossRef]
- Blitzblau, R.; Wright, A.; Arya, R.; Broadwater, G.; Pura, J.; Hardenbergh, P.H.; Borges-Neto, S.; Wong, T.; Marks, L.; Horton, J. Are Long-term Cardiac Outcomes Predicted by Short-term Postradiation Cardiac Perfusion Deficits: An 8–15 Year Follow-up of a Prospective Study. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, S106–S107. [Google Scholar] [CrossRef]
- Eber, J.; Nannini, S.; Chambrelant, I.; Le Fèvre, C.; Noël, G.; Antoni, D. Impact of thoracic irradiation on cardiac structures. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2022, 26, 526–536. [Google Scholar]
- Jacob, S.; Camilleri, J.; Derreumaux, S.; Walker, V.; Lairez, O.; Lapeyre, M.; Bruguière, E.; Pathak, A.; Bernier, M.-O.; Laurier, D.; et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: A dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiat. Oncol. 2019, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Herr, A.C.; Borm, K.J.; Trott, K.R.; Molls, M.; Oechsner, M.; Combs, S.E. Tangential Field Radiotherapy for Breast Cancer—The Dose to the Heart and Heart Subvolumes: What Structures Must Be Contoured in Future Clinical Trials? Front. Oncol. 2017, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loap, P.; Tkatchenko, N.; Goudjil, F.; Ribeiro, M.; Baron, B.; Fourquet, A.; Kirova, Y. Cardiac substructure exposure in breast radiotherapy: A comparison between intensity modulated proton therapy and volumetric modulated arc therapy. Acta Oncol. 2021, 60, 1038–1044. [Google Scholar] [CrossRef]
- Chen, C.; Qin, C.; Qiu, H.; Tarroni, G.; Duan, J.; Bai, W.; Rueckert, D. Deep Learning for Cardiac Image Segmentation: A Review. Front. Cardiovasc. Med. 2020, 7, 25. [Google Scholar] [CrossRef]
- Morris, E.D.; Ghanem, A.I.; Dong, M.; Pantelic, M.V.; Walker, E.M.; Glide-Hurst, C.K. Cardiac substructure segmentation with deep learning for improved cardiac sparing. Med. Phys. 2020, 47, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Berrington de Gonzalez, A.; Gilbert, E.; Curtis, R.; Inskip, P.; Kleinerman, R.; Morton, L.; Rajaraman, P.; Little, M. Second Solid Cancers After Radiation Therapy: A Systematic Review of the Epidemiologic Studies of the Radiation Dose-Response Relationship. Int. J. Radiat. Oncol. 2013, 86, 224–233. [Google Scholar] [CrossRef] [Green Version]
- De Gonzalez, A.B.; Curtis, R.E.; Kry, S.F.; Gilbert, E.; Lamart, S.; Berg, C.D.; Stovall, M.; Ron, E. The proportion of second cancers attributable to radiotherapy treatment in adults: A prospective cohort study in the US SEER cancer registries. Lancet Oncol. 2011, 12, 353–360. [Google Scholar] [CrossRef] [Green Version]

