Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical Features and Treatment of Low-Grade Neuroendocrine Tumors
3. Clinical Features and Treatment for High-Grade Neuroendocrine Neoplasms
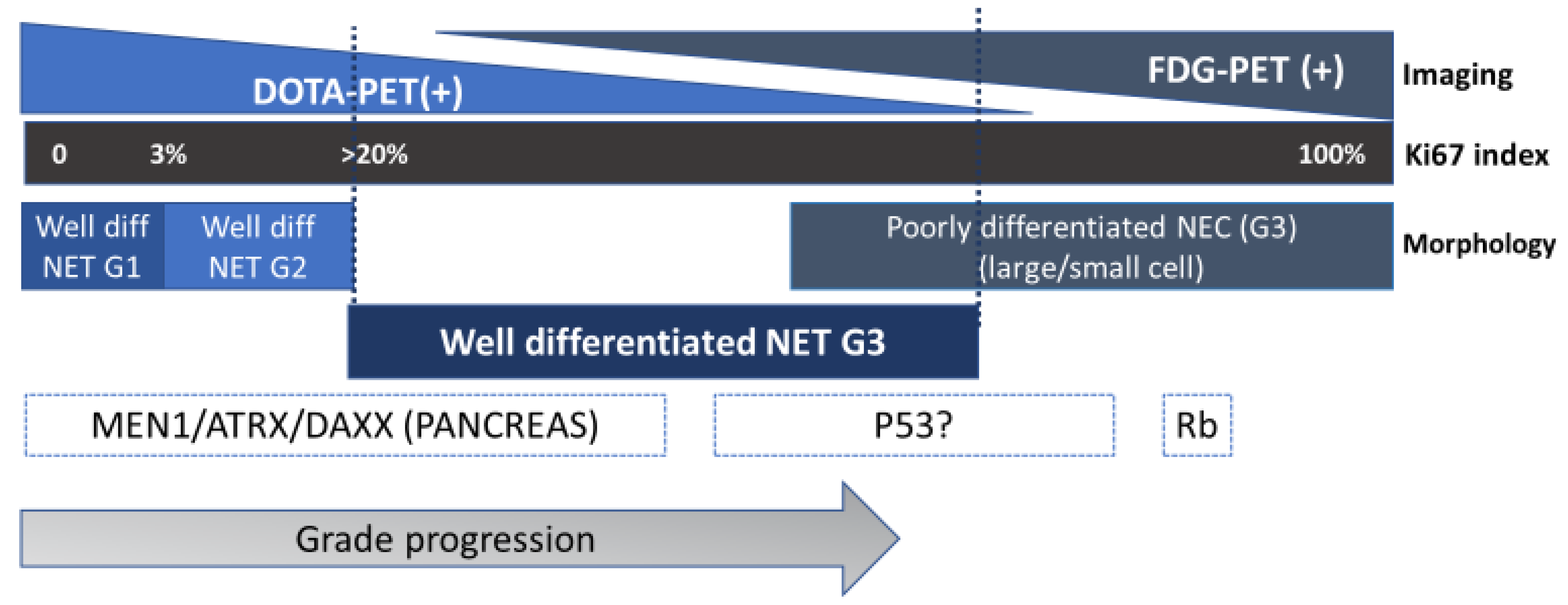
3.1. Grade 3 Neuroendocrine Tumors
3.2. Grade 3 Neuroendocrine Carcinomas
4. Diagnostic Challenges Related to GEP-NEN Classification
4.1. FNA vs. Core Biopsy vs. Surgical Sample
4.2. G3NENs with Ambiguous Morphology
5. Intrapatient Heterogeneity in GEP-NENs—Histopathologic Classification and Proliferation Rate
5.1. Heterogeneity of Grade within Primary Tumors at Baseline
5.2. Heterogeneity between Primary Tumors and Synchronous Metastases
5.3. Heterogeneity of Ki-67 in Synchronous Metastatic Deposits
| Reference | N | Site of Origin | Time Between Biopsies (mo) | Type of Heterogeneity | Description | Grade Change, (n) | |
|---|---|---|---|---|---|---|---|
| Ki67 index N (%) | Grade N (%) | ||||||
| Grillo [7] | 60 | GEP-NET # (52% SB; 27% P) | Not defined | 22/60 (37%) | 3/60 (5%) | Discordance within primary tumor | G2 → G1 (2) (NOS) G3 → G2 (1) (NOS) |
| Grillo [7] | 8 | Not defined | 3/8 (37%) | Discordance within multiple primary tumors | Larger tumors (1–2 cm) G2, smaller tumors G1 | ||
| Grillo [7] | 47 | ≤4 months | 24/47 (51%) | 11/47 (23%) | Variable Ki67 index +/− higher grade in synchronous metastases vs. primary tumors | G1→ G2 (8) (NOS) G2→ G3 (2) (both P) | |
| Grillo [7] | 60 | Not defined | 31/60 (52%)-distant 10/44 (23%)-locoregional | Grade discordance in metastases vs. primary tumors | |||
| Keck [71] | 103 | Resected GEP-NET ^ (77% SB; 20% P) | Not defined-resected primary tumors with “concurrent” metastases | 25/103 (24%) & | Higher grade in liver or lymph node metastases vs. resected primary tumors | G1→ G2 (24) (NOS) G2→ G3 (1) (NOS) | |
| 10/103 (10%) & | Lower grade in metastases vs. resected primary tumors | G2 →G1 (10) (NOS) | |||||
| 9/38 (24%) & | Discordance in synchronous primary tumors (NOS) | (NOS) | |||||
| 8/20 (40%) | Discordance in synchronous liver metastases | (NOS) | |||||
| Yang [8] | 41 (45 tumors) | Resected NET mix * (29% P; 27% SB) | Same day-liver metastases | 21/45 tumors (47%) | Discordance (G1 and G2) within single liver metastases | 91% of G2 cases heterogeneous (NOS) | |
| Shi [74] | 27 (188 liver lesions) 20 primary tumors | Resected Liver mets (SB) | ≈50% synchronous | 13/20 (65%) | Ki67 discordance in resected liver metastases ≥1 cm; Grade discordance between primary and liver metastases | N = 27 with liver mets, 10 (37%) only G1, 9 (33%) G2 +/-G1 tumors, 8 (30%) G3 +/- G1/2 G1→ G2 +/-G1 (6/17) G1→ G3 +/-G1/2 (5/17) G2→G2 (1/3) G2→ G3 (2/3) | |
| Shi [73] | 35 | GEP NEN NET mix (27.7% P; 26.7% R) | 71% synchronous | 19/35 (54.3%) | 4/35 (11%) | Higher grade in metastases vs. primary tumors | G1→ G2 (3/35) G1→G3 (1/35) |
| 1/35 (2%) | Lower grade in metastases compared to primary tumors | G2→G1 (1/35) | |||||
5.4. Grade Progression and Grade Migration over Time
5.5. Treatment-Related Changes in High-Grade Tumors
6. Heterogeneity in GEP-NENS: Molecular Features
6.1. Heterogeneity of Molecular Features—Primary vs. Metastasis
6.2. Heterogeneity of Molecular Features—Changes over Time
7. Heterogeneity in GEP-NENs—Functional Imaging with 18F-FDG (18-Fluorine Fluorodeoxyglucose) vs. 68Ga DOTATATE PET
