The Bone Microenvironment Soil in Prostate Cancer Metastasis: An miRNA Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Bone Metastasis Microenvironment
3. Exosomes and the Future Bone Metastatic Niche
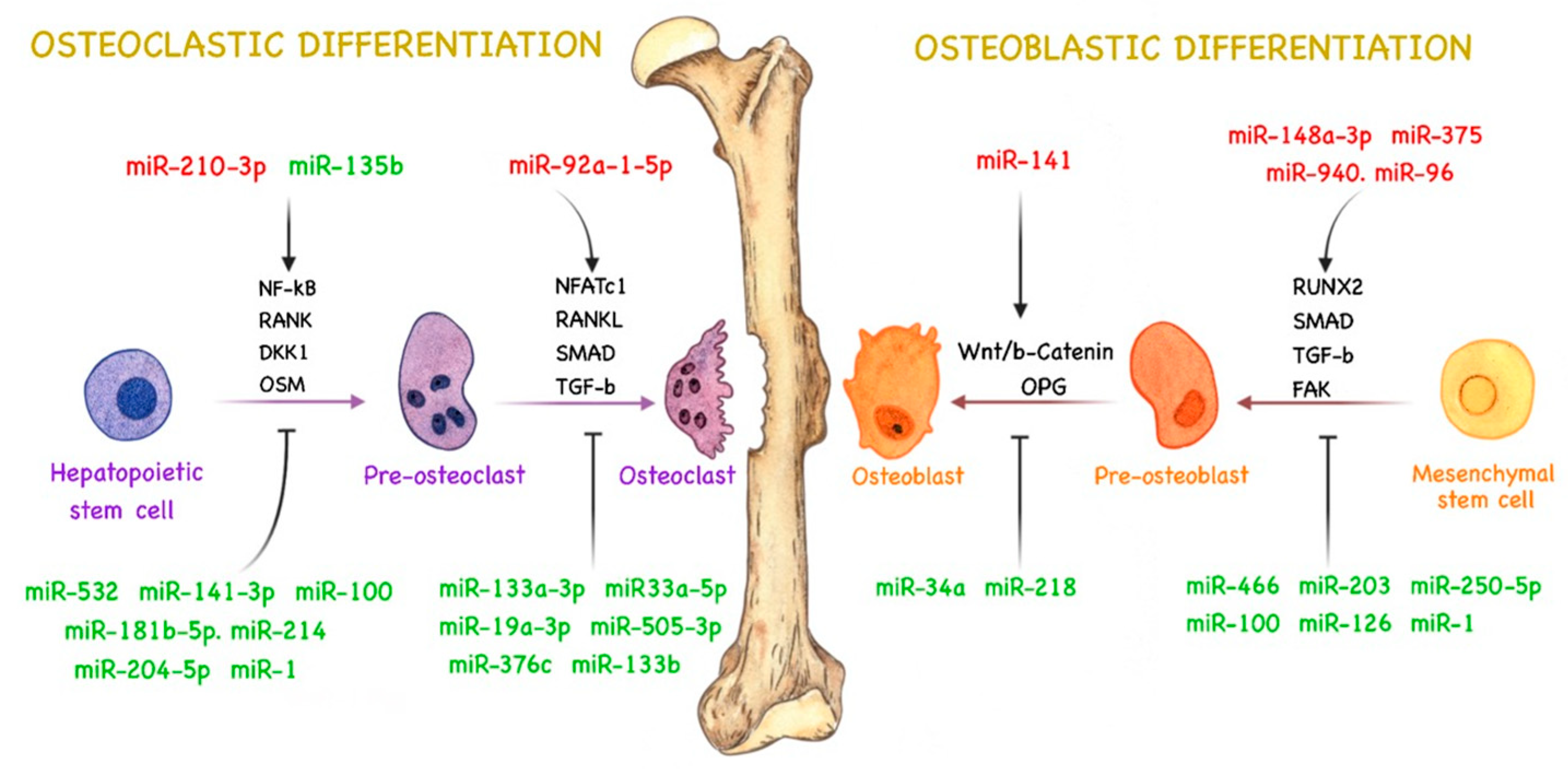
4. miRNAs and Bone Remodeling
4.1. miRNAs Related to Osteoblast Activity in PCa
4.2. miRNAs Related to Osteoclast Activity in PCa
4.3. miRNAs Related to Both Types of Bone Metastasis
5. miRNAs as Potential Therapeutic Targets
6. Diagnosis
| Expression | miRNA | Target | Bone Remodeling Signaling Pathways | Reference |
|---|---|---|---|---|
| Upregulated | miR-221 | SOCS1 | Stimulates cell proliferation, migration, and EMT via SOCS1 downregulation, E-cadherin expression, and activation of the RAS/RAF/MEK/ERK pathway. | [223,225] |
| miR-181a | TGIF2 and KLF17 | Stimulates EMT, invasion, and migration by suppressing TGIF2, inhibits KLF17 and promotes bone metastasis by activating EMT. | [226,228] | |
| miR-199a-5p | PIAS3 and TGF-β | Decreases PIAS3 expression, which increases AKT2 expression and enhances EMT to promote metastasis; activates TGF-β, promoting EMT, invasion, and migration. | [237,238] | |
| miR-425-5p | HSPB8 | Suppresses HSPB8, increasing bone metastasis. | [239] | |
| miR-378-3p | Promotes osteolytic progression by activation of the DYRK1a/NFATC1/ANGPTL2 pathway. | [241] | ||
| Downregulated | miR-23b | HIP1R | Stimulates invasion due to HIP1R overexpression. | [223,224] |
| miR-188-5p | LAPTM4B | Promotes cell proliferation, invasion, and migration by downregulating LAPTM4B. | [229] | |
| miR-543 | eNOS | Increases eNOS expression, promoting cell migration and invasion. | [230] | |
| miR-335 | eNOS | Increases eNOS expression, promoting cell migration and invasion. | [230] | |
| miR-320a | LAMP1 | Increases cell migration and invasion via LAMP1 overexpression. | [232] | |
| miR-194 | BMP1 and CDH2 | Increases cell invasion via overexpression of MMP2 and MMP9 by targeting BMP1, decreases cell death and apoptosis via CDH2 overexpression. | [233,234] | |
| miR-127-3p | PSMB5 | Promotes PSMB5 overexpression, increasing bone metastasis. | [235] | |
| let-7a-5p | TGF-β | Activates the TGF-β pathway, promoting EMT, invasion, and migration. | [238] |
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.; Webber, M.; Benayahu, D.; Kleinman, H.K. Osteonectin promotes prostate cancer cell migration and invasion: A possible mechanism for metastasis to bone. Cancer Res. 1999, 59, 4453–4457. [Google Scholar] [PubMed]
- Theriault, R.L. Biology of bone metastases. Cancer Control 2012, 19, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; MacVicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef] [Green Version]
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Mundy, G.R. Mechanisms of Bone Metastasis. Cancer 1997, 80, 1546–1556. [Google Scholar] [CrossRef]
- Pond, G.R.; Sonpavde, G.; de Wit, R.; Eisenberger, M.A.; Tannock, I.F.; Armstrong, A.J. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur. Urol. 2014, 65, 3–6. [Google Scholar] [CrossRef]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Giaze, T.R.; Chin, K.Y.; Mohamed, N.; Ima-Nirwana, S. Prostate cancer and bone metastases: The underlying mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, G.; Taipaleenmäki, H.; Stein, G.S.; Stein, J.L.; Lian, J.B. MicroRNAs in the control of metastatic bone disease. Trends Endocrinol. Metab. 2014, 25, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoni, E.; van der Pluijm, G. The role of microRNAs in bone metastasis. J. Bone Oncol. 2016, 5, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “seed and Soil” Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Santos, G.G.; Nitadori-Hoshin, A.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Martins, V.R.; Dias, M.S.; Hainaut, P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 2013, 25, 66–75. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Croucher, P.I.; McDonald, M.M.; Martin, T.J. Bone metastasis: The importance of the neighbourhood. Nat. Rev. Cancer 2016, 16, 373–386. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Recent Advances in Osteoclast Biological Behavior. Front. Cell Dev. Biol. 2021, 9, 788680. [Google Scholar] [CrossRef] [PubMed]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2011, 13, 27–38. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.L.; Prideaux, M.; Bonewald, L.F. The osteocyte: An endocrine cell... and more. Endocr. Rev. 2013, 34, 658–690. [Google Scholar] [CrossRef] [Green Version]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Hägnäs, G.; Annala, M.; et al. Author Correction: The evolutionary history of lethal metastatic prostate cancer. Nature 2020, 584, E18. [Google Scholar] [CrossRef] [PubMed]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Guise, T.; Kang, Y. The Biology of Bone Metastasis. Cold Spring Harb. Perspect. Med. 2018, 8, a031252. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingsley, L.A.; Fournier, P.G.; Chirgwin, J.M.; Guise, T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [Green Version]
- Ell, B.; Kang, Y. SnapShot: Bone Metastasis. Cell 2012, 151, 690.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpal, M.; Yan, J.; Lu, X.; Xu, S.; Lerit, D.A.; Kang, Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat. Med. 2009, 15, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.; Jamal, R.; Abu, N. Cancer-Derived Exosomes as Effectors of Key Inflammation-Related Players. Front. Immunol. 2019, 10, 2103. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, H.; Heikamp, E.; Turley, H.; Dragovic, R.; Thomas, P.; Oon, C.E.; Leek, R.; Edelmann, M.; Kessler, B.; Sainson, R.C.; et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood 2010, 116, 2385–2394. [Google Scholar] [CrossRef] [Green Version]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2012, 32, 2747–2755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Dutta, A.; Li, J.; Lu, H.; Akech, J.; Pratap, J.; Wang, T.; Zerlanko, B.J.; FitzGerald, T.J.; Jiang, Z.; Birbe, R.; et al. Integrin αvβ6 promotes an osteolytic program in cancer cells by upregulating MMP2. Cancer Res. 2014, 74, 1598–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xinxin, Y.; Niansong, Q.; Shukuan, L.; Yuheng, L.; Weijia, S.; Jianwei, L.; Ruikai, D.; Guohui, Z.; Caizhi, L.; Guotao, Y.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef]
- Raimondo, S.; Saieva, L.; Vicario, E.; Pucci, M.; Toscani, D.; Manno, M.; Raccosta, S.; Giuliani, N.; Alessandro, R. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J. Hematol. Oncol. 2019, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Gil-Bazo, I.; Rolfo, C.; Alessandro, R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef] [Green Version]