| Volume | Dosimetric Parameter | DIBH-3DCRT | FB-3DCRT | FB-HT | p-Value | ||
|---|---|---|---|---|---|---|---|
| DIBH-3DCRT vs. FB-3DCRT | DIBH-3DCRT vs. FB-HT | FB-3DCRT vs. FB-HT | |||||
| PTV | D98% (Gy) | 37.4 ± 0.4 | 37.5 ± 0.9 | 37.5 ± 0.5 | 0.97 | 0.48 | 0.68 |
| V38.1 Gy (%) | 96.2 ± 1.0 | 95.5 ± 3.3 | 96.9 ± 1.1 | 0.97 | 0.27 | 0.27 | |
| D2% (Gy) | 42.5 ± 0.3 | 42.6 ± 1.1 | 40.9 ± 0.3 | 0.12 | 1.08 | 1.08 | |
| Dmax (Gy) | 43.6 ± 0.3 | 43.9 ± 1.6 | 42.7 ± 0.6 | 0.43 | <0.01 | <0.01 | |
| Dmean (Gy) | 40.3 ± 0.3 | 40.3 ± 0.6 | 39.7 ± 0.2 | 0.54 | <0.01 | <0.01 | |
| Heart | Dmean (Gy) | 1.0 ± 0.4 | 2.1 ± 1.1 | 1.6 ± 0.2 | <0.01 | <0.01 | 0.47 |
| Dmax (Gy) | 20.3 ± 15.6 | 37.1 ± 7.9 | 16.6 ± 5.0 | <0.01 | 0.74 | <0.01 | |
| V35.0 Gy (%) | 0.2 ± 0.5 | 4.3 ± 9.2 | 0.0 ± 0.0 | 0.03 | 0.07 | <0.01 | |
| Ascending aorta | Dmean (Gy) | 0.5 ± 0.1 | 0.6 ± 0.1 | 2.1 ± 1.1 | 0.32 | <0.01 | <0.01 |
| Dmax (Gy) | 1.0 ± 0.2 | 1.1 ± 0.2 | 7.4 ± 4.3 | 0.05 | <0.01 | <0.01 | |
| Descending aorta | Dmean (Gy) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.9 ± 0.2 | 0.26 | <0.01 | <0.01 |
| Dmax (Gy) | 0.5 ± 0.2 | 0.6 ± 0.1 | 1.8 ± 0.5 | 0.36 | <0.01 | <0.01 | |
| Circumflex artery | Dmean (Gy) | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.3 ± 0.3 | 0.08 | <0.01 | <0.01 |
| Dmax (Gy) | 0.8 ± 0.2 | 0.9 ± 0.3 | 1.7 ± 0.4 | 0.14 | <0.01 | <0.01 | |
| Main coronary artery | Dmean (Gy) | 0.5 ± 0.1 | 0.7 ± 0.2 | 1.2 ± 0.2 | 0.08 | <0.01 | <0.01 |
| Dmax (Gy) | 0.7 ± 0.2 | 0.9 ± 0.2 | 1.5 ± 0.3 | 0.06 | <0.01 | <0.01 | |
| Right coronary artery | Dmean (Gy) | 0.6 ± 0.2 | 0.7 ± 0.2 | 2.3 ± 0.6 | 0.1 | <0.01 | <0.01 |
| Dmax (Gy) | 0.8 ± 0.2 | 1.0 ± 0.3 | 4.8 ± 2.5 | 0.12 | <0.01 | <0.01 | |
| LADA | Dmean (Gy) | 4.3 ± 3.7 | 13.3 ± 8.8 | 3.7 ± 1.3 | 0.04 | 0.23 | <0.01 |
| Dmax (Gy) | 15.4 ± 15.7 | 32.0 ± 13.5 | 9.9 ± 5.7 | <0.01 | 0.91 | <0.01 | |
| V26.5 Gy (%) | 4.9 ± 9.5 | 27.3 ± 24.4 | 0.0 ± 0.0 | 0.02 | 0.08 | <0.01 | |
| V35.3 Gy (%) | 3.7 ± 7.9 | 22.2 ± 20.9 | 0.0 ± 0.0 | <0.01 | 0.17 | <0.01 | |
| Pulmonary artery | Dmean (Gy) | 0.8 ± 0.1 | 0.9 ± 0.3 | 1.6 ± 0.4 | 0.34 | <0.01 | <0.01 |
| Dmax (Gy) | 2.4 ± 0.5 | 5.3 ± 6.0 | 4.4 ± 2.1 | <0.01 | <0.01 | <0.01 | |
| Left atrium | Dmean (Gy) | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.01 | 0.27 | <0.01 | <0.01 |
| Dmax (Gy) | 1.1 ± 0.3 | 1.6 ± 1.1 | 2.3 ± 0.7 | 0.38 | <0.01 | <0.01 | |
| Right atrium | Dmean (Gy) | 0.3 ± 0.1 | 0.4 ± 0.1 | 1.3 ± 0.3 | 0.11 | <0.01 | <0.01 |
| Dmax (Gy) | 0.7 ± 0.2 | 1.0 ± 0.4 | 5.6 ± 2.2 | 0.02 | <0.01 | <0.01 | |
