Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
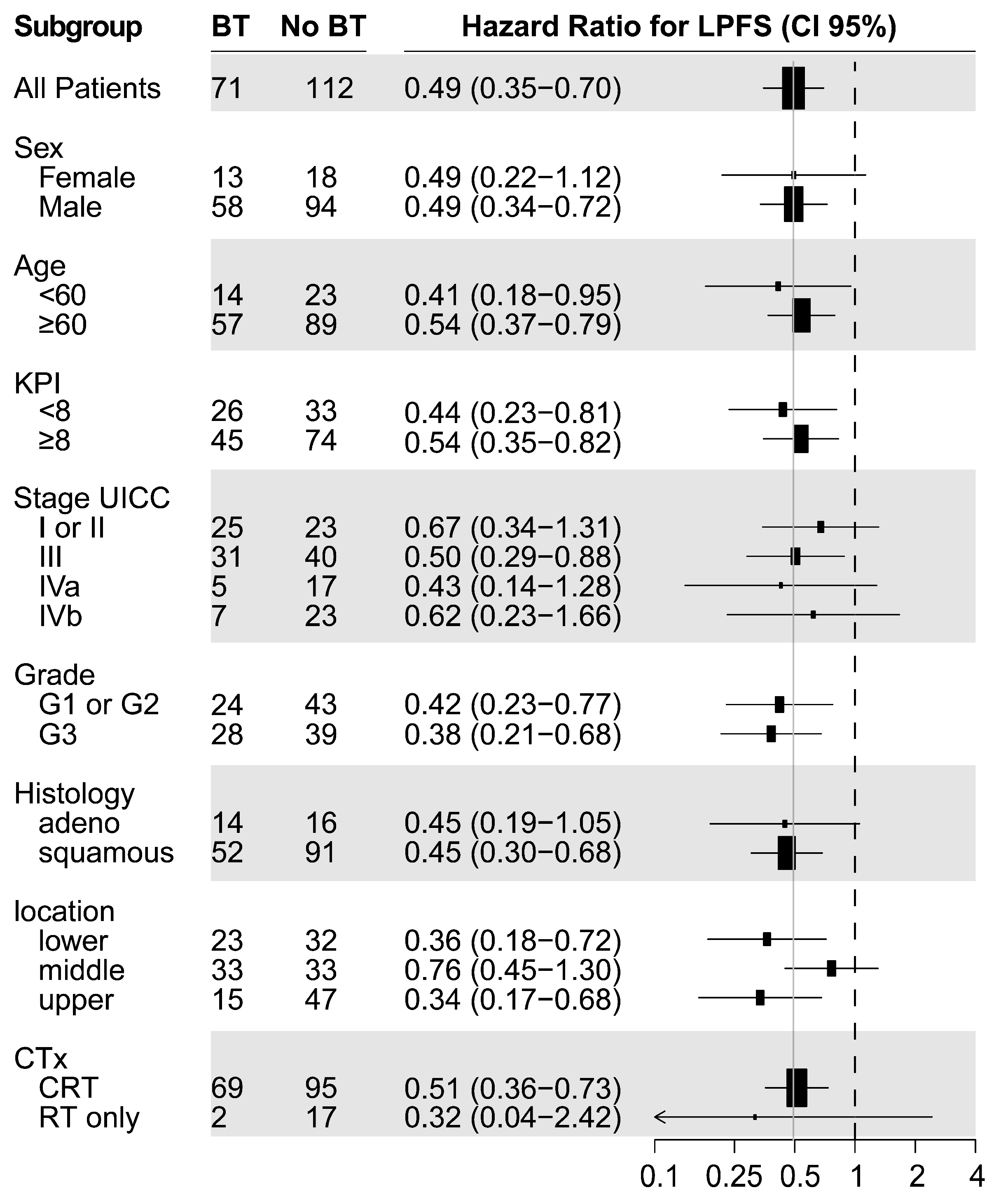
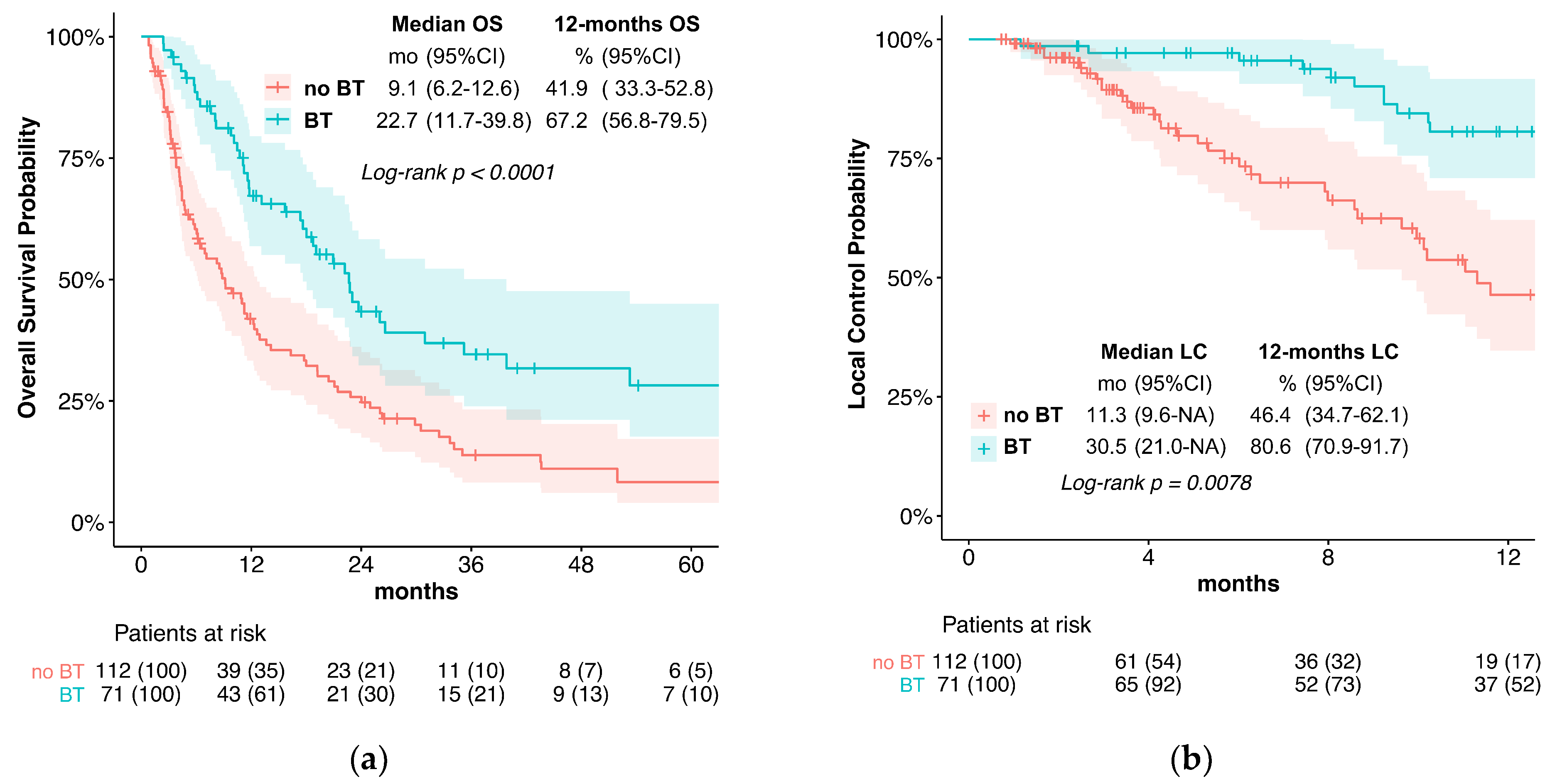
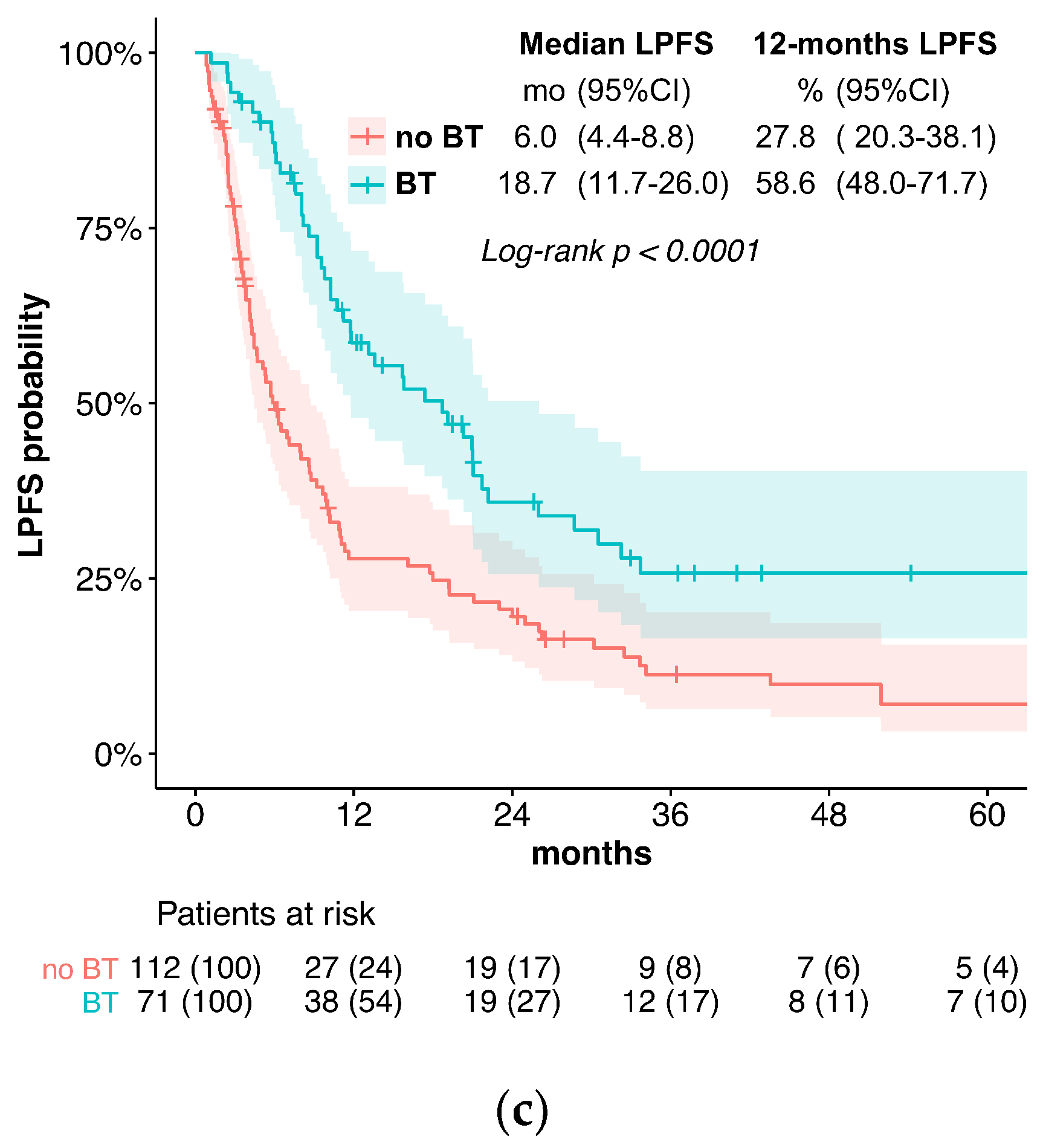
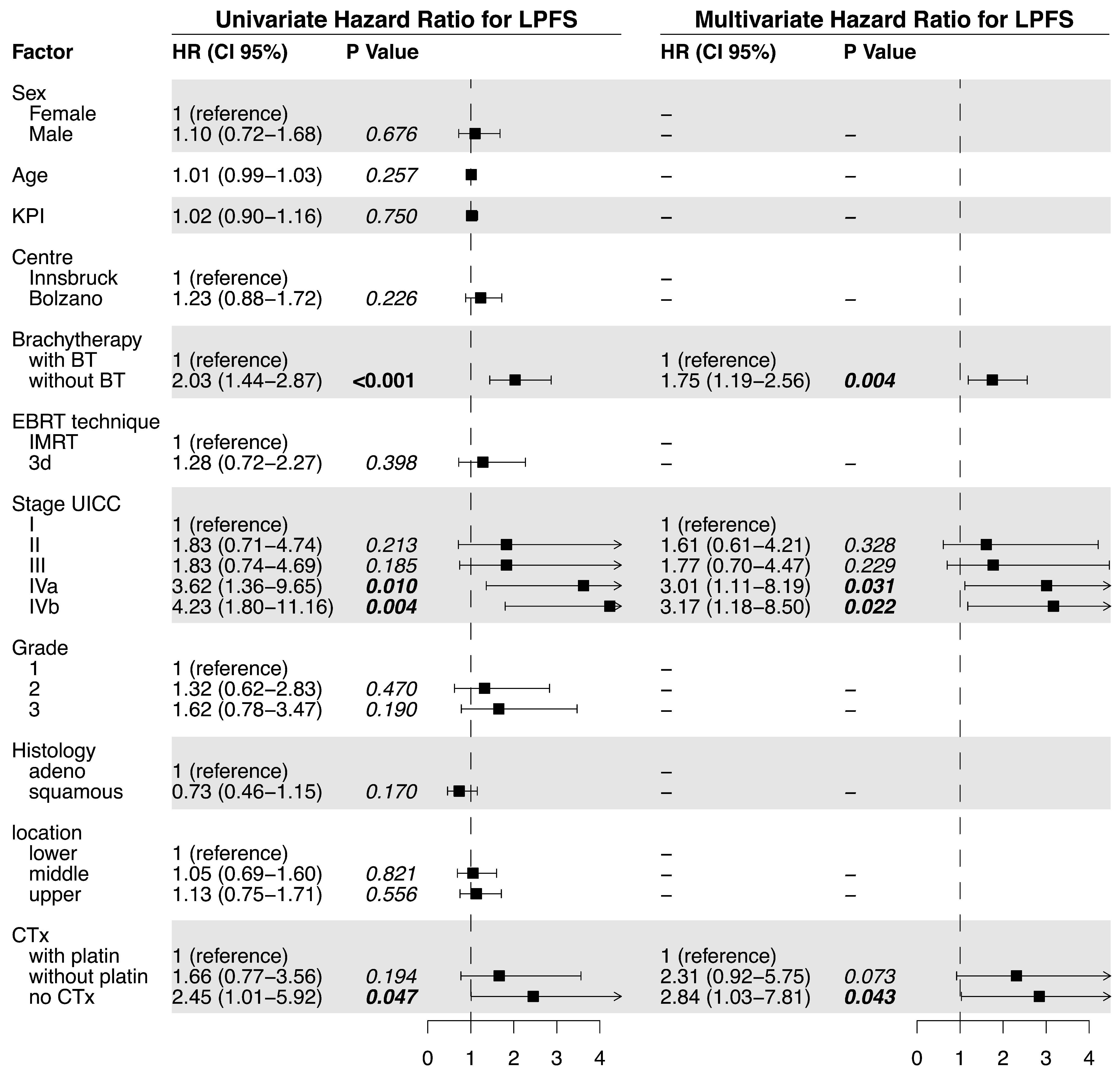
3. Results
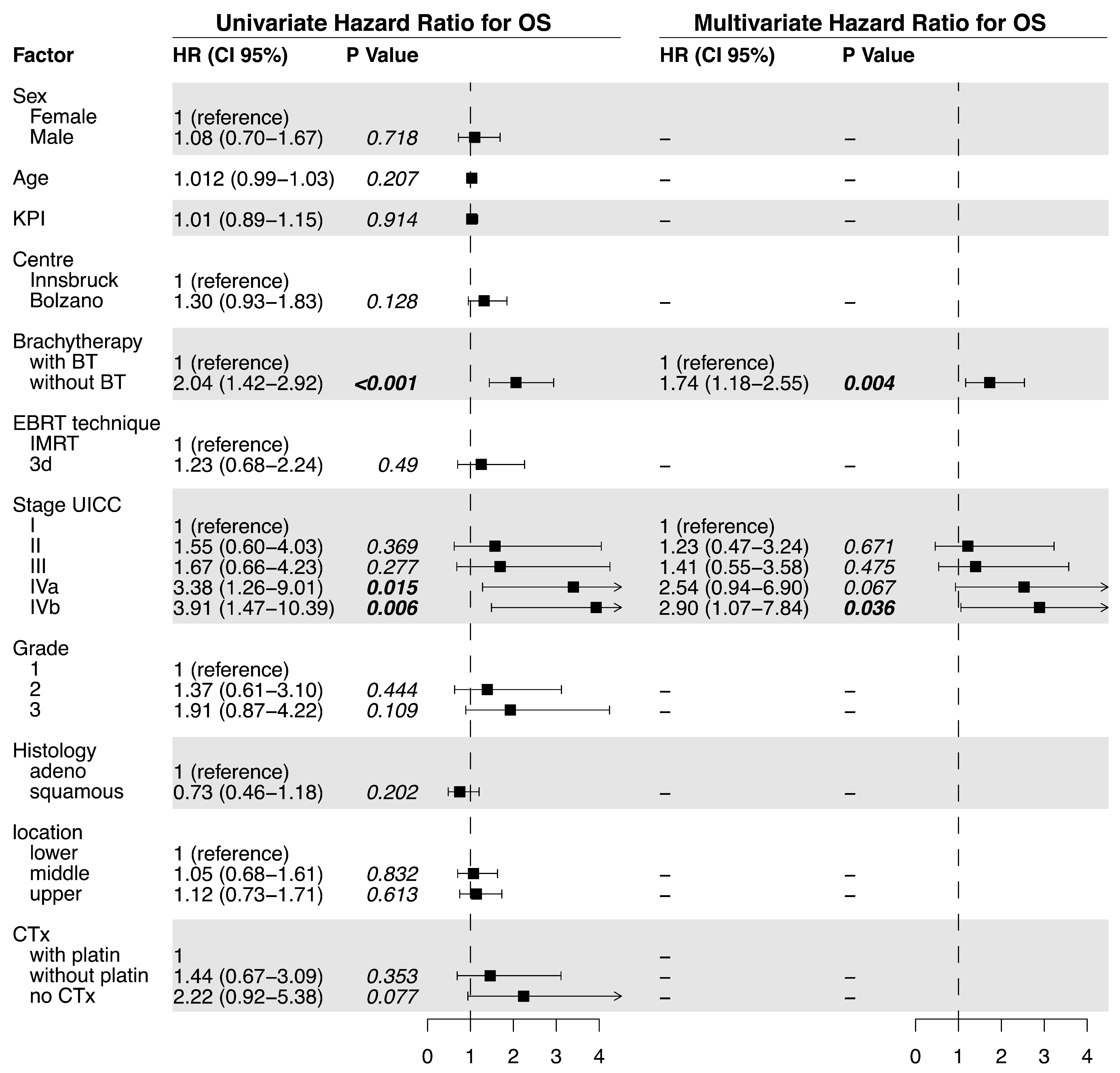
3.1. Outcome
3.2. Treatment-Related Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



| Acute Toxicities Grade | Without BT | With BT |
|---|---|---|
| esophagitis * | ||
| none | 21 (18.8%) | 4 (5.6%) |
| 1 | 40 (35.7%) | 37 (52.1%) |
| 2 | 42 (37.5%) | 23 (32.4%) |
| 3 | 9 (8.0%) | 7 (9.9%) |
| 4–5 | 0 (0%) | 0 (0%) |
| esophageal perforation | ||
| none | 110 (97.2%) | 71 (100.0%) |
| 1–4 | 0 (0%) | 0 (0%) |
| 5 | 2 (1.8%) | 0 (0%) |
| dermatitis | ||
| none | 47 (42.0%) | 20 (28.2%) |
| 1 | 47 (42.0%) | 41 (57.7%) |
| 2 | 18 (16.1%) | 10 (14.1%) |
| ≥3 | 0 (0%) | 0 (0%) |
| hematologic toxicity | ||
| none | 89 (79.5%) | 60 (84.5%) |
| 1 | 15 (13.4%) | 7 (9.9%) |
| 2 | 5 (4.5%) | 2 (2.8%) |
| 3 | 2 (1.8%) | 2 (2.8%) |
| 4 | 1 (0.9%) | 0 (0%) |
| 5 | 0 (0%) | 0 (0%) |
| pneumonitis (≤90 days) | ||
| none | 106 (94.6%) | 64 (90.1%) |
| 1 | 2 (1.8%) | 0 (0%) |
| 2 | 3 (2.7%) | 6 (8.5%) |
| 3 | 1 (0.9%) | 0 (0%) |
| 4 | 0 (0%) | 0 (0%) |
| 5 | 0 (0%) | 1 (1.4%) |
| Late Toxicities Grade | Without BT | With BT |
|---|---|---|
| pneumonitis (>90 days) | ||
| none | 109 (97.3%) | 65 (91.1%) |
| 1 | 2 (1.8%) | 5 (7.0%) |
| 2 | 1 (0.9%) | 1 (1.4%) |
| ≥3 | 0 (0%) | 0 (0%) |
| esophageal fistula | ||
| none | 106 (94.6%) | 66 (93.0%) |
| 1 | 2 (1.8%) | 0 (0%) |
| 2 | 2 (1.8%) | 0 (0%) |
| 3 | 1 (0.9%) | 5 (7.0%) |
| 4 | 0 (0%) | 0 (0%) |
| 5 | 1 (0.9%) | 0 (0%) |
| esophageal perforation | ||
| none | 110 (98.2%) | 70 (98.6%) |
| 2 | 0 (0%) | 0 (0%) |
| 3 | 2 (1.8%) | 0 (0%) |
| 4 | 0 (0%) | 1 (1.4%) |
| 5 | 0 (0%) | 0 (0%) |
| esophageal bleeding | ||
| none | 107 (95.5%) | 59 (83.1%) |
| 1 | 0 (0%) | 2 (2.8%) |
| 2 | 2 (1.8%) | 3 (4.2%) |
| 3 | 1 (0.9%) | 3 (4.2%) |
| 4 | 1 (0.9%) | 1 (1.4%) |
| 5 | 1 (0.9%) | 3 (4.2%) |
| esophageal stenosis * | ||
| none | 101 (90.2%) | 55 (77.5%) |
| 1 | 2 (1.8%) | 7 (9.9%) |
| 2 | 3 (2.7%) | 2 (2.8%) |
| 3 | 6 (5.4%) | 7 (9.9%) |
| ≥4 | 0 (0%) | 0 (0%) |
References
