A Personalized Risk Model for Azacitidine Outcome in Myelodysplastic Syndrome and Other Myeloid Neoplasms Identified by Machine Learning Model Utilizing Real-World Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Response Assessment
2.3. Construction of Prediction Model Using Random Survival Forest Machine Learning Algorithm
2.4. Statistical Analysis
3. Results
3.1. Personalized Risk Model to Predict Outcome of Patients Treated with Azacitidine
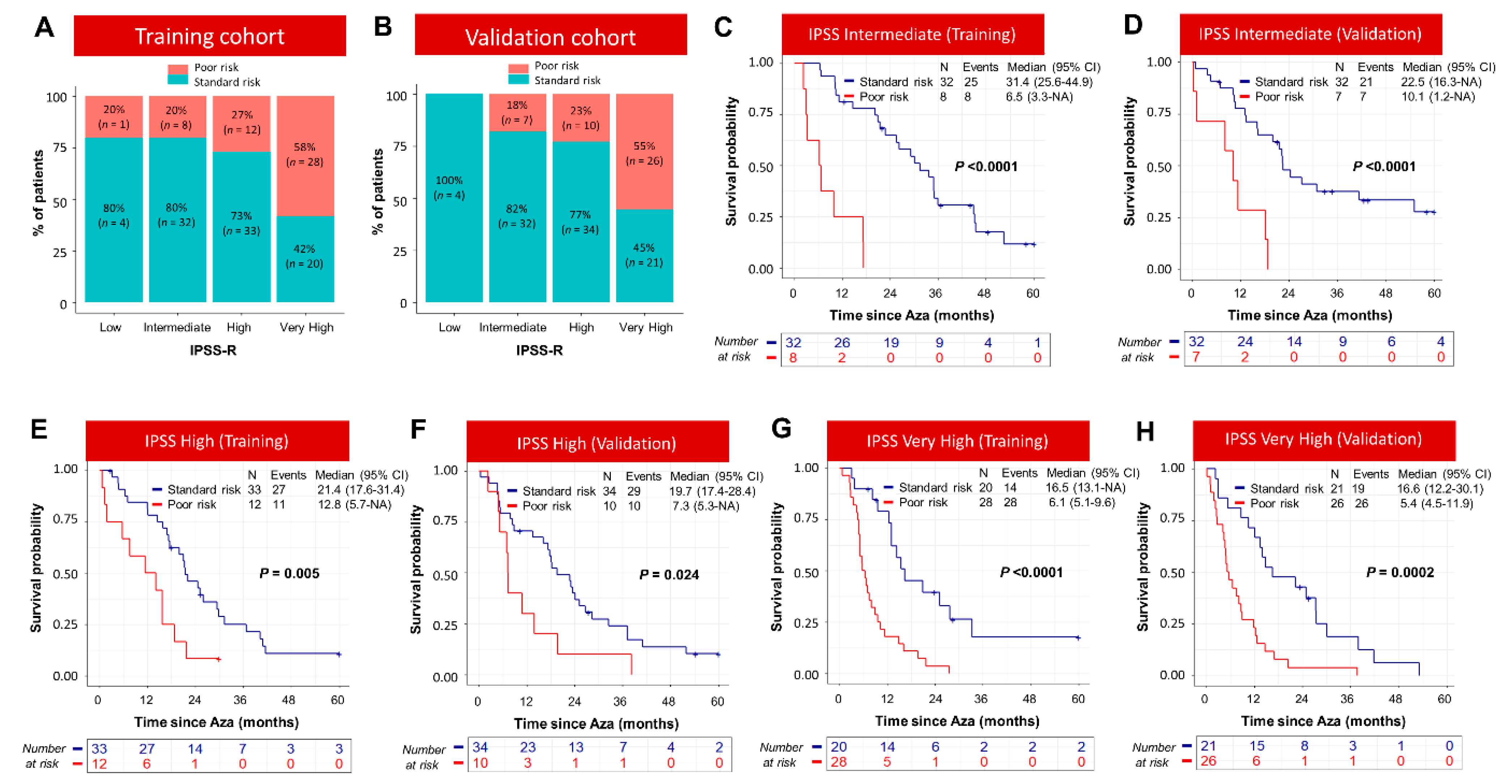
3.2. Integration of Somatic Mutation Information into the Prognostic Model Based on Clinical Variables Identified Three Distinct Groups of Patients with Significant Survival Differences
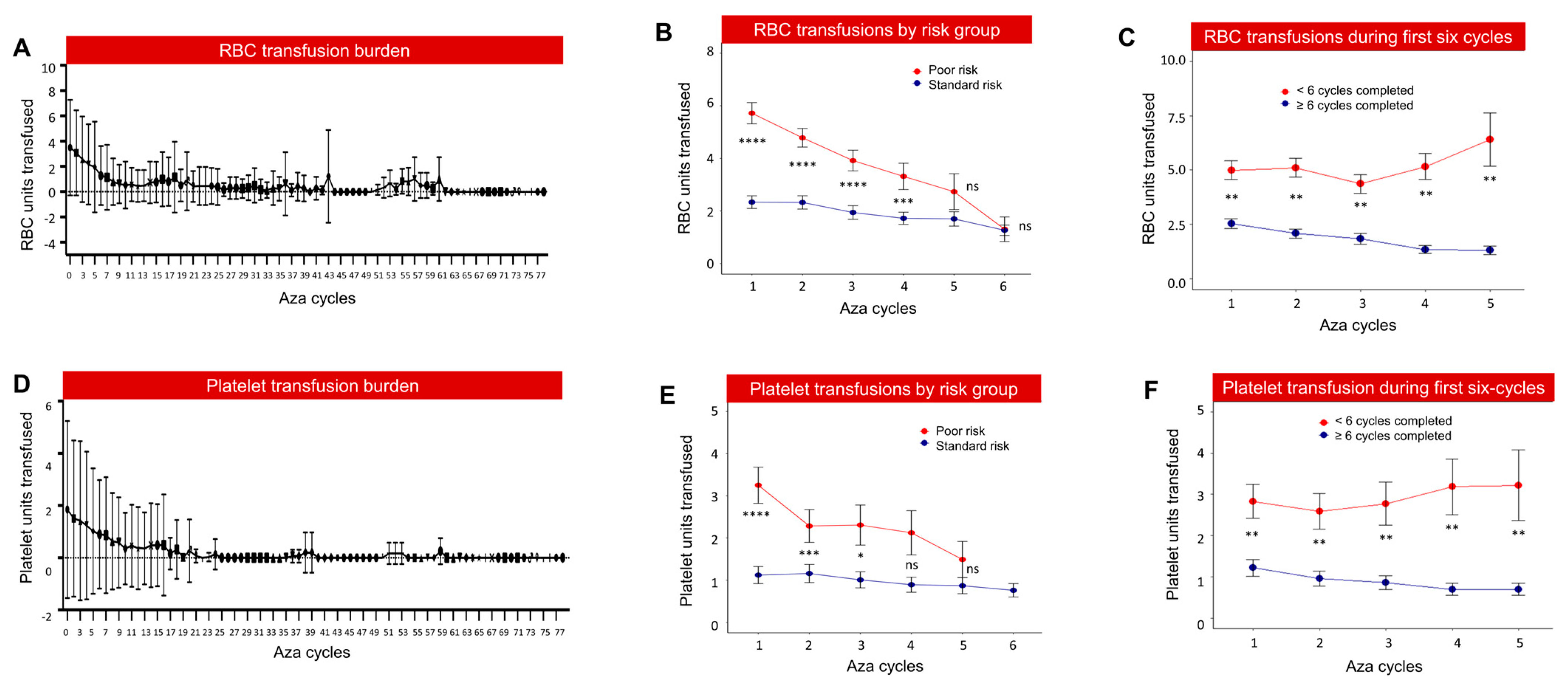
3.3. High Transfusion Burden and Healthcare Utilization in Poor-Risk Patients
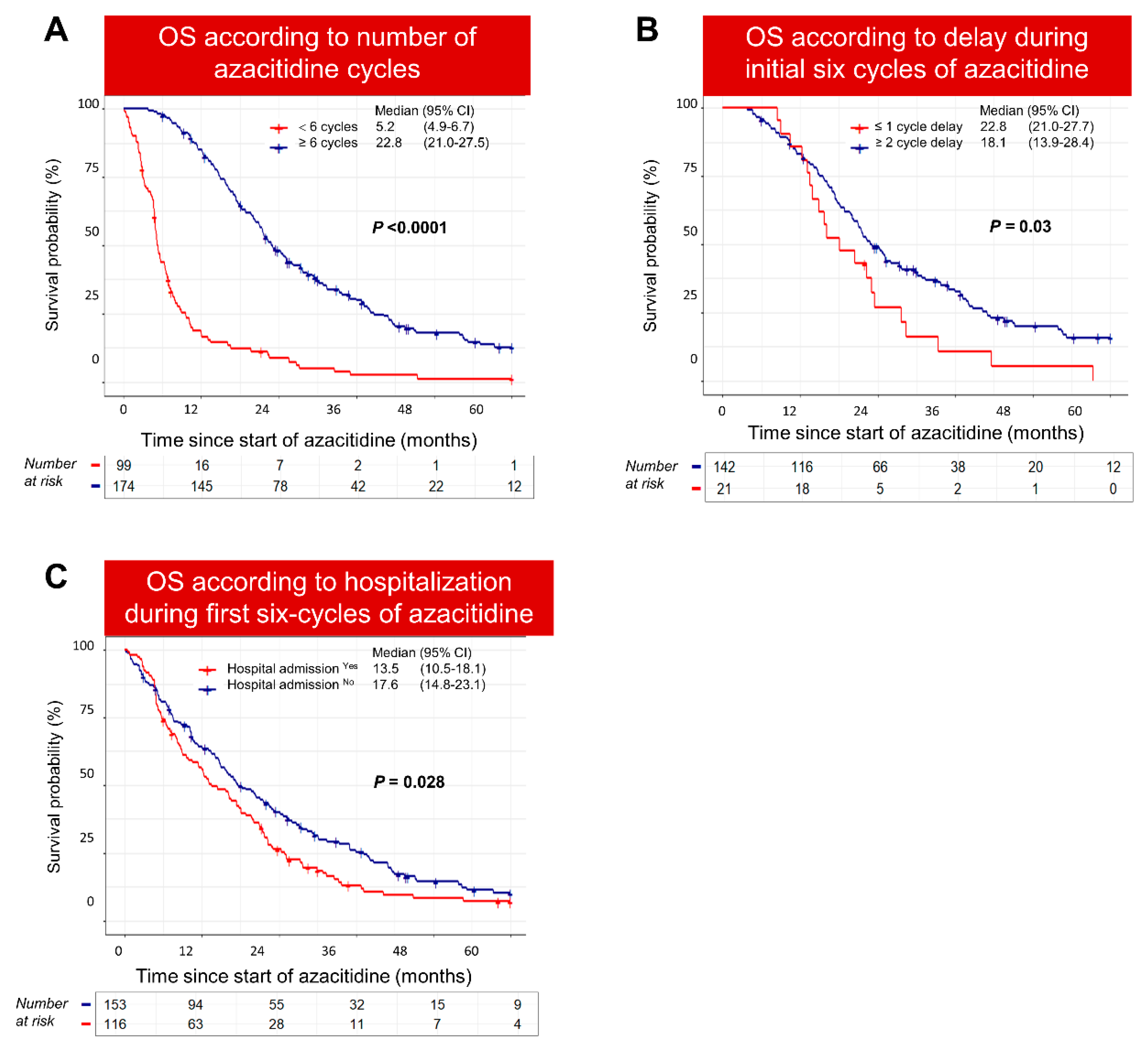
3.4. Poor-Risk Patients Were Less Likely to Complete Six Cycles of Azacitidine
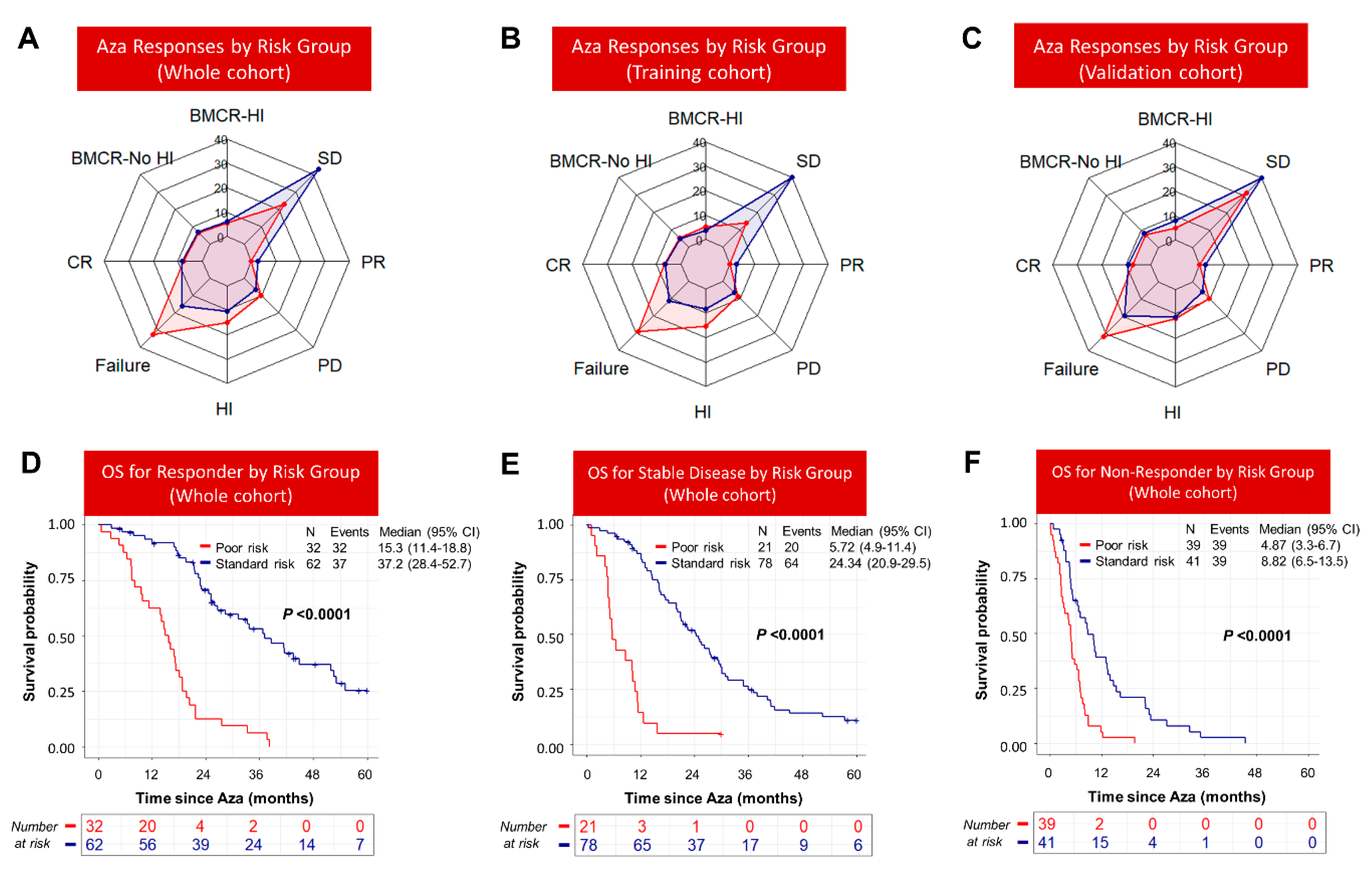
3.5. Response to Azacitidine Did Not Completely Abrogate Inferior Survival of Poor-Risk Patients
3.6. Poor Survival after Azacitidine Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, T.; Martínez-Camblor, P.; Sánchez-García, J.; de Paz, R.; Luño, E.; Nomdedeu, B.; Ardanaz, M.T.; Pedro, C.; Amigo, M.L.; Xicoy, B.; et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: Results from the Spanish registry. Leukemia 2015, 29, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Bravo, G.; Garcia-Manero, G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2018, 93, 129–147. [Google Scholar] [CrossRef]
