Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pancreatic Cancer Risk Factors
3. Pancreatic Cancer Epidemiology
4. Pancreatic Cancer Screening Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizot, C.; Dragomir, M.; Macacu, A.; Koechlin, A.; Bota, M.; Boyle, P. Global burden of pancreas cancer: Regional disparities in incidence, mortality, and survival. J. Health Inequal. 2019, 5, 96–112. [Google Scholar] [CrossRef]
- Thallinger, C.; Belina, I.; Comanescu, A.; Cufer, T.; Jassem, J.; Kiesewetter, B.; Markaroff, L.; Ott, R.; Polinski, B.; Rasinar, R.; et al. Limitations of cancer care in Central and South-Eastern Europe: Results of the international conference organized by the Central European Cooperative Oncology Group (CECOG). J. Health Inequal. 2020, 6, 139–152. [Google Scholar] [CrossRef]
- Pancreatic Cancer Treatment. Available online: https://www.cancer.gov/types/pancreatic/patient/pancreatic-treatment-pdq (accessed on 16 June 2023).
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Becker, A.E.; Hernandez, Y.G.; Frucht, H.; Lucas, A.L. Pancreatic ductal adenocarcinoma: Risk factors, screening, and early detection. World J. Gastroenterol. 2014, 20, 11182–11198. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 20 June 2023).
- Grover, S.; Syngal, S. Hereditary pancreatic cancer. Gastroenterology 2010, 139, 1076–1080.e2. [Google Scholar] [CrossRef] [Green Version]
- Brune, K.A.; Lau, B.; Palmisano, E.; Canto, M.; Goggins, M.G.; Hruban, R.H.; Klein, A.P. Importance of age of onset in pancreatic cancer kindreds. J. Natl. Cancer Inst. 2010, 102, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Lai, E.; Ziranu, P.; Spanu, D.; Dubois, M.; Pretta, A.; Tolu, S.; Camera, S.; Liscia, N.; Mariani, S.; Persano, M. BRCA-mutant pancreatic ductal adenocarcinoma. Br. J. Cancer 2021, 125, 1321–1332. [Google Scholar] [CrossRef]
- Korsse, S.E.; Harinck, F.; van Lier, M.G.; Biermann, K.; Offerhaus, G.J.; Krak, N.; Looman, C.W.; van Veelen, W.; Kuipers, E.J.; Wagner, A.; et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: A large cohort study and implications for surveillance. J. Med. Genet. 2013, 50, 59–64. [Google Scholar] [CrossRef]
- De Snoo, F.A.; Bishop, D.T.; Bergman, W.; van Leeuwen, I.; van der Drift, C.; van Nieuwpoort, F.A.; Out-Luiting, C.J.; Vasen, H.F.; ter Huurne, J.A.; Frants, R.R.; et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin. Cancer Res. 2008, 14, 7151–7157. [Google Scholar] [CrossRef] [Green Version]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Ojajärvi, I.A.; Partanen, T.J.; Ahlbom, A.; Boffetta, P.; Hakulinen, T.; Jourenkova, N.; Kauppinen, T.P.; Kogevinas, M.; Porta, M.; Vainio, H.U.; et al. Occupational exposures and pancreatic cancer: A meta-analysis. Occup. Environ. Med. 2000, 57, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosetti, C.; Bertuccio, P.; Negri, E.; La Vecchia, C.; Zeegers, M.P.; Boffetta, P. Pancreatic cancer: Overview of descriptive epidemiology. Mol. Carcinog. 2012, 51, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.S.; Berger, N.A. Impact of bariatric surgery on cancer risk reduction. Ann. Transl. Med. 2020, 8, S13. [Google Scholar] [CrossRef]
- WHO European Regional Obesity Report. 2022. Available online: https://apps.who.int/iris/handle/10665/353747 (accessed on 3 April 2023).