| Pericardium | Dmean (Gy) | 1.5 ± 0.9 | 3.6 ± 2.0 | 2.2 ± 0.3 | <0.01 | 0.03 | 0.2 |
| Dmax (Gy) | 20.3 ± 15.6 | 36.9 ± 8.3 | 16.5 ± 4.9 | 0.01 | 0.85 | <0.01 | |
| Superior vena cava | Dmean (Gy) | 0.4 ± 0.1 | 0.4 ± 0.1 | 2.5 ± 1.7 | 0.73 | <0.01 | <0.01 |
| Dmax (Gy) | 0.5 ± 0.1 | 0.6 ± 0.2 | 5.0 ± 2.8 | 0.65 | <0.01 | <0.01 | |
| Left ventricle | Dmean (Gy) | 1.1 ± 0.4 | 2.8 ± 1.5 | 1.6 ± 0.2 | <0.01 | <0.01 | 0.21 |
| Dmax (Gy) | 15.8 ± 15.4 | 35.5 ± 10.2 | 10.8 ± 5.0 | <0.01 | 0.74 | <0.01 | |
| V4.4 Gy (%) | 1.1 ± 1.6 | 7.6 ± 5.2 | 0.7 ± 0.6 | <0.01 | 0.42 | <0.01 | |
| V20.3 Gy (%) | 0.3 ± 0.5 | 3.8 ± 3.3 | 0.0 ± 0.0 | <0.01 | 0.08 | <0.01 | |
| Right ventricle | Dmean (Gy) | 1.0 ± 0.6 | 3.3 ± 2.5 | 2.1 ± 0.4 | <0.01 | <0.01 | 0.53 |
| Dmax (Gy) | 12.0 ± 15.3 | 34.7 ± 11.1 | 13.6 ± 4.5 | <0.01 | 0.09 | <0.01 | |
| Right breast | Dmean (Gy) | 0.3 ± 0.1 | 0.3 ± 0.1 | 3.6 ± 0.5 | 0.62 | <0.01 | <0.01 |
| Left lung | Dmean (Gy) | 4.8 ± 0.7 | 5.1 ± 0.9 | 3.2 ± 0.6 | 0.31 | <0.01 | <0.01 |
| V15.0 Gy (%) | 9.9 ± 1.9 | 10.8 ± 2.6 | 1.4 ± 0.8 | 0.44 | <0.01 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eber, J.; Schmitt, M.; Dehaynin, N.; Le Fèvre, C.; Antoni, D.; Noël, G. Evaluation of Cardiac Substructures Exposure of DIBH-3DCRT, FB-HT, and FB-3DCRT in Hypofractionated Radiotherapy for Left-Sided Breast Cancer after Breast-Conserving Surgery: An In Silico Planning Study. Cancers 2023, 15, 3406. https://doi.org/10.3390/cancers15133406
Eber J, Schmitt M, Dehaynin N, Le Fèvre C, Antoni D, Noël G. Evaluation of Cardiac Substructures Exposure of DIBH-3DCRT, FB-HT, and FB-3DCRT in Hypofractionated Radiotherapy for Left-Sided Breast Cancer after Breast-Conserving Surgery: An In Silico Planning Study. Cancers. 2023; 15(13):3406. https://doi.org/10.3390/cancers15133406
Chicago/Turabian StyleEber, Jordan, Martin Schmitt, Nicolas Dehaynin, Clara Le Fèvre, Delphine Antoni, and Georges Noël. 2023. "Evaluation of Cardiac Substructures Exposure of DIBH-3DCRT, FB-HT, and FB-3DCRT in Hypofractionated Radiotherapy for Left-Sided Breast Cancer after Breast-Conserving Surgery: An In Silico Planning Study" Cancers 15, no. 13: 3406. https://doi.org/10.3390/cancers15133406
APA StyleEber, J., Schmitt, M., Dehaynin, N., Le Fèvre, C., Antoni, D., & Noël, G. (2023). Evaluation of Cardiac Substructures Exposure of DIBH-3DCRT, FB-HT, and FB-3DCRT in Hypofractionated Radiotherapy for Left-Sided Breast Cancer after Breast-Conserving Surgery: An In Silico Planning Study. Cancers, 15(13), 3406. https://doi.org/10.3390/cancers15133406







