Simple Summary
The tumor microenvironment plays an important role in drug resistance and supports/promotes tumorigenesis. Stromal modulators in combination with nanomedicine therapeutics have recently been investigated for reprogramming the tumor microenvironment. Here, we review the major stromal components and recent advances in the use of stroma-targeted therapies for cancer treatment.
Abstract
The tumor stroma, or the microenvironment surrounding solid tumors, can significantly impact the effectiveness of cancer therapies. The tumor microenvironment is characterized by high interstitial pressure, a consequence of leaky vasculature, and dense stroma created by excessive deposition of various macromolecules such as collagen, fibronectin, and hyaluronic acid (HA). In addition, non-cancerous cells such as cancer-associated fibroblasts (CAFs) and the extracellular matrix (ECM) itself can promote tumor growth. In recent years, there has been increased interest in combining standard cancer treatments with stromal-targeting strategies or stromal modulators to improve therapeutic outcomes. Furthermore, the use of nanomedicine, which can improve the delivery and retention of drugs in the tumor, has been proposed to target the stroma. This review focuses on how different stromal components contribute to tumor progression and impede chemotherapeutic delivery. Additionally, this review highlights recent advancements in nanomedicine-based stromal modulation and discusses potential future directions for developing more effective stroma-targeted cancer therapies.
1. Introduction
Cancer remains a leading cause of global morbidity and mortality despite advances in targeted therapeutics. The main reason for treatment failures is drug resistance. Genetic factors such as mutations and copy number variations are a key source of resistance but are not the only determinants [1]. Stroma comprises a collection of cells and matrix components that support the delivery of nutrients and the survival of functional cells [2]. In cancer, the stroma plays a crucial role in tumorigenesis [3], tumor growth [4], metastasis [5], and resistance to chemotherapy [6]. Additionally, elevated interstitial fluid pressure (IFP) and dense tumor stroma in the tumor microenvironment (TME) contributes to high transport resistance [7]. Further, overexpression of drug efflux transporters, such as ATP binding cassette (ABC) proteins [8], and the deregulation of drug-associated tumor targets [9,10] allow tumor cells to escape the cytotoxicity of anticancer drugs.
Another important source of drug resistance is tumor heterogeneity, which can arise from both intrinsic and extrinsic factors. Intrinsic factors primarily result from variations among cancer cell populations within a tumor (intra-tumor heterogeneity) or among patients (inter-tumor heterogeneity). These variations can be attributed to the differences in genetic mutations, copy number variants, RNA expression, and protein abundance [11]. Extrinsic factors arise from the changes in the surrounding environment of cancer cells owing to the presence of fibroblasts, vasculature, and extracellular matrix (ECM). Tumor heterogeneity resulting from the combination of intrinsic and/or extrinsic factors hinders targeted therapy and the identification of biomarkers for selecting appropriate patient populations to maximize the therapeutic efficacy of chemo- and immunotherapeutics [12]. TME alterations, such as increased immunosuppression, secretion of growth factors, various paracrine cytokines, and chemokines to modulate the recruitment, infiltration, polarization, and function of immune cells within it further contributes to tumor progression [13]. Furthermore, current traditional anticancer therapies primarily focus on targeting and eliminating cancer cells, often overlooking the role of the surrounding normal cells and matrix components of TME, resulting in marginal progress in clinical outcomes [14]. Therefore, incorporating considerations for tumor heterogeneity into therapeutic approaches holds promise in enhancing the efficacy of current conventional therapies.
Recently, there has been considerable interest in developing anticancer strategies that combine conventional therapies with TME modulators to address drug resistance [15,16]. Figure 1 summarizes different TME modulators currently under investigation. It was found that combination therapies involving TME modulators resulted in higher tumor growth inhibition (TGI) when compared to the groups receiving anticancer drugs without TME modulators. Among the various TME modulators, stromal modulators (e.g., TGF-β inhibitors, hedgehog inhibitors, among others) have demonstrated greater (>70%) tumor inhibition and have advanced to clinical trials for further evaluation [17]. Notably, several U.S. Food and Drug Administration (FDA) approved drugs, including losartan, GDC-0449 (vismodegib), and arsenic trioxide, originally indicated for hypertension, basal cell carcinoma, and refractory or relapsed acute promyelocytic leukemia, respectively, have demonstrated improved anticancer efficacy when delivered in combination with TME modulators [18,19,20,21,22]. Modulation of stroma to overcome immunosuppression can further enhance the efficacy of immunotherapy and other therapeutic modalities such as radiation therapy [23,24,25].
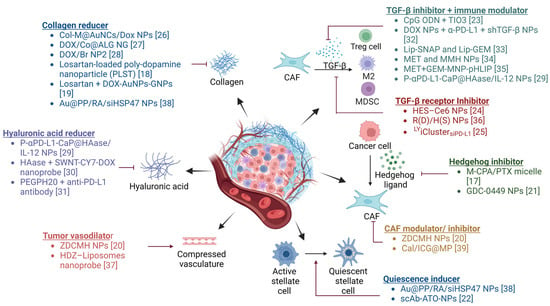
Figure 1.
TME modulator combination therapies [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Various agents that modulate the production of stromal components and/or signaling in the TME have been investigated to overcome resistance to anticancer therapies. Created with BioRender.com. Abbreviations: CAF: cancer-associated fibroblast, Treg: regulatory T cell, M2: M2 phenotype microphage, MDSC: myeloid-derived suppressor cell, Col-M: collagenase-functionalized biomimetic, AuNCs: Au nanocages, DOX: doxorubicin, NP: nanoparticle, Co: collagenase, ALG: alginic acid, NG: nanogel, Br: bromelain, DOX-AuNPs-GNPs: doxorubicin-loaded gold nanoparticles (DOX-AuNPs) onto matrix metalloproteinase-2 (MMP-2)-degradable gelatin nanoparticles (GNPs), Au@PP: mPEG-d-PEI-coated mercaptoundecanoic acid-capped AuNPs, RA: retinoic acid, siHSP47: small interfering collagen-specific molecular chaperone RNA, P-αPD-L1-CaP@HAase/IL-12 NPs: polyethylene glycol shieldable nanodrug P-αPD-L1-calcium phosphate@HAase/IL-12 nanoparticles, HAase: hyaluronidase, SWNT-CY7-DOX: single-walled carbon nanotubes- cyanine7—doxorubicin, PEGPH20: PEGylated human hyaluronidase, ZDCMH NPs: hyaluronic acid gel shell-coated zinc phthalocyanine and bromopentacarbonylmanganese(I) and losartan-coloaded mesoporous silica nanoparticles, HDZ: hydralazine, CpG ODN: cytidine phosphate guanosine (CpG) oligodinucleotide (ODN), shTGF-β: short hairpin RNA-targeting transforming growth factor β, Lip-SNAP: nitric oxide (NO) donor S-nitroso-N-acetylpenicillamine (SNAP)-loaded liposomes, Lip-GEM: gemcitabine (GEM)-loaded liposomes, MET: metformin, MMH NPs: MIL-100/mitoxantrone/hyaluronic acid nanoparticles, GEM-MNP-pHLIP: Gemcitabine (GEM) and pH (low) insertion peptide (pHLIP) comodified magnetic nanoparticles, HES-Ce6 NPs: hydroxyethyl starch-chlorin e6 conjugate self-assembled nanoparticles, R(D)/H(S) NPs: doxorubicin loaded rotaxane/SB431542 loaded heparin coated nanoparticles, LYiClustersiPD-L1: LY2157299 and siRNA targeting PD-L1 (siPD-L1) clustered nanoparticle, M-CPA/PTX micelle: cyclopamine (CPA) and paclitaxel (PTX) polymeric micelle, ZDCMH NPs: zinc phthalocyanine (ZnPc, a photosensitizer), bromopentacarbonylmanganese(I) (COMn, a CO donor), and losartan (Dup, a CAF inhibitor) were coloaded inside mesoporous silica nanoparticles (MSNs), Cal/ICG@MPs: tumor cell-derived microparticles co-delivering calcipotriol and Indocyanine green, Au@PP/RA/siHSP47 NPs: all-trans retinoic acid (RA) and siRNA targeting heat shock protein 47 (siHSP47) PEGylated polyethylenimine (PEI)-coated gold nanoparticles, scAb-ATO-NPs: single-chain antibody of fibroblast activation protein-α (scAbFAP-α) modified arsenic trioxide (ATO)-loaded nanoparticles. Created with BioRender.com.
