State of the Art of Pharmacological Activators of p53 in Ocular Malignancies
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Role of p53 in Tissue and Ocular Homeostasis
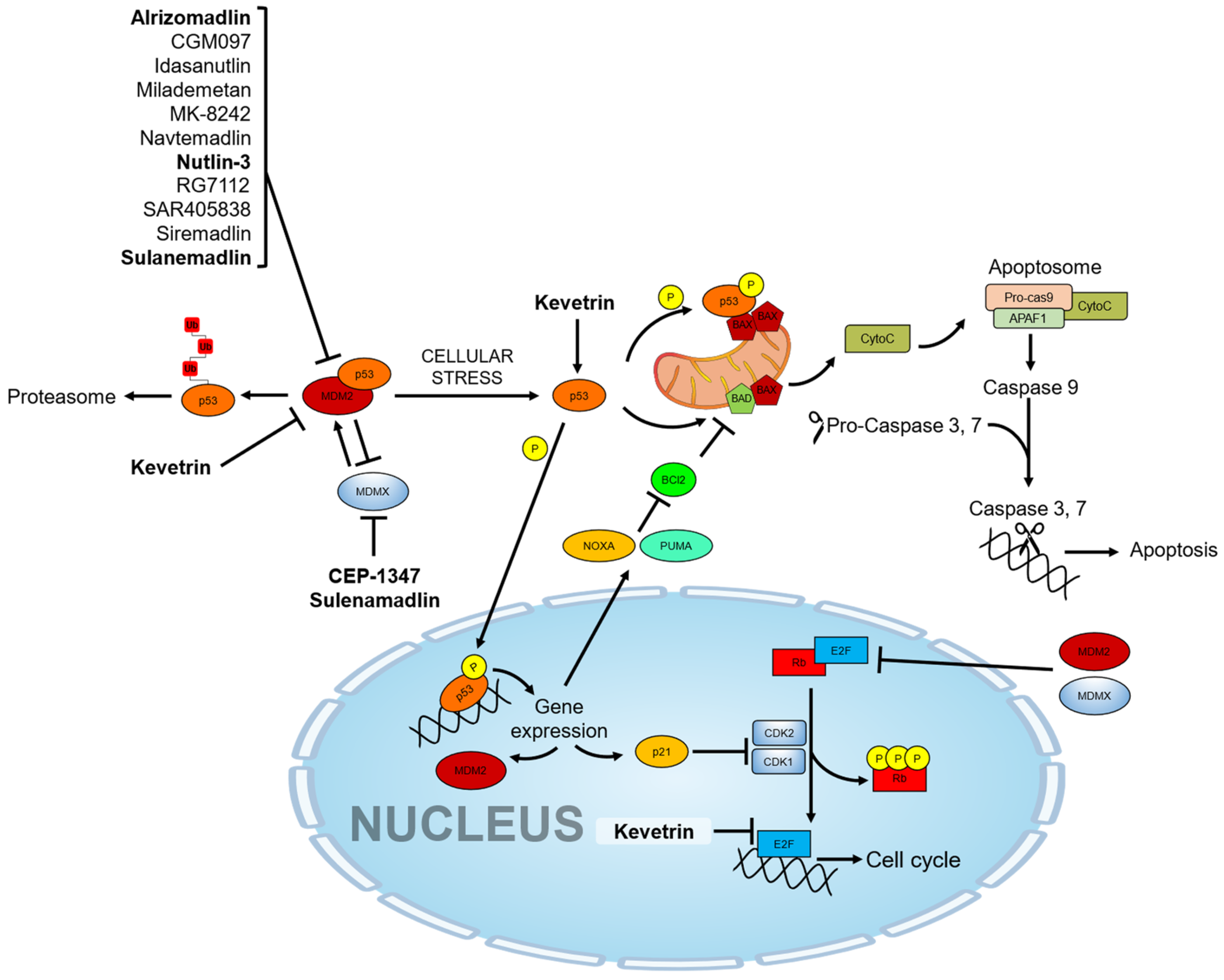
3. Pharmacological Activators of the p53 Pathway
4. Role of p53 Therapy in Pterygium
5. Role of p53 Therapy in Conjunctival Melanoma
6. Role of p53 Therapy for Retinoblastoma
7. Role of p53 in Uveal Melanoma
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neupane, R.; Gaudana, R.; Boddu, S.H.S. Imaging Techniques in the Diagnosis and Management of Ocular Tumors: Prospects and Challenges. AAPS J. 2018, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, E.; Topham, A.; Singh, A.D. Incidence of retinoblastoma in the USA: 1975-2004. Br. J. Ophthalmol. 2009, 93, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Seregard, S.; Lundell, G.; Svedberg, H.; Kivela, T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: Advantages of birth cohort analysis. Ophthalmology 2004, 111, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017, 31, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Roelofsen, C.D.M.; Wierenga, A.P.A.; van Duinen, S.; Verdijk, R.M.; Bleeker, J.; Marinkovic, M.; Luyten, G.P.M.; Jager, M.J. Five Decades of Enucleations for Uveal Melanoma in One Center: More Tumors with High Risk Factors, No Improvement in Survival over Time. Ocul. Oncol. Pathol. 2021, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; He, Q.; Lu, M. Global, regional, and national burden of blindness and vision loss due to common eye diseases along with its attributable risk factors from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Aging 2021, 13, 19614–19642. [Google Scholar] [CrossRef]
- Sarkar, P.; Mehtani, A.; Gandhi, H.C.; Bhalla, J.S.; Tapariya, S. Paraneoplastic ocular syndrome: A pandora’s box of underlying malignancies. Eye 2022, 36, 1355–1367. [Google Scholar] [CrossRef]
- Aboudehen, K.; Hilliard, S.; Saifudeen, Z.; El-Dahr, S.S. Mechanisms of p53 activation and physiological relevance in the developing kidney. Am. J. Physiol. Renal Physiol. 2012, 302, F928–F940. [Google Scholar] [CrossRef]
- Saifudeen, Z.; Dipp, S.; El-Dahr, S.S. A role for p53 in terminal epithelial cell differentiation. J. Clin. Investig. 2002, 109, 1021–1030. [Google Scholar] [CrossRef]
- Jacobs, W.B.; Kaplan, D.R.; Miller, F.D. The p53 family in nervous system development and disease. J. Neurochem. 2006, 97, 1571–1584. [Google Scholar] [CrossRef]
- Eischen, C.M. Genome Stability Requires p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026096. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, H.; Ji, Y.; Zhou, Q.; Du, Z.; Tian, L.; Jiang, Y.; Yao, K.; Zhou, Z. Targeting p53-MDM2 interaction by small-molecule inhibitors: Learning from MDM2 inhibitors in clinical trials. J. Hematol. Oncol. 2022, 15, 91. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, J.; Lei, H. The roles of mouse double minute 2 (MDM2) oncoprotein in ocular diseases: A review. Exp. Eye Res. 2022, 217, 108910. [Google Scholar] [CrossRef] [PubMed]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Melloni, E.; Marchesi, E.; Preti, L.; Casciano, F.; Rimondi, E.; Romani, A.; Secchiero, P.; Navacchia, M.L.; Perrone, D. Synthesis and Biological Investigation of Bile Acid-Paclitaxel Hybrids. Molecules 2022, 27, 471. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Casciano, F.; Stevanin, C.; Maietti, A.; Tedeschi, P.; Secchiero, P.; Marchetti, N.; Voltan, R. Anticancer Activity of Aqueous Extracts from Asparagus officinalis L. Byproduct on Breast Cancer Cells. Molecules 2021, 26, 6369. [Google Scholar] [CrossRef] [PubMed]
- Morshed, A.; Paul, S.; Hossain, A.; Basak, T.; Hossain, M.S.; Hasan, M.M.; Hasibuzzaman, M.A.; Rahaman, T.I.; Mia, M.A.R.; Shing, P.; et al. Baicalein as Promising Anticancer Agent: A Comprehensive Analysis on Molecular Mechanisms and Therapeutic Perspectives. Cancers 2023, 15, 2128. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Campbell, H.; Drummond, C.J.; Li, K.; Murray, K.; Slatter, T.; Bourdon, J.C.; Braithwaite, A.W. Adaptive homeostasis and the p53 isoform network. EMBO Rep. 2021, 22, e53085. [Google Scholar] [CrossRef]
- Blander, G.; Zalle, N.; Leal, J.F.; Bar-Or, R.L.; Yu, C.E.; Oren, M. The Werner syndrome protein contributes to induction of p53 by DNA damage. FASEB J. 2000, 14, 2138–2140. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Tong, J.; Jha, A.; Risnik, D.; Lizardo, D.; Lu, X.; Goel, A.; Opresko, P.L.; Yu, J.; Zhang, L. Synthetical lethality of Werner helicase and mismatch repair deficiency is mediated by p53 and PUMA in colon cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2211775119. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Maezawa, Y.; Nishijima, D.; Iwamoto, E.; Takeda, J.; Kanamori, T.; Yamaga, M.; Mishina, T.; Takeda, Y.; Izumi, S.; et al. A high prevalence of myeloid malignancies in progeria with Werner syndrome is associated with p53 insufficiency. Exp. Hematol. 2022, 109, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Oshima, J.; von Kobbe, C.; Cheng, W.H.; Leistritz, D.F.; Bohr, V.A. The clinical characteristics of Werner syndrome: Molecular and biochemical diagnosis. Hum. Genet. 2008, 124, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lee, L.; Hanson, N.B.; Lenaerts, C.; Hoehn, H.; Poot, M.; Rubin, C.D.; Chen, D.F.; Yang, C.C.; Juch, H.; et al. The spectrum of WRN mutations in Werner syndrome patients. Hum. Mutat. 2006, 27, 558–567. [Google Scholar] [CrossRef]
- Reichel, M.B.; Ali, R.R.; D’Esposito, F.; Clarke, A.R.; Luthert, P.J.; Bhattacharya, S.S.; Hunt, D.M. High frequency of persistent hyperplastic primary vitreous and cataracts in p53-deficient mice. Cell Death Differ. 1998, 5, 156–162. [Google Scholar] [CrossRef]
- Damato, B. Does ocular treatment of uveal melanoma influence survival? Br. J. Cancer 2010, 103, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Shih, C.Y.; Rosen, L.; Steiner, A.; Milman, T.; Udell, I.J. Recurrence of Ocular Surface Squamous Neoplasia Treated With Excisional Biopsy and Cryotherapy. Am. J. Ophthalmol. 2015, 160, 213–219.e1. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; He, W. Clinical features and factors affecting prognosis and partial deterioration of ocular papilloma: A retrospective study of 298 cases. Graefes Arch. Clin. Exp. Ophthalmol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Garcia Tirado, A.; Boto de Los Bueis, A.; Rivas Jara, L. Ocular surface changes in recurrent pterygium cases post-operatively treated with 5-fluorouracil subconjunctival injections. Eur. J. Ophthalmol. 2019, 29, 9–14. [Google Scholar] [CrossRef]