- Li, F.X.; Liu, J.J.; Xu, F.; Lin, X.; Zhong, J.Y.; Wu, F.; Yuan, L.Q. Role of tumor-derived exosomes in bone metastasis. Oncol. Lett. 2019, 18, 3935–3945. [Google Scholar] [CrossRef]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef]
- Probert, C.; Dottorini, T.; Speakman, A.; Hunt, S.; Nafee, T.; Fazeli, A.; Wood, S.; Brown, J.E.; James, V. Communication of prostate cancer cells with bone cells via extracellular vesicle RNA; a potential mechanism of metastasis. Oncogene 2018, 38, 1751–1763. [Google Scholar] [CrossRef]
- Morrissey, C.; Lai, J.S.; Brown, L.G.; Wang, Y.C.; Roudier, M.P.; Coleman, I.M.; Gulati, R.; Vakar-Lopez, F.; True, L.D.; Corey, E.; et al. The expression of osteoclastogenesis-associated factors and osteoblast response to osteolytic prostate cancer cells. Prostate 2010, 70, 412–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, T.; Ito, Y.; Ohtsuki, Y.; Ando, M.; Tsukamasa, Y.; Yamada, N.; Naoe, T.; Akao, Y. Microvesicles released from hormone-refractory prostate cancer cells facilitate mouse pre-osteoblast differentiation. J. Mol. Histol. 2012, 43, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, T.; Lundholm, M.; Widmark, A.; Persson, E. Tumor Cell-Derived Exosomes from the Prostate Cancer Cell Line TRAMP-C1 Impair Osteoclast Formation and Differentiation. PLoS ONE 2016, 11, e0166284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inder, K.L.; Ruelcke, J.E.; Petelin, L.; Moon, H.; Choi, E.; Rae, J.; Blumenthal, A.; Hutmacher, D.; Saunders, N.A.; Stow, J.L.; et al. Cavin-1/PTRF alters prostate cancer cell-derived extracellular vesicle content and internalization to attenuate extracellular vesicle-mediated osteoclastogenesis and osteoblast proliferation. J. Extracell. Vesicles 2014, 3, 23784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morhayim, J.; van de Peppel, J.; Demmers, J.A.; Kocer, G.; Nigg, A.L.; van Driel, M.; Chiba, H.; van Leeuwen, J.P. Proteomic signatures of extracellular vesicles secreted by nonmineralizing and mineralizing human osteoblasts and stimulation of tumor cell growth. FASEB J. 2014, 29, 274–285. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff’s law: The bone modeling problem. Anat. Rec. 1990, 226, 403–413. [Google Scholar] [CrossRef]
- Hsu, H.; Lacey, D.L.; Dunstan, C.R.; Solovyev, I.; Colombero, A.; Timms, E.; Tan, H.L.; Elliott, G.; Kelley, M.J.; Sarosi, I.; et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 1999, 96, 3540–3545. [Google Scholar] [CrossRef]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Gonçalves, F. Bone metastases: An overview. Oncol. Rev. 2017, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, C.J.; Lin, S.H. Osteoblasts in prostate cancer metastasis to bone. Nat. Rev. Cancer 2005, 5, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rummel, K.; Benson, J.; Roller, L. Prostate adenocarcinoma with osteolytic metastases: Case report and review of the literature. Radiol. Case Rep. 2021, 16, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nishimura, K.; Oka, D.; Nakai, Y.; Shiba, M.; Tokizane, T.; Arai, Y.; Nakayama, M.; Shimizu, K.; Takaha, N.; et al. Prostate cancer mediates osteoclastogenesis through two different pathways. Cancer Lett. 2005, 223, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Keller, E.T.; Brown, J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J. Cell. Biochem. 2004, 91, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Whang, P.G.; Schwarz, E.M.; Gamradt, S.C.; Dougall, W.C.; Lieberman, J.R. The effects of RANK blockade and osteoclast depletion in a model of pure osteoblastic prostate cancer metastasis in bone. J. Orthop. Res. 2005, 23, 1475–1483. [Google Scholar] [CrossRef]
- Jin, R.; Sterling, J.A.; Edwards, J.R.; DeGraff, D.J.; Lee, C.; Park, S.I.; Matusik, R.J. Activation of NF-kappa B Signaling Promotes Growth of Prostate Cancer Cells in Bone. PLoS ONE 2013, 8, e60983. [Google Scholar] [CrossRef] [Green Version]
- Jing, D.; Hao, J.; Shen, Y.; Tang, G.; Li, M.L.; Huang, S.H.; Zhao, Z.H. The role of microRNAs in bone remodeling. Int. J. Oral. Sci. 2015, 7, 131–143. [Google Scholar] [CrossRef] [Green Version]
- Taipaleenmäki, H. Regulation of Bone Metabolism by microRNAs. Curr. Osteoporos. Rep. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Dang, L.; Wu, X.; Li, D.; Ren, Q.; Lu, A.; Zhang, G. microRNA-Mediated Regulation of Bone Remodeling: A Brief Review. JBMR Plus 2019, 3, e10213. [Google Scholar] [CrossRef]
- Hashimoto, K.; Ochi, H.; Sunamura, S.; Kosaka, N.; Mabuchi, Y.; Fukuda, T.; Yao, K.; Kanda, H.; Ae, K.; Okawa, A.; et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA 2018, 115, 2204–2209. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Stice, S.L. Mesenchymal stem cells: Applications in cell and gene therapy. Front. Cord. Blood Sci. 2009, 8, 97–122. [Google Scholar] [CrossRef]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone Marrow Stromal Stem Cells: Nature, Biology, and Potential Applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T. Osteoimmunology—Regulation of Osteoblast Differentiation by Runx2; Choi, Y., Ed.; Springer: Philadelphia, PA, USA, 2010; Volume 658, pp. 43–49. [Google Scholar]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akech, J.; Wixted, J.J.; Bedard, K.; Deen, M.v.d.; Hussain, S.; Guise, T.A.; Wijnen, A.J.v.; Stein, J.L.; Languino, L.R.; Altieri, D.C.; et al. Runx2 Association with Progression of Prostate Cancer in Patients: Mechanisms Mediating Bone Osteolysis and Osteoblastic Metastatic Lesions. Oncogene 2010, 29, 811–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colden, M.; Dar, A.A.; Saini, S.; Dahiya, P.V.; Shahryari, V.; Yamamura, S.; Tanaka, Y.; Stein, G.; Dahiya, R.; Majid, S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017, 8, e2572. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Majid, S.; Yamamura, S.; Tabatabai, L.; Suh, S.O.; Shahryari, V.; Chen, Y.; Deng, G.; Tanaka, Y.; Dahiya, R. Regulatory role of mir-203 in prostate cancer progression and metastasis. Clin. Cancer Res. 2011, 17, 5287–5298. [Google Scholar] [CrossRef] [Green Version]
- Koeneman, K.S.; Yeung, F.; Chung, L.W.K. Osteomimetic properties of prostate cancer cells: A hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate 1999, 39, 246–261. [Google Scholar] [CrossRef]
- Holleville, N.; Matéos, S.; Bontoux, M.; Bollerot, K.; Monsoro-Burq, A.H. Dlx5 drives Runx2 expression and osteogenic differentiation in developing cranial suture mesenchyme. Dev. Biol. 2007, 304, 860–874. [Google Scholar] [CrossRef]