- Wang, H.; Maitra, A.; Wang, H. Obesity, Intrapancreatic Fatty Infiltration, and Pancreatic Cancer. Clin. Cancer Res. 2015, 21, 3369–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracci, P.M. Obesity and pancreatic cancer: Overview of epidemiologic evidence and biologic mechanisms. Mol. Carcinog. 2012, 51, 53–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, A.A.; Helzlsouer, K.J.; Kooperberg, C.; Shu, X.O.; Steplowski, E.; Bueno-de-Mesquita, H.B.; Fuchs, C.S.; Gross, M.D.; Jacobs, E.J.; Lacroix, A.Z.; et al. Anthropometric measures, body mass index, and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch. Intern. Med. 2010, 170, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Genkinger, J.M.; Spiegelman, D.; Anderson, K.E.; Bernstein, L.; van den Brandt, P.A.; Calle, E.E.; English, D.R.; Folsom, A.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer. 2011, 129, 1708–1717. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009, 301, 2553–2562. [Google Scholar] [CrossRef] [Green Version]
- Pisani, P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch. Physiol. Biochem. 2008, 114, 63–70. [Google Scholar] [CrossRef]
- Badowska-Kozakiewicz, A.; Fudalej, M.; Kwaśniewska, D.; Durlik, M.; Nasierowska-Guttmejer, A.; Mormul, A.; Włoszek, E.; Czerw, A.; Banaś, T.; Deptała, A. Diabetes Mellitus and Pancreatic Ductal Adenocarcinoma—Prevalence, Clinicopathological Variables, and Clinical Outcomes. Cancers 2022, 14, 2840. [Google Scholar] [CrossRef]
- Quoc Lam, B.; Shrivastava, S.K.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. The Impact of Obesity and Diabetes Mellitus on Pancreatic Cancer: Molecular Mechanisms and Clinical Perspectives. J. Cell Mol. Med. 2020, 24, 7706–7716. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic Cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Stram, D.O.; Porcel, J.; Chari, S.T.; Maskarinec, G.; Le Marchand, L.; Wilkens, L.R.; Haiman, C.A.; Pandol, S.J.; Monroe, K.R. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J. Natl. Cancer Inst. 2019, 111, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Korc, M.; Jeon, C.Y.; Edderkaoui, M.; Pandol, S.J.; Petrov, M.S.; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). Tobacco and alcohol as risk factors for pancreatic cancer. Best. Pract. Res. Clin. Gastroenterol. 2017, 31, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann. Oncol. 2012, 23, 1880–1888, Erratum in Ann. Oncol. 2012, 23, 2773. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Holly, E.A.; Wang, F.; Bracci, P.M. Cigarette, cigar and pipe smoking, passive smoke exposure, and risk of pancreatic cancer: A population-based study in the San Francisco Bay Area. BMC Cancer. 2011, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Yu, C.; Han, Z.; Xu, S.; Li, D.; Meng, X.; Chen, D. Environmental tobacco smoke and pancreatic cancer: A case-control study. Int. J. Clin. Exp. Med. 2015, 8, 16729–16732. [Google Scholar]
- Global Cancer Observatory Statistic Data. Available online: https://gco.iarc.fr/today/home (accessed on 3 April 2023).
- Hartwig, W.; Strobel, O.; Hinz, U.; Fritz, S.; Hackert, T.; Roth, C.; Büchler, M.W.; Werner, J. CA19-9 in potentially resectable pancreatic cancer: Perspective to adjust surgical and perioperative therapy. Ann. Surg. Oncol. 2013, 20, 2188–2196. [Google Scholar] [CrossRef]
- Canto, M.I.; Hruban, R.H.; Fishman, E.K.; Kamel, I.R.; Schulick, R.; Zhang, Z.; Topazian, M.; Takahashi, N.; Fletcher, J.; Petersen, G.; et al. American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012, 142, 796–804, quiz e14-5. [Google Scholar] [CrossRef] [Green Version]
- Soriano, A.; Castells, A.; Ayuso, C.; Ayuso, J.R.; De Caralt, M.T.; Ginès, M.À.; Real, M.I.; Gilabert, R.; Quintó, L.; Trilla, A.; et al. Preoperative Staging and Tumor Resectability Assessment of Pancreatic Cancer: Prospective Study Comparing Endoscopic Ultrasonography, Helical Computed Tomography, Magnetic Resonance Imaging, and Angiography. Am. J. Gastroenterol. 2004, 99, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2019, 54, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Yamashita, Y.; Kitano, M. Endoscopic ultrasound for early diagnosis of pancreatic cancer. Diagnostics. 2019, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Overbeek, K.A.; Levink, I.J.M.; Koopmann, B.D.M.; Harinck, F.; Konings, I.C.A.W.; Ausems, M.G.E.M.; Wagner, A.; Fockens, P.; van Eijck, C.H.; Koerkamp, B.G.; et al. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 2022, 71, 1152–1160. [Google Scholar] [CrossRef]
- Rhee, H.; Park, M.-S. The Role of Imaging in Current Treatment Strategies for Pancreatic Adenocarcinoma. Korean J. Radiol. 2021, 22, 23–40. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Pancreatic Cancer US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019, 322, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Waleleng, B.J.; Adiwinata, R.; Wenas, N.T.; Haroen, H.; Rotty, L.; Gosal, F.; Rotty, L.; Winarta, J.; Waleleng, A.; Simadibrata, M. Screening of pancreatic cancer: Target population, optimal timing and how? Ann Med Surg 2022, 84, 104814. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Sawhney, M.S.; Calderwood, A.H.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. Prepared by: ASGE STANDARDS OF PRACTICE COMMITTEE. ASGE guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Summary and recommendations. Gastrointest. Endosc. 2022, 95, 817–826. [Google Scholar] [CrossRef]
- Available online: https://www.cancerresearchuk.org/about-cancer/pancreatic-cancer/getting-diagnosed/screening (accessed on 17 April 2023).