- Linzer, D.I.; Maltzman, W.; Levine, A.J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology 1979, 98, 308–318. [Google Scholar] [CrossRef]
- DeLeo, A.B.; Jay, G.; Appella, E.; Dubois, G.C.; Law, L.W.; Old, L.J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc. Natl. Acad. Sci. USA 1979, 76, 2420–2424. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.V.; Pim, D.C.; Bulbrook, R.D. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int. J. Cancer 1982, 30, 403–408. [Google Scholar] [CrossRef]
- Crawford, L. The 53,000-dalton cellular protein and its role in transformation. Int. Rev. Exp. Pathol. 1983, 25, 1–50. [Google Scholar] [PubMed]
- Wolf, D.; Rotter, V. Inactivation of p53 gene expression by an insertion of Moloney murine leukemia virus-like DNA sequences. Mol. Cell Biol. 1984, 4, 1402–1410. [Google Scholar]
- Wolf, D.; Rotter, V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc. Natl. Acad. Sci. USA 1985, 82, 790–794. [Google Scholar] [CrossRef]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.W.; Zaika, A.; Moll, U.M. Nuclear and cytoplasmic degradation of endogenous p53 and HDM2 occurs during down-regulation of the p53 response after multiple types of DNA damage. FASEB J. 2003, 17, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- do Patrocinio, A.B.; Rodrigues, V.; Guidi Magalhaes, L. P53: Stability from the Ubiquitin-Proteasome System and Specific 26S Proteasome Inhibitors. ACS Omega 2022, 7, 3836–3843. [Google Scholar] [CrossRef]
- Ringshausen, I.; O’Shea, C.C.; Finch, A.J.; Swigart, L.B.; Evan, G.I. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell 2006, 10, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yan, Z.; Liao, X.; Li, Y.; Yang, J.; Wang, Z.G.; Zuo, Y.; Kawai, H.; Shadfan, M.; Ganapathy, S.; et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 12001–12006. [Google Scholar] [CrossRef] [PubMed]
- de Rozieres, S.; Maya, R.; Oren, M.; Lozano, G. The loss of mdm2 induces p53-mediated apoptosis. Oncogene 2000, 19, 1691–1697. [Google Scholar] [CrossRef]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef]
- Lopez-Pajares, V.; Kim, M.M.; Yuan, Z.M. Phosphorylation of MDMX mediated by Akt leads to stabilization and induces 14-3-3 binding. J. Biol. Chem. 2008, 283, 13707–13713. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Qu, R.; Liu, D.; Xiong, X.; Liang, T.; Zhao, Y. The Cross Talk Between p53 and mTOR Pathways in Response to Physiological and Genotoxic Stresses. Front. Cell Dev Biol. 2021, 9, 775507. [Google Scholar] [CrossRef]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Casciano, F.; Zauli, E.; Rimondi, E.; Mura, M.; Previati, M.; Busin, M.; Zauli, G. The role of the mTOR pathway in diabetic retinopathy. Front. Med. 2022, 9, 973856. [Google Scholar] [CrossRef]
- Qin, X.; Zou, H. The role of lipopolysaccharides in diabetic retinopathy. BMC Ophthalmol. 2022, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Zauli, E.; Casciano, F.; Secchiero, P.; Zauli, G.; Fields, M.; Melloni, E. Palmitic Acid Induced a Long-Lasting Lipotoxic Insult in Human Retinal Pigment Epithelial Cells, which Is Partially Counteracted by TRAIL. Antioxidants 2022, 11, 2340. [Google Scholar] [CrossRef] [PubMed]
- Gurel, Z.; Zaro, B.W.; Pratt, M.R.; Sheibani, N. Identification of O-GlcNAc modification targets in mouse retinal pericytes: Implication of p53 in pathogenesis of diabetic retinopathy. PLoS ONE 2014, 9, e95561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhao, H.; Chen, B. DJ-1/PARK7 inhibits high glucose-induced oxidative stress to prevent retinal pericyte apoptosis via the PI3K/AKT/mTOR signaling pathway. Exp. Eye Res. 2019, 189, 107830. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, H.; Zhang, Q. Procyanidin protects human retinal pigment epithelial cells from high glucose by inhibiting autophagy. Environ. Toxicol. 2022, 37, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Gu, W. p53 post-translational modification: Deregulated in tumorigenesis. Trends Mol. Med. 2010, 16, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tavana, O.; Gu, W. p53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell. Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef]
- Holley, A.K.; St Clair, D.K. Watching the watcher: Regulation of p53 by mitochondria. Future Oncol. 2009, 5, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chaiswing, L.; Velez, J.M.; Batinic-Haberle, I.; Colburn, N.H.; Oberley, T.D.; St Clair, D.K. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res. 2005, 65, 3745–3750. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef]
- Secchiero, P.; Barbarotto, E.; Tiribelli, M.; Zerbinati, C.; di Iasio, M.G.; Gonelli, A.; Cavazzini, F.; Campioni, D.; Fanin, R.; Cuneo, A.; et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL). Blood 2006, 107, 4122–4129. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, K.S.; Yoo, Y.H.; Kwon, T.K. Nutlin-3, a small-molecule MDM2 inhibitor, sensitizes Caki cells to TRAIL-induced apoptosis through p53-mediated PUMA upregulation and ROS-mediated DR5 upregulation. Anticancer Drugs 2013, 24, 260–269. [Google Scholar] [CrossRef]
- Valente, L.J.; Aubrey, B.J.; Herold, M.J.; Kelly, G.L.; Happo, L.; Scott, C.L.; Newbold, A.; Johnstone, R.W.; Huang, D.C.; Vassilev, L.T.; et al. Therapeutic Response to Non-genotoxic Activation of p53 by Nutlin3a Is Driven by PUMA-Mediated Apoptosis in Lymphoma Cells. Cell Rep. 2016, 14, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, J.; Liao, J.; Huang, Y.; Gan, Y.; Larisch, S.; Zeng, S.X.; Lu, H.; Zhou, X. p53 induces ARTS to promote mitochondrial apoptosis. Cell Death Dis. 2021, 12, 204. [Google Scholar] [CrossRef]
- Li, L.; Song, M.; Zhou, J.; Sun, X.; Lei, Y. Ambient particulate matter exposure causes visual dysfunction and retinal neuronal degeneration. Ecotoxicol. Environ. Saf. 2022, 247, 114231. [Google Scholar] [CrossRef] [PubMed]
- Loughery, J.; Cox, M.; Smith, L.M.; Meek, D.W. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res. 2014, 42, 7666–7680. [Google Scholar] [CrossRef] [PubMed]