- Tai, H.C.; Chang, A.C.; Yu, H.J.; Huang, C.Y.; Tsai, Y.C.; Lai, Y.W.; Sun, H.L.; Tang, C.H.; Wang, S.W. Osteoblast-derived WISP-1 increases VCAM-1 expression and enhances prostate cancer metastasis by down-regulating miR-126. Oncotarget 2014, 5, 7589–7598. [Google Scholar] [CrossRef] [Green Version]
- French, D.M.; Kaul, R.J.; D’Souza, A.L.; Crowley, C.W.; Bao, M.; Frantz, G.D.; Filvaroff, E.H.; Desnoyers, L. WISP-1 is an osteoblastic regulator expressed during skeletal development and fracture repair. Am. J. Pathol. 2004, 165, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Siu, M.K.; Tsai, Y.C.; Chang, Y.S.; Yin, J.J.; Suau, F.; Chen, W.Y.; Liu, Y.N. Transforming growth factor-β promotes prostate bone metastasis through induction of microRNA-96 and activation of the mTOR pathway. Oncogene 2014, 34, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Long, F. MTOR signaling in skeletal development and disease. Bone Res. 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, G.; Haflidadóttir, B.S.; Järemo, H.; Persson, M.; Ivkovic, T.C.; Wikström, P.; Ceder, Y. Regulation of cell-cell adhesion in prostate cancer cells by microRNA-96 through upregulation of E-Cadherin and EpCAM. Carcinogenesis 2020, 41, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Yang, J.; Vazquez, E.S.; Rose, D.; Vakar-Lopez, F.; Mathew, P.; Lopez, A.; Logothetis, C.J.; Lin, S.H.; Navone, N.M. Low-density lipoprotein receptor-related protein 5 (LRP5) mediates the prostate cancer-induced formation of new bone. Oncogene 2008, 27, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, Z.; Zielske, S.P.; Ibrahim, K.G.; Cackowski, F.C. Wnt and β-catenin signaling in the bone metastasis of prostate cancer. Life 2021, 11, 1099. [Google Scholar] [CrossRef]
- Li, F.; Gu, C.; Tian, F.; Jia, Z.; Meng, Z.; Ding, Y.; Yang, J. miR-218 impedes IL-6-induced prostate cancer cell proliferation and invasion via suppression of LGR4 expression. Oncol. Rep. 2016, 35, 2859–2865. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Fan, M.; Zhang, X. MicroRNA-218 inhibits prostate cancer cell growth and promotes apoptosis by repressing TPD52 expression. Biochem. Biophys. Res. Commun. 2015, 456, 804–809. [Google Scholar] [CrossRef]
- Nishikawa, R.; Goto, Y.; Sakamoto, S.; Chiyomaru, T.; Enokida, H.; Kojima, S.; Kinoshita, T.; Yamamoto, N.; Nakagawa, M.; Naya, Y.; et al. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 2014, 105, 802–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Zhao, X.; Zhang, H.; Yuan, H.; Zhu, M.; Sun, Q.; Lai, X.; Wang, Y.; Huang, J.; Yan, J.; et al. MiR-130b suppresses prostate cancer metastasis through down-regulation of MMP2. Mol. Carcinog. 2014, 54, 1292–1300. [Google Scholar] [CrossRef]
- Mulholland, D.J.; Kobayashi, N.; Ruscetti, M.; Zhi, A.; Tran, L.M.; Huang, J.; Gleave, M.; Wu, H. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res. 2012, 72, 1878–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galceran, J.; Fariñas, I.; Depew, M.J.; Clevers, H.; Grosschedl, R. Wnt3a−/−-like phenotype and limb deficiency in Lef1−/−Tcf1−/− mice. Genes. Dev. 1999, 13, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Liu, S.-Y.; Chang, Y.-S.; Yin, J.J.; Yeh, H.-l.; Mouhieddine, T.H.; Hadadeh, O.; Abou-Kheir, W.; Liu, Y.-N. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget 2015, 6, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Mo, C.; Huang, B.; Zhuang, J.; Jiang, S.; Guo, S.; Mao, X. LncRNA nuclear-enriched abundant transcript 1 shuttled by prostate cancer cells-secreted exosomes initiates osteoblastic phenotypes in the bone metastatic microenvironment via miR-205-5p/runt-related transcription factor 2/splicing factor proline- and glu. Clin. Transl. Med. 2021, 11, e493. [Google Scholar] [CrossRef]
- Hu, N.; Feng, C.; Jiang, Y.; Miao, Q.; Liu, H. Regulative effect of mir-205 on osteogenic differentiation of bone mesenchymal stem cells (BMSCs): Possible role of SATB2/Runx2 and ERK/MAPK pathway. Int. J. Mol. Sci. 2015, 16, 10491–10506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, S.H.; Cheng, J.W.; Chen, G.; Huang, Z.G.; Gu, Y.Y.; Yan, H.B.; He, M.L. Downregulation of miRNA-205 Expression and Biological Mechanism in Prostate Cancer Tumorigenesis and Bone Metastasis. BioMed Res. Int. 2020, 2020, 6037434. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 9483–9495. [Google Scholar] [CrossRef] [Green Version]
- Clay, M.R.; Halloran, M.C. Rho activation is apically restricted by arhgap1 in neural crest cells and drives epithelial-to-mesenchymal transition. Development 2013, 140, 3198–3209. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, G.; Yuhong, M.; Huiyun, L.I.N.; Yinglin, L.U. MAG-2 promotes invasion, mobility and adherence capability of lung cancer cells by MMP-2, CD44 and intracellular calcium in vitro. Oncol. Rep. 2009, 21, 697–706. [Google Scholar] [CrossRef]
- Yu, L.; Sui, B.; Fan, W.; Lei, L.; Zhou, L.; Yang, L.; Diao, Y.; Zhang, Y.; Li, Z.; Liu, J.; et al. Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J. Extracell. Vesicles 2021, 10, e12056. [Google Scholar] [CrossRef]
- Li, S.; Ye, Y.; Wang, S.; Wang, J. Exosomal miR-375 promotes the activity of osteoblasts in prostate cancer. Blood Genom. 2018, 2, 241–248. [Google Scholar] [CrossRef]
- Li, S.L.; An, N.; Liu, B.; Wang, S.Y.; Wang, J.J.; Ye, Y. Exosomes from LNCaP cells promote osteoblast activity through miR-375 transfer. Oncol. Lett. 2019, 17, 4463–4473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor. Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; Yasuda, H.; Yano, K.; Morinaga, T.; Higashio, K. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem. Biophys. Res. Commun. 1998, 253, 395–400. [Google Scholar] [CrossRef]
- Darnay, B.G.; Ni, J.; Moore, P.A.; Aggarwal, B.B. Activation of NF-κB by rank requires tumor necrosis factor receptor- associated factor (TRAF) 6 and NF-κB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 1999, 274, 7724–7731. [Google Scholar] [CrossRef] [Green Version]
- Ren, D.; Yang, Q.; Dai, Y.; Guo, W.; Du, H.; Song, L.; Peng, X. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-κB signaling pathway. Mol. Cancer 2017, 16, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wa, Q.; Zou, C.; Lin, Z.; Huang, S.; Peng, X.; Yang, C.; Ren, D.; Xu, D.; Guo, Y.; Liao, Z.; et al. Ectopic Expression of miR-532-3p Suppresses Bone Metastasis of Prostate Cancer Cells via Inactivating NF-κB Signaling. Mol. Ther.-Oncolytics 2020, 17, 267–277. [Google Scholar] [CrossRef]