- Cooper, J.S.; Guo, M.D.; Herskovic, A.; Macdonald, J.S.; Martenson, J.J.A., Jr.; Al-Sarraf, M.; Byhardt, R.; Russell, A.H.; Beitler, J.J.; Spencer, S.; et al. Chemoradiotherapy of Locally Advanced Esophageal Cancer: Long-term follow-up of a prospective randomized trial (Rtog 85-01). JAMA 1999, 281, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A.; Kelsen, D.P. Int 0123 (Radiation Therapy Oncology Group 94-05) Phase Iii Trial of Combined-Modality Therapy for Esophageal Cancer: High-Dose Versus Standard-Dose Radiation Therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Laarhoven, H.W.M.; van Hooft, J.E.; van Os, R.M.; Geijsen, E.D.; Henegouwen, M.I.v.B.; Hulshof, M.C.C.M. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: Locoregional recurrence pattern. Dis. Esophagus 2015, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Teoh, A.Y.B.; Chiu, P.W.Y.; Yeung, W.K.; Liu, S.Y.W.; Wong, S.K.H.; Ng, E.K.W. Long-term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: Results from a randomized controlled trial. Ann. Oncol. 2013, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.; Settle, S.H.; Amini, A.; Xiao, L.; Suzuki, A.; Hayashi, Y.; Hofstetter, W.; Komaki, R.; Liao, Z.; Ajani, J.A. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer 2012, 118, 2632–2640. [Google Scholar] [CrossRef]
- Hulshof, M.C.C.M.; Geijsen, E.D.; Rozema, T.; Oppedijk, V.; Buijsen, J.; Neelis, K.J.; Nuyttens, J.J.M.E.; van der Sangen, M.J.C.; Jeene, P.M.; Reinders, J.G.; et al. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (Artdeco Study). J. Clin. Oncol. 2021, 39, 2816–2824. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Winter, K.; I Kocha, W.; Coia, L.R.; Herskovic, A.; Graham, M. A phase I/II study of external beam radiation, brachytherapy, and concurrent chemotherapy for patients with localized carcinoma of the esophagus (Radiation Therapy Oncology Group Study 9207): Final report. Cancer 2000, 88, 988–995. [Google Scholar] [CrossRef]
- Song, T.; Liang, X.; Fang, M.; Wu, S. High-dose versus conventional-dose irradiation in cisplatin-based definitive concurrent chemoradiotherapy for esophageal cancer: A systematic review and pooled analysis. Expert Rev. Anticancer Ther. 2015, 15, 1157–1169. [Google Scholar] [CrossRef]
- Gaspar, L.E.; Nag, S.; Herskovic, A.; Mantravadi, R.; Speiser, B.; The Clinical Research Committee, American Brachytherapy Society. American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 127–132. [Google Scholar] [CrossRef]