- NICE Guideline [NG85]. Pancreatic Cancer in Adults: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng85/chapter/Recommendations#people-with-inherited-high-risk-of-pancreatic-cancer (accessed on 17 April 2023).
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Dbouk, M.; Katona, B.W.; Brand, R.E.; Chak, A.; Syngal, S.; Farrell, J.J.; Kastrinos, F.; Stoffel, E.M.; Blackford, A.L.; Rustgi, A.K.; et al. The Multicenter Cancer of Pancreas Screening Study: Impact on Stage and Survival. J. Clin. Oncol. 2022, 40, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xu, C.F.; Wan, X.Y.; Zhu, H.T.; Yu, C.H.; Li, Y.M. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J. Gastroenterol. 2015, 21, 8678–8686. [Google Scholar] [CrossRef] [PubMed]
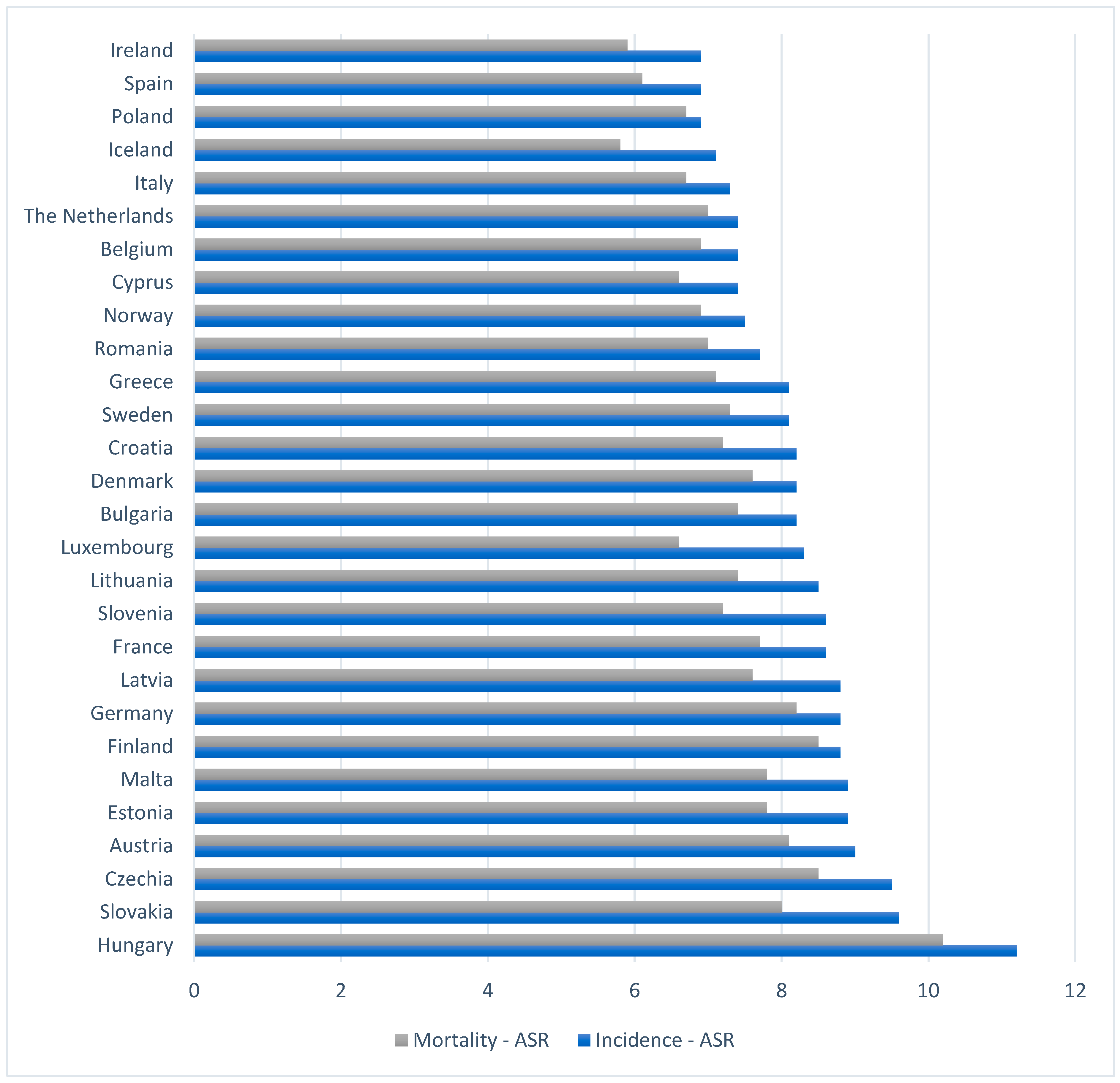
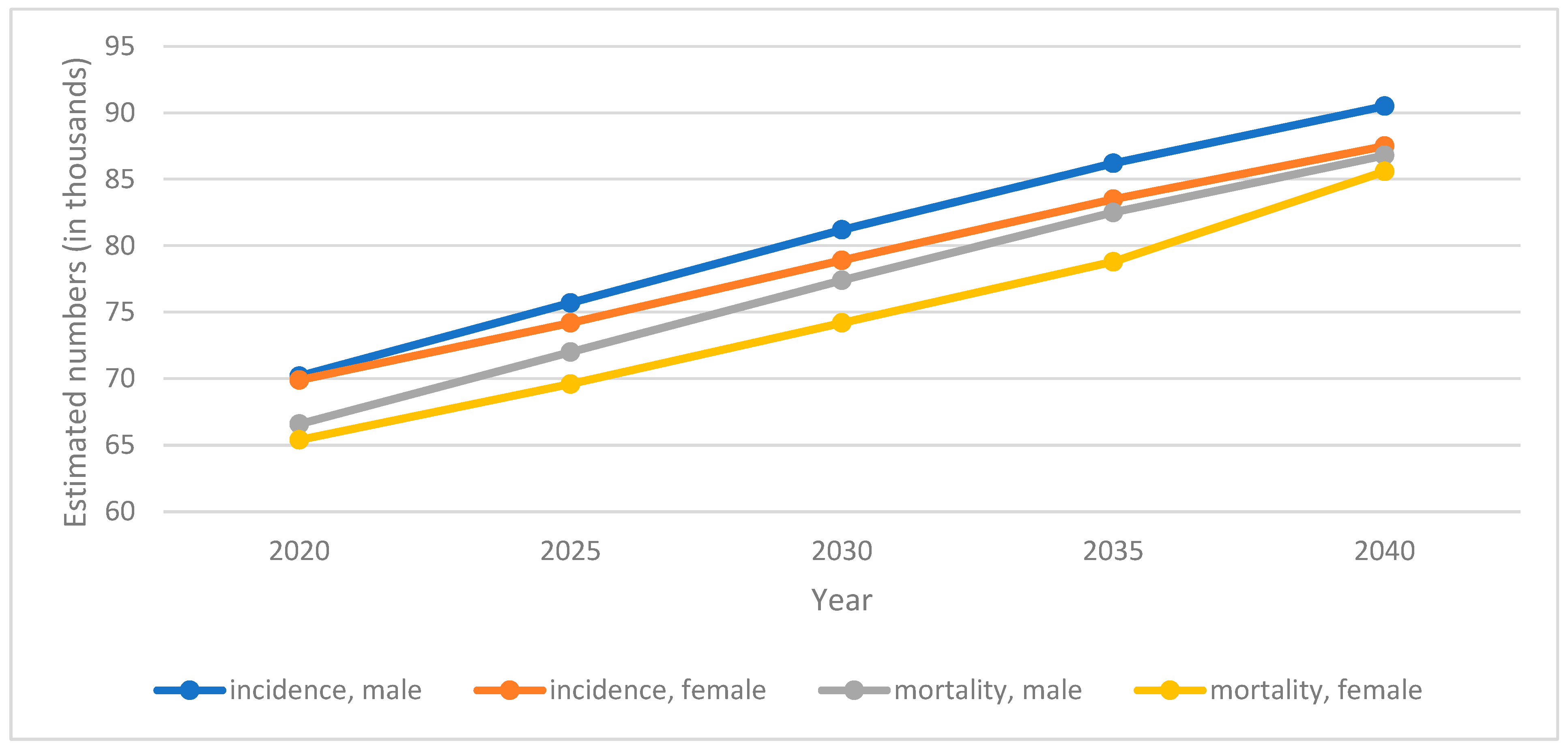
| World | Europe | ||
|---|---|---|---|
| Incidence | Number (95% UIs) | 495,773 (488,953.0–502,688.0) | 140,116 (136,953.0–143,352.0) |
| Crude rate a | 6.4 | 18.7 | |
| ASR a | 4.9 | 7.8 | |
| Cumulative risk b | 1.63 | 2.31 | |
| Mortality | Number (95% UIs) | 466,003 (459,505.0–472,593.0) | 132,134 (129,563.0–134,756.0) |
| Crude rate a | 6.0 | 17.6 | |
| ASR a | 4.5 | 7.2 | |
| Cumulative risk b | 1.58 | 2.21 | |
| Institution | Genetic Factors | Medical History | Testing Method | Time Pattern of Screening |
|---|---|---|---|---|