- Wa, Q.; Huang, S.; Pan, J.; Tang, Y.; He, S.; Fu, X.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; et al. miR-204-5p Represses Bone Metastasis via Inactivating NF-κB Signaling in Prostate Cancer. Mol. Ther.-Nucleic Acids 2019, 18, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lin, Y.; Guo, Z.; Cheng, J.; Huang, J.; Deng, L.; Liao, W.; Chen, Z.; Liu, Z.G.; Su, B. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2001, 2, 620–624. [Google Scholar] [CrossRef]
- Terzic, J.J.; Marinovic-Terzic, I.I.; Ikeda, F.F.; Dikic, I.I. Ubiquitin signals in the NF-kappaB pathway. Biochem. Soc. Trans. 2007, 35, 942–945. [Google Scholar] [CrossRef]
- Duan, Y.; Tan, Z.; Yang, M.; Li, J.; Liu, C.; Wang, C.; Zhang, F.; Jin, Y.; Wang, Y.; Zhu, L. PC-3-Derived Exosomes Inhibit Osteoclast Differentiation by Downregulating miR-214 and Blocking NF-kB Signaling Pathway. BioMed Res. Int. 2019, 2019, 8650846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.-C.; Tang, C.-H.; Lin, L.-W.; Tsai, C.-H.; Chu, C.-Y.; Lin, T.-H.; Huang, Y.-L. Thrombospondin-2 promotes prostate cancer bone metastasis by the up-regulation of matrix metalloproteinase-2 through down-regulating miR-376c expression. J. Hematol. Oncol. 2017, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakides, T.R.; Zhu, Y.H.; Smith, L.T.; Bain, S.D.; Yang, Z.; Lin, M.T.; Danielson, K.G.; Iozzo, R.V.; LaMarca, M.; McKinney, C.E.; et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J. Cell Biol. 1998, 140, 419–430. [Google Scholar] [CrossRef]
- Bornstein, P.; Armstrong, L.C.; Hankenson, K.D.; Kyriakides, T.R.; Yang, Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000, 19, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Jay, P.R.; Centrella, M.; Lorenzo, J.; Bruce, A.G.; Horowitz, M.C. Oncostatin-M: A new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology 1996, 137, 1151–1158. [Google Scholar] [CrossRef] [Green Version]
- Goyden, J.; Tawara, K.; Hedeen, D.; Willey, J.S.; Oxford, J.T.; Jorcyk, C.L. The effect of OSM on MC3T3-E1 osteoblastic cells in simulated microgravity with radiation. PLoS ONE 2015, 10, e0127230. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Zhan, R.; Chen, S.; Deng, J.; Shi, J.; Wang, W. miR-181b/Oncostatin m axis inhibits prostate cancer bone metastasis via modulating osteoclast differentiation. J. Cell. Biochem. 2019, 121, 1664–1674. [Google Scholar] [CrossRef]
- Tang, Y.; Pan, J.; Huang, S.; Peng, X.; Zou, X.; Luo, Y.; Ren, D.; Zhang, X.; Li, R.; He, P.; et al. Downregulation of miR-133a-3p promotes prostate cancer bone metastasis via activating PI3K/AKT signaling. J. Exp. Clin. Cancer Res. 2018, 37, 160. [Google Scholar] [CrossRef]
- Li, N.; Xue, W.; Yuan, H.; Dong, B.; Ding, Y.; Liu, Y.; Jiang, M.; Kan, S.; Sun, T.; Ren, J.; et al. AKT-mediated stabilization of histone methyltransferase WHSC1 promotes prostate cancer metastasis. J. Clin. Investig. 2017, 127, 1284–1302. [Google Scholar] [CrossRef] [Green Version]
- Shariat, S.F.; Shalev, M.; Menesses-Diaz, A.; Kim, I.Y.; Kattan, M.W.; Wheeler, T.M.; Slawin, K.M. Preoperative Plasma Levels of Transforming Growth Factor Beta1 (TGF-B1) Strongly Predict Progression in Patients Undergoing Radical Prostatectomy. J. Clin. Oncol. 2001, 19, 2856–2864. [Google Scholar] [CrossRef]
- Jones, E.; Pu, H.; Kyprianou, N. Targeting TGF-β in prostate cancer: Therapeutic possibilities during tumor progression. Expert. Opin. Ther. Targets 2009, 13, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Mundy, G.R. Role of transforming growth factor-beta in bone remodeling. Clin. Orthop. Relat. Res. 1990, 250, 261–276. [Google Scholar] [CrossRef]
- Oursler, M.J. Osteoclast synthesis and secretion and activation of latent transforming growth factor β. J. Bone Miner. Res. 1994, 9, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol. Med. 2005, 11, 76–81. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-β1-induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption and Formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [Green Version]
- Fox, S.W.; Lovibond, A.C. Current insights into the role of transforming growth factor-β in bone resorption. Mol. Cell. Endocrinol. 2005, 243, 19–26. [Google Scholar] [CrossRef]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef]
- Fennen, M.; Pap, T.; Dankbar, B. Smad-dependent mechanisms of inflammatory bone destruction. Arthritis Res. Ther. 2016, 18, 279. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zou, C.; Tang, Y.; Wa, Q.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; Huang, Y.; Liao, Z.; et al. miR-582-3p and miR-582-5p Suppress Prostate Cancer Metastasis to Bone by Repressing TGF-β Signaling. Mol. Ther.-Nucleic Acids 2019, 16, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Wu, B.; Huang, S.; Peng, X.; Li, X.; Huang, X.; Zhou, W.; Xie, P.; He, P. Downregulation of miR-505-3p predicts poor bone metastasis-free survival in prostate cancer. Oncol. Rep. 2019, 41, 57–66. [Google Scholar] [CrossRef]
- Wa, Q.; Li, L.; Lin, H.; Peng, X.; Ren, D.; Huang, Y.; He, P.; Huang, S. Downregulation of MIR-19a-3p promotes invasion, migration and bone metastasis via activating TGF-β signaling in prostate cancer. Oncol. Rep. 2018, 39, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Wa, Q.; Pan, J.; Peng, X.; Ren, D.; Li, Q.; Dai, Y.; Yang, Q.; Huang, Y.; Zhang, X.; et al. Transcriptional downregulation of miR-133b by REST promotes prostate cancer metastasis to bone via activating TGF-β signaling article. Cell Death Dis. 2018, 9, 779. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Li, S.L.; Wang, J.J. miR-100-5p Downregulates mTOR to Suppress the Proliferation, Migration, and Invasion of Prostate Cancer Cells. Front. Oncol. 2020, 10, 578948. [Google Scholar] [CrossRef]
- Porkka, K.P.; Pfeiffer, M.J.; Waltering, K.K.; Vessella, R.L.; Tammela, T.L.J.; Visakorpi, T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007, 67, 6130–6135. [Google Scholar] [CrossRef] [Green Version]
- Tong, A.W.; Fulgham, P.; Jay, C.; Chen, P.; Khalil, I.; Liu, S.; Senzer, N.; Eklund, A.C.; Han, J.; Nemunaitis, J. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009, 16, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lee, Y.S.; Malhotra, A.; Kim, H.K.; Matecic, M.; Evans, C.; Jensen, R.V.; Moskaluk, C.A.; Dutta, A. miR-99 Family of MicroRNAs Suppresses the Expression of Prostate-Specific Antigen and Prostate Cancer Cell Proliferation. Cancer Res. 2011, 71, 1313–1324. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ren, D.; Guo, W.; Wang, Z.; Huang, S.; Du, H.; Song, L.; Peng, X. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2. Int. J. Oncol. 2014, 45, 362–372. [Google Scholar] [CrossRef] [Green Version]