- Aubry, A.; Pearson, J.D.; Huang, K.; Livne-Bar, I.; Ahmad, M.; Jagadeesan, M.; Khetan, V.; Ketela, T.; Brown, K.R.; Yu, T.; et al. Functional genomics identifies new synergistic therapies for retinoblastoma. Oncogene 2020, 39, 5338–5357. [Google Scholar] [CrossRef]
- Pant, V.; Xiong, S.; Jackson, J.G.; Post, S.M.; Abbas, H.A.; Quintas-Cardama, A.; Hamir, A.N.; Lozano, G. The p53-Mdm2 feedback loop protects against DNA damage by inhibiting p53 activity but is dispensable for p53 stability, development, and longevity. Genes Dev. 2013, 27, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Hamard, P.J.; Tonnessen, C.; Manfredi, J.J. E2F7, a novel target, is up-regulated by p53 and mediates DNA damage-dependent transcriptional repression. Genes Dev. 2012, 26, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Mansilla, S.F.; de la Vega, M.B.; Calzetta, N.L.; Siri, S.O.; Gottifredi, V. CDK-Independent and PCNA-Dependent Functions of p21 in DNA Replication. Genes 2020, 11, 593. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Koivomagi, M.; Skotheim, J.M. Cyclin D-Cdk4,6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770.e4. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Linn, P.; Kohno, S.; Sheng, J.; Kulathunga, N.; Yu, H.; Zhang, Z.; Voon, D.; Watanabe, Y.; Takahashi, C. Targeting RB1 Loss in Cancers. Cancers 2021, 13, 3737. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.D.; Dyer, M.A. Genetic and Epigenetic Discoveries in Human Retinoblastoma. Crit. Rev. Oncog. 2015, 20, 217–225. [Google Scholar] [CrossRef]
- Romani, A.; Zauli, E.; Zauli, G.; AlMesfer, S.; Al-Swailem, S.; Voltan, R. MDM2 inhibitors-mediated disruption of mitochondrial metabolism: A novel therapeutic strategy for retinoblastoma. Front. Oncol. 2022, 12, 1000677. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Wang, J.; Thomas, H.R.; Li, Z.; Yeo, N.C.F.; Scott, H.E.; Dang, N.; Hossain, M.I.; Andrabi, S.A.; Parant, J.M. Puma, noxa, p53, and p63 differentially mediate stress pathway induced apoptosis. Cell Death Dis. 2021, 12, 659. [Google Scholar] [CrossRef]
- Roufayel, R.; Younes, K.; Al-Sabi, A.; Murshid, N. BH3-Only Proteins Noxa and Puma Are Key Regulators of Induced Apoptosis. Life 2022, 12, 256. [Google Scholar] [CrossRef] [PubMed]
- Li, M. The role of P53 up-regulated modulator of apoptosis (PUMA) in ovarian development, cardiovascular and neurodegenerative diseases. Apoptosis 2021, 26, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, K.; Hertlein, V.; Jenner, A.; Dellmann, T.; Gojkovic, M.; Pena-Blanco, A.; Dadsena, S.; Wajngarten, N.; Danial, J.S.H.; Thevathasan, J.V.; et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol. Cell 2022, 82, 933–949.e9. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H. Structural features of caspase-activating complexes. Int. J. Mol. Sci. 2012, 13, 4807–4818. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Lazebnik, Y. Caspase-9 and APAF-1 form an active holoenzyme. Genes Dev. 1999, 13, 3179–3184. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [PubMed]
- Marchenko, N.D.; Moll, U.M. Mitochondrial death functions of p53. Mol. Cell. Oncol. 2014, 1, e955995. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Wang, J.; Zhang, T.; Xu, D.; Hu, W.; Feng, Z. The Interplay Between Tumor Suppressor p53 and Hypoxia Signaling Pathways in Cancer. Front. Cell Dev. Biol. 2021, 9, 648808. [Google Scholar] [CrossRef]
- Nieminen, A.L.; Qanungo, S.; Schneider, E.A.; Jiang, B.H.; Agani, F.H. Mdm2 and HIF-1alpha interaction in tumor cells during hypoxia. J. Cell. Physiol. 2005, 204, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mammadzada, P.; Corredoira, P.M.; Andre, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef]
- Lee, D.; Kunimi, H.; Negishi, K.; Kurihara, T. Degeneration of retinal ganglion cells in hypoxic responses: Hypoxia-inducible factor inhibition, a new therapeutic insight. Neural Regen. Res. 2022, 17, 2230–2231. [Google Scholar] [PubMed]
- Rosenbaum, D.M.; Rosenbaum, P.S.; Gupta, H.; Singh, M.; Aggarwal, A.; Hall, D.H.; Roth, S.; Kessler, J.A. The role of the p53 protein in the selective vulnerability of the inner retina to transient ischemia. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2132–2139. [Google Scholar]
- Kaur, C.; Foulds, W.S.; Ling, E.A. Hypoxia-ischemia and retinal ganglion cell damage. Clin. Ophthalmol. 2008, 2, 879–889. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Raj, S.; DePamphilis, M.L. Developmental Acquisition of p53 Functions. Genes 2021, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, Y.; Xiong, S.; Williams-Villalobo, A.E. A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer. Biology 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.D.; Pozniak, C.D.; Walsh, G.S. Neuronal life and death: An essential role for the p53 family. Cell Death Differ. 2000, 7, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Casciano, F.; Bianchi, N.; Borin, M.; Vellani, V.; Secchiero, P.; Bergamini, C.M.; Capsoni, S.; Pignatelli, A. Characterization by Gene Expression Analysis of Two Groups of Dopaminergic Cells Isolated from the Mouse Olfactory Bulb. Biology 2023, 12, 367. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Sanya, O.J. Bax modulates neuronal survival while p53 is unaltered after Cytochrome C induced oxidative stress in the adult olfactory bulb in vivo. Ann. Neurosci. 2015, 22, 19–25. [Google Scholar] [CrossRef]
- Fatt, M.P.; Cancino, G.I.; Miller, F.D.; Kaplan, D.R. p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death Differ. 2014, 21, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.; Brobst, D.E.; Ivanovic, I.; Sherry, D.M.; Al-Ubaidi, M.R. p53 selectively regulates developmental apoptosis of rod photoreceptors. PLoS ONE 2013, 8, e67381. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.; Brobst, D.E.; Saadi, A.; Ivanovic, I.; Al-Ubaidi, M.R. Pattern of expression of p53, its family members, and regulators during early ocular development and in the post-mitotic retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4821–4831. [Google Scholar] [CrossRef]
- Tendler, Y.; Panshin, A. Features of p53 protein distribution in the corneal epithelium and corneal tear film. Sci. Rep. 2020, 10, 10051. [Google Scholar] [CrossRef]