Nanomedicine has been investigated extensively, mainly for applications in cancer drug targeting. Due to the relatively leaky vasculature in tumors compared to healthy tissues, where the range of inter-endothelial cell gaps in tumors is between 200 and 1200 nm [40], nanoparticles in this size range can traverse through these gaps and improve tumor drug accumulation. Nanoparticles including liposomes [41], inorganic nanoparticles [42], polymeric nanoparticles [43], solid lipid nanoparticles [44], micelles [45], inclusion complexes with cyclodextrins [46], and dendrimers [47] have been investigated to deliver chemotherapeutics to tumor tissues, allowing for a reduction in the systemic toxicity. Moreover, because of the progress made in chemical and material sciences, nanoparticles can now be fabricated with high payload capacity and sustained drug release. Nanomedicine can overcome the limitations of conventional approaches such as low bioavailability, low targeted cell membrane penetration, and unintended drug degradation in circulation [48]. Multifunctional nanomedicine systems can be designed using a variety of techniques, including surface targeting ligand modification [49], surface property alteration [50], material composition selection [51,52], physical property optimization [53] and co-delivery of several therapeutic drugs [54,55]. Beyond the field of cancer therapies, the use of nanomedicine in other disease areas, including lung disease [56], cardiovascular disease [57], and bone and cartilage disorders [58], has resulted in improved effectiveness as well. Currently, the FDA and European Medicines Agency (EMA) have authorized over 80 nanomedicine therapeutics [59], and more than 60 clinical studies are ongoing (clinicaltrials.gov, accessed May 2023).
In this review, we first provide an overview of the function of stroma and its role in cancer progression. We focus on three non-cellular components of stroma, collagen, fibronectin (FN), hyaluronic acid (HA), and one key stromal cell type, CAFs. Finally, this review focuses on the use of nanomedicine-based stromal modulation to improve tumor penetration and hence efficacy.
2. Stroma: Components and Role in Physiology and Pathology
The stroma is a complex microenvironment that surrounds and supports the proliferation and survival of cells in the body. It is composed of diverse cells, including vascular cells, infiltrating immune cells, and fibroblasts [60]. Vascular cells, such as endothelial cells and pericytes, are responsible for maintaining the integrity and function of blood vessels, which is essential for the transport of nutrients and oxygen to the tissue. Infiltrating immune cells, such as B-cells, T-cells, natural killer (NK) cells, innate lymphoid cells, dendritic cells, and macrophages, play a critical role in generating an immune response against microbial pathogens and malignant cells. Immune cells are attracted to the site of injury or damage through complex cytokine signaling and migrate through the blood vessels to eliminate pathogens and promote tissue repair [61]. Fibroblasts are the major source of ECM components, including collagen, elastin, HA, and other glycosaminoglycans, which play a vital role in maintaining the structural integrity of the stroma [62]. The interactions between these different types of stromal cells are critical for maintaining healthy homeostasis in the microenvironment. However, when homeostasis is disrupted, various diseases such as cancer [63], chronic inflammation [64], and bone marrow disorders with impaired hematopoiesis can occur [65].
The functions of stroma can be divided into two main categories: cytokine network regulation and tissue structure maintenance. Cytokine network regulation controls how cells respond to external and internal stimuli, such as cell damage and healing. For example, when cells are damaged, injured cells initiate the clotting cascade to stop bleeding and begin tissue repair through a series of events, including vasoconstriction, platelet aggregation, and fibrin deposition [66]. These sequential events are controlled by multiple cytokines secreted by stromal cells, including platelet-derived growth factor (PDGF), transforming growth factors α and β (TGF-α and TGF-β), and fibroblast growth factor-2 (FGF-2) [66]. For tissue structure maintenance, fibroblasts increase the stiffness of the stroma by secreting ECM components and soften the stroma matrix by secreting matrix metalloproteinases (MMPs) to break the linkages between ECM components. However, an imbalance in cytokine networks and structure maintenance can lead to many diseases [67]. Keloid scarring is an example of uncontrolled ECM overproduction resulting from collagen types I and III overproduction at the injury site [68,69]. Therefore, a balanced cytokine network and proper tissue structure maintenance are critical for maintaining tissue health and homeostasis [70,71].
3. Role of Stroma in Cancer Progression
In healthy tissues, the stroma plays a significant role in maintaining tissue structure and function through the regulation of blood vessel formation and the facilitation of immune cell migration and function [72,73,74,75,76]. However, in the presence of cancer, the stroma can be altered and can contribute to tumor growth. CAFs are a phenotype created and activated by tumorigenic signals released by cancer cells [77]. CAFs, in turn, secrete various proteins, including growth factors, cytokines, chemokines, and ECM components, that remodel the tissue structure, promote tumor proliferation, and contribute to therapy resistance [78]. Unlike in normal tissue, where repair processes are tightly regulated, this balance is disrupted in cancer, which is characterized by an overproduction of ECM components and cytokines [79]. This results in a modified stromal environment that supports cancer progression. Figure 2 illustrates how the stroma can contribute to cancer progression.

Figure 2.
Stroma alteration and its impact on cancer progression. Cancer cells release a variety of cytokines, such as TGF-β, PDGF, and other growth factors, which activate fibroblasts to become CAFs [77]. The CAFs secrete a range of growth factors, cytokines, and chemokines that support tumor growth, induce cancer stemness, and promote migration. Additionally, the overproduction of ECM components by CAFs results in an increase in ECM stiffness and a reduction in blood perfusion. Hypoxia, diminished tissue oxygen levels created by reduced blood flow, leads to a shift in cancer cell metabolism, including upregulation of glycolysis, acidification of the ECM, and initiation of angiogenesis, cell migration, and the formation of an immunosuppressive TME [80,81,82,83]. Abbreviations: TGF-β, transforming growth factor beta; PDGF, platelet-derived growth factor; EGF, epidermal growth factor; CAFs, cancer-associated fibroblasts; VEGF, vascular endothelial growth factor; IL-6, interleukin 6; CXCL-12, C-X-C motif chemokine-12; ECM, extracellular matrix; HA, hyaluronic acid; TME, tumor microenvironment. Created with BioRender.com.
In the following sections, we introduce how collagen, fibronectin, HA, and CAFs impact cancer progression, respectively.
3.1. Collagen
Proliferation, invasion, metastasis, and apoptosis of cancer cells have all been demonstrated to be significantly influenced by collagens, particularly beaded filament type VI and fibrillar collagens [84]. The role of collagen in different types of cancers is shown in Table 1. Collagen type I plays a critical role in the desmoplastic reaction, leading to an increase in fibroblasts and ECM components, and has been shown to contribute to chemoresistance in pancreatic ductal adenocarcinoma (PDAC) [85]. In the desmoplastic reaction, collagen I supports cell proliferation and reduces the sensitivity of tumor cells to anticancer drugs [86]. Previous studies show that sensitivity to 5-fluorouracil (5-FU) was reduced in pancreatic cell lines in the presence of collagen I [85]. Additionally, collagens are also associated with metastasis [87]. Studies have shown that collagen V activated α2β1-integrin and promoted migration of pancreatic cancer cell lines, where higher α2β1-integrin expression increased the likelihood of migration and invasion [88]. Unlike most collagens secreted by myofibroblasts, collagen IV is produced by pancreatic cancer cells and is localized on the cancer cell surface. Upregulation of collagen IV enhances α1β1-integrin and α2β1-integrin binding on the cancer cell surface, promoting PDAC migration and growth [89]. Furthermore, collagens can indirectly impact metastasis through cadherins. Cadherins, which are cell–cell adhesion molecules, are regulated by collagens. The more stable E-cadherin/catenin complex formation between epithelial cells leads to reduced cell migration. Research has found that collagens can disturb the stabilization of the E-cadherin/catenin complex and the presence of collagen types I and III promoted metastasis three- to fivefold in vivo [90].

Table 1.
Pathological functions of collagen in tumor progression.
Type I collagen has been shown to affect metastasis, as exposure to type I collagen results in a more invasive behavior in tumor cells [90]. Barcus et al. demonstrated that the number of circulating tumor cells increased as compared to that in wild-type (wt) mice in an in vivo breast cancer model with accumulated type I collagen distribution. Furthermore, the metastatic lesions were much larger than in the wt mice [100].
Recently, Papanicolaou et al. identified four critical matrisomal clusters via single-cell transcriptomics and proteomics and demonstrated how the structure of collagen I is altered by CAF-secreted collagen XII, resulting in a pro-invasive milieu that supports metastatic spread [101]. Additionally, their studies suggest that collagen XII could be used as a biomarker in breast cancer patients at high risk of metastatic recurrence [101].