- Hutvagner, G.; Simard, M.J. Argonaute proteins: Key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008, 9, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Sugatani, T.; Hruska, K.A. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J. Biol. Chem. 2009, 284, 4667–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Tao, L.; Zhong, H.; Zhao, S.; Yu, Y.; Yu, B.; Chen, X.; Gao, J.; Wang, R. MiR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol. Lett. 2016, 11, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.L.; Kaczmarek, M.; Keegan, A.D.; Tondravi, M. IL-4 suppresses osteoclast development and mature osteoclast function by a STAT6-dependent mechanism: Irreversible inhibition of the differentiation program activated by RANKL. Blood 2003, 102, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Abu-Amer, Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-κB. J. Clin. Investig. 2001, 107, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivan, M.; Garcia, M.; Suárez, L.; Guiu, M.; Gros, L.; Méndez, O.; Rigau, M.; Reventós, J.; Segura, M.F.; Torres, I.D.; et al. Loss of microRNA-135b Enhances Bone Metastasis in ProstateCancer and Predicts Aggressiveness in Human Prostate Samples. Cancers 2021, 13, 6202. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.L.; Fang, L.; Lake, R.G.; Agarwa, S.; et al. EGR1 Regulates Angiogenic and Osteoclastogenic Factors in Prostate Cancer and Promotes Metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef]
- Hagberg Thulin, M.; Jennbacken, K.; Damber, J.E.; Welén, K. Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies. Clin. Exp. Metastasis 2014, 31, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Traish, A.M.; Morgentaler, A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: A potential molecular switch for tumour growth. Br. J. Cancer 2009, 101, 1949–1956. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-S.; Chen, W.-Y.; Yin, J.J.; Sheppard-Tillman, H.; Huang, J.; Liu, Y.-N. EGF Receptor Promotes Prostate Cancer Bone Metastasis by Downregulating miR-1 and Activating TWIST1. Cancer Res. 2015, 75, 3077–3086. [Google Scholar] [CrossRef] [Green Version]
- Yuen, H.F.; Kwok, W.K.; Chan, K.K.; Chua, C.W.; Chan, Y.P.; Chu, Y.Y.; Wong, Y.C.; Wang, X.; Chan, K.W. TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis 2008, 29, 1509–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Wa, Q.; Pan, J.; Peng, X.; Ren, D.; Huang, Y.; Chen, X.; Tang, Y. Downregulation of miR-141-3p promotes bone metastasis via activating NF-κB signaling in prostate cancer. J. Exp. Clin. Cancer Res. 2017, 36, 173. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-L.; Qin, X.-J.; Cao, D.-L.; Zhu, Y.; Yao, X.-D.; Zhang, S.-L.; Dai, B.; Ye, D.-W. An elevated serum miR-141 level in patients withbone-metastatic prostate cancer is correlated with morebone lesions.pdf. Asian J. Androl. 2013, 15, 231–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Li, S.L.; Ma, Y.Y.; Diao, Y.J.; Yang, L.; Su, M.Q.; Li, Z.; Ji, Y.; Wang, J.; Lei, L.; et al. Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget 2017, 8, 94834–94849. [Google Scholar] [CrossRef] [Green Version]
- Akimoto, S.; Inomiya, H.; Furuya, Y.; Akakura, K.; Ito, H. Prognostic value of the serum levels of bone formation and bone resorption markers in prostate cancer patients with bone metastasis. Eur. Urol. 1998, 34, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Yang, J. Patisiran for the treatment of hereditary transthyretin-mediated amyloidosis. Expert. Rev. Clin. Pharmacol. 2019, 12, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther. Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.; Patkaniowska, A.; Urlaub, H.; Lührmann, R.; Tuschl, T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 2002, 110, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, D.S.; Hutvágner, G.; Du, T.; Xu, Z.; Aronin, N.; Zamore, P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003, 115, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, A.G.; Brown, D.; Stoudemire, J.; Lammers, P. Developing therapeutic microRNAs for cancer. Gene Ther. 2011, 18, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC 2019, 30, 114–127. [Google Scholar]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef] [Green Version]
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.; Vallely, M.P.; et al. Restoring expression of miR-16: A novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. 2013, 24, 3128–3135. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Therapeutic advances of miRNAs: A preclinical and clinical update. J. Adv. Res. 2020, 28, 127–138. [Google Scholar] [CrossRef]
- Qin, W.; Zhao, B.; Shi, Y.; Yao, C.; Jin, L.; Jin, Y. BMPRII is a direct target of miR-21. Acta Biochim. Biophys. Sin. 2009, 41, 618–623. [Google Scholar] [CrossRef]
- Gururajan, M.; Josson, S.; Chu, G.C.; Lu, C.L.; Lu, Y.T.; Haga, C.L.; Zhau, H.E.; Liu, C.; Lichterman, J.; Duan, P.; et al. miR-154* and miR-379 in the DLK1-DIO3 microRNA mega-cluster regulate epithelial to mesenchymal transition and bone metastasis of prostate cancer. Clin. Cancer Res. 2014, 20, 6559–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josson, S.; Gururajan, M.; Hu, P.; Shao, C.; Chu, G.Y.; Zhau, H.E.; Liu, C.; Lao, K.; Lu, C.L.; Lu, Y.T.; et al. miR-409-3p/-5p promotes tumorigenesis, epithelial-to-mesenchymal transition, and bone metastasis of human prostate cancer. Clin. Cancer Res. 2014, 20, 4636–4646. [Google Scholar] [CrossRef] [Green Version]
- Iscaife, A.; Reis, S.T.; Morais, D.R.; Viana, N.I.; da Silva, I.A.; Pimenta, R.; Bordini, A.; Dip, N.; Srougi, M.; Leite, K.R.M. Treating metastatic prostate cancer with microRNA-145. Apoptosis 2018, 23, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furesi, G.; de Jesus Domingues, A.M.; Alexopoulou, D.; Dahl, A.; Hackl, M.; Schmidt, J.R.; Kalkhof, S.; Kurth, T.; Taipaleenmäki, H.; Conrad, S.; et al. Exosomal miRNAs from Prostate Cancer Impair Osteoblast Function in Mice. Int. J. Mol. Sci. 2022, 23, 1285. [Google Scholar] [CrossRef]