- Tendler, Y.; Weisinger, G.; Coleman, R.; Diamond, E.; Lischinsky, S.; Kerner, H.; Rotter, V.; Zinder, O. Tissue-specific p53 expression in the nervous system. Brain Res. Mol. Brain Res. 1999, 72, 40–46. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, H.Y.; Lee, H.W.; Kim, H.J.; Lee, E.; Cho, S.S.; Baik, S.H.; Lee, K.H. In situ localization of p53, bcl-2 and bax mRNAs in rat ocular tissue. Neuroreport 1999, 10, 2165–2167. [Google Scholar] [CrossRef]
- Pokroy, R.; Tendler, Y.; Pollack, A.; Zinder, O.; Weisinger, G. p53 expression in the normal murine eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1736–1741. [Google Scholar]
- Li, J.J.; Yi, S.; Wei, L. Ocular Microbiota and Intraocular Inflammation. Front. Immunol. 2020, 11, 609765. [Google Scholar] [CrossRef] [PubMed]
- Jaki Mekjavic, P.; Tipton, M.J.; Mekjavic, I.B. The eye in extreme environments. Exp. Physiol. 2021, 106, 52–64. [Google Scholar] [CrossRef]
- Blindness, G.B.D.; Vision Impairment, C.; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar]
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ramberg, I.; Heegaard, S. Human Papillomavirus Related Neoplasia of the Ocular Adnexa. Viruses 2021, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.W.; Arruda, J.T.; Silva, R.E.; Moura, K.K. TP53 gene expression, codon 72 polymorphism and human papillomavirus DNA associated with pterygium. Genet. Mol. Res. 2008, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chang, C.C.; Chiang, C.C.; Yeh, K.T.; Chen, P.L.; Chang, C.H.; Chou, M.C.; Lee, H.; Cheng, Y.W. HPV infection and p53 inactivation in pterygium. Mol. Vis. 2009, 15, 1092–1097. [Google Scholar]
- Dushku, N.; Hatcher, S.L.; Albert, D.M.; Reid, T.W. p53 expression and relation to human papillomavirus infection in pingueculae, pterygia, and limbal tumors. Arch. Ophthalmol. 1999, 117, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharmacother. 2020, 130, 110577. [Google Scholar] [CrossRef]
- Song, W.; Zhu, R.; Gao, W.; Xing, C.; Yang, L. Blue Light Induces RPE Cell Necroptosis, Which Can Be Inhibited by Minocycline. Front. Med. 2022, 9, 831463. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Hafezi, F.; Lansel, N.; Hegi, M.E.; Wenzel, A.; Grimm, C.; Niemeyer, G.; Reme, C.E. Light-induced cell death of retinal photoreceptors in the absence of p53. Investig. Ophthalmol. Vis. Sci. 1998, 39, 846–849. [Google Scholar]
- Lansel, N.; Hafezi, F.; Marti, A.; Hegi, M.; Reme, C.; Niemeyer, G. The mouse ERG before and after light damage is independent of p53. Doc. Ophthalmol. 1998, 96, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.L.; Deng, W.L.; Huang, N.; Wang, Y.Y.; Lei, X.L.; Xu, Z.Q.; Hu, D.N.; Cai, J.Q.; Lu, F.; Jin, Z.B. Upregulation of GADD45alpha in light-damaged retinal pigment epithelial cells. Cell Death Discov. 2016, 2, 16013. [Google Scholar] [CrossRef] [PubMed]
- Westlund, B.S.; Cai, B.; Zhou, J.; Sparrow, J.R. Involvement of c-Abl, p53 and the MAP kinase JNK in the cell death program initiated in A2E-laden ARPE-19 cells by exposure to blue light. Apoptosis 2009, 14, 31–41. [Google Scholar] [CrossRef]
- Lyu, Y.; Tschulakow, A.V.; Wang, K.; Brash, D.E.; Schraermeyer, U. Chemiexcitation and melanin in photoreceptor disc turnover and prevention of macular degeneration. Proc. Natl. Acad. Sci. USA 2023, 120, e2216935120. [Google Scholar] [CrossRef]
- Fietz, A.; Hurst, J.; Schnichels, S. Out of the Shadow: Blue Light Exposure Induces Apoptosis in Muller Cells. Int. J. Mol. Sci. 2022, 23, 14540. [Google Scholar] [CrossRef]
- Alvarez-Barrios, A.; Alvarez, L.; Garcia, M.; Artime, E.; Pereiro, R.; Gonzalez-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Wallace, D.M.; O’Brien, C.J.; Cotter, T.G. A novel antioxidant function for the tumor-suppressor gene p53 in the retinal ganglion cell. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4237–4244. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.J.; Gallego-Pinazo, R.; de Hoz, R.; Pinazo-Duran, M.D.; Rojas, B.; Ramirez, A.I.; Serrano, M.; Ramirez, J.M. "Super p53" mice display retinal astroglial changes. PLoS ONE 2013, 8, e65446. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Ahmed, T.; Suzumura, A.; Terasaki, H. Oxidative Stress-Induced Cellular Senescence in Aging Retina and Age-Related Macular Degeneration. Antioxidants 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Zhang, X.; Srivenugopal, K.S.; Wang, M.H.; Wang, W.; Zhang, R. Targeting MDM2-p53 interaction for cancer therapy: Are we there yet? Curr. Med. Chem. 2014, 21, 553–574. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Maki, C.G. Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr. Pharm. Des. 2011, 17, 560–568. [Google Scholar] [CrossRef]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef]
- Neochoritis, C.; Estrada-Ortiz, N.; Khoury, K.; Dömling, A. p53–MDM2 and MDMX Antagonists. Annu. Rep. Med. Chem. 2014, 49, 167–187. [Google Scholar]
- Agnoletto, C.; Brunelli, L.; Melloni, E.; Pastorelli, R.; Casciano, F.; Rimondi, E.; Rigolin, G.M.; Cuneo, A.; Secchiero, P.; Zauli, G. The anti-leukemic activity of sodium dichloroacetate in p53mutated/null cells is mediated by a p53-independent ILF3/p21 pathway. Oncotarget 2015, 6, 2385–2396. [Google Scholar] [CrossRef]
- Secchiero, P.; Bosco, R.; Celeghini, C.; Zauli, G. Recent advances in the therapeutic perspectives of Nutlin-3. Curr. Pharm. Des. 2011, 17, 569–577. [Google Scholar] [CrossRef]
- Zauli, G.; AlHilali, S.; Al-Swailem, S.; Secchiero, P.; Voltan, R. Therapeutic potential of the MDM2 inhibitor Nutlin-3 in counteracting SARS-CoV-2 infection of the eye through p53 activation. Front. Med. 2022, 9, 902713. [Google Scholar] [CrossRef]
- Milani, D.; Caruso, L.; Zauli, E.; Al Owaifeer, A.M.; Secchiero, P.; Zauli, G.; Gemmati, D.; Tisato, V. p53/NF-kB Balance in SARS-CoV-2 Infection: From OMICs, Genomics and Pharmacogenomics Insights to Tailored Therapeutic Perspectives (COVIDomics). Front. Pharmacol. 2022, 13, 871583. [Google Scholar] [CrossRef]
- Lodi, G.; Gentili, V.; Casciano, F.; Romani, A.; Zauli, G.; Secchiero, P.; Zauli, E.; Simioni, C.; Beltrami, S.; Fernandez, M.; et al. Cell cycle block by p53 activation reduces SARS-CoV-2 release in infected alveolar basal epithelial A549-hACE2 cells. Front. Pharmacol. 2022, 13, 1018761. [Google Scholar] [CrossRef]
- Tisato, V.; Voltan, R.; Gonelli, A.; Secchiero, P.; Zauli, G. MDM2/X inhibitors under clinical evaluation: Perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 2017, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, H.; Thuraisamy, A. MDM2/P53 Inhibitors as Sensitizing Agents for Cancer Chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Chen, Z.-S., Yang, D.-H., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 4, pp. 243–266. [Google Scholar]