- Stomper, J.; Lubbert, M. Can we predict responsiveness to hypomethylating agents in AML? Semin. Hematol. 2019, 56, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Stahl, M.; Sekeres, M.A.; Steensma, D.P.; Komrokji, R.S.; Gore, S.D. A call for action: Increasing enrollment of untreated patients with higher-risk myelodysplastic syndromes in first-line clinical trials. Cancer 2017, 123, 3662–3672. [Google Scholar] [CrossRef]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Beyne-Rauzy, O.; Turlure, P.; Vey, N.; Recher, C.; Dartigeas, C.; Legros, L.; et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011, 117, 403–411. [Google Scholar] [CrossRef] [PubMed]
- van der Helm, L.H.; Alhan, C.; Wijermans, P.W.; van Marwijk Kooy, M.; Schaafsma, R.; Biemond, B.J.; Beeker, A.; Hoogendoorn, M.; van Rees, B.P.; de Weerdt, O.; et al. Platelet doubling after the first azacitidine cycle is a promising predictor for response in myelodysplastic syndromes (MDS), chronic myelomonocytic leukaemia (CMML) and acute myeloid leukaemia (AML) patients in the Dutch azacitidine compassionate named patient programme. Br. J. Haematol. 2011, 155, 599–606. [Google Scholar] [CrossRef]
- García-Delgado, R.; de Miguel, D.; Bailén, A.; González, J.R.; Bargay, J.; Falantes, J.F.; Andreu, R.; Ramos, F.; Tormo, M.; Brunet, S.; et al. Effectiveness and safety of different azacitidine dosage regimens in patients with myelodysplastic syndromes or acute myeloid leukemia. Leuk. Res. 2014, 38, 744–750. [Google Scholar] [CrossRef]