- Bonci, D.; Coppola, V.; Musumeci, M.; Addario, A.; Giuffrida, R.; Memeo, L.; D’Urso, L.; Pagliuca, A.; Biffoni, M.; Labbaye, C.; et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008, 14, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Li, X.; Zhu, C. miR-27b and miR-34a enhance docetaxel sensitivity of prostate cancer cells through inhibiting epithelial-to-mesenchymal transition by targeting ZEB1. Biomed. Pharmacother. 2017, 97, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Ottman, R.; Levy, J.; Grizzle, W.E.; Chakrabarti, R. The other face of miR-17-92a cluster, exhibiting tumor suppressor effects in prostate cancer. Oncotarget 2016, 7, 73739–73753. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhang, X.W.; Liu, C.H.; Lu, K.; Huang, Y.Q.; Wang, Y.D.; Xing, L.; Zhang, L.J.; Liu, N.; Jiang, H.; et al. miRNA-30a functions as a tumor suppressor by downregulating cyclin E2 expression in castration-resistant prostate cancer. Mol. Med. Rep. 2016, 14, 2077–2084. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.V.; Veliceasa, D.; Vinokour, E.; Volpert, O.V. miR-200b inhibits prostate cancer EMT, growth and metastasis. PLoS ONE 2013, 8, e83991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, V.; Yadav, A.; Tiwari, R.; Nigam, S.; Goel, S.; Carskadon, S.; Gupta, N.; Goel, A.; Palanisamy, N.; Ateeq, B. Epigenetic Silencing of miRNA-338-5p and miRNA-421 Drives SPINK1-Positive Prostate Cancer. Clin. Cancer Res. 2018, 25, 2755–2768. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhou, W.; Jiang, H.; Xiang, Z.; Wang, L. miR-1303 promotes the proliferation, migration and invasion of prostate cancer cells through regulating the Wnt/β-catenin pathway by targeting DKK3. Exp. Ther. Med. 2019, 18, 4747–4757. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Tao, T.; Wang, Y.; Fang, F.; Huang, Y.; Chen, S.; Zhu, W.; Chen, M. hsa-miR-135a-1 inhibits prostate cancer cell growth and migration by targeting EGFR. Tumour Biol. 2016, 37, 14141–14151. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Boya, V.K.N.; Kashyap, V.K.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy. Cancers 2018, 10, 289. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Dhar, S.; Campanelli, G.; Butt, N.A.; Schallheim, J.M.; Gomez, C.R.; Levenson, A.S. MTA1 drives malignant progression and bone metastasis in prostate cancer. Mol. Oncol. 2018, 12, 1596–1607. [Google Scholar] [CrossRef] [Green Version]
- Dhar, S.; Kumar, A.; Gomez, C.R.; Akhtar, I.; Hancock, J.C.; Lage, J.M.; Pound, C.R.; Levenson, A.S. MTA1-activated Epi-microRNA-22 regulates E-cadherin and prostate cancer invasiveness. FEBS Lett. 2017, 591, 924–933. [Google Scholar] [CrossRef] [Green Version]
- Levenson, A.S. Dietary stilbenes as modulators of specific miRNAs in prostate cancer. Front. Pharmacol. 2022, 13, 970280. [Google Scholar] [CrossRef]
- Hemani, R.; Patel, I.; Inamdar, N.; Campanelli, G.; Donovan, V.; Kumar, A.; Levenson, A.S. Dietary Pterostilbene for MTA1-Targeted Interception in High-Risk Premalignant Prostate Cancer. Cancer Prev. Res. 2022, 15, 87–100. [Google Scholar] [CrossRef]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, T.; Patel, I.; Kumar, A.; Donovan, V.; Levenson, A.S. Grape Powder Supplementation Attenuates Prostate Neoplasia Associated with Pten Haploinsufficiency in Mice Fed High-Fat Diet. Mol. Nutr. Food Res. 2020, 64, e2000326. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, F.; Shen, L.; Tang, X.; Du, C.; Sun, Z.; Ding, H.; Chen, J.; Shen, B. Biomarker microRNAs for prostate cancer metastasis: Screened with a network vulnerability analysis model. J. Transl. Med. 2018, 16, 134. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; La Manna, F.; Bonollo, F.; Sampson, N.; Alberts, I.L.; Mingels, C.; Afshar-Oromieh, A.; Thalmann, G.N.; Karkampouna, S. Tumor microenvironment mechanisms and bone metastatic disease progression of prostate cancer. Cancer Lett. 2022, 530, 156–169. [Google Scholar] [CrossRef]
- Body, J.J. Metastatic bone disease: Clinical and therapeutic aspects. Bone 1992, 13, S57–S62. [Google Scholar] [CrossRef]
- Cleeland, C.S. The measurement of pain from metastatic bone disease: Capturing the patient’s experience. Clin. Cancer Res. 2006, 12, 6236–6242. [Google Scholar] [CrossRef] [Green Version]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- Coleman, R.E.; Brown, J.; Holen, I. Bone Metastases, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 809–830.e803. [Google Scholar]
- Sailer, V.; Schiffman, M.H.; Kossai, M.; Cyrta, J.; Beg, S.; Sullivan, B.; Pua, B.B.; Lee, K.S.; Talenfeld, A.D.; Nanus, D.M.; et al. Bone Biopsy Protocol for Advanced Prostate Cancer in the Era of Precision Medicine. Cancer 2018, 124, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Quiroz-Munoz, M.; Izadmehr, S.; Arumugam, D.; Wong, B.; Kirschenbaum, A.; Levine, A.C. Mechanisms of osteoblastic bone metastasis in prostate cancer: Role of prostatic acid phosphatase. J. Endocr. Soc. 2019, 3, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonpavde, G.; Pond, G.R.; Berry, W.R.; de Wit, R.; Armstrong, A.J.; Eisenberger, M.A.; Tannock, I.F. Serum alkaline phosphatase changes predict survival independent of PSA changes in men with castration-resistant prostate cancer and bone metastasis receiving chemotherapy. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.A.; Álvarez-Carrión, L.; Gutiérrez-Rojas, I.; Alonso, V. Role of calcium signaling in prostate cancer progression: Effects on cancer hallmarks and bone metastatic mechanisms. Cancers 2020, 12, 1071. [Google Scholar] [CrossRef]
- Hodara, E.; Morrison, G.; Cunha, A.; Zainfeld, D.; Xu, T.; Xu, Y.; Dempsey, P.W.; Pagano, P.C.; Bischoff, F.; Khurana, A.; et al. Multiparametric liquid biopsy analysis in metastatic prostate cancer. JCI Insight 2019, 4, e125529. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kuai, Y.; Zhu, R.; Zhou, C.; Tao, Y.; Han, W.; Chen, Q. Prognosis of prostate cancer and bone metastasis pattern of patients: A SEER-based study and a local hospital based study from China. Sci. Rep. 2020, 10, 9104. [Google Scholar] [CrossRef]
- Gravis, G.; Boher, J.M.; Fizazi, K.; Joly, F.; Priou, F.; Marino, P.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Prognostic Factors for Survival in Noncastrate Metastatic Prostate Cancer: Validation of the Glass Model and Development of a Novel Simplified Prognostic Model. Eur. Urol. 2015, 68, 196–204. [Google Scholar] [CrossRef]
- Bonci, D.; Coppola, V.; Patrizii, M.; Addario, A.; Cannistraci, A.; Francescangeli, F.; Pecci, R.; Muto, G.; Collura, D.; Bedini, R.; et al. A microRNA code for prostate cancer metastasis. Oncogene 2016, 35, 1180–1192. [Google Scholar] [CrossRef] [Green Version]
- Stephan, C.; Ralla, B.; Jung, K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochim. Biophys. Acta 2014, 1846, 99–112. [Google Scholar] [CrossRef]
- Cucchiara, V.; Cooperberg, M.R.; Dall’Era, M.; Lin, D.W.; Montorsi, F.; Schalken, J.A.; Evans, C.P. Genomic Markers in Prostate Cancer Decision Making. Eur. Urol. 2018, 73, 572–582. [Google Scholar] [CrossRef]