Wang et al. demonstrated the role of the interaction between collagen and mutant proto-oncogenes in cancer progression [102,103]. It was shown that silencing of Kras expression significantly reduced collagen I deposition in renal fibrosis, and together with the epithelial–mesenchymal transition (EMT) regulator, SNAIL, mutant Kras increased collagen formation by pancreatic cancer stellate cells (PCAS) [102,103].
Senthebane et al. demonstrated that esophageal squamous cell carcinoma (ESCC) tissues had elevated expression levels of laminins, FN, and collagen when compared to normal tissue [104]. Further decellularization of ECMs abrogated the effect of chemotherapeutics such as epirubicin, 5-fluorouracil, and cisplatin on cell proliferation, cell cycle, and drug-induced apoptosis. Furthermore, the use of chemotherapeutics in combination with anti-fibronectin agents on collagen-deficient ECMs enhanced cancer cells’ susceptibility to chemotherapeutics by 30 to 50% and led to a decrease in tumor invasion and a reduction in the colony formation [104].
3.2. Fibronectin (FN)
Multiple studies have shown the critical role played by FN in cancer cell migration, growth, and metastasis as well as drug resistance. The formation of FN networks in CAFs is known to influence the direction of cancer cell migration [105]. Unlike the “mesh-like” FN network produced by normal fibroblasts, CAFs produce a “parallel-like” network that guides cancer cell migration. Studies have also shown that depletion of FN can inhibit the proliferation of PDAC cell lines and increase apoptosis by up to 50% [106]. Additionally, FN has been found to induce chemoresistance by activating the ERK1/2 phosphorylation pathway [107]. Cao et al. showed that FN was overexpressed in gall bladder cancer (GBC) tissues and was linked to poor prognosis in individuals with GBC [108]. Additionally, the authors showed that the GBC-SD and NOZ cells, secreted higher levels of MMP-9 when incubated with recombinant FN. Further, animal studies demonstrated that treatment with FN promoted GBC cell proliferation and metastasis, suggesting the involvement of FN in cell proliferation and invasion via activation of the mTOR signaling cascade during GBC progression [108].
In another study, Von Au et al. demonstrated that circulating FN facilitates the growth of bone metastases by promoting neo-angiogenesis in transgenic mice [109]. Furthermore, they showed that circulating FN enhances its own local production via a positive feedback loop as well as the production of vascular endothelial growth factor (VEGF) that is retained in the matrix [109]. Both FN and VEGF then cooperate in neo-angiogenesis. Additionally, they showed that FN content in tumors correlates with the number of blood vessels and tumor growth in mouse models [109]. Ou and colleagues demonstrated the critical function that FN plays in renal cell cancer (RCC) [110]. In human Caki-1 and RCC 786-O cells, RNA interference (RNAi)-based silencing of FN resulted in decreased cell growth as well as clonogenic cell growth in long-term cultures [110]. In addition, silencing FN in 786-O cells reduced their chemotactic migration toward 10% fetal bovine serum and wound healing relative to the control cells. Further analysis demonstrated reduced expression of vimentin and cyclin D1 as well as TGF-β1 production following FN gene silencing. The administration of exogenous FN and TGF-β1 to the silenced cells reversed the effects on cell proliferation and migration. These cells had elevated levels of TGF- β1, cyclin D1, and vimentin expression [110]. Table 2 summarizes the pathological functions of FN in solid tumors.

Table 2.
Pathological functions of FN in solid tumors.
3.3. Hyaluronic Acid (HA)
High overexpression of HA has been linked to poor prognosis in patients with pancreatic, colorectal, prostate, and ovarian cancer [117,118]. Higher expression of HA has also been shown to be a potential biomarker of metastasis in breast cancer patients [119,120]. HA is produced by HA synthase (HAS1, HAS2, and HAS3) and degraded by hyaluronidases (HYAL1 and HYAL2). It is also a well-known ligand of both a cell surface HA-binding transmembrane glycoprotein (CD44) and a receptor for HA-mediated motility (RHAMM) [121]. CD44 is often overexpressed on the surface of cancer cells and is viewed as a biomarker in many solid tumors [122]. For cell motility, cancer cells secrete hyaluronidases to degrade HA into smaller molecules and cause cleavage of CD44, inducing filopodia formation and promoting cell migration [123]. Importantly, the HA-CD44 interaction is known to activate downstream EGFR-mediated pathways, which promote cancer cell proliferation and migration [124]. A study by Song et al. demonstrated that silencing HAS2 and HAS3 resulted in a reduction in HA synthesis, which subsequently inhibited the proliferation of non-small cell lung cancer cells and reduced CD44, RHAMM, EGFR, Akt, and ERK expression [125]. Recently, HA-CD44 interactions have been utilized in HA-conjugated nanoparticles to improve anticancer efficacy via enhanced internalization of nanoparticles in CD44-overexpressed tumor cells [126,127]. Additionally, CD44 is a well-known stem cell marker and is associated with chemoresistance [128]. A study using athymic nude mice found that mice implanted orthotopically with CD44high pancreatic cancer cell lines were more resistant to gemcitabine compared to those implanted with CD44low pancreatic cancer cell lines [129]. Ricciardelli et al. observed that about 75% of ovarian cancer patients had increased serum HA levels after carboplatin treatment [130]. Similarly, in their in vitro study, HA, HAS2, HAS3, and ABCC2 expression increased after carboplatin treatment [130]. Furthermore, the authors found that different forms of CD44 display distinctive features. Cells with prominent levels of the standard form of CD44 tend to have the EMT phenotype, such as fibroblast-like appearance, high vimentin, and low E-cadherin. Conversely, high levels of CD44 variant isoforms display more epithelial-like phenotypes [129]. Table 3 summarizes the ways in which HA influences tumor progression.

Table 3.
Pathological functions of HA in tumor progression.
3.4. Cancer-Associated Fibroblasts (CAFs)
CAFs are key players in modulating the TME and play a crucial role in the regulation of tumor structure [84,138]. CAFs secrete growth factors and cytokines, such as TGF-β, VEGF, interleukin-6 (IL-6), interleukin-8 (IL-8), CC-chemokine ligand 5 (CCL5), C-X-C motif chemokine ligand (CXCL) 8, and CXCL12, which results in an immunosuppressive environment [139,140,141]. Different cell populations can give rise to CAFs, including endothelial cells, pericytes, adipocytes, stellate cells, tissue-resident fibroblasts, epithelial cells, fibrocytes, and mesenchymal stem cells [142,143]. CAF markers include alpha smooth muscle actin (αSMA), fibroblast activation protein alpha (FAP-α), actin alpha 2 (ACTA2), fibroblast-specific protein 1 (FSP1), and platelet-derived growth factor receptor alpha and/or beta (PDGFRα/β) [139]. The expression level of these markers depends on the origin and surrounding environment [144]. Furthermore, the functions and heterogeneity of CAFs are dependent on the cancer stage, tumor type, and spatial location. For example, breast cancer has four subtypes of CAFs—CAF-S1, CAF-S2, CAF-S3, and CAF-S4—with distinguishable functions. CAF-S1 regulates Treg-mediated immunosuppression and CAF-S4 is involved in cell contractility [145,146]. Based on spatial locations, CAFs in pancreatic cancer can be categorized into myofibroblastic CAFs (My-CAFs), inflammatory CAFs (ICAFs), and antigen presenting CAFs (Ap-CAFs). My-CAFs are located near cancer cells and express high levels of αSMA. In contrast, ICAFs are located farther away from cancer cells, express relatively low levels of αSMA, and secrete high levels of cytokines and chemokines [147]. Ap-CAFs mainly regulate immune response. The main function of My-CAFs is ECM construction and remodeling by ECM components (e.g., collagen, FN, elastin, tenascin) and enzyme secretion (e.g., MMPs) [143]. ICAFs tend to secrete immune-relevant cytokines (e.g., IL-6, IL-11 and leukemia inhibitory factor, CXCL1, and CXCL2), which may promote cancer progression [148], migration [149], and chemoresistance [150]. Ap-CAFs tend to regulate immune response (e.g., CD4+ T-cell deactivation) [151]. Although different phenotypes of CAFs function distinctly, the phenotypes of CAFs can be interchangeable according to their location and biochemical microenvironment [152].