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- WHO. WHO Classification of Digestive System Tumours, 5th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Endocrine and Neuroendocrine Tumours; IARC: Lyon, France, 2022. [Google Scholar]
- Nuñez-Valdovinos, B.; Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Capdevila, J.; Castaño-Pascual, Á.; Benavent, M.; Pi Barrio, J.J.; Teule, A.; Alonso, V.; Custodio, A.; et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist 2018, 23, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milione, M.; Maisonneuve, P.; Spada, F.; Pellegrinelli, A.; Spaggiari, P.; Albarello, L.; Pisa, E.; Barberis, M.; Vanoli, A.; Buzzoni, R.; et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology 2017, 104, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Albertelli, M.; Brisigotti, M.P.; Borra, T.; Boschetti, M.; Fiocca, R.; Ferone, D.; Mastracci, L. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology 2016, 103, 452–459. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am. J. Surg. Pathol. 2011, 35, 853–860. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Liu, C.; Yang, J.; Lin, Q.; Zheng, S.; Chen, C.; Zhou, Q.; Chen, R. Single-cell RNA sequencing reveals spatiotemporal heterogeneity and malignant progression in pancreatic neuroendocrine tumor. Int. J. Biol. Sci. 2021, 17, 3760–3775. [Google Scholar] [CrossRef]
- Raj, N.; Shah, R.; Stadler, Z.; Mukherjee, S.; Chou, J.; Untch, B.; Li, J.; Kelly, V.; Saltz, L.B.; Mandelker, D.; et al. Real-Time Genomic Characterization of Metastatic Pancreatic Neuroendocrine Tumors Has Prognostic Implications and Identifies Potential Germline Actionability. JCO Precis. Oncol. 2018, 2018. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, J.; Li, J.; Shen, L.; Li, N.; Zhu, H.; Zhai, S.; Zhang, Y.; Yang, Z.; Lu, M. Clinical and Prognostic Value of PET/CT Imaging with Combination of (68)Ga-DOTATATE and (18)F-FDG in Gastroenteropancreatic Neuroendocrine Neoplasms. Contrast. Media Mol. Imaging 2018, 2018, 2340389. [Google Scholar] [CrossRef] [Green Version]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.-M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Boyar Cetinkaya, R.; Vatn, M.; Aabakken, L.; Bergestuen, D.S.; Thiis-Evensen, E. Survival and prognostic factors in well-differentiated pancreatic neuroendocrine tumors. Scand. J. Gastroenterol. 2014, 49, 734–741. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Chan, D.L.; Dixon, M.; Law, C.H.L.; Koujanian, S.; Beyfuss, K.A.; Singh, S.; Myrehaug, S.; Hallet, J. Outcomes of Cytoreductive Surgery for Metastatic Low-Grade Neuroendocrine Tumors in the Setting of Extrahepatic Metastases. Ann. Surg. Oncol. 2018, 25, 1768–1774. [Google Scholar] [CrossRef]
- Scott, A.T.; Breheny, P.J.; Keck, K.J.; Bellizzi, A.M.; Dillon, J.S.; O’Dorisio, T.M.; Howe, J.R. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2019, 165, 166–175. [Google Scholar] [CrossRef]
- Oberg, K. Endocrine tumors of the gastrointestinal tract: Systemic treatment. Anti-Cancer Drugs 1994, 5, 503–519. [Google Scholar]