- Papai, Z.; Chen, L.C.; Da Costa, D.; Blotner, S.; Vazvaei, F.; Gleave, M.; Jones, R.; Zhi, J. A single-center, open-label study investigating the excretion balance, pharmacokinetics, metabolism, and absolute bioavailability of a single oral dose of [(14)C]-labeled idasanutlin and an intravenous tracer dose of [(13)C]-labeled idasanutlin in a single cohort of patients with solid tumors. Cancer Chemother. Pharmacol. 2019, 84, 93–103. [Google Scholar] [PubMed]
- Zauli, G.; Tisato, V.; Secchiero, P. Rationale for Considering Oral Idasanutlin as a Therapeutic Option for COVID-19 Patients. Front. Pharmacol. 2020, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.; Wovkulich, P.; Pizzolato, G.; Lovey, A.; Ding, Q.; Jiang, N.; Liu, J.J.; Zhao, C.; Glenn, K.; Wen, Y.; et al. Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med. Chem. Lett. 2013, 4, 466–469. [Google Scholar] [CrossRef]
- McKean, M.; Tolcher, A.W.; Reeves, J.A.; Chmielowski, B.; Shaheen, M.F.; Beck, J.T.; Orloff, M.M.; Somaiah, N.; Van Tine, B.A.; Drabick, J.J.; et al. Newly updated activity results of alrizomadlin (APG-115), a novel MDM2/p53 inhibitor, plus pembrolizumab: Phase 2 study in adults and children with various solid tumors. J. Clin. Oncol. 2022, 40, 9517. [Google Scholar] [CrossRef]
- Bill, K.L.; Garnett, J.; Meaux, I.; Ma, X.; Creighton, C.J.; Bolshakov, S.; Barriere, C.; Debussche, L.; Lazar, A.J.; Prudner, B.C.; et al. SAR405838: A Novel and Potent Inhibitor of the MDM2:p53 Axis for the Treatment of Dedifferentiated Liposarcoma. Clin. Cancer Res. 2016, 22, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.J.; Banerji, U.; Mahipal, A.; Somaiah, N.; Hirsch, H.; Fancourt, C.; Johnson-Levonas, A.O.; Lam, R.; Meister, A.K.; Russo, G.; et al. Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, R.; De Matteis, S.; Carloni, S.; Bruno, S.; Abbati, G.; Capelli, L.; Ghetti, M.; Bochicchio, M.T.; Liverani, C.; Mercatali, L.; et al. Kevetrin induces apoptosis in TP53 wild-type and mutant acute myeloid leukemia cells. Oncol. Rep. 2020, 44, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; DeAngelo, D.J.; Chromik, J.; Chatterjee, M.; Bauer, S.; Lin, C.C.; Suarez, C.; de Vos, F.; Steeghs, N.; Cassier, P.A.; et al. Results from a First-in-Human Phase I Study of Siremadlin (HDM201) in Patients with Advanced Wild-Type TP53 Solid Tumors and Acute Leukemia. Clin. Cancer Res. 2022, 28, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Fujiwara, Y.; Nakano, K.; Shimizu, T.; Tomomatsu, J.; Koyama, T.; Ogura, M.; Tachibana, M.; Kakurai, Y.; Yamashita, T.; et al. Safety and pharmacokinetics of milademetan, a MDM2 inhibitor, in Japanese patients with solid tumors: A phase I study. Cancer Sci. 2021, 112, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Demetri, G.D.; Halilovic, E.; Dummer, R.; Meille, C.; Tan, D.S.W.; Guerreiro, N.; Jullion, A.; Ferretti, S.; Jeay, S.; et al. Pharmacokinetic-pharmacodynamic guided optimisation of dose and schedule of CGM097, an HDM2 inhibitor, in preclinical and clinical studies. Br. J. Cancer 2021, 125, 687–698. [Google Scholar] [CrossRef]
- Gluck, W.L.; Gounder, M.M.; Frank, R.; Eskens, F.; Blay, J.Y.; Cassier, P.A.; Soria, J.C.; Chawla, S.; de Weger, V.; Wagner, A.J.; et al. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced P53 wild-type solid tumors or multiple myeloma. Investig. New Drugs 2020, 38, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Togashi, K.; Okada, M.; Suzuki, S.; Sanomachi, T.; Seino, S.; Yamamoto, M.; Yamashita, H.; Kitanaka, C. Inhibition of Retinoblastoma Cell Growth by CEP1347 Through Activation of the P53 Pathway. Anticancer Res. 2020, 40, 4961–4968. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Xie, L.; He, X.; Bai, J. Progress in the pathogenesis of pterygium. Curr. Eye Res. 2013, 38, 1191–1197. [Google Scholar] [CrossRef]
- Threlfall, T.J.; English, D.R. Sun exposure and pterygium of the eye: A dose-response curve. Am. J. Ophthalmol. 1999, 128, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Reisman, D.; McFadden, J.W.; Lu, G. Loss of heterozygosity and p53 expression in Pterygium. Cancer Lett. 2004, 206, 77–83. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Cheng, Y.W.; Lee, H.; Tsai, F.J.; Tseng, S.H.; Chang, K.C. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol. Vis. 2005, 11, 50–55. [Google Scholar]
- Spandidos, D.A.; Sourvinos, G.; Kiaris, H.; Tsamparlakis, J. Microsatellite instability and loss of heterozygosity in human pterygia. Br. J. Ophthalmol. 1997, 81, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.; Lim, A.S.; Goh, H.S.; Smith, D.R. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am. J. Ophthalmol. 1997, 123, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Onur, C.; Orhan, D.; Orhan, M.; Dizbay Sak, S.; Tulunay, O.; Irkec, M. Expression of p53 protein in pterygium. Eur. J. Ophthalmol. 1998, 8, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Dushku, N.; Reid, T.W. P53 expression in altered limbal basal cells of pingueculae, pterygia, and limbal tumors. Curr. Eye Res. 1997, 16, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Chowers, I.; Pe’er, J.; Zamir, E.; Livni, N.; Ilsar, M.; Frucht-Pery, J. Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology 2001, 108, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Chang, K.C.; Lin, C.L.; Lee, H.; Tsai, F.J.; Cheng, Y.W.; Tseng, S.H. p53 Expression in pterygium by immunohistochemical analysis: A series report of 127 cases and review of the literature. Cornea 2005, 24, 583–586. [Google Scholar] [CrossRef]
- Weinstein, O.; Rosenthal, G.; Zirkin, H.; Monos, T.; Lifshitz, T.; Argov, S. Overexpression of p53 tumor suppressor gene in pterygia. Eye 2002, 16, 619–621. [Google Scholar] [CrossRef]
- Tan, D.T.; Tang, W.Y.; Liu, Y.P.; Goh, H.S.; Smith, D.R. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br. J. Ophthalmol. 2000, 84, 212–216. [Google Scholar] [CrossRef]
- Martins, T.G.; Costa, A.L.; Alves, M.R.; Chammas, R.; Schor, P. Mitomycin C in pterygium treatment. Int. J. Ophthalmol. 2016, 9, 465–468. [Google Scholar]
- Cao, D.; Chu, W.K.; Ng, T.K.; Yip, Y.W.Y.; Young, A.L.; Pang, C.P.; Jhanji, V. Cellular Proliferation and Migration of Human Pterygium Cells: Mitomycin Versus Small-Molecule Inhibitors. Cornea 2018, 37, 760–766. [Google Scholar] [CrossRef]