- Gangat, N.; Patnaik, M.M.; Begna, K.; Al-Kali, A.; Litzow, M.R.; Ketterling, R.P.; Hanson, C.A.; Pardanani, A.D.; Tefferi, A. Survival trends in primary myelodysplastic syndromes: A comparative analysis of 1000 patients by year of diagnosis and treatment. Blood Cancer J. 2016, 6, e414. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Davidoff, A.J.; Long, J.B.; Hu, X.; Wang, R.; Ma, X.; Gross, C.P.; Abel, G.A.; Huntington, S.F.; Podoltsev, N.A.; et al. Comparative clinical effectiveness of azacitidine versus decitabine in older patients with myelodysplastic syndromes. Br. J. Haematol. 2016, 175, 829–840. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Sekeres, M.A.; Garcia-Manero, G.; Steensma, D.P.; Zell, K.; Barnard, J.; Ali, N.A.; Zimmerman, C.; Roboz, G.; DeZern, A.; et al. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia 2016, 30, 649–657. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Wang, R.; Gross, C.P.; Gore, S.D.; Huntington, S.F.; Prebet, T.; Abel, G.A.; Davidoff, A.J.; Ma, X. Modest improvement in survival of patients with refractory anemia with excess blasts in the hypomethylating agents era in the United States. Leuk. Lymphoma 2017, 58, 982–985. [Google Scholar] [CrossRef]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. Ann. Appl. Stat. 2008, 2, 820, 841–860. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the Yield of Medical Tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Hiwase, D.K.; Singhal, D.; Strupp, C.; Chhetri, R.; Kutyna, M.M.; Wee, L.A.; Harrison, P.B.; Nath, S.V.; Wickham, N.; Hui, C.-H.; et al. Dynamic assessment of RBC-transfusion dependency improves the prognostic value of the revised-IPSS in MDS patients. Am. J. Hematol. 2017, 92, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Grinblatt, D.L.; Sekeres, M.A.; Komrokji, R.S.; Swern, A.S.; Sullivan, K.A.; Narang, M. Patients with myelodysplastic syndromes treated with azacitidine in clinical practice: The AVIDA registry. Leuk. Lymphoma 2015, 56, 887–895. [Google Scholar] [CrossRef]