4. Mechanisms of Stroma-Mediated Therapy Resistance
4.1. Barrier to Tumor Cell Drug Accumulation
The tumor stroma limits the efficacy of chemotherapy through several mechanisms. The high IFP observed in the tumor stroma impedes the convective distribution of chemotherapeutics to cancer cells [153,154]. Further, the tumor ECM is more rigid than normal tissue ECM, which limits the diffusive transport of chemotherapeutics to the site of action [155]. The blood vessels in the tumor tissue are compressed by the rigid tumor ECM, which limits perfusion to tumors, resulting in a reduced drug delivery [156]. Provenzano et al. observed limited penetration of doxorubicin into the tumor due to high IFP. Coadministration of doxorubicin with PEGylated human hyaluronidase (PEGPH20) was found to be effective in degrading stromal HA and normalizing IFP, resulting in a greater than sixfold increase in doxorubicin delivery to tumor cells in pancreatic cancer mouse models [153]. In addition, tumor tissue was less rigid and highly vascularized following treatment with PEGPH20 [153].
The strong binding of the ECM proteins to cancer cells allows cancer cells to escape cytotoxic drugs through a process known as cell-adhesion-mediated drug resistance (CAM-DR) [79]. This CAM-DR is mediated by various adhesion molecules, such as integrins [157], C-X-C motif chemokine receptors (CXCR) [158], and β-catenin [159]. Waldschmidt et al. observed increased cytotoxicity when co-culturing stromal cells and multiple myeloma cells with bortezomib and the CXCR4 inhibitor plerixafor [160]. Ding et al. demonstrated improved tumor inhibition when temozolomide (TMZ) was co-administered with oroxylin A, a β-catenin pathway inhibitor, compared to that with just TMZ in a glioblastoma xenograft mouse model [161]. Moreover, proteins such as periostin secreted by pancreatic stellate cells (PSCs) and stroma have been shown to promote carcinogenesis and resist gemcitabine, a chemotherapy drug commonly used in the treatment of pancreatic cancer [162].
4.2. Expression of Factors That Contribute to Chemotherapy Resistance
Chemotherapy alone is often not sufficient to eliminate the entire cancer cell population, as cancer cells can develop resistance mechanisms and receive support from the tumor microenvironment. The stroma can activate a stress response in response to DNA damage caused by chemotherapy, leading to the secretion of growth factors and cytokines that promote tumor cell survival, proliferation, migration, invasion, and metastasis [79]. This creates a complex network of interactions between cancer cells and the stroma that can promote chemoresistance and impede the efficacy of chemotherapy [163].
The stromal cells secrete enzymes (for, e.g., cytochrome P450) that degrade drugs and reduce their therapeutic activity [164]. It has been reported that CAFs secrete growth factors and cytokines leading to drug resistance and relapse in tumors [165]. Amin et al. found that the viability of neuroendocrine (NT-3) cells in response to everolimus treatment was increased by about 27% when cultured in CAF-conditioned media [166]. Lu et al. found that the stiffness of the ECM due to high expression of Calponin-1 in CAFs resulted in 5-FU resistance in gastric tumor cells [167]. Interestingly, a synergistic interaction of CAF-triggered growth factors with hypoxia-inducible factor (HIF-1α) was found to increase chemotherapy resistance and enhance the stemness of cancer stem cells in colorectal cancer [168]. In addition to these, the accumulation of CAFs in tumor tissues can promote drug resistance by inducing the expression of insulin-like growth factor 2 (IGF2) and insulin-like growth factor receptor-1 (IGF-1R) signaling, which leads to P-glycoprotein expression and increased drug efflux [169]. In addition to CAFs, tumor-associated macrophages (TAMs) also contribute to drug resistance through various signaling mechanisms. For instance, 5-FU treatment in gastric cancer promotes the accumulation of reactive oxygen species (ROS) that activates HIF-1α signaling. This, in turn, stimulates the TAMs to produce growth differentiation factor 15 (GDF15) that contributes to drug resistance by enhancing tumor cell fatty acid β-oxidation [170].
4.3. Resistance to Radiation Therapy
Radiation therapy, like chemotherapy, can lead to DNA damage and fibrosis in the ECM, resulting in survival and proliferation of stromal cells and resistance of cancer cells to radiotherapy [171]. Additionally, radiation activates CAFs, leading to the promotion of EMT and cancer cell invasiveness while inhibiting the interaction between CAFs and pancreatic cells through the CXCL12/CXCR4 pathway [172]. Studies have shown that ionizing radiation can also trigger an upregulation of integrin expression in fibroblasts, as seen in in vitro and in vivo models, and patient samples [173]. For example, radiation was shown to activate focal adhesion kinase (FAK) and upregulate β1 integrin in PSCs, providing protection to pancreatic cancer cells [174]. In another report, Yao and colleagues demonstrated α5β1 integrin upregulation in pancreatic tumor xenografts (Panc-1, MiaPaCa-2, and BxPC-3) that were exposed to radiation [175]. Moreover, stromal fibroblasts exposed to irradiation develop an ability to secrete chemoresistance-inducing factors [176]. Co-culturing pancreatic cancer cells with irradiated fibroblasts has been shown to significantly enhance cancer cell invasiveness through the activation of the hepatocyte growth factor (HGF)/c-Met signaling pathway [177].
4.4. Hypoxia
A key feature of solid tumors is abnormal vasculature, which results in reduced and uneven supply of oxygen [178]. The consequent hypoxia induces further changes in stromal cell biology within the TME that promotes tumor progression and drug resistance [179,180,181]. Stromal cells in the TME undergo several metabolic derangements following activation of hypoxia inducible factor (HIF) and utilize the “Warburg effect” to reduce their dependence on oxygen levels [182]. Cancer cells and CAFs secrete IL-6 to induce HIF-1α expression that modulates downstream genes responsible for chemotherapeutic resistance in cancer cells [183,184]. In addition, HIF contributes to ECM homeostasis, as evidenced by the involvement of HIF-target genes in the synthesis, modification, and degradation of collagen [185,186]. Furthermore, hypoxia tolerant cells have shown to be resistant against most anticancer therapeutics including photodynamic therapy (PDT) [187], immunotherapy (IT) [188], radiotherapy (RT) [189,190], and chemotherapy (CT) [180,191,192]. Hypoxia confers chemotherapy resistance to cancer cells by regulating cell cycle arrest [193], inhibiting senescence and cancer cell apoptosis, and controlling mitochondrial activity, p53, and autophagy [194,195]. Additionally, hypoxia contributes to chemoresistance by upregulating the p-glycoprotein drug efflux pump [196].
5. Strategies for Targeting Stromal Interactions
To overcome ECM-associated transport barriers, various strategies have been developed to target signaling pathways associated with the ECM, including inhibition of TGF-β and Hedgehog (Hh) signaling pathways as well as stromal depletion and regulation of CAFs. Additionally, these strategies in combination with nanomedicine provide a more effective way to deliver anticancer drugs while minimizing toxicity to normal cells [197,198].
Nanoparticles formulated using proteins [199,200,201], lipids [202,203,204], and polymers [205,206,207] have been commonly used to encapsulate chemotherapeutic drugs. Several nano-formulations including Abraxane®, Genexol-PM, Caelyx, and Onivyde have been approved for treating metastatic cancer [208]. In addition, nanoparticles formulated using inorganic materials such as gold [209], silver [210], and mesoporous silica [211] have demonstrated great potential for implementing novel modalities such as photodynamic and photothermal therapy [212,213,214]. For cancer therapy, use of nano-delivery systems allows cellular toxins [215], radioisotopes [216], and chemotherapeutic agents [217,218] to be targeted to a greater extent to the tumor cells with minimal effects on normal cells. However, these systems rely on passive accumulation in the tumor through the enhanced permeation and retention (EPR) effect and have demonstrated only limited therapeutic benefit in clinical practice [219]. The EPR effect is not observed in all tumors and the targeting effectiveness is low (typically, less than 5% of the administered dose accumulates in the tumor) [220,221]. As discussed in previous sections, greatly elevated IFP and dense tumor stroma are barriers to the uniform distribution of drugs and nanocarriers within the tumor tissue [222,223]. Thus, modulating TME to improve the delivery of nanocarriers has been pursued.
Nanomedicine approaches to normalize the hypoxic regions by the delivery of agents such as nitric oxide [224], HIF inhibitors, and oxygen have been utilized to target hypoxic solid tumors [225]. Perfluorocarbons (PFCs), which are made by combining fluorine and carbon molecules, have high oxygen-carrying potential and high biocompatibility and, therefore, have been used in anti-hypoxic nanotherapeutics [226]. Furthermore, PFCs have oxygen shuttling and donating capabilities similar to hemoglobin (Hb) [226]. Further, nanocarriers have been used to control drug release in the TME by conjugating TME-responsive ligands on the surface of nanocarriers or utilizing TME-responsive linkers [227,228]. In the following sections, we introduce examples of strategies targeting stromal interactions to improve anticancer efficacy.