- Plöckinger, U.; Wiedenmann, B. Biotherapy. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 145–162. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.E.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.H.; Goldner, W.S.; Benson, A.B.; Bergsland, E.; Blaszkowsky, L.S.; Brock, P.; Chan, J.; Das, S.; Dickson, P.V.; Fanta, P.; et al. Neuroendocrine and Adrenal Tumors, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 839–868. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.S. Hepatic-directed Therapies in Patients with Neuroendocrine Tumors. Hematol. Oncol. Clin. N. Am. 2016, 30, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, R.A.; Beyer, D.T.; Chauhan, A.; Boudreaux, J.P.; Wang, Y.Z.; Woltering, E.A. The Role of Capecitabine/Temozolomide in Metastatic Neuroendocrine Tumors. Oncologist 2016, 21, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, P.L.; Graham, N.T.; Catalano, P.J.; Nimeiri, H.S.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Martin, B.A.; Yao, J.C.; Kulke, M.H.; et al. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J. Clin. Oncol. 2023, 41, 1359–1369. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib Malate for the Treatment of Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Rindi, G.; Klöppel, G.; Alhman, H.; Caplin, M.; Couvelard, A.; de Herder, W.W.; Erikssson, B.; Falchetti, A.; Falconi, M.; Komminoth, P.; et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Archiv. 2006, 449, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Basturk, O.; Yang, Z.; Tang, L.H.; Hruban, R.H.; Adsay, V.; McCall, C.M.; Krasinskas, A.M.; Jang, K.-T.; Frankel, W.L.; Balci, S.; et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am. J. Surg. Pathol. 2015, 39, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Coriat, R.; Walter, T.; Terris, B.; Couvelard, A.; Ruszniewski, P. Gastroenteropancreatic Well-Differentiated Grade 3 Neuroendocrine Tumors: Review and Position Statement. Oncologist 2016, 21, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Velayoudom-Cephise, F.L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T.; et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer. 2013, 20, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Kasajima, A.; Konukiewitz, B.; Schlitter, A.M.; Weichert, W.; Klöppel, G. An analysis of 130 neuroendocrine tumors G3 regarding prevalence, origin, metastasis, and diagnostic features. Virchows Arch. 2022, 480, 359–368. [Google Scholar] [CrossRef]
- Elvebakken, H.; Perren, A.; Scoazec, J.Y.; Tang, L.H.; Federspiel, B.; Klimstra, D.S.; Vestermark, L.W.; Ali, A.S.; Zlobec, I.; Myklebust, T.; et al. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology 2021, 111, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Raj, N.; Valentino, E.; Capanu, M.; Tang, L.H.; Basturk, O.; Untch, B.R.; Allen, P.J.; Klimstra, D.S.; Reidy-Lagunes, D. Treatment Response and Outcomes of Grade 3 Pancreatic Neuroendocrine Neoplasms Based on Morphology: Well Differentiated Versus Poorly Differentiated. Pancreas 2017, 46, 296–301. [Google Scholar] [CrossRef]
- Venizelos, A.; Elvebakken, H.; Perren, A.; Nikolaienko, O.; Deng, W.; Lothe, I.M.B.; Couvelard, A.; Hjortland, G.O.; Sundlöv, A.; Svensson, J.; et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr.-Relat. Cancer 2022, 29, 1–14. [Google Scholar] [CrossRef]
- Yachida, S.; Totoki, Y.; Noë, M.; Nakatani, Y.; Horie, M.; Kawasaki, K.; Nakamura, H.; Saito-Adachi, M.; Suzuki, M.; Takai, E.; et al. Comprehensive Genomic Profiling of Neuroendocrine Carcinomas of the Gastrointestinal System. Cancer Discov. 2022, 12, 692–711. [Google Scholar] [CrossRef]
- Öberg, K.; Knigge, U.; Kwekkeboom, D.; Perren, A. ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23 (Suppl. S7), vii124–vii130. [Google Scholar] [CrossRef]