- Galalae, R.; Pötter, R.; Van Limbergen, E. Oesophageal Cancer Brachytherapy. In The Gec Estro Handbook of Brachytherapy —Part II: Clinical Practice, 2nd ed.; Van Limbergen, R.P.E., Hoskin, P., Baltas, D., Eds.; The European SocieTy for Radiotherapy and Oncology (ESTRO): Brussels, Belgium, 2019; pp. 3–23. [Google Scholar]
- Rovirosa, Á.; Tagliaferri, L.; Chicheł, A.; Lancellotta, V.; Zhang, Y.; Antelo, G.; Hoskin, P.; van der Steen-Banasik, E.; Biete, A.; Kovács, G. Why is a very easy, useful, old technique underused? An overview of esophageal brachytherapy—Interventional radiotherapy. J. Contemp. Brachytherapy 2022, 14, 299–309. [Google Scholar] [CrossRef]
- Lancellotta, V.; Cellini, F.; Fionda, B.; De Sanctis, V.; Vidali, C.; Fusco, V.; Frassine, F.; Tomasini, D.; Vavassori, A.; Gambacorta, M.A.; et al. The role of interventional radiotherapy (brachytherapy) in stage I esophageal cancer: An AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7589–7597. [Google Scholar] [CrossRef]
- Yorozu, A.; Dokiya, T.; Oki, Y.; Suzuki, T. Curative radiotherapy with high-dose-rate brachytherapy boost for localized esophageal carcinoma: Dose-effect relationship of brachytherapy with the balloon type applicator system. Radiother. Oncol. 1999, 51, 133–139. [Google Scholar] [CrossRef]
- Murakami, M.; Kuroda, Y.; Okamoto, Y.; Kono, K.; Yoden, E.; Kusumi, F.; Hajiro, K.; Matsusue, S.; Takeda, H. Neoadjuvant concurrent chemoradiotherapy followed by definitive high-dose radiotherapy or surgery for operable thoracic esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1049–1059. [Google Scholar] [CrossRef]
- Okawa, T.; Dokiya, T.; Nishio, M.; Hishikawa, Y.; Morita, K.; Japanese Society of Therapeutic Radiology and Oncology (JASTRO) Study Group. Multi-institutional randomized trial of external radiotherapy with and without intraluminal brachytherapy for esophageal cancer in Japan. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 623–628. [Google Scholar] [CrossRef]
- Pasquier, D.; Mirabel, X.; Adenis, A.; Rezvoy, N.; Hecquet, G.; Fournier, C.; Coche-Dequeant, B.; Prevost, B.; Castelain, B.; Lartigau, E. External beam radiation therapy followed by high-dose-rate brachytherapy for inoperable superficial esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1456–1461. [Google Scholar] [CrossRef]
- Yamada, K.; Murakami, M.; Okamoto, Y.; Okuno, Y.; Nakajima, T.; Kusumi, F.; Takakuwa, H.; Matsusue, S. Treatment results of chemoradiotherapy for clinical stage I (T1N0M0) esophageal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1106–1111. [Google Scholar] [CrossRef]