5.1. Targeting Signaling Pathways
5.1.1. TGF-β
In the tumor stroma, TGF-β signaling promotes many cancer progression events, such as activation of CAFs [229], generation of an immunosuppressive environment [230,231], regulation of EMT [232,233,234], angiogenesis [235,236], and reprogramming of cancer metabolism [237]. Therapies that inhibit TGF-β signaling have been shown to significantly improve tumor suppression compared to single therapies. Table 4 summarizes TGF-β-targeted combination strategies.

Table 4.
TGF-β-Targeted Combination Strategies.
Pei et al. administered fraxinellone, a TGF-β signaling regulator, in CGKRK-modified nanoparticles (Frax-NP-CGKRK) to evaluate tumor inhibition and found that fraxinellone nanoparticles regulated the TGF-β signaling pathway and were involved in the inactivation of CAFs and M2 macrophage polarization. The combination therapy of fraxinellone nanoparticles and siKras gene resulted in twofold higher tumor inhibition compared to siKras gene therapy alone. Compared to simultaneous combination therapy, sequential combination therapy (fraxinellone nanoparticles followed by siKras gene therapy) resulted in better tumor inhibition and longer median survival [242]. Similar results were reported by Feng et al. where i.v. administration of CAF targeted α-mangostin nanoparticles, followed by acid-triggered triptolide micelles, resulted in a prolonged survival (50 days) and significantly higher tumor inhibition (p<0.001) compared to the combination delivery of the chemotherapeutic agent and CAF-targeted agent (38 days) in an orthotopic pancreatic mice model [238].
Another example is all-trans retinoic acid (ATRA), which has been shown to induce PSC quiescence and disable TGF-β activation [243]. Han et al. co-delivered ATRA, gemcitabine, and small interfering RNA targeting heat shock protein 47 (siHSP47) and achieved about a 75% reduction in tumor weight in the Panc-1/PSC subcutaneous mouse model compared to the saline-treated control group [38].
Combination therapies targeting TGF- β and the hypoxic tumor environment have also been investigated using nanomedicine approaches. Liu et al. developed Gd-metallofullerenol (Gd@C82(OH)22) nanoparticles to target both TGF-β and HIF-1α in mice models of triple-negative breast cancer (TNBC) [244]. These nanoparticles demonstrated preferential penetration of TME and delivery of targeted therapeutics to tumor cells only [244].
5.1.2. Hedgehog Signaling
Hedgehog (Hh) signaling has been described as the “Achilles’ heel” in cancer due to its crucial functions, including promoting tumor proliferation, cancer stem cell renewal, and ECM component synthesis [245,246]. Studies have demonstrated that combining a Hh inhibitor with current standard cancer therapy improves efficacy and alters the TME. For example, Zhao et al. co-delivered micelles encapsulating cyclopamine, an Hh inhibitor, and paclitaxel and observed several TME changes, such as hypoxia reduction, vascular normalization, and stroma reshaping in orthotopic PDAC tumor models [17].
Similarly, Wang et al. co-delivered SN-38, the active metabolite of irinotecan, and GDC-0449, an Hh inhibitor, to treat fibroblast-enriched PDAC and observed reductions in multiple fibroblast activation markers, such as α-SMA, collagen, and glioma-associated oncogene transcription factor-1 [21]. Furthermore, other sonic Hh inhibitors, such as vismodegib [247] and NVP-LDE225 [247,248], have shown synergistic effects when combined with chemotherapy or biologics in treating solid tumors. For example, the combination of vismodegib and gemcitabine significantly delayed pancreatic tumor growth compared to gemcitabine monotherapy in animal models. Additionally, the combination of vismodegib with Abraxane® or Doxil® resulted in a better antitumor effect than monotherapy [247].
Another drug shown to target stroma is IPI-926 (Infinity Pharmaceuticals). IPI-926 targets the transmembrane protein Smoothened (Smo), thereby inhibiting the Hh signaling pathway, and has shown therapeutic promise against various Hh-dependent malignancies. For example, mouse models of pancreatic cancer treated with IPI-926 demonstrate a reduction in tumor-associated stromal tissue and an increase in intra-tumoral vessel density [249]. Furthermore, IPI-926 improved the efficacy of simultaneously dosed systemic drugs such as gemcitabine, which led to a decrease in tumor burden and improved survival in mouse tumor models [249].
5.2. Stromal Depletion
The overproduction of ECM components in solid tumors increases the stiffness of stroma, which creates a transport barrier for the delivery of drugs and the infiltration of immune cells. To address this issue, antifibrotic agents have been investigated to reduce matrix stiffness and improve the delivery efficiency of chemotherapeutics.
One of the key developments in targeting stroma has been the development of PEGPH20, a recombinant version of the human hyaluronidase enzyme [250,251]. In multiple solid tumors, treatment with PEGPH20 significantly degraded HA, a glycosaminoglycan and a major component of the tumor stroma. For example, patients with HA-high metastatic PDAC experienced a twofold increase in progression-free survival and an improvement in overall survival when given PEGPH20 together with Abraxane® and gemcitabine, according to a phase 2 clinical study [252]. However, the results from a subsequent phase III clinical trial did not show a significant improvement in overall survival in HA-high patients [253]. In another study, the combination of PEGPH20 with Pembrolizumab (Pembro) was shown to be effective in patients with advanced or metastatic non-small cell lung cancer [254]. A study by Morosi et al. in 2021, demonstrated that restructuring the stroma of HA-rich tumors by depleting HA with PEGPH20 pre-treatment is a potentially effective method to increase the therapeutic effectiveness of anticancer medications without increasing their toxicity [255]. Though a number of studies have demonstrated that PEGPH20 may be useful as a therapeutic agent, the thromboembolic consequences of PEGPH20 need to be taken into consideration.
Another approach to improving tumor perfusion and drug delivery is the use of tissue plasminogen activator (tPA). Kirtane et al. used tPA to improve the distribution of Doxil® into the core of the tumor without affecting distribution in normal tissues [256]. Banerjee et al. utilized MinnelideTM, a water-soluble analog of triptolide, to reduce stroma components in human-tumor-derived xenografts. This approach resulted in a significant reduction in collagen stabilization, HA synthase expression (p < 0.05), CAFs, and tumor cell viability [257]. In another study, Zhu et al. developed polyethylene glycol (PEG), 2-deoxyglucose (DG), and fluorescently tagged CdTe quantum dots (Qds) to deliver siRNA against HIF-1α [258]. These nanocarriers were found to rupture and release their siRNA load in hypoxic TME thereby improving the tumor perfusion and antitumor efficacy [258]. Qds have also been utilized for image-guided delivery of diagnostic and therapeutic agents [258]. PFC@PLGA-red blood cell membrane (RBCM) nanoparticles were used to deliver oxygen to the interior hypoxic regions [259]. Song et al. developed near-infrared light-activated PFC-loaded Bi2Se3 nanoparticles to promote oxygenation and overcome radiotherapy resistance in hypoxic tumors [226].
The use of antifibrotic agents can also lead to a less immunosuppressive tumor microenvironment. Elahi-Gedwillo et al. reported a correlation between the content of HA in the stroma and the infiltration of immune cells. They observed that treatment with halofuginone, an antifibrotic agent, increased immune cell infiltration more than threefold compared to the control group. After 24 h of treatment, CD8+CD3+ cells, CD11b+ cells, and iNOS+ cells increased by about twofold, fivefold, and tenfold, respectively [260]. Another antifibrotic agent, fraxinellone, has also been shown to convert the TME to a less immunosuppressive state by increasing T-cell and NK cell populations, interferon-gamma (IFN-γ) secretion, and decreasing the number of myeloid-derived suppressor cells [92]. These findings suggest that antifibrotic agents may be useful in enhancing the effectiveness of immunotherapy for cancer.
Nanomedicine approaches have been investigated to target the desmoplastic stromal environment and deliver chemotherapeutic agents following the depletion of resistant stromal matrix. Han et al. investigated a two-step strategy to deliver metformin followed by administration of gemcitabine conjugated iron oxide nanoparticles for pancreatic cancer therapy [35]. Metformin administration was found to induce stromal disruption and reduce TGF-β expression that resulted in the suppression of pancreatic stellate cell (PSC) activity. Further, inhibition of PSC desmoplastic activity to form collagen and α-smooth muscle actin resulted in improved delivery of magnetic nanoparticles immobilized with gemcitabine. This two-step approach resulted in greater than 90% inhibition of tumor growth in orthotopic and subcutaneous mouse models of pancreatic cancer. Similarly, Chen et al. investigated a two-step sequential delivery of S-nitroso-N-acetylpenicillamine (SNAP), a nitric oxide donor-loaded liposomes (Lip-SNAP), followed by administration of gemcitabine-loaded liposomes in a mouse model of PDAC [33]. Inhibition of desmoplastic stroma production by Lip-SNAP resulted in the enhanced penetration of gemcitabine liposomes and improved therapeutic efficacy. In another study, a mesoporous silica nanoparticle (MSNP) carrier system loaded with gemcitabine and paclitaxel demonstrated improved efficacy of gemcitabine due to the dual effect of paclitaxel in inducing tumor stroma suppression and inhibiting the expression of the GEM-inactivating enzyme cytidine deaminase (CDA) in a PANC-1 orthotopic model [261]. Table 5 summarizes the combination strategies targeted towards depletion of stromal matrix components.