- Lithgow, K.; Venkataraman, H.; Hughes, S.; Shah, H.; Kemp-Blake, J.; Vickrage, S.; Smith, S.; Humphries, S.; Elshafie, M.; Taniere, P.; et al. Well-differentiated gastroenteropancreatic G3 NET: Findings from a large single centre cohort. Sci. Rep. 2021, 11, 17947. [Google Scholar] [CrossRef]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef]
- Sorbye, H.; Strosberg, J.; Baudin, E.; Klimstra, D.S.; Yao, J.C. Gastroenteropancreatic high-grade neuroendocrine carcinoma. Cancer 2014, 120, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2018, 124, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive Genomic Profiling of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Clin. Cancer. Res. 2020, 26, 5943–5951. [Google Scholar] [CrossRef]
- Alese, O.B.; Jiang, R.; Shaib, W.; Wu, C.; Akce, M.; Behera, M.; El-Rayes, B.F. High-Grade Gastrointestinal Neuroendocrine Carcinoma Management and Outcomes: A National Cancer Database Study. Oncologist 2019, 24, 911–920. [Google Scholar] [CrossRef] [Green Version]
- McNamara, M.G.; Swain, J.; Craig, Z.; Sharma, R.; Faluyi, O.O.; Wadsley, J.; Morgan, C.; Wall, L.R.; Chau, I.; Reed, N.; et al. NET-02: A multicenter, randomized, phase II trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients (pts) with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma (PD-EP-NEC). J. Clin. Oncol. 2022, 40, 4005. [Google Scholar] [CrossRef]
- Walter, T.; Lievre, A.; Coriat, R.; Malka, D.; Elhajbi, F.; Di Fiore, F.; Hentic, O.; Smith, D.; Hautefeuille, V.; Roquin, G.; et al. Bevacizumab plus FOLFIRI after failure of platinum–etoposide first-line chemotherapy in patients with advanced neuroendocrine carcinoma (PRODIGE 41-BEVANEC): A randomised, multicentre, non-comparative, open-label, phase 2 trial. Lancet Oncol. 2023, 24, 297–306. [Google Scholar] [CrossRef]
- McGarrah, P.W.; Leventakos, K.; Hobday, T.J.; Molina, J.R.; Finnes, H.D.; Westin, G.F.; Halfdanarson, T.R. Efficacy of Second-Line Chemotherapy in Extrapulmonary Neuroendocrine Carcinoma. Pancreas 2020, 49, 529–533. [Google Scholar] [CrossRef]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Capdevila, J.; Teule, A.; López, C.; García-Carbonero, R.; Benavent, M.; Custodio, A.; Cubillo, A.; Alonso, V.; Gordoa, T.A.; Carmona-Bayonas, A.; et al. 1157O A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann. Oncol. 2020, 31, S770–S771. [Google Scholar] [CrossRef]
- Al-Toubah, T.; Cives, M.; Strosberg, J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl. Gastroenterol. Hepatol. 2020, 5, 54. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Thorburn, D.; Toumpanakis, C.; Frazzoni, L.; Johnson, G.; Vessal, S.; Luong, T.V.; Caplin, M.; Pereira, S.P. Endoscopic ultrasound-guided fine-needle aspiration vs. fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1393–E1399. [Google Scholar] [CrossRef] [Green Version]
- Rossi, R.E.; Elvevi, A.; Gallo, C.; Palermo, A.; Invernizzi, P.; Massironi, S. Endoscopic techniques for diagnosis and treatment of gastro-entero-pancreatic neuroendocrine neoplasms: Where we are. World J. Gastroentrology 2022, 28, 3258. [Google Scholar] [CrossRef]
- Larghi, A.; Capurso, G.; Carnuccio, A.; Ricci, R.; Alfieri, S.; Galasso, D.; Lugli, F.; Bianchi, A.; Panzuto, F.; De Marinis, L.; et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest. Endosc. 2012, 76, 570–577. [Google Scholar] [CrossRef]
- Vinayek, R.; Capurso, G.; Larghi, A. Grading of EUS-FNA cytologic specimens from patients with pancreatic neuroendocrine neoplasms: It is time move to tissue core biopsy? Gland. Surg. 2014, 3, 222–225. [Google Scholar] [CrossRef]