- Tamaki, T.; Ishikawa, H.; Takahashi, T.; Tamaki, Y.; Kitamoto, Y.; Okamoto, M.; Noda, S.-E.; Katoh, H.; Shirai, K.; Sakurai, H.; et al. Comparison of efficacy and safety of low-dose-rate vs. high-dose-rate intraluminal brachytherapy boost in patients with superficial esophageal cancer. Brachytherapy 2012, 11, 130–136. [Google Scholar] [CrossRef]
- Hishikawa, Y.; Kamikonya, N.; Tanaka, S.; Miura, T. Radiotherapy of esophageal carcinoma: Role of high-dose-rate intracavitary irradiation. Radiother. Oncol. 1987, 9, 13–20. [Google Scholar] [CrossRef]
- Murakami, Y.; Nagata, Y.; Nishibuchi, I.; Kimura, T.; Kenjo, M.; Kaneyasu, Y.; Okabe, T.; Hashimoto, Y.; Akagi, Y. Long-term outcomes of intraluminal brachytherapy in combination with external beam radiotherapy for superficial esophageal cancer. Int. J. Clin. Oncol. 2012, 17, 263–271. [Google Scholar] [CrossRef]
- Ishikawa, H.; Sakurai, H.; Yamakawa, M.; Saito, Y.; Nakayama, Y.; Kitamoto, Y.; Okamoto, M.; Harada, K.; Hasegawa, M.; Nakano, T. Clinical Outcomes and Prognostic Factors for Patients With Early Esophageal Squamous Cell Carcinoma Treated With Definitive Radiation Therapy Alone. J. Clin. Gastroenterol. 2005, 39, 495–500. [Google Scholar] [CrossRef]
- Vuong, T.; Szego, P.; David, M.; Evans, M.; Parent, J.; Mayrand, S.; Corns, R.; Burtin, P.; Faria, S.; Devic, S. The safety and usefulness of high-dose-rate endoluminal brachytherapy as a boost in the treatment of patients with esophageal cancer with external beam radiation with or without chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 758–764. [Google Scholar] [CrossRef]
- Calais, G.; Dorval, E.; Louisot, P.; Bourlier, P.; Klein, V.; Chapet, S.; Reynaud-Bougnoux, A.; Huten, N.; de Calan, L.; Aget, H.; et al. Radiotherapy with high dose rate brachytherapy boost and concomitant chemotherapy for stages IIB and III esophageal carcinoma: Results of a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Tessa, M.; Rotta, P.; Ragona, R.; Sola, B.; Grassini, M.; Nassisi, D.; Sciacero, P.; Airoldi, M.; Filippi, A.R.; Gianello, L.; et al. Concomitant Chemotherapy and External Radiotherapy plus Brachytherapy for Locally Advanced Esophageal Cancer Results of a Retrospective Multicenter Study. Tumori J. 2005, 91, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kissel, M.; Chirat, E.; Annede, P.; Burtin, P.; Fumagalli, I.; Bronsart, E.; Mignot, F.; Schernberg, A.; Dumas, I.; Haie-Meder, C.; et al. Esophageal brachytherapy: Institut Gustave Roussy’s experience. Brachytherapy 2020, 19, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Safaei, A.M.; Ghalehtaki, R.; Khanjani, N.; Farazmand, B.; Babaei, M.; Esmati, E. High-dose-rate intraluminal brachytherapy prior to external radiochemotherapy in locally advanced esophageal cancer: Preliminary results. J. Contemp. Brachytherapy 2017, 9, 30–35. [Google Scholar] [CrossRef]
- Patonay, P.; Naszály, A.; Mayer, A. Simultaneous Radiochemotherapy and Endoluminal Hdr Brachytherapy in Esophageal Cancer. Strahlenther. Onkol. 2007, 183, 94–98. [Google Scholar] [CrossRef]
- Hujala, K.; Sipilä, J.; Minn, H.; Ruotsalainen, P.; Grenman, R. Combined external and intraluminal radiotherapy in the treatment of advanced oesophageal cancer. Radiother. Oncol. 2002, 64, 41–45. [Google Scholar] [CrossRef]
- Muijs, C.T.; Beukema, J.C.; Mul, V.E.; Plukker, J.T.; Sijtsema, N.M.; Langendijk, J.A. External beam radiotherapy combined with intraluminal brachytherapy in esophageal carcinoma. Radiother. Oncol. 2012, 102, 303–308. [Google Scholar] [CrossRef]
- Taal, B.; Aleman, B.; Koning, C.; Boot, H. High dose rate brachytherapy before external beam irradiation in inoperable oesophageal cancer. Br. J. Cancer 1996, 74, 1452–1457. [Google Scholar] [CrossRef]
- Someya, M.; Sakata, K.-I.; Saito, A.; Nagakura, H.; Oouchi, A.; Hareyama, M. Results of external irradiation and low-dose-rate intraluminal brachytherapy for esophageal cancer. Acta Oncol. 2002, 41, 63–68. [Google Scholar] [CrossRef]
- Laskar, S.G.; Lewis, S.; Agarwal, J.P.; Mishra, S.; Mehta, S.; Patil, P. Combined brachytherapy and external beam radiation: An effective approach for palliation in esophageal cancer. J. Contemp. Brachytherapy 2015, 7, 453–461. [Google Scholar] [CrossRef]
- Aggarwal, A.; Harrison, M.; Glynne-Jones, R.; Sinha-Ray, R.; Cooper, D.; Hoskin, P. Combination External Beam Radiotherapy and Intraluminal Brachytherapy for Non-radical Treatment of Oesophageal Carcinoma in Patients not Suitable for Surgery or Chemoradiation. Clin. Oncol. 2015, 27, 56–64. [Google Scholar] [CrossRef]
- Carrizosa, M.C.L.; Ots, P.M.S.; Pérez, A.R.; Sotoca, A.; Garrido, J.S.; de Miguel, M.M. High dose rate brachytherapy (HDR-BT) in locally advanced oesophageal cancer. Clinic response and survival related to biological equivalent dose (BED). Clin. Transl. Oncol. 2007, 9, 385–391. [Google Scholar] [CrossRef]