- Steensma, D.P. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J. 2018, 8, 47. [Google Scholar] [CrossRef]
- Singhal, D.; Wee, L.Y.A.; Kutyna, M.M.; Chhetri, R.; Geoghegan, J.; Schreiber, A.W.; Feng, J.; Wang, P.P.; Babic, M.; Parker, W.T.; et al. The mutational burden of therapy-related myeloid neoplasms is similar to primary myelodysplastic syndrome but has a distinctive distribution. Leukemia 2019, 33, 2842–2853. [Google Scholar] [CrossRef]
- Hiwase, D.; Hahn, C.; Tran, E.N.H.; Chhetri, R.; Baranwal, A.; Al-Kali, A.; Sharplin, K.; Ladon, D.; Hollins, R.; Greipp, P.; et al. TP53 mutation in therapy-related myeloid neoplasm defines a distinct molecular subtype. Blood 2023, 141, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Tran, E.N.H.; Shah, S.; Chhetri, R.; Baranwal, A.; Ladon, D.; Shultz, C.; Al-Kali, A.; Brown, A.L.; Chen, D.; et al. TP53 mutation variant allele frequency of >/=10% is associated with poor prognosis in therapy-related myeloid neoplasms. Blood Cancer J. 2023, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Tuechler, H.; Greenberg Peter, L.; Hasserjian Robert, P.; Arango Ossa Juan, E.; Nannya, Y.; Devlin Sean, M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef]
- Nazha, A.; Komrokji, R.; Meggendorfer, M.; Jia, X.; Radakovich, N.; Shreve, J.; Hilton, C.B.; Nagata, Y.; Hamilton, B.K.; Mukherjee, S.; et al. Personalized Prediction Model to Risk Stratify Patients With Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 3737–3746. [Google Scholar] [CrossRef]