- Fujimori, N.; Osoegawa, T.; Lee, L.; Tachibana, Y.; Aso, A.; Kubo, H.; Kawabe, K.; Igarashi, H.; Nakamura, K.; Oda, Y.; et al. Efficacy of endoscopic ultrasonography and endoscopic ultrasonography-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors. Scand. J. Gastroenterol. 2016, 51, 245–252. [Google Scholar] [CrossRef]
- Weynand, B.; Borbath, I.; Bernard, V.; Sempoux, C.; Gigot, J.F.; Hubert, C.; Lannoy, V.; Deprez, P.H.; Jouret-Mourin, A. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: High reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 2014, 25, 389–395. [Google Scholar] [CrossRef]
- Chung, Y.R.; Jang, M.H.; Park, S.Y.; Gong, G.; Jung, W.-H.; The Korean Breast Pathology Ki-67 Study Group. Interobserver Variability of Ki-67 Measurement in Breast Cancer. J. Pathol. Transl. Med. 2016, 50, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.H.; Basturk, O.; Sue, J.J.; Klimstra, D.S. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am. J. Surg. Pathol. 2016, 40, 1192–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klöppel, G. Neuroendocrine Neoplasms: Dichotomy, Origin and Classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Untch, B.R.; Reidy, D.L.; O’Reilly, E.; Dhall, D.; Jih, L.; Basturk, O.; Allen, P.J.; Klimstra, D.S. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1011–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Shao, Y.; Yi, X.; Newey, P.J.; Li, D.; Ding, K.; Gastrointestinal Cancer Evolution Study Group. Metastatic Timing and Genetic Heterogeneity in the Evolution of a Pancreatic Neuroendocrine Tumor. Off. J. Am. Coll. Gastroenterol.|ACG 2021, 116, 844–847. [Google Scholar] [CrossRef]
- Pedraza-Arévalo, S.; Gahete, M.D.; Alors-Pérez, E.; Luque, R.M.; Castaño, J.P. Multilayered heterogeneity as an intrinsic hallmark of neuroendocrine tumors. Rev. Endocr. Metab. Disord. 2018, 19, 179–192. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, Q.; Han, C.; Zhen, D.; Lin, R. Variability of the Ki-67 proliferation index in gastroenteropancreatic neuroendocrine neoplasms–a single-center retrospective study. BMC Endocr. Disord. 2018, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Hallet, J.; Rowsell, C.; Law, C.H. Variability of Ki67 labeling index in multiple neuroendocrine tumors specimens over the course of the disease. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2014, 40, 1517–1522. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Poppinga, J.; Lehman, B.; Maurer, E.; Ramaswamy, A.; Grass, A.; Di Fazio, P.; Rinke, A.; Denkert, C.; Bartsch, D.K. Frequency and Prognostic Significance of Intertumoural Heterogeneity in Multifocal Jejunoileal Neuroendocrine Tumours. Cancers 2022, 14, 3963. [Google Scholar] [CrossRef]
- Keck, K.J.; Choi, A.; Maxwell, J.E.; Li, G.; O’Dorisio, T.M.; Breheny, P.; Bellizzi, A.M.; Howe, J.R. Increased Grade in Neuroendocrine Tumor Metastases Negatively Impacts Survival. Ann. Surg. Oncol. 2017, 24, 2206–2212. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, C.; Wang, X.; Ding, X.; Zhang, B.; Yang, Z.; Wang, Y.; Sheng, W.; Huang, D. Digital Image Analysis of Ki67 Heterogeneity Improves the Diagnosis and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Mod. Pathol. 2023, 36, 100017. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Zhang, Q.; Qi, C.; Yao, H.; Lin, R. Clinicopathological heterogeneity between primary and metastatic sites of gastroenteropancreatic neuroendocrine neoplasm. Diagn. Pathol. 2020, 15, 108. [Google Scholar] [CrossRef]