- Ye, M.; Han, D.; Mao, Z.; Cheng, G. A prospective study of radical external beam radiotherapy versus external beam radiotherapy combined with intraluminal brachytherapy for primary esophageal cancer. Brachytherapy 2022, 21, 703–711. [Google Scholar] [CrossRef]
- Nishimura, Y.; Okuno, Y.; Ono, K.; Mitsumori, M.; Nagata, Y.; Hiraoka, M. External beam radiation therapy with or without high-dose-rate intraluminal brachytherapy for patients with superficial esophageal carcinoma. Cancer 1999, 86, 220–228. [Google Scholar] [CrossRef]
- Sur, R.K.; Singh, D.P.; Sharma, S.C.; Singh, M.T.; Kochhar, R.; Negi, P.S.; Sethi, T.; Patel, F.; Ayyagari, S.; Bhatia, S.; et al. Radiation therapy of esophageal cancer: Role of high dose rate brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 1992, 22, 1043–1046. [Google Scholar] [CrossRef]
- Banerjee, S.; Sharan, K.; Fernandes, D.J.; Saxena, P.P.; Sathian, B. Treatment outcomes after intraluminal brachytherapy following definitive chemoradiotherapy in patients with esophageal cancer. J. Cancer Res. Ther. 2014, 10, 337–341. [Google Scholar] [CrossRef]
- Folkert, M.R.; Cohen, G.N.; Wu, A.J.; Gerdes, H.; Schattner, M.A.; Markowitz, A.J.; Ludwig, E.; Ilson, D.H.; Bains, M.S.; Zelefsky, M.J.; et al. Endoluminal high-dose-rate brachytherapy for early stage and recurrent esophageal cancer in medically inoperable patients. Brachytherapy 2013, 12, 463–470. [Google Scholar] [CrossRef]
- Kim, H.J.; Suh, Y.-G.; Lee, Y.C.; Kil Lee, S.; Shin, S.K.; Cho, B.C.; Lee, C.G. Dose-Response Relationship between Radiation Dose and Loco-regional Control in Patients with Stage II-III Esophageal Cancer Treated with Definitive Chemoradiotherapy. Cancer Res. Treat. 2017, 49, 669–677. [Google Scholar] [CrossRef]
- Brower, J.V.; Chen, S.; Bassetti, M.F.; Yu, M.; Harari, P.M.; Ritter, M.A.; Baschnagel, A.M. Radiation Dose Escalation in Esophageal Cancer Revisited: A Contemporary Analysis of the National Cancer Data Base, 2004 to 2012. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 985–993. [Google Scholar] [CrossRef]