- Isager, P.; Engholm, G.; Overgaard, J.; Storm, H. Uveal and conjunctival malignant melanoma in denmark 1943-97: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006, 13, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Markowitz, J.S.; Belinsky, I.; Schwartzstein, H.; George, N.S.; Lally, S.E.; Mashayekhi, A.; Shields, J.A. Conjunctival melanoma: Outcomes based on tumor origin in 382 consecutive cases. Ophthalmology 2011, 118, 389–395.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Mashayekhi, A.; Marr, B.P.; Benavides, R.; Thangappan, A.; Phan, L.; Eagle, R.C., Jr. Primary acquired melanosis of the conjunctiva: Risks for progression to melanoma in 311 eyes. The 2006 Lorenz E. Zimmerman lecture. Ophthalmology 2008, 115, 511–519.e2. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Gunduz, K.; Cater, J.; Mercado, G.V.; Gross, N.; Lally, B. Conjunctival melanoma: Risk factors for recurrence, exenteration, metastasis, and death in 150 consecutive patients. Arch. Ophthalmol. 2000, 118, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Finger, P.T.; Fili, M.; Damato, B.; Coupland, S.E.; Heimann, H.; Kenawy, N.; Brouwer, N.J.; Marinkovic, M.; Van Duinen, S.G.; et al. Conjunctival melanoma treatment outcomes in 288 patients: A multicentre international data-sharing study. Br. J. Ophthalmol. 2021, 105, 1358–1364. [Google Scholar] [CrossRef]
- Seregard, S. Cell growth and p53 expression in primary acquired melanosis and conjunctival melanoma. J. Clin. Pathol. 1996, 49, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Cismas, S.; Crudden, C.; Trocme, E.; Worrall, C.; Suleymanova, N.; Lin, T.; Zheng, H.; Seregard, S.; Girnita, A.; et al. IGF-1R is a molecular determinant for response to p53 reactivation therapy in conjunctival melanoma. Oncogene 2022, 41, 600–611. [Google Scholar] [CrossRef]
- Global Retinoblastoma Study Group; Fabian, I.D.; Abdallah, E.; Abdullahi, S.U.; Abdulqader, R.A.; Adamou Boubacar, S.; Ademola-Popoola, D.S.; Adio, A.; Afshar, A.R.; Aggarwal, P.; et al. Global Retinoblastoma Presentation and Analysis by National Income Level. JAMA Oncol. 2020, 6, 685–695. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Orbach, D.B.; VanderVeen, D. Retinoblastoma. Pediatr. Clin. N. Am. 2015, 62, 201–223. [Google Scholar] [CrossRef]
- Fernandes, A.G.; Pollock, B.D.; Rabito, F.A. Retinoblastoma in the United States: A 40-Year Incidence and Survival Analysis. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 182–188. [Google Scholar] [CrossRef]
- Zhang, J.; Benavente, C.A.; McEvoy, J.; Flores-Otero, J.; Ding, L.; Chen, X.; Ulyanov, A.; Wu, G.; Wilson, M.; Wang, J.; et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012, 481, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.A.; Gardner, H.A.; Gallie, B.L. Segregation of chromosome 13 in retinoblastoma. Lancet 1978, 1, 989. [Google Scholar] [CrossRef] [PubMed]
- Sidle, A.; Palaty, C.; Dirks, P.; Wiggan, O.; Kiess, M.; Gill, R.M.; Wong, A.K.; Hamel, P.A. Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit. Rev. Biochem. Mol. Biol. 1996, 31, 237–271. [Google Scholar] [CrossRef] [PubMed]
- Wallace, V.A. Cancer biology: Second step to retinal tumours. Nature 2006, 444, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.A.; Tsao, H. Melanoma and genetics. Clin. Dermatol. 2009, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, A. Understanding signal transduction pathways to overcome targeted therapy resistance in glioblastoma. In Glioblastoma Resistance to Chemotherapy: Molecular Mechanisms and Innovative Reversal Strategies; Paulmurugan, R., Massoud, T.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 15, pp. 547–585. [Google Scholar]
- Sdek, P.; Ying, H.; Chang, D.L.; Qiu, W.; Zheng, H.; Touitou, R.; Allday, M.J.; Xiao, Z.X. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol. Cell 2005, 20, 699–708. [Google Scholar] [CrossRef]
- Tang, Y.A.; Lin, R.K.; Tsai, Y.T.; Hsu, H.S.; Yang, Y.C.; Chen, C.Y.; Wang, Y.C. MDM2 overexpression deregulates the transcriptional control of RB/E2F leading to DNA methyltransferase 3A overexpression in lung cancer. Clin. Cancer Res. 2012, 18, 4325–4333. [Google Scholar] [CrossRef]
- Hernandez-Monge, J.; Rousset-Roman, A.B.; Medina-Medina, I.; Olivares-Illana, V. Dual function of MDM2 and MDMX toward the tumor suppressors p53 and RB. Genes Cancer 2016, 7, 278–287. [Google Scholar] [CrossRef]
- Grace, C.R.; Ban, D.; Min, J.; Mayasundari, A.; Min, L.; Finch, K.E.; Griffiths, L.; Bharatham, N.; Bashford, D.; Kiplin Guy, R.; et al. Monitoring Ligand-Induced Protein Ordering in Drug Discovery. J. Mol. Biol. 2016, 428, 1290–1303. [Google Scholar] [CrossRef]
- Marine, J.C.; Jochemsen, A.G. Mdmx as an essential regulator of p53 activity. Biochem. Biophys. Res. Commun. 2005, 331, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Rimondi, E.; Melloni, E.; Rigolin, G.M.; Casciano, F.; Arcidiacono, M.V.; Celeghini, C.; Cuneo, A.; Zauli, G.; Secchiero, P. Ibrutinib synergizes with MDM-2 inhibitors in promoting cytotoxicity in B chronic lymphocytic leukemia. Oncotarget 2016, 7, 70623–70638. [Google Scholar] [CrossRef] [PubMed]
- Elison, J.R.; Cobrinik, D.; Claros, N.; Abramson, D.H.; Lee, T.C. Small molecule inhibition of HDM2 leads to p53-mediated cell death in retinoblastoma cells. Arch. Ophthalmol. 2006, 124, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Deo, D.; Xia, M.; Vassilev, L.T. Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol. Cancer Res. 2009, 7, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Brennan, R.C.; Federico, S.; Bradley, C.; Zhang, J.; Flores-Otero, J.; Wilson, M.; Stewart, C.; Zhu, F.; Guy, K.; Dyer, M.A. Targeting the p53 pathway in retinoblastoma with subconjunctival Nutlin-3a. Cancer Res. 2011, 71, 4205–4213. [Google Scholar] [CrossRef] [PubMed]
- Lenos, K.; de Lange, J.; Teunisse, A.F.; Lodder, K.; Verlaan-de Vries, M.; Wiercinska, E.; van der Burg, M.J.; Szuhai, K.; Jochemsen, A.G. Oncogenic functions of hMDMX in in vitro transformation of primary human fibroblasts and embryonic retinoblasts. Mol. Cancer 2011, 10, 111. [Google Scholar] [CrossRef]