- Mytsyk, Y.; Nakonechnyi, Y.; Dosenko, V.; Kowal, P.; Pietrus, M.; Gazdikova, K.; Labudova, M.; Caprnda, M.; Prosecky, R.; Dragasek, J.; et al. The performance and limitations of PCA3, TMPRSS2:ERG, HOXC6 and DLX1 urinary markers combined in the improvement of prostate cancer diagnostics. Clin. Biochem. 2023, 116, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hessels, D.; Klein Gunnewiek, J.M.; van Oort, I.; Karthaus, H.F.; van Leenders, G.J.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur. Urol. 2003, 44, 8–15, discussion 15–16. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Chow, C.W.; Schirra, H.J.; Richards, R.; Buck, M.; Selth, L.A.; Doi, S.A.; Samaratunga, H.; Perry-Keene, J.; Payton, D.; et al. Diagnostic performance of expression of PCA3, Hepsin and miR biomarkers inejaculate in combination with serum PSA for the detection of prostate cancer. Prostate 2015, 75, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Schiffer, E.; von Wilcke, P.; Bauer, H.W.; Leung, H.; Siwy, J.; Ulrici, W.; Paasch, U.; Horn, L.C.; Stolzenburg, J.U. Seminal plasma as a source of prostate cancer peptide biomarker candidates for detection of indolent and advanced disease. PLoS ONE 2013, 8, e67514. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, T.; Straus, J.; Ritter, M.A.; Jarrard, D.F.; Huang, W. Semen AMACR protein as a novel method for detecting prostate cancer. Urol. Oncol. 2018, 36, e531–e532. [Google Scholar] [CrossRef]
- Barceló, M.; Castells, M.; Pérez-Riba, M.; Bassas, L.; Vigués, F.; Larriba, S. Seminal plasma microRNAs improve diagnosis/prognosis of prostate cancer in men with moderately altered prostate-specific antigen. Am. J. Transl. Res. 2020, 12, 2041–2051. [Google Scholar]
- Larson, J.; Ozen, M.O.; Kohli, M.; Akin, D.; Demirci, U. Systematic Analysis of Tissue-Derived and Biofluid Extracellular Vesicle miRNAs Associated with Prostate Cancer. Adv. Biol. 2023, 7, e2200327. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Xuan, C.; Chen, Q.; Xiang, Q.; Wang, J.; Liu, Y.; Luo, L.; Zhao, S.; Deng, Y.; et al. Identifying the key genes and microRNAs in prostate cancer bone metastasis by bioinformatics analysis. FEBS Open Bio 2020, 10, 674–688. [Google Scholar] [CrossRef]
- Peng, X.; Guo, W.; Liu, T.; Wang, X.; Tu, X.a.; Xiong, D.; Chen, S.; Lai, Y.; Du, H.; Chen, G.; et al. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS ONE 2011, 6, e20341. [Google Scholar] [CrossRef]
- Huang, S.; Guo, W.; Tang, Y.; Ren, D.; Zou, X.; Peng, X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol. Rep. 2012, 28, 1831–1837. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Yang, M.; Chen, S.; Balk, S.; Pomerantz, M.; Hsieh, C.L.; Brown, M.; Lee, G.S.M.; Kantoff, P.W. The altered expression of MiR-221/-222 and MiR-23b/-27b is associated with the development of human castration resistant prostate cancer. Prostate 2012, 72, 1093–1103. [Google Scholar] [CrossRef] [Green Version]
- Rice, M.A.; Ishteiwy, R.A.; Magani, F.; Udayakumar, T.; Reiner, T.; Yates, T.J.; Miller, P.; Perez-Stable, C.; Rai, P.; Verdun, R.; et al. The microRNA-23b/-27b cluster suppresses prostate cancer metastasis via Huntingtin-interacting protein 1-related. Oncogene 2016, 35, 4752–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, N.; Ma, G.; Zhang, J.; Zhu, W. MIR-221-5p enhances cell proliferation and metastasis through post-transcriptional regulation of SOCS1 in human prostate cancer. BMC Urol. 2018, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Zhiping, C.; Shijun, T.; Linhui, W.; Yapei, W.; Lianxi, Q.; Qiang, D. MiR-181a promotes epithelial to mesenchymal transition of prostate cancer cells by targeting TGIF2. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4835–4843. [Google Scholar] [PubMed]
- Hu, W.; Yan, F.; Ru, Y.; Xia, M.; Yan, G.; Zhang, M.; Wang, H.; Wu, G.; Yao, L.; Shen, L.; et al. MIIP inhibits EMT and cell invasion in prostate cancer through miR-181a/b-5p-KLF17 axis. Am. J. Cancer Res. 2020, 10, 630–647. [Google Scholar] [PubMed]
- Wang, Y.; Fang, Y.X.; Dong, B.; Du, X.; Wang, J.; Wang, X.; Gao, W.Q.; Xue, W. Discovery of extracellular vesicles derived miR-181a-5p in patient’s serum as an indicator for bone-metastatic prostate cancer. Theranostics 2020, 11, 878–892. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, S.; Zhang, T.; Wang, A.; Liu, R.; Guo, J.; Wang, Y.; Xu, Y. miR-188-5p inhibits tumour growth and metastasis in prostate cancer by repressing LAPTM4B expression. Oncotarget 2015, 6, 6092–6104. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Liu, X.; Liu, Y.; Yang, J.; Lv, G.; Dong, S. MicroRNA-335 and -543 suppress bone metastasis in prostate cancer via targeting endothelial nitric oxide synthase. Int. J. Mol. Med. 2015, 36, 1417–1425. [Google Scholar] [CrossRef] [Green Version]
- Damodaran, C.; Das, T.P.; Papu John, A.M.S.; Suman, S.; Kolluru, V.; Morris, T.J.; Faber, E.N.; Rai, S.N.; Messer, J.C.; Alatassi, H.; et al. miR-301a expression: A prognostic marker for prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 336.e13–336.e20. [Google Scholar] [CrossRef] [Green Version]
- Okato, A.; Goto, Y.; Kurozumi, A.; Kato, M.; Kojima, S.; Matsushita, R.; Yonemori, M.; Miyamoto, K.; Ichikawa, T.; Seki, N. Direct regulation of LAMP1 by tumor-suppressive microRNA-320a in prostate cancer. Int. J. Oncol. 2016, 49, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shu, L.; Kim, H.; Khor, T.O.; Wu, R.; Li, W.; Kong, A.N.T. Phenethyl isothiocyanate (PEITC) suppresses prostate cancer cell invasion epigenetically through regulating microRNA-194. Mol. Nutr. Food Res. 2016, 60, 1427–1436. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Wu, R.; Wu, L.; Tian, X.; Zhang, Z. microRNA-194 regulates cell viability and apoptosis by targeting CDH2 in prostatic cancer. OncoTargets Ther. 2018, 11, 4837–4844. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Du, W.; Zhang, H.; Wang, Y.; Li, K.; Meng, Y.; Wang, J. Transcriptional downregulation of miR-127-3p by CTCF promotes prostate cancer bone metastasis by targeting PSMB5. FEBS Lett. 2020, 594, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, S.; Du, Q.; Shao, M. miR-146a and miR-152 in prostate cancer and clinicopatho- logical parameters. JBUON 2019, 24, 1692–1699. [Google Scholar]
- Tseng, J.C.; Huang, S.H.; Lin, C.Y.; Wang, B.J.; Huang, S.F.; Shen, Y.Y.; Chuu, C.P. ROR2 suppresses metastasis of prostate cancer via regulation of miR-199a-5p–PIAS3–AKT2 signaling axis. Cell Death Dis. 2020, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Alwhaibi, A.; Parvathagiri, V.; Verma, A.; Artham, S.; Adil, M.S.; Somanath, P.R. Regulation of Let-7a-5p and miR-199a-5p Expression by Akt1 Modulates Prostate Cancer Epithelial-to-Mesenchymal Transition via the Transforming Growth Factor-β Pathway. Cancers 2022, 14, 1625. [Google Scholar] [CrossRef]