- Zhang, W.; Luo, Y.; Wang, X.; Han, G.; Wang, P.; Yuan, W.; Dai, S.-B. Dose-escalated radiotherapy improved survival for esophageal cancer patients with a clinical complete response after standard-dose radiotherapy with concurrent chemotherapy. Cancer Manag. Res. 2018, 10, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Matoska, T.; Banerjee, A.; Shreenivas, A.; Jurkowski, L.; Shukla, M.E.; Gore, E.M.; Linsky, P.; Gasparri, M.; George, B.; Johnstone, C.; et al. Definitive Chemoradiation Associated with Improved Survival Outcomes in Patients with Synchronous Oligometastatic Esophageal Cancer. Cancers 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall | Without Brachytherapy | With Brachytherapy |
|---|---|---|---|
| Patients | 183 | 112 | 71 |
| Centre | |||
| Innsbruck | 117 (63.9%) | 49 (43.8%) | 68 (95.8%) |
| Bolzano | 66 (36.1%) | 63 (56.3%) | 3 (4.2%) |
| Sex | |||
| female | 31 (16.9%) | 18 (16.1%) | 13 (18.3%) |
| male | 152 (83.1%) | 94 (83.9%) | 58 (81.7%) |
| age | |||
| median (IQR) | 69 (61–76) | 69.5 (61–78) | 68 (61–75) |
| KPS * | |||
| 10 | 21 (11.5%) | 10 (8.9%) | 11 (15.5%) |
| 9 | 53 (29%) | 27(24.1%) | 26 (36.6%) |
| 8 | 45 (24.6%) | 37 (33.0%) | 8 (11.3%) |
| 7 | 38 (20.8%) | 24 (21.4%) | 14 (19.7%) |
| 6 | 12 (6.6%) | 4 (3.6%) | 8 (11.3%) |
| 5 | 7 (3.8%) | 4 (3.6%) | 3 (4.2%) |
| 4 | 2 (1.1%) | 1 (0.9%) | 1 (1.4%) |
| not assessed | 5 (2.7%) | 5 (4.5%) | 0 (0%) |
| Histology | |||
| squamous | 143 (78.1%) | 91 (81.3%) | 52 (73.2%) |
| adeno | 30 (16.4%) | 16 (14.3%) | 14 (19.7%) |
| other | 10 (5.5%) | 5 (4.5%) | 5 (7.0%) |
| Localization | |||
| upper third | 62 (33.9%) | 47 (42.0%) | 15 (21.1%) |
| middle third | 66 (36.1%) | 33 (29.5%) | 33 (46.5%) |
| lower third | 55 (30.1%) | 32 (28.6%) | 23 (32.4%) |
| Stage (UICC) * | |||
| I | 15 (9.4%) | 6 (6.3%) | 9 (14.1%) |
| II | 28 (17.5%) | 11 (11.5%) | 17 (26.6%) |
| III | 87 (54.4%) | 56 (58.3%) | 31 (48.4%) |
| IV | 30 (18.8%) | 23 (24.0%) | 7 (10.9%) |
| EBRT technique | |||
| 3D | 162 (88.5%) | 101 (90.2%) | 61 (85.9%) |
| IMRT | 7 (3.8%) | 0 (0%) | 7 (9.9%) |
| VMAT | 14 (7.7%) | 11 (9.8%) | 3 (4.2%) |
| Combined chemotherapy * | |||
| with platinum | 13 (7.1%) | 11 (9.8%) | 2 (2.8%) |
| without platinum | 151 (82.5%) | 84 (75.0%) | 67 (94.4%) |
| no chemotherapy | 19 (10.4%) | 17 (15.2%) | 2 (2.8%) |
| HR (95% CI) | p | |
|---|---|---|
| Sex | ||
| Female | 1 | |
| Male | 2.48 (0.99–6.2) | 0.052 |
| Age (higher vs. lower) | 1.00 (0.97–1.03) | 0.997 |
| KPI (lower vs. higher) | 1.18 (0.96–1.46) | 0.109 |
| Centre | ||
| Innsbruck | 1 | |
| Bolzano | 0.94 (0.54–1.63) | 0.818 |
| Brachytherapy | ||