- Laurie, N.A.; Donovan, S.L.; Shih, C.S.; Zhang, J.; Mills, N.; Fuller, C.; Teunisse, A.; Lam, S.; Ramos, Y.; Mohan, A.; et al. Inactivation of the p53 pathway in retinoblastoma. Nature 2006, 444, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, Y.; Nakagawa-Saito, Y.; Togashi, K.; Suzuki, S.; Sugai, A.; Matsuda, K.I.; Sonoda, Y.; Kitanaka, C.; Okada, M. CEP-1347 Targets MDM4 Protein Expression to Activate p53 and Inhibit the Growth of Glioma Cells. Anticancer Res. 2022, 42, 4727–4733. [Google Scholar] [CrossRef]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Shields, C.L.; Furuta, M.; Thangappan, A.; Nagori, S.; Mashayekhi, A.; Lally, D.R.; Kelly, C.C.; Rudich, D.S.; Nagori, A.V.; Wakade, O.A.; et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch. Ophthalmol. 2009, 127, 989–998. [Google Scholar] [CrossRef]
- Branisteanu, D.E.; Porumb-Andrese, E.; Starica, A.; Munteanu, A.C.; Toader, M.P.; Zemba, M.; Porumb, V.; Cozmin, M.; Moraru, A.D.; Nicolescu, A.C.; et al. Differences and Similarities in Epidemiology and Risk Factors for Cutaneous and Uveal Melanoma. Medicina 2023, 59, 943. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.A.; Brock, A.L. Radiation therapy for uveal melanoma: A review of treatment methods available in 2021. Curr. Opin. Ophthalmol. 2021, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch. Ophthalmol. 2006, 124, 1684–1693. [Google Scholar]
- Gragoudas, E.S. Proton beam irradiation of uveal melanomas: The first 30 years. The Weisenfeld Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Shields, C.L.; Rojanaporn, D.; Badal, J.; Devisetty, L.; Emrich, J.; Komarnicky, L.; Shields, J.A. Scleral necrosis after plaque radiotherapy of uveal melanoma: A case-control study. Ophthalmology 2013, 120, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, K.; Shields, C.L.; Shields, J.A.; Cater, J.; Freire, J.E.; Brady, L.W. Radiation retinopathy following plaque radiotherapy for posterior uveal melanoma. Arch. Ophthalmol. 1999, 117, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, C.; Kim, D.W.; Gombos, D.S.; Oba, J.; Qin, Y.; Williams, M.D.; Esmaeli, B.; Grimm, E.A.; Wargo, J.A.; Woodman, S.E.; et al. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer 2016, 122, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Kastan, M.B.; Onyekwere, O.; Sidransky, D.; Vogelstein, B.; Craig, R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991, 51, 6304–6311. [Google Scholar] [CrossRef]
- Sun, Y.; Tran, B.N.; Worley, L.A.; Delston, R.B.; Harbour, J.W. Functional analysis of the p53 pathway in response to ionizing radiation in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1561–1564. [Google Scholar] [CrossRef]
- Chana, J.S.; Wilson, G.D.; Cree, I.A.; Alexander, R.A.; Myatt, N.; Neale, M.; Foss, A.J.; Hungerford, J.L. c-myc, p53, and Bcl-2 expression and clinical outcome in uveal melanoma. Br. J. Ophthalmol. 1999, 83, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Zemba, M.; Dumitrescu, O.M.; Gheorghe, A.G.; Radu, M.; Ionescu, M.A.; Vatafu, A.; Dinu, V. Ocular Complications of Radiotherapy in Uveal Melanoma. Cancers 2023, 15, 333. [Google Scholar] [CrossRef] [PubMed]
- Decaudin, D.; Frisch Dit Leitz, E.; Nemati, F.; Tarin, M.; Naguez, A.; Zerara, M.; Marande, B.; Vivet-Noguer, R.; Halilovic, E.; Fabre, C.; et al. Preclinical evaluation of drug combinations identifies co-inhibition of Bcl-2/XL/W and MDM2 as a potential therapy in uveal melanoma. Eur. J. Cancer 2020, 126, 93–103. [Google Scholar] [CrossRef]
- Aguilar, A.; Lu, J.; Liu, L.; Du, D.; Bernard, D.; McEachern, D.; Przybranowski, S.; Li, X.; Luo, R.; Wen, B.; et al. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. [Google Scholar]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Tolcher, A.W.; Reeves, J.A.; McKean, M.; Chmielowski, B.; Beck, J.T.; Shaheen, M.F.; Somaiah, N.; Wilson, M.; Spira, A.I.; Drabick, J.J.; et al. Preliminary results of a phase II study of alrizomadlin (APG-115), a novel, small-molecule MDM2 inhibitor, in combination with pembrolizumab in patients (pts) with unresectable or metastatic melanoma or advanced solid tumors that have failed immuno-oncologic (I-O) drugs. J. Clin. Oncol. 2021, 39, 2506. [Google Scholar]
- Attardi, L.D.; Reczek, E.E.; Cosmas, C.; Demicco, E.G.; McCurrach, M.E.; Lowe, S.W.; Jacks, T. PERP, an apoptosis-associated target of p53, is a novel member of the PMP-22/gas3 family. Genes Dev. 2000, 14, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.; Spiller, D.; White, M.R.; Grierson, I.; Paraoan, L. PERP expression stabilizes active p53 via modulation of p53-MDM2 interaction in uveal melanoma cells. Cell Death Dis. 2011, 2, e136. [Google Scholar] [CrossRef] [PubMed]
- Paraoan, L.; Gray, D.; Hiscott, P.; Ebrahimi, B.; Damato, B.; Grierson, I. Expression of p53-induced apoptosis effector PERP in primary uveal melanomas: Downregulation is associated with aggressive type. Exp. Eye Res. 2006, 83, 911–919. [Google Scholar] [CrossRef]

| Drug | Conditions | Phases, N | Status | NCT Number |
|---|---|---|---|---|
| Idasanutlin (RG7388, RO5503781) | ||||
| PV | II, 27 | Terminated | NCT03287245 | |
| PV, ET | I, 13 | Completed | NCT02407080 | |
| AML | I/II, 24 | Terminated | NCT03850535 | |
| AML | I, 88 | Completed | NCT02670044 | |
| AML | III, 447 | Terminated | NCT02545283 | |
| AML, Acute Lymphoblastic Leukemia, Neuroblastoma | I/II, 183 | Recruiting | NCT04029688 | |
| AML | I, 122 | Completed | NCT01773408 | |
| NHL | I/II, 25 | Terminated | NCT02624986 | |
| NHL | I/II, 29 | Terminated | NCT03135262 | |
| Recurrent Plasma Cell Myeloma | I/II, 33 | Active, not recruiting | NCT02633059 | |
| CC | I/II, 94 | Terminated | NCT03555149 | |
| BC | I/II, 12 | Terminated | NCT03566485 | |
| ST | I, 8 | Completed | NCT02828930 | |
| ST | I, 48 | Completed | NCT03362723 | |
| ST | II, 770 | Recruiting | NCT04589845 | |
| GB | I/II, 350 | Recruiting | NCT03158389 | |
| ST | I, 99 | Completed | NCT01462175 | |
| ST | I, 61 | Completed | NCT01901172 | |
| Nutlin (RO5045337, RG7112) | ||||
| PV, ET | n.a., 131 | Completed | NCT01970930 | |
| AML | I, 43 | Completed | NCT01635296 | |
| Hematologic Cancers | I, 116 | Completed | NCT00623870 | |
| AML | I, 11 | Completed | NCT01677780 | |
| Sarcoma | I, 23 | Completed | NCT01605526 | |
| Sarcoma | I, 20 | Completed | NCT01143740 | |
| ST | I, 76 | Completed | NCT01164033 | |
| ST | I, 106 | Completed | NCT00559533 | |
| Alrizomadlin (APG-115) | ||||
| T-Prolymphocytic Leukemia | II, 36 | Recruiting | NCT04496349 | |
| AML, Chronic Myelomonocytic Leukemia, MDS | I/II, 69 | Recruiting | NCT04358393 | |
| AML, MDS | I, 102 | Recruiting | NCT04275518 | |