- Nazha, A.; Sekeres, M.A.; Bejar, R.; Rauh, M.J.; Othus, M.; Komrokji, R.S.; Barnard, J.; Hilton, C.B.; Kerr, C.M.; Steensma, D.P.; et al. Genomic Biomarkers to Predict Resistance to Hypomethylating Agents in Patients With Myelodysplastic Syndromes Using Artificial Intelligence. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Stahl, M.; DeVeaux, M.; Giri, S.; Huntington, S.; Podoltsev, N.; Wang, R.; Ma, X.; Davidoff, A.J.; Gore, S.D. Counseling patients with higher-risk MDS regarding survival with azacitidine therapy: Are we using realistic estimates? Blood Cancer J. 2018, 8, 55. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Stahl, M.; Hu, X.; Wang, R.; Huntington, S.F.; Podoltsev, N.A.; Gore, S.D.; Ma, X.; Davidoff, A.J. Long-term survival of older patients with MDS treated with HMA therapy without subsequent stem cell transplantation. Blood 2018, 131, 818–821. [Google Scholar] [CrossRef]
- Garcia, J.S.; Swords, R.T.; Roboz, G.J.; Jacoby, M.A.; Garcia-Manero, G.; Hong, W.J.; Yang, X.; Zhou, Y.; Platzbecker, U.; Steensma, D.P.; et al. A systematic review of higher-risk myelodysplastic syndromes clinical trials to determine the benchmark of azacitidine and explore alternative endpoints for overall survival. Leuk. Res. 2021, 104, 106555. [Google Scholar] [CrossRef]
- Ishwaran, H.; Lauer, M.S.; Blackstone, E.H.; Lu, M.; Kogalur, U.B. randomForestSRC: Random Survival Forests Vignette. 