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.C.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am. J. Clin. Pathol. 2015, 143, 398–404. [Google Scholar] [CrossRef] [Green Version]
- Botling, J.; Lamarca, A.; Bajic, D.; Norlén, O.; Lönngren, V.; Kjaer, J.; Eriksson, B.; Welin, S.; Hellman, P.; Rindi, G.; et al. High-Grade Progression Confers Poor Survival in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 891–898. [Google Scholar] [CrossRef]
- Panzuto, F.; Cicchese, N.; Partelli, S.; Rinzivillo, M.; Capurso, G.; Merola, E.; Manzoni, M.; Pucci, E.; Iannicelli, E.; Pilozzi, E.; et al. Impact of Ki67 re-assessment at time of disease progression in patients with pancreatic neuroendocrine neoplasms. PLoS ONE 2017, 12, e0179445. [Google Scholar] [CrossRef] [Green Version]
- Merola, E.; Perren, A.; Rinke, A.; Zerbi, A.; McNamara, M.G.; Arsenic, R.; Fazio, N.; de Herder, W.; Valle, J.W.; Gress, T.M.; et al. High rate of Ki-67 increase in entero-pancreatic NET relapses after surgery with curative intent. J. Neuroendocrinol. 2022, 34, e13193. [Google Scholar] [CrossRef]
- Vyas, M.; Tang, L.H.; Rekhtman, N.; Klimstra, D.S. Alterations in Ki67 Labeling Following Treatment of Poorly Differentiated Neuroendocrine Carcinomas: A Potential Diagnostic Pitfall. Am. J. Surg. Pathol. 2021, 45, 25–34. [Google Scholar] [CrossRef]
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci. Rep. 2018, 8, 3811. [Google Scholar] [CrossRef] [Green Version]
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef]
- Kayani, I.; Bomanji, J.B.; Groves, A.; Conway, G.; Gacinovic, S.; Win, T.; Dickson, J.; Caplin, M.; Ell, P.J. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 2008, 112, 2447–2455. [Google Scholar] [CrossRef]
- Panagiotidis, E.; Alshammari, A.; Michopoulou, S.; Skoura, E.; Naik, K.; Maragkoudakis, E.; Mohmaduvesh, M.; Al-Harbi, M.; Belda, M.; Caplin, M.E.; et al. Comparison of the Impact of 68Ga-DOTATATE and 68F-FDG PET/CT on Clinical Management in Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Q.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Baum, R.P. Prognostic Value of (18)F-FDG PET/CT in a Large Cohort of Patients with Advanced Metastatic Neuroendocrine Neoplasms Treated with Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2020, 61, 1560–1569. [Google Scholar] [CrossRef]
- Adnan, A.; Basu, S. Discordance Between Histopathologic Grading and Dual-Tracer PET/CT Findings in Metastatic NETs and Outcome of (177)Lu-DOTATATE PRRT: Does In Vivo Molecular PET Perform Better from the Viewpoint of Prediction of Tumor Biology? J. Nucl. Med. Technol. 2022, 50, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Barnes, T.; Diakos, C.; Khasraw, M.; Samra, J.; et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics 2017, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, D.L.; Hayes, A.R.; Karfis, I.; Conner, A.; Furtado O’Mahony, L.; Mileva, M.; Bernard, E.; Roach, P.; Marin, G.; Pavlakis, N.; et al. Dual [(68)Ga]DOTATATE and [(18)F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: A multicentre validation of the NETPET score. Br. J. Cancer 2023, 128, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Nakamoto, R.; Moore, S.E.; Pantel, A.R.; Eads, J.R.; Aparici, C.M.; Pryma, D.A. Combined Quantification of (18)F-FDG and (68)Ga-DOTATATE PET/CT for Prognosis in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. Acad. Radiol. 2022, 29, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- De Hosson, L.D.; van der Loo–van der Schaaf, A.M.; Boellaard, R.; van Snick, J.H.; de Vries, E.G.E.; Brouwers, A.H.; Walenkamp, A.M.E. Interlesional Heterogeneity of Metastatic Neuroendocrine Tumors Based on 18F-DOPA PET/CT. Clin. Nucl. Med. 2019, 44, 612–619. [Google Scholar] [CrossRef]