| International Cancer of the Pancreas Screening (CAPS) Consortium | BRCA1, BRCA2, PALB2 gene mutation (age 45–50); Peutz–Jeghers syndrome (age 40); FAMMM syndrome; hereditary pancreatitis with a mutation in the PRSS1 gene (age 40); HNPCC syndrome; ataxia–telangiectasia syndrome | >1 first-degree relative with pancreatic cancer; 1 first-degree relative and 1 second/third-degree relative; >2 second-degree relatives; >3 relatives with pancreatic cancer, regardless of the degree of relationship | MRI/MRCP + EUS + fasting glucose and/or HbAlc; MRI/MRCP or EUS during follow-up examination; CT for a solid tumour or asymptomatic stenosis of the pancreatic duct of unknown aetiology | 12-month intervals when there are no concerning pancreas lesions; 3 or 6 months if concerning abnormalities for which immediate surgery is not indicated (solid or cystic lesion size ≤ 5 mm, MDP dilation ≤ 10 mm) |
| American Society for Gastrointestinal Endoscopy (ASGE) | BRCA1, BRCA2, PALB2 gene mutation (age 50 a or 10 years less b); FAMMM syndrome (age 40 a or 10 years less b); Peutz–Jeghers syndrome (age 35 a or 10 years less b); Lynch syndrome (age 50 a or 10 years less b); hereditary pancreatitis (age 40 a) | at least 1 first-degree relative with pancreatic cancer (age 50 a or 10 years earlier b); | EUS preferred in people from very high risk groups (with Peutz–Jeghers syndrome, FAMMM syndrome) c EUS + panendoscopy/colonoscopy—Lynch syndrome, Peutz–Jeghers syndrome c; MRI—in people at increased risk of an adverse event related to anaesthesia or invasiveness of the examination | Annual screening to be performed |