Table 5.
Stromal-Matrix-Targeted Strategies.
In another study, PEGylated polyethylenimine-coated gold nanoparticles were investigated for TME-responsive delivery of all-trans retinoic acid and siRNA targeting heat shock protein 47 [38]. Gold nanoshells resulted in improved accumulation of chemotherapeutics and improved efficacy, by inducing quiescence of PSCs and inhibiting hyperplasia of the ECM. Recently, Karimnia and colleagues tested in vitro heterocellular 3D co-culture models in combination with imaging and microrheology to investigate the effect of photodynamic stromal depletion in pancreatic cancer [263]. This approach resulted in improved delivery of miR-21-5P (oncomiR)-loaded nanoparticles in pancreatic ductal adenocarcinoma. Mardhian and colleagues [262] demonstrated the efficacy of relaxin-2 conjugated superparamagnetic iron oxide nanoparticles (SPIONs) to reduce fibrosis by modulating the pSmad2 signaling pathway. SPIONs resulted in enhanced tumor cell kill by reducing the expression of stromal components (collagen I, CD31, and desmin) and improving the delivery of gemcitabine [262]. Kang et al. utilized the endohedral metallofullerenol Gd@C82(OH)22 nanoparticles system to inhibit the activity of the matrix metalloproteinases (MMPs) and demonstrated the efficacy of Gd nanoparticles in mouse xenograft models of PDAC [264]. As demonstrated through these multiple studies, nanomedicine strategies have resulted in the improved delivery and penetration of chemotherapeutics in the presence of stromal depleting agents.
5.3. Regulation of CAFs
CAFs have gained increased attention in recent years due to their role in regulating tumor–stroma crosstalk [265], producing ECM components [84], and creating an immunosuppressive TME [139]. CAF-like cell models have been created from normal stromal cells such as stellate cells by treating them with TGF-β [266,267]. Drug delivery systems conjugated with CAF-targeting ligands that have high binding affinities to receptors or proteases of CAFs have been investigated to specifically target CAFs. For example, Chen et al. decorated FH-peptide (FHKHKSPALSPVGGG), a tenascin C targeting peptide [268], on navitoclax-loaded nanoliposomes and showed that the system had higher cytotoxicity on CAFs than on tumor cells [267]. Similarly, Guo et al. observed that more FH nanobubbles were located around CAFs compared to non-targeted nanobubbles [266]. Table 6 summarizes different CAF targeting strategies that have been pursued previously.

Table 6.
CAF-Targeting Strategies.
An alternative strategy for controlling therapeutic release specifically surrounding CAFs is using environment-responsive peptides. FAP-α is a protease secreted specifically by CAFs. Utilizing such a protease allows for active control of drug release since the peptide is mainly cleaved by CAFs. This method has been used to control the release of smaller particles from larger carriers to enhance penetration of particles in solid tumors, known as a size-shrinkage strategy. For example, Yu et al. used liposomes with FAP-α-responsive cleavable amphiphilic peptide (CAP) to control the release of small albumin-paclitaxel complexes (about 7.9 nm diameter) surrounding CAFs, resulting in increased drug penetration in the tumor, from 100 μm to 500–1000 μm [269]. Similarly, Zang et al. utilized FAP-α-responsive CAP in their drug delivery system to control doxorubicin release in the TME. This control release system significantly reduced CAF marker expression, such as α-SMA, FAP-α, and TGF-β levels and further inhibited tumor growth [270]. These results showed that CAF-targeting peptides successfully improved drug penetration due to the depletion of the CAF stromal barrier.
The angiotensin II receptor antagonist losartan was shown to inhibit type I collagen production by CAFs in various desmoplastic models of human breast, pancreatic, and skin cancers in a dose-dependent manner and to enhance the distribution and efficacy of therapeutic nanoparticles [19,273]. However, concerns about cancer recurrence and metastasis resulting from the depletion of CAFs have been observed [274,275]. Therefore, CAF modulation has become an alternative way to avoid the risk of cancer recurrence. For example, Cun et al. utilized 18β-glycyrrhetinic acid (GA) to reduce Wnt16 expression in CAFs located around blood vessels, since Wnt16 causes resistance to chemotherapy and can facilitate metastasis. Utilizing a size-switching strategy controlled by MMP-2, which is present in high concentrations in the tumor microenvironment, the team was able to demonstrate enhanced accumulation of GA-loaded large particles (~88.0 ± 1.1 nm) around blood vessels to suppress Wnt16 expression in CAFs and penetration of gemcitabine conjugated small particles (~28.7 ± 4.1 nm) to deep tumor locations. In vivo antitumor efficacy data showed that this approach resulted in significant tumor growth inhibition compared to controls and no tumor recurrence was observed eight days after the last dose [271].
Tumor cells and CAFs communicate through chemokines and cytokines. CXCL12, a secretory chemokine produced by CAFs, has been shown to play a role in tumor cell invasion [276,277], migration [278], and angiogenesis [279]. To target this communication, Lang et al. developed an siRNA delivery system that downregulates CXCL12 expression in CAFs. This system consisted of an FAP-α antibody for targeting CAFs, a cell-penetrating peptide, and siCXCL12. The delivery system was found to be effective in targeting the CAFs, as evidenced by increased G0/G1 phase arrest of CAFs, inhibition of tumor cell migration, and downregulation of several cytokines involved in cell proliferation, angiogenesis, and cell migration. Additionally, the study found that adding a CAF-targeting ligand significantly improved the gene silencing in CAFs. In vitro studies showed that inclusion of the FAP-α antibody increased inhibition of CXCL12 mRNA expression, α-SMA expression, cell migration, and endothelial cell tube formation compared to that with the non-specific IgG. Furthermore, the number of tumor metastases were significantly decreased by the FAP-α antibody conjugated system compared to that with the negative control siRNA nanocomplex [272]. Overall, these stroma modulation strategies have demonstrated strong antitumor, anti-metastatic, and anti-angiogenic effects and have highlighted the importance of stroma–tumor communication in promoting cancer progression.
When formulating therapies related to CAFs, there are several important factors to consider. First, the order of administration should be considered. For example, studies have shown that administering TGF-β interference therapy prior to antitumor therapeutic agents leads to greater therapeutic efficacy than following co-administration [238,242]. Second, the composition of nanoparticles could be important, as shown by the fact that gold nanoparticles can convert activated CAFs to a quiescent state [280]. Third, the risk of drug tolerance should be considered. For example, high doses (100 mg/kg once to twice daily) of Hh inhibitors have been reported to increase the risk of EMT due to over-ablation of the ECM and CAFs, and long-term use of high doses of Hh inhibitors increases the risk of developing Hh inhibitor tolerance [281,282]. A quantitative modeling approach to determine a suitable drug holiday approach may be a solution to reduce the chance of pharmacodynamic tolerance and to determine the optimal dosing regimen for combination therapy [248].
6. Conclusions and Future Directions
It is well established that TME plays a crucial role in cancer progression. Targeting the stroma has shown promising results for improving anticancer efficacy. However, previous studies suggest that stromal ablation can carry potential risks of tumor recurrence and metastasis [274]. One potential solution to mitigate these risks is through stromal modulation or re-education. A deeper understanding of the changes in the TME and the effects of products generated after targeting the stroma can provide insights for improving current strategies. For example, knowledge of the increased LMW-HA promoting tumor angiogenesis after hyaluronidase therapy can inform the use of combination therapies to overcome unwanted effects [283]. Additionally, utilizing single-cell sequencing to decipher the heterogeneity of stromal cell populations [284] and quantitative pharmacological analysis to describe the kinetic relationships between stromal cell populations, ECM reduction/production, and cancer metastasis after stroma targeting treatments can provide a foundation for reducing unwanted effects such as cancer metastasis and angiogenesis. Another area that has not been explored in depth is the potential use of nanomedicine strategies to modulate stroma that supports the growth of metastatic lesions. Recent studies suggest that vascularized organs such as lungs and liver are preconditioned by primary tumors to facilitate the ‘seeding’ of tumor cells in these distant sites [285,286]. Developing strategies to interfere with these interactions could help develop new therapeutic approaches to prevent and/or treat metastases. In conclusion, modulating the TME by targeting the stroma presents an opportunity to treat cancer more effectively.