- Rode, M.P.; Silva, A.H.; Cisilotto, J.; Rosolen, D.; Creczynski-Pasa, T.B. miR-425-5p as an exosomal biomarker for metastatic prostate cancer. Cell Signal 2021, 87, 110113. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hou, J.; Li, X.; Zhou, J.; Luo, B.; Liang, S.; Lo, R.; Wong, T.M.; Kuang, G.-M. Exosome-Derived miRNAs as Potential Biomarkers for Prostate Bone Metastasis. Int. J. Gen. Med. 2022, 15, 5369–5383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Du, X.; Wang, X.; Xiao, H.; Jing, N.; Xue, W.; Dong, B.; Gao, W.Q.; Fang, Y.X. Tumor-derived miR-378a-3p-containing extracellular vesicles promote osteolysis by activating the Dyrk1a/Nfatc1/Angptl2 axis for bone metastasis. Cancer Lett. 2022, 526, 76–90. [Google Scholar] [CrossRef]

| Expression | miRNA | Target | Bone Remodeling Signaling Pathways | Reference |
|---|---|---|---|---|
| Upregulated | miR-96 | AKT1S1 | Inhibits AKT1S1 expression, inducing MTOR kinase activity and osteoblast differentiation. | [83] |
| miR-96 | E-cadherin and EPCAM | Enhances PCa cell–cell interactions and their ability to bind to osteoblasts by upregulating E-cadherin and EPCAM expression. | [85] | |
| miR-940 | ARHGAP1 and FAM134A | Promotes differentiation of mesenchymal stem cells to osteoblasts. | [70] | |
| miR-148a-3p | - | Induces osteogenic differentiation. | [101] | |
| miR-375 | - | Enhances osteoprotegerin, RUNX2, osteopontin, and bone sialoprotein expression in LNCaP cells, stimulating osteoblast differentiation and function. | [102,103] | |
| Downregulated | miR-446 | RUNX2 | Suppresses PCa proliferation and bone metastasis through regulation of osteogenic factors, such as RUNX2, osteopontin, osteocalcin, ANGPT1, ANGPT4, MMP11, Fyn, pAKT, FAK, and vimentin. | [77] |
| miR-203 | RUNX2 | Negatively regulates RUNX2 expression, suppressing bone formation and metastasis. | [78] | |
| miR-126 | VCAM-1 | Osteoblast-derived WISP-1 induces miR-126 downregulation via αvβ1 integrin, FAK, and p38 signaling pathways, leading to migration and VCAM-1 expression in metastatic PCa cells. | [81] | |
| miR-218 | LGR4 | Suppresses IL-6-induced cell proliferation and invasion via downregulation of LGR4. | [88] | |
| miR-34a | TCL7 | Inhibits TCF7 expression, downregulating the Wnt/β-catenin pathway. | [94] | |
| miR-205-5p | RUNX2 | Negatively regulates RUNX2 expression, suppressing osteogenic differentiation in hBMSC. | [95,96] |
| Expression | miRNA | Target | Bone Remodeling Signaling Pathways | Reference |
|---|---|---|---|---|
| Upregulated | miR-92a-1-5p | COL1A1 | Induces type I collagen degradation by targeting COL1A1, stimulating bone ECM degradation and bone resorption. | [101] |
| miR-210-3p | TNIP1 and SOCS1 | Promotes osteoclast differentiation by sustained activation of the NF-κB signaling pathway through TNIP1 and SOCS1 inhibition. | [107] | |
| Downregulated | miR-532-3p | TRAF1, TRAF2, and TRAF3 | Suppresses NF-κB activation via downregulation of TRAF1, TRAF2, and TRAF3. | [108] |
| miR-204-5p | TRAF1, TAB3, and MAP3K3 | Suppresses NF-κB activation via downregulation of TRAF1, TAB3, and MAP3K3. | [109] | |
| miR-214 | Promotes NF-κB activity, leading to osteoclast differentiation. | [112] | ||
| miR-376c | MMP2 | Negatively regulates MMP2 expression, suppressing matrix degradation and osteoclastogenesis. | [113] | |
| miR-181b-5p | OSM | Inhibits OSM expression, decreases IL-6 and AREG, and increases osteoprotegerin, suppressing osteoclast differentiation. | [118] | |
| miR-133a-3p | EGFR, FGFR1, IGF1R, and MET | Directly inhibits cytokine receptors of the PI3K/AKT signaling pathway, minimizing stimulation of osteolytic bone lesions. | [119] | |
| miR-582-3p and miR-582-5p | SMAD2, SMAD4, TGFβRI, and TGFβRII | Inhibits TGF-β signaling activity by downregulating SMAD2, SMAD4, TGFβRI, and TGFβRII, reducing bone osteolytic metastasis. | [132] | |
| miR-505-3p | SMAD2 and SMAD3 | Inhibits TGF-β signaling activity via downregulation of SMAD2 and SMAD3, reducing invasion and bone metastasis. | [133] | |
| miR-19a-3p | SMAD2 and SMAD4 | Inhibits TGF-β signaling activity via downregulation of SMAD2 and SMAD4, reducing osteolytic bone lesions. | [134] | |
| miR-133b | TGFβRI and TGRFβRII | Inhibits TGF-β signaling activity via downregulation of TGFβRI and TGRFβRII, reducing osteolytic bone lesions. | [135] | |
| miR-33a-5p | TGFβRI | Inhibits TGF-β signaling activity via downregulation of TGFβRI, reducing osteolytic bone lesions. | [49] |
| Expression | miRNA | Target | Bone Remodeling Signaling Pathways | Reference |
|---|---|---|---|---|
| Upregulated | 141-3p | DLC1 | Activates the p38MAPK pathway, increasing osteoprotegerin/RANKL expression and osteoblast maturation via downregulation of DLC1. | [155] |
| Downregulated | 141-3p | TRAF5 and TRAF6 | Suppresses NF-κB activation via downregulation of TRAF5 and TRAF6. | [153] |
| miR-100 | AGO2 | Suppresses osteoclast differentiation and function by impairing miRNA pathways through AGO2 inhibition. | [141,143] | |
| miR-100-5p | MTOR | Inhibits osteoblast differentiation and function via MTOR downregulation. | [137] | |
| miR-135b | STAT6 | Promotes osteoclast activity and bone resorption by stimulating RANKL-activated signaling and NF-κB activity via downregulation of STAT6. | [144] | |
| miR-135b | VIT1b, JAKMIP2, PLAG1, and PDGFA | Implicates osteogenesis in PCa via regulation of VIT1b, JAKMIP2, PLAG1, and PDGFA genes. | [147] | |
| miR-1 | TWIST1 | Regulates TWSIT1 expression, which promotes PCa bone remodeling by regulating DKK1 and RUNX2 expression. | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigol, A.N.; Rode, M.P.; da Luz Efe, F.; Saleh, N.A.; Creczynski-Pasa, T.B. The Bone Microenvironment Soil in Prostate Cancer Metastasis: An miRNA Approach. Cancers 2023, 15, 4027. https://doi.org/10.3390/cancers15164027
Prigol AN, Rode MP, da Luz Efe F, Saleh NA, Creczynski-Pasa TB. The Bone Microenvironment Soil in Prostate Cancer Metastasis: An miRNA Approach. Cancers. 2023; 15(16):4027. https://doi.org/10.3390/cancers15164027
Chicago/Turabian StylePrigol, Anne Natalie, Michele Patrícia Rode, Fernanda da Luz Efe, Najla Adel Saleh, and Tânia Beatriz Creczynski-Pasa. 2023. "The Bone Microenvironment Soil in Prostate Cancer Metastasis: An miRNA Approach" Cancers 15, no. 16: 4027. https://doi.org/10.3390/cancers15164027
APA StylePrigol, A. N., Rode, M. P., da Luz Efe, F., Saleh, N. A., & Creczynski-Pasa, T. B. (2023). The Bone Microenvironment Soil in Prostate Cancer Metastasis: An miRNA Approach. Cancers, 15(16), 4027. https://doi.org/10.3390/cancers15164027