| American Gastroenterological Association | BRCA1, BRCA2, PALB2, ATM gene mutation (age 50 a or 10 years less b); Peutz–Jeghers syndrome (age 35 a); hereditary pancreatitis with a mutation in the PRSS1 gene (age 40 a); FAMMM syndrome (age 40 a); Lynch syndrome | >2 first-degree relatives who do not meet the criteria for other hereditary cancer syndromes | EUS, MRI, EUS + MRI | 12-month intervals when there are no concerning pancreas lesions; In low risk lesions, EUS every 6-12 months; For indeterminate lesions, EUS every 3–6 months; 3-month interval for high-risk lesions (if resection is not planned) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. https://doi.org/10.3390/cancers15143634
Partyka O, Pajewska M, Kwaśniewska D, Czerw A, Deptała A, Budzik M, Cipora E, Gąska I, Gazdowicz L, Mielnik A, et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers. 2023; 15(14):3634. https://doi.org/10.3390/cancers15143634
Chicago/Turabian StylePartyka, Olga, Monika Pajewska, Daria Kwaśniewska, Aleksandra Czerw, Andrzej Deptała, Michał Budzik, Elżbieta Cipora, Izabela Gąska, Lucyna Gazdowicz, Aneta Mielnik, and et al. 2023. "Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations" Cancers 15, no. 14: 3634. https://doi.org/10.3390/cancers15143634
APA StylePartyka, O., Pajewska, M., Kwaśniewska, D., Czerw, A., Deptała, A., Budzik, M., Cipora, E., Gąska, I., Gazdowicz, L., Mielnik, A., Sygit, K., Sygit, M., Krzych-Fałta, E., Schneider-Matyka, D., Grochans, S., Cybulska, A. M., Drobnik, J., Bandurska, E., Ciećko, W., ... Kozłowski, R. (2023). Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers, 15(14), 3634. https://doi.org/10.3390/cancers15143634