Author Contributions
Conceptualization, S.P.; writing the first draft, M.-C.S. and S.K.N.; writing, review, and editing, M.-C.S., S.K.N., P.K.D. and S.P.; supervision, S.P.; funding acquisition, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funding support for SP from the OVPR (Temple University), Basser Cancer Center and American Cancer Society (RSG-22-123-01-ET).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, X.; Li, L.; Li, Z.; Xie, K. Targeted destruction of the orchestration of the pancreatic stroma and tumor cells in pancreatic cancer cases: Molecular basis for therapeutic implications. Cytokine Growth Factor Rev. 2012, 23, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Honan, A.M.; Chen, Z. Stromal Cells Underlining the Paths From Autoimmunity, Inflammation to Cancer With Roles Beyond Structural and Nutritional Support. Front. Cell Dev. Biol. 2021, 9, 658984. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Gieniec, K.A.; Lannagan, T.R.M.; Wang, T.; Asai, N.; Mizutani, Y.; Iida, T.; Ando, R.; Thomas, E.M.; Sakai, A.; et al. The Origin and Contribution of Cancer-Associated Fibroblasts in Colorectal Carcinogenesis. Gastroenterology 2022, 162, 890–906. [Google Scholar] [CrossRef]
- Davidson, S.; Efremova, M.; Riedel, A.; Mahata, B.; Pramanik, J.; Huuhtanen, J.; Kar, G.; Vento-Tormo, R.; Hagai, T.; Chen, X.; et al. Single-Cell RNA Sequencing Reveals a Dynamic Stromal Niche That Supports Tumor Growth. Cell Rep. 2020, 31, 107628. [Google Scholar] [CrossRef]
- Guo, S.; Deng, C.X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef]
- Ria, R.; Vacca, A. Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 613. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bell, D.W.; Gore, I.; Okimoto, R.A.; Godin-Heymann, N.; Sordella, R.; Mulloy, R.; Sharma, S.V.; Brannigan, B.W.; Mohapatra, G.; Settleman, J.; et al. Inherited susceptibility to lung cancer may be associated with the T790M drug resistance mutation in EGFR. Nat. Genet. 2005, 37, 1315–1316. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef]
- Sun, X.X.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Campisi, J.; Higano, C.; Beer, T.M.; Porter, P.; Coleman, I.; True, L.; Nelson, P.S. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat. Med. 2012, 18, 1359–1368. [Google Scholar] [CrossRef]
- Vickman, R.E.; Faget, D.V.; Beachy, P.; Beebe, D.; Bhowmick, N.A.; Cukierman, E.; Deng, W.M.; Granneman, J.G.; Hildesheim, J.; Kalluri, R.; et al. Deconstructing tumor heterogeneity: The stromal perspective. Oncotarget 2020, 11, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Jeong, J.H.; Chen, Z.; Chen, Z.; Luo, J.L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020, 13, 57–69. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, H.; Hsiao, C.H.; Chow, D.S.; Koay, E.J.; Kang, Y.; Wen, X.; Huang, Q.; Ma, Y.; Bankson, J.A.; et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials 2018, 159, 215–228. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles loaded with an angiotensin antagonist for an improved photothermal cancer therapy. Biomater. Sci. 2020, 8, 1658–1668. [Google Scholar] [CrossRef]
- Cun, X.; Ruan, S.; Chen, J.; Zhang, L.; Li, J.; He, Q.; Gao, H. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater. 2016, 31, 186–196. [Google Scholar] [CrossRef]
- Yao, H.; Xu, K.; Zhou, J.; Zhou, L.; Wei, S. A Tumor Microenvironment Destroyer for Efficient Cancer Suppression. ACS Biomater. Sci. Eng. 2020, 6, 450–462. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Zhou, Q.; Sui, M.; Lu, Z.; Zhou, Z.; Tang, J.; Miao, Y.; Zheng, M.; Wang, W.; et al. Terminating the criminal collaboration in pancreatic cancer: Nanoparticle-based synergistic therapy for overcoming fibroblast-induced drug resistance. Biomaterials 2017, 144, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, H.; Yang, K.; Han, S.; Chen, S.; Li, Y.; Chen, S.; Huang, K.; Lian, G.; Li, J. Arsenic trioxide-loaded nanoparticles enhance the chemosensitivity of gemcitabine in pancreatic cancer via the reversal of pancreatic stellate cell desmoplasia by targeting the AP4/galectin-1 pathway. Biomater. Sci. 2022, 10, 5989–6002. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Qu, K.; Wang, Y.; Wang, Z.; Lu, W.; Yu, Y.; Wang, L. Immunotherapy for lung cancer combining the oligodeoxynucleotides of TLR9 agonist and TGF-beta2 inhibitor. Cancer Immunol. Immunother. 2023, 72, 1103–1120. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liu, X.; Liu, S.; Xiao, C.; Zhang, Z.; Li, S.; Li, Z.; Yang, X. Transforming growth factor-beta blockade modulates tumor mechanical microenvironments for enhanced antitumor efficacy of photodynamic therapy. Nanoscale 2021, 13, 9989–10001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Z.; Du, X.; Chen, S.; Zhang, W.; Wang, J.; Li, H.; He, X.; Cao, J.; Wang, J. Co-inhibition of the TGF-beta pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater. Sci. 2020, 8, 5121–5132. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhang, J.G.; Zhou, Q.M.; Yu, J.N.; Lu, Y.F.; Wang, X.J.; Zhou, J.P.; Ding, X.F.; Du, Y.Z.; Yu, R.S. Extracellular matrix modulating enzyme functionalized biomimetic Au nanoplatform-mediated enhanced tumor penetration and synergistic antitumor therapy for pancreatic cancer. J. Nanobiotechnol. 2022, 20, 524. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; He, L.; Cheng, X.; Yan, G.; Wang, J.; Tang, R. Hybrid pH-sensitive nanogels surface-functionalized with collagenase for enhanced tumor penetration. J. Colloid Interface Sci. 2018, 525, 269–281. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Xu, X.; Fang, Q.; Tang, R. pH-sensitive bromelain nanoparticles by ortho ester crosslinkage for enhanced doxorubicin penetration in solid tumor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 113, 111004. [Google Scholar] [CrossRef]
- Xiao, Z.; Tan, Y.; Cai, Y.; Huang, J.; Wang, X.; Li, B.; Lin, L.; Wang, Y.; Shuai, X.; Zhu, K. Nanodrug removes physical barrier to promote T-cell infiltration for enhanced cancer immunotherapy. J. Control. Release 2023, 356, 360–372. [Google Scholar] [CrossRef]
- Fan, Y.F.; Shang, W.T.; Lu, G.H.; Guo, K.X.; Deng, H.; Zhu, X.H.; Wang, C.C.; Tian, J. Decreasing hyaluronic acid combined with drug-loaded nanoprobes improve the delivery and efficacy of chemotherapeutic drugs for pancreatic cancer. Cancer Lett. 2021, 523, 1–9. [Google Scholar] [CrossRef]
- Clift, R.; Souratha, J.; Garrovillo, S.A.; Zimmerman, S.; Blouw, B. Remodeling the Tumor Microenvironment Sensitizes Breast Tumors to Anti-Programmed Death-Ligand 1 Immunotherapy. Cancer Res. 2019, 79, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, Z.; Ni, W.; Feng, Y.; Guo, X.; Meng, M.; Yuan, Y.; Lin, L.; Chen, J.; Tian, H.; et al. Novel Cocktail Therapy Based on a Nanocarrier with an Efficient Transcytosis Property Reverses the Dynamically Deteriorating Tumor Microenvironment for Enhanced Immunotherapy. Nano Lett. 2022, 22, 7220–7229. [Google Scholar] [CrossRef]