| Lymphoma, ST | I, 50 | Completed | NCT02935907 | |
| Neuroblastoma, ST | I, 100 | Recruiting | NCT05701306 | |
| Liposarcoma, ST | I/II, 92 | Recruiting | NCT04785196 | |
| Uveal Melanoma, Melanoma, ST | I/II, 224 | Recruiting | NCT03611868 | |
| Salivary Gland Cancer | I/II, 34 | Recruiting | NCT03781986 | |
| SAR405838 (MI-77301) | ||||
| ST | I, 26 | Completed | NCT01985191 | |
| ST | I, 77 | Completed | NCT01636479 | |
| MK-8242 | ||||
| AML | I, 26 | Terminated | NCT01451437 | |
| ST | I, 48 | Terminated | NCT01463696 | |
| Kevetrin | ||||
| Ovarian Cancer | II, 2 | Completed | NCT03042702 | |
| ST | I, 48 | Completed | NCT01664000 | |
| Sulanemadlin (ALRN-6924) | ||||
| BC | I, 6 | Terminated | NCT05622058 | |
| AML, MDS | I, 55 | Completed | NCT02909972 | |
| BC, ST | I, 35 | Active, not recruiting | NCT03725436 | |
| Retinoblastoma, Leukemia, Lymphoma, Brain Tumor, ST | I, 69 | Active, not recruiting | NCT03654716 | |
| Lymphoma, ST | I/II, 149 | Completed | NCT02264613 | |
| Lung Cancer | I, 35 | Terminated | NCT04022876 | |
| Siremadlin (HDM201) | ||||
| Hepatic Impairment | I, 48 | Recruiting | NCT05599932 | |
| AML, Allogeneic Stem Cell Transplantation | I/II, 38 | Recruiting | NCT05447663 | |
| Sarcoma | I/II, 58 | Recruiting | NCT05180695 | |
| AML | I, 2 | Terminated | NCT04496999 | |
| CC, ST | I, 24 | Recruiting | NCT03714958 | |
| Liposarcoma | I, 74 | Completed | NCT02343172 | |
| AML | I/II, 56 | Recruiting | NCT05155709 | |
| ST | I, 208 | Completed | NCT02143635 | |
| AML, MDS | I, 52 | Active, not recruiting | NCT03940352 | |
| AML | I/II, 0 | Withdrawn | NCT03760445 | |
| Uveal Melanoma | I, 107 | Terminated | NCT02601378 | |
| Myelofibrosis | I/II, 45 | Active, not recruiting | NCT04097821 | |
| ST | II, 425 | Recruiting | NCT04116541 | |
| CC, Lung Cancer, BC, Renal Cell Carcinoma | I, 298 | Completed | NCT02890069 | |
| Milademetan (RAIN-32, DS-3032) | ||||
| AML | I, 14 | Completed | NCT03671564 | |
| AML | I, 10 | Terminated | NCT03552029 | |
| AML, Myelodysplastic Syndrome | I, 74 | Terminated | NCT02319369 | |
| AML | I/II, 21 | Completed | NCT03634228 | |
| Lymphoma, ST | I, 108 | Completed | NCT01877382 | |
| ST | II, 65 | Recruiting | NCT05012397 | |
| Liposarcoma | III, 160 | Active, not recruiting | NCT04979442 | |
| CGM097 (NVP-CGM097) | ||||
| ST | I, 51 | Completed | NCT01760525 | |
| Navtemadlin (AMG-232, KRT-232) | ||||
| AML | I/II, 18 | Active, not recruiting | NCT04669067 | |
| AML | I, 48 | Suspended | NCT03041688 | |
| AML | I, 36 | Completed | NCT02016729 | |
| AML | I, 24 | Suspended | NCT04190550 | |
| AML | I/II, 86 | Recruiting | NCT04113616 | |
| Chronic Myeloid Leukemia | I/II, 109 | Recruiting | NCT04835584 | |
| Chronic Lymphocytic Leukemia, NHL | I/II, 84 | Recruiting | NCT04502394 | |
| PM, PV, ET | I/II, 116 | Recruiting | NCT04640532 | |
| PM, PV, ET | II, 52 | Recruiting | NCT04878003 | |
| PM, PV, ET | II/III, 385 | Recruiting | NCT03662126 | |
| Myelofibrosis | I/II, 36 | Recruiting | NCT04485260 | |
| PV | II, 20 | Unknown status | NCT03669965 | |
| Plasma Cell Myeloma | I, 40 | Recruiting | NCT03031730 | |
| GB, Multiple Myeloma, ST | I, 107 | Completed | NCT01723020 | |
| Small cell Lung Cancer | II, 38 | Recruiting | NCT05027867 | |
| Non Small Lung Cancer | I/II, 92 | Not yet recruiting | NCT05705466 | |
| Merkel Cell Carcinoma | I/II, 115 | Recruiting | NCT03787602 | |
| GB, Gliosarcoma | I, 86 | Suspended | NCT03107780 | |
| Melanoma, ST | I, 31 | Completed | NCT02110355 | |
| Sarcoma | I, 46 | Active, not recruiting | NCT03217266 | |
| Endometrial Cancer | II/III, 268 | Not yet recruiting | NCT05797831 | |
| Drugs | Synonyms | Molecular Formula | Molecular Weight (g/mol) | Mechanism of Action |
|---|---|---|---|---|
| Alrizomadlin | APG-115 | C34H38Cl2FN3O4 | 642.6 | Blocks HDM2 interaction with p53 |
| CGM097 | NVP-CGM097 | C38H47ClN4O4 | 659.3 | Blocks HDM2 interaction with p53 |
| Idasanutlin | RG7388, RO5503781 | C31H29Cl2F2N3O4 | 616.5 | Blocks MDM2 interaction with p53 |
| Kevetrin | 4-Isothioureidobutyronitrile, thioureidobutyronitrile | C5H9N3S | 143.21 | Induce activation of p53; Alters the E3 ligase processivity of MDM2; Induces p21 and PUMA |
| Milademetan | RAIN-32, DS-3032 | C30H34Cl2FN5O4 | 618.5 | Blocks MDM2 interaction with p53; Inhibits proteasome-mediated enzymatic degradation of p53; Restores p53 transcriptional activity and signaling |
| MK-8242 | C41H47F6N3O7S | 839.9 | Blocks MDM2 interaction with p53; Restores p53 signaling | |
| Navtemadlin | AMG-232, KRT-232 | C28H35Cl2NO5S | 568.6 | Blocks MDM2 interaction with p53; Restores p53 transcriptional activity |
| RG7112 | RO5045337 | C38H48Cl2N4O4S | 727.8 | Blocks MDM2 interaction with p53; Stabilizes the p53 protein; Induces p53 target genes such as CDKN1A, NOXA, PUMA, Fas, and BAX |
| SAR405838 | MI-77301 | C29H34Cl2FN3O3 | 562.5 | Blocks MDM2 interaction with p53; Inhibits proteasome-mediated enzymatic degradation of p53; Restores p53 transcriptional activity and signaling |
| Siremadlin | HDM201 | C26H24Cl2N6O4 | 555.4 | Blocks MDM2 interaction with p53; Inhibits proteasome-mediated enzymatic degradation of p53; Restores p53 transcriptional activity and signaling |
| Sulanemadlin | ALRN-6924 | C95H140N20O23 | 1930.2 | Blocks HDM2 interaction with p53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casciano, F.; Zauli, E.; Busin, M.; Caruso, L.; AlMesfer, S.; Al-Swailem, S.; Zauli, G.; Yu, A.C. State of the Art of Pharmacological Activators of p53 in Ocular Malignancies. Cancers 2023, 15, 3593. https://doi.org/10.3390/cancers15143593
Casciano F, Zauli E, Busin M, Caruso L, AlMesfer S, Al-Swailem S, Zauli G, Yu AC. State of the Art of Pharmacological Activators of p53 in Ocular Malignancies. Cancers. 2023; 15(14):3593. https://doi.org/10.3390/cancers15143593
Chicago/Turabian StyleCasciano, Fabio, Enrico Zauli, Massimo Busin, Lorenzo Caruso, Saleh AlMesfer, Samar Al-Swailem, Giorgio Zauli, and Angeli Christy Yu. 2023. "State of the Art of Pharmacological Activators of p53 in Ocular Malignancies" Cancers 15, no. 14: 3593. https://doi.org/10.3390/cancers15143593
APA StyleCasciano, F., Zauli, E., Busin, M., Caruso, L., AlMesfer, S., Al-Swailem, S., Zauli, G., & Yu, A. C. (2023). State of the Art of Pharmacological Activators of p53 in Ocular Malignancies. Cancers, 15(14), 3593. https://doi.org/10.3390/cancers15143593