- Assi, H.A.; Hornbacker, K.; Shaheen, S.; Wittenberg, T.; Silberman, R.; Kunz, P.L. Rapid Progression After 177Lu-DOTATATE in Patients With Neuroendocrine Tumors. Pancreas 2021, 50, 890–894. [Google Scholar] [CrossRef]
- Ireland, A.S.; Micinski, A.M.; Kastner, D.W.; Guo, B.; Wait, S.J.; Spainhower, K.B.; Conley, C.C.; Chen, O.S.; Guthrie, M.R.; Soltero, D.; et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate. Cancer Cell 2020, 38, 60–78.e12. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Dong, B.; Miao, J.; Wang, Y.; Luo, W.; Ji, Z.; Lai, H.; Zhang, M.; Cheng, X.; Wang, J.; Fang, Y.; et al. Single-cell analysis supports a luminal-neuroendocrine transdifferentiation in human prostate cancer. Commun. Biol. 2020, 3, 778. [Google Scholar] [CrossRef]
- Hope, T.A.; Pavel, M.; Bergsland, E.K. Neuroendocrine Tumors and Peptide Receptor Radionuclide Therapy: When Is the Right Time? J. Clin. Oncol. 2022, 40, 2818–2829. [Google Scholar] [CrossRef]
- Sorbye, H.; Grande, E.; Pavel, M.; Tesselaar, M.; Fazio, N.; Reed, N.S.; Knigge, U.; Christ, E.; Ambrosini, V.; Couvelard, A.; et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for digestive neuroendocrine carcinoma. J. Neuroendocrinol. 2023, 35, e13249. [Google Scholar] [CrossRef]
- Sun, F.; Grenert, J.P.; Tan, L.; Van Ziffle, J.; Joseph, N.M.; Mulvey, C.K.; Bergsland, E. Checkpoint Inhibitor Immunotherapy to Treat Temozolomide-Associated Hypermutation in Advanced Atypical Carcinoid Tumor of the Lung. JCO Precis. Oncol. 2022, 6, e2200009. [Google Scholar] [CrossRef]
| Terminology | Differentiation | Mitotic Count | Ki-67 Index |
|---|---|---|---|
| Grade 1 NET | Well differentiated | <2/2 mm2 and/or | <3% |
| Grade 2 NET | Well differentiated | 2–20/2 mm2. and/or | 3–20% |
| Grade 3 NET | Well differentiated | >20/2 mm2 and/or | >20% |
| Neuroendocrine carcinoma (NEC) | Poorly differentiated Small-cell cytomorphology Large-cell cytomorphology | >20/2 mm2 and/or | >20% |
| Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) | Well or poorly differentiated component (≥30%) | Variable | Variable |
| Reference | N | Site of Origin | Definition of Metachronous (mo) | Type of Heterogeneity | Description | Grade Change, (n) | |
|---|---|---|---|---|---|---|---|
| Ki67 Index N (%) | Grade Change N (%) | ||||||
| Singh [69] | 43 | NET mix ^ (47% SB; 14% P) (baseline grade NOS) | Not explicitly Defined & (13/16 cases of grade change metachronous; range 229–2613 days between samples) (3/16 cases of grade change synchronous; range 111–143 days) | 12/43 (28%) | Higher grade over time (9/12 initial biopsies of the primary site; 11/12 follow-up biopsies of the metastatic site) | G1→G2(5) (3 SB, 1P, 1R) G1→G3(5) ** G2→G3(2) ** | |
| 4/43 (9%) | Lower grade over time (3/4 had biopsies of the metastatic site followed by the primary site; ¼ had serial biopsies of the metastatic site) | G3 → G1 (1) (1 SB) G2→ G1 (3) (1 SB, 1 P, 1R) | |||||
| Grillo [7] | 12 | GEP-NET # (52% SB; 27% P) (93% G1/2) | >4 mo | 10/12 (83%) | Higher grade over time in metastases | G1→ G2 (9) (NOS) G2→G3 (1) (1 P) | |