| with BT | 1 | |
| without BT * | 2.00 (1.19–3.36) | 0.009 |
| EBRT technique | ||
| IMRT/VMAT | 1 | |
| 3D | 1.51 (0.61–3.78) | 0.374 |
| Stage UICC | ||
| I | 1 | |
| II | 3.48 (0.45–26.66) | 0.230 |
| III | 3.54 (0.48–26.19) | 0.215 |
| IVa * | 9.28 (1.18–73.15) | 0.035 |
| IVb | 6.97 (0.89–54.78) | 0.065 |
| Grade | ||
| 1 | 1 | |
| 2 | 1.49 (0.5–4.25) | 0.487 |
| 3 | 1.07 (0.36–3.20) | 0.898 |
| Histology | ||
| adeno | 1 | |
| squamous | 0.69 (0.36–1.3) | 0.252 |
| location | ||
| lower | 1 | |
| middle | 1.14 (0.62–2.12) | 0.669 |
| upper | 1.13 (0.59–2.14) | 0.716 |
| Chemotherapy | ||
| with platin | 1 | |
| without platin | 2.55 (0.62–10.47) | 0.194 |
| no chemotherapy | 1.86 (0.34–10.19) | 0.472 |
| Acute Toxicities Grade | Without BT | With BT |
|---|---|---|
| ≥1 | 104 (92.9%) | 69 (97.2%) |
| ≥2 | 74 (66.1%) | 39 (54.9%) |
| ≥3 | 19 (17.0%) | 11 (15.5%) |
| ≥4 | 3 (2.7%) | 1 (1.4%) |
| 5 | 2 (1.8%) | 1 (1.4%) |
| Late Toxicities Grade | Without BT | With BT |
|---|---|---|
| ≥1 * | 21 (18.8%) | 31 (43.7%) |
| ≥2 ** | 16 (14.3%) | 21 (29.6%) |
| ≥3 ** | 10 (8.9%) | 16 (22.5%) |
| ≥4 | 3 (2.7%) | 4 (5.6%) |
| 5 | 2 (1.8%) | 3 (4.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangesius, J.; Hörmandinger, K.; Jäger, R.; Skvortsov, S.; Plankensteiner, M.; Maffei, M.; Seppi, T.; Dejaco, D.; Santer, M.; Sarcletti, M.; et al. Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma. Cancers 2023, 15, 3594. https://doi.org/10.3390/cancers15143594
Mangesius J, Hörmandinger K, Jäger R, Skvortsov S, Plankensteiner M, Maffei M, Seppi T, Dejaco D, Santer M, Sarcletti M, et al. Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma. Cancers. 2023; 15(14):3594. https://doi.org/10.3390/cancers15143594
Chicago/Turabian StyleMangesius, Julian, Katharina Hörmandinger, Robert Jäger, Sergej Skvortsov, Marlen Plankensteiner, Martin Maffei, Thomas Seppi, Daniel Dejaco, Matthias Santer, Manuel Sarcletti, and et al. 2023. "Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma" Cancers 15, no. 14: 3594. https://doi.org/10.3390/cancers15143594
APA StyleMangesius, J., Hörmandinger, K., Jäger, R., Skvortsov, S., Plankensteiner, M., Maffei, M., Seppi, T., Dejaco, D., Santer, M., Sarcletti, M., & Ganswindt, U. (2023). Chemoradiotherapy Combined with Brachytherapy for the Definitive Treatment of Esophageal Carcinoma. Cancers, 15(14), 3594. https://doi.org/10.3390/cancers15143594