2021. Available online: http://randomforestsrc.org/articles/survival.html (accessed on 10 March 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharplin, K.; Proudman, W.; Chhetri, R.; Tran, E.N.H.; Choong, J.; Kutyna, M.; Selby, P.; Sapio, A.; Friel, O.; Khanna, S.; et al. A Personalized Risk Model for Azacitidine Outcome in Myelodysplastic Syndrome and Other Myeloid Neoplasms Identified by Machine Learning Model Utilizing Real-World Data. Cancers 2023, 15, 4019. https://doi.org/10.3390/cancers15164019
Sharplin K, Proudman W, Chhetri R, Tran ENH, Choong J, Kutyna M, Selby P, Sapio A, Friel O, Khanna S, et al. A Personalized Risk Model for Azacitidine Outcome in Myelodysplastic Syndrome and Other Myeloid Neoplasms Identified by Machine Learning Model Utilizing Real-World Data. Cancers. 2023; 15(16):4019. https://doi.org/10.3390/cancers15164019
Chicago/Turabian StyleSharplin, Kirsty, William Proudman, Rakchha Chhetri, Elizabeth Ngoc Hoa Tran, Jamie Choong, Monika Kutyna, Philip Selby, Aidan Sapio, Oisin Friel, Shreyas Khanna, and et al. 2023. "A Personalized Risk Model for Azacitidine Outcome in Myelodysplastic Syndrome and Other Myeloid Neoplasms Identified by Machine Learning Model Utilizing Real-World Data" Cancers 15, no. 16: 4019. https://doi.org/10.3390/cancers15164019
APA StyleSharplin, K., Proudman, W., Chhetri, R., Tran, E. N. H., Choong, J., Kutyna, M., Selby, P., Sapio, A., Friel, O., Khanna, S., Singhal, D., Damin, M., Ross, D., Yeung, D., Thomas, D., Kok, C. H., & Hiwase, D. (2023). A Personalized Risk Model for Azacitidine Outcome in Myelodysplastic Syndrome and Other Myeloid Neoplasms Identified by Machine Learning Model Utilizing Real-World Data. Cancers, 15(16), 4019. https://doi.org/10.3390/cancers15164019