| Botling [75] | 46 | Pancreas NET (96% G1/2) | Not defined * | 76% with Ki67 index | 34/59 samples (58%) | Ki67 index +/− higher grade in metastases vs. original biopsy (52% with G1/2 to G3 NETs) | G1→G2 (7) G1/G2→G3(27) (all P) |
| 4/59 samples (7%) | Lower grade over time in metastases vs. original biopsies | ||||||
| Panzuto [76] | 43 | G1 /G2 GEP-NET ^^ (56% P; 44% SB) | & (range of 3–148 mo for pancreas; 5–128 mo for SB) | 28/43 (65%) with Ki67 index (71% P; 29% SB) | 12/43 (28%) | Ki 67 index and/or higher grade at disease progression (10/24 (41.7%) of P cases and 2/19 (10.5%) of the SB cases had grade progression) | G1→G2 (8) (6P; 2 SB) G2→G3 (4) (all P; 4/24, 16.7%) |
| 2/43 (5%) | Lower grade at progression | G2→G1(2) (SB only) | |||||
| Merola [77] | 47 | G1/G2 GEP-NET (43% P; 57% SB) | 4–176 mo | 31/47 (65%) with Ki-67 index | Higher grade in 34% of cases over time | ||
| Study | N | Tumor Type | Site | Proportion of Patients/Lesions with Heterogeneity | Types of Scans Compared | Conclusions |
|---|---|---|---|---|---|---|
| Chan [85] | 62 | G1–G3 NET | NET mix (P = 39%, midgut = 32%) | 33/62 (53%) | 68Ga- DOTATATE and 18F-FDG PET scan. | Dual imaging is prognostic |
| Zhang [11] | 83 | GEP-NEN | P = 27%, GI = 42%, Unknown = 13% | 37/83 (44%) | 68Ga-DOTATATE and 18F-FDG PET scan | Dual imaging is prognostic, especially if Ki-67 > 10%. |
| Kayani [81] | 38 (303 lesions) | G1–G3 NEN | Lung = 6 GEP = 28 Unknown = 4 | 71/303 (23%) | 68Ga-DOTATATE and 18F-FDG PET scan. | Dual imaging facilitates the characterization of intermediate and high-grade NETs (and the heterogeneity within and between tumor sites) |
| Graf [80] | 65 | G1/G2 NET | NET mix (ileum = 36.9%, P = 24.6%) | 28/65 (43%) | SSTR expression by 68Ga-DOTA PET imaging | Heterogeneous SSTR expression by 68Ga-DOTATATE or 68Ga-DOTATOC PET imaging is prognostic in G1/2 NETs |
| Zhang [83] | 495 | G1/G2/G3 NEN | NET mix (P = 199, midgut = 139, rectal = 20, lung = 38, stomach = 8, unknown/other = 91) | 382/495(77%) | 68Ga-DOTATATE and 18F-FDG PET scan in patients before PRRT | Positive lesions in 18F-FDG PET imaging is an independent prognostic factor in patients treated with PRRT. |
| Chan [86] | 319 | G1/G2/G3 Unknown NEN | NET mix (midgut = 52%, P = 36%, hindgut/rectum = 7%, other = 5%) | 193/319 (63%) | 68Ga- DOTATATE and 18F-FDG PET scan | Dual imaging as prognostic. |
| Adnan [84] | 36 | G1–G3 NET/NEC | NET mix | 68Ga-DOTATATE and 18F-FDG PET scan in patients before PRRT and WHO grading | Dual imaging showed better prognostication in PRRT than WHO grading, differentiation, and immunohistochemistry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varghese, D.G.; Del Rivero, J.; Bergsland, E. Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2023, 15, 3712. https://doi.org/10.3390/cancers15143712
Varghese DG, Del Rivero J, Bergsland E. Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers. 2023; 15(14):3712. https://doi.org/10.3390/cancers15143712
Chicago/Turabian StyleVarghese, Diana Grace, Jaydira Del Rivero, and Emily Bergsland. 2023. "Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors" Cancers 15, no. 14: 3712. https://doi.org/10.3390/cancers15143712
APA StyleVarghese, D. G., Del Rivero, J., & Bergsland, E. (2023). Grade Progression and Intrapatient Tumor Heterogeneity as Potential Contributors to Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers, 15(14), 3712. https://doi.org/10.3390/cancers15143712