- Chen, X.; Jia, F.; Li, Y.; Deng, Y.; Huang, Y.; Liu, W.; Jin, Q.; Ji, J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials 2020, 246, 119999. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wu, J.; Feng, Y.; Hu, Y.; Liu, H.; Chen, J.; Chen, F.; Tian, H. Metformin reprograms tumor microenvironment and boosts chemoimmunotherapy in colorectal cancer. Biomater. Sci. 2022, 10, 5596–5607. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Hou, Y.; Chen, X.; Zhang, P.; Kang, M.; Jin, Q.; Ji, J.; Gao, M. Metformin-Induced Stromal Depletion to Enhance the Penetration of Gemcitabine-Loaded Magnetic Nanoparticles for Pancreatic Cancer Targeted Therapy. J. Am. Chem. Soc. 2020, 142, 4944–4954. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, T.; Yang, J.; Hu, Z.; Wang, Z.; Xu, R.; Ma, S.; Wei, Y.; Shen, Q. Hierarchically Releasing Bio-Responsive Nanoparticles for Complete Tumor Microenvironment Modulation via TGF-beta Pathway Inhibition and TAF Reduction. ACS Appl. Mater. Interfaces 2021, 13, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, W.; Shen, L.; Qiu, N.; Hu, M.; Liu, Y.; Liu, Q.; Huang, L. Vasodilator Hydralazine Promotes Nanoparticle Penetration in Advanced Desmoplastic Tumors. ACS Nano 2019, 13, 1751–1763. [Google Scholar] [CrossRef]
- Han, X.; Li, Y.; Xu, Y.; Zhao, X.; Zhang, Y.; Yang, X.; Wang, Y.; Zhao, R.; Anderson, G.J.; Zhao, Y.; et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat. Commun. 2018, 9, 3390. [Google Scholar] [CrossRef]
- Li, X.; Yong, T.; Wei, Z.; Bie, N.; Zhang, X.; Zhan, G.; Li, J.; Qin, J.; Yu, J.; Zhang, B.; et al. Reversing insufficient photothermal therapy-induced tumor relapse and metastasis by regulating cancer-associated fibroblasts. Nat. Commun. 2022, 13, 2794. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; De Gaetano, F.; Larsen, K.L.; Pignatello, R.; Mazzaglia, A.; Puglisi, G.; Ventura, C.A. A physico-chemical study on amphiphilic cyclodextrin/liposomes nanoassemblies with drug carrier potential. J. Liposome Res. 2020, 30, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Xiao, X.; Teng, F.; Shi, C.; Chen, J.; Wu, S.; Wang, B.; Meng, X.; Essiet Imeh, A.; Li, W. Polymeric nanoparticles-Promising carriers for cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1024143. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Celesti, C.; Paladini, G.; Venuti, V.; Cristiano, M.C.; Paolino, D.; Iannazzo, D.; Strano, V.; Gueli, A.M.; Tommasini, S.; et al. Solid Lipid Nanoparticles Containing Morin: Preparation, Characterization, and Ex Vivo Permeation Studies. Pharmaceutics 2023, 15, 1605. [Google Scholar] [CrossRef]
- Aqeel, R.; Srivastava, N.; Kushwaha, P. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistara, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef]
- Crintea, A.; Motofelea, A.C.; Sovrea, A.S.; Constantin, A.M.; Crivii, C.B.; Carpa, R.; Dutu, A.G. Dendrimers: Advancements and Potential Applications in Cancer Diagnosis and Treatment-An Overview. Pharmaceutics 2023, 15, 1406. [Google Scholar] [CrossRef]
- Yang, C.; Han, M.; Li, R.; Zhou, L.; Zhang, Y.; Duan, L.; Su, S.; Li, M.; Wang, Q.; Chen, T.; et al. Curcumin Nanoparticles Inhibiting Ferroptosis for the Enhanced Treatment of Intracerebral Hemorrhage. Int. J. Nanomed. 2021, 16, 8049–8065. [Google Scholar] [CrossRef]
- Scheeren, L.E.; Nogueira-Librelotto, D.R.; Mathes, D.; Pillat, M.M.; Macedo, L.B.; Mitjans, M.; Vinardell, M.P.; Rolim, C.M.B. Multifunctional PLGA nanoparticles combining transferrin-targetability and pH-stimuli sensitivity enhanced doxorubicin intracellular delivery and in vitro antineoplastic activity in MDR tumor cells. Toxicol. In Vitro 2021, 75, 105192. [Google Scholar] [CrossRef]
- Becicka, W.M.; Bielecki, P.A.; Lorkowski, M.E.; Moon, T.J.; Zhang, Y.; Atukorale, P.U.; Covarrubias, G.; Karathanasis, E. The effect of PEGylation on the efficacy and uptake of an immunostimulatory nanoparticle in the tumor immune microenvironment. Nanoscale Adv. 2021, 3, 4961–4972. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liu, R.; Zhou, Y.; Gao, H. Size-Tunable Strategies for a Tumor Targeted Drug Delivery System. ACS Cent. Sci. 2020, 6, 100–116. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Liu, M.; Shen, S.; Wen, D.; Li, M.; Li, T.; Chen, X.; Gu, Z.; Mo, R. Hierarchical Nanoassemblies-Assisted Combinational Delivery of Cytotoxic Protein and Antibiotic for Cancer Treatment. Nano Lett. 2018, 18, 2294–2303. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, X.; Zeng, Y.; Lin, D.; Wu, J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021, 14, 2067–2089. [Google Scholar] [CrossRef]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Qiao, K.; Xu, L.; Tang, J.; Wang, Q.; Lim, K.S.; Hooper, G.; Woodfield, T.B.F.; Liu, G.; Tian, K.; Zhang, W.; et al. The advances in nanomedicine for bone and cartilage repair. J. Nanobiotechnol. 2022, 20, 141. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Wu, P.H.; Shen, Y.A.; Kuo, C.C.; Wang, W.J.; Chen, Y.C.; Lee, H.L.; Chiou, J.F. Recent Advances in Metal-Based NanoEnhancers for Particle Therapy. Nanomaterials 2023, 13, 1011. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Donnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Taylor & Francis Group: Abingdon, UK, 2017; Chapter 1. [Google Scholar]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Kadaba, R.; Birke, H.; Wang, J.; Hooper, S.; Andl, C.D.; Di Maggio, F.; Soylu, E.; Ghallab, M.; Bor, D.; Froeling, F.E.; et al. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J. Pathol. 2013, 230, 107–117. [Google Scholar] [CrossRef]
- Naylor, A.J.; Filer, A.; Buckley, C.D. The role of stromal cells in the persistence of chronic inflammation. Clin. Exp. Immunol. 2013, 171, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Chatterjee, S.; Basak, P.; Das, P.; Pereira, J.A.; Dutta, R.K.; Chaklader, M.; Chaudhuri, S.; Law, S. The bone marrow stem stromal imbalance--a key feature of disease progression in case of myelodysplastic mouse model. J. Stem Cells 2010, 5, 49–64. [Google Scholar] [PubMed]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing an overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Stone, M.J.; Hayward, J.A.; Huang, C.; Huma, Z.E.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef]
- Ashcroft, K.J.; Syed, F.; Bayat, A. Site-specific keloid fibroblasts alter the behaviour of normal skin and normal scar fibroblasts through paracrine signalling. PLoS ONE 2013, 8, e75600. [Google Scholar] [CrossRef] [PubMed]
- Syed, F.; Ahmadi, E.; Iqbal, S.A.; Singh, S.; McGrouther, D.A.; Bayat, A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen I and III compared with intralesional and extralesional sites: Clinical implications for lesional site-directed therapy. Br. J. Dermatol. 2011, 164, 83–96. [Google Scholar] [CrossRef]
- Morris, R.M.; Mortimer, T.O.; O’Neill, K.L. Cytokines: Can Cancer Get the Message? Cancers 2022, 14, 2178. [Google Scholar] [CrossRef]
- Bissell, M.J.; Kenny, P.A.; Radisky, D.C. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: The role of extracellular matrix and its degrading enzymes. Cold Spring Harb. Symp. Quant. Biol. 2005, 70, 343–356. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Manetti, M. Molecular Morphology and Function of Stromal Cells. Int. J. Mol. Sci. 2021, 22, 13422. [Google Scholar] [CrossRef]
- Ramaglia, V.; Florescu, A.; Zuo, M.; Sheikh-Mohamed, S.; Gommerman, J.L. Stromal Cell-Mediated Coordination of Immune Cell Recruitment, Retention, and Function in Brain-Adjacent Regions. J. Immunol. 2021, 206, 282–291. [Google Scholar] [CrossRef]
- Mueller, S.N.; Germain, R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 2009, 9, 618–629. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Falcone, I.; Cognetti, F.; Milella, M.; Ciuffreda, L. Advances in Tumor-Stroma Interactions: Emerging Role of Cytokine Network in Colorectal and Pancreatic Cancer. J. Oncol. 2019, 2019, 5373580. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; de Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, S.; Jeong, A.L.; Han, S.; Ka, H.I.; Lim, J.S.; Lee, M.S.; Yoon, D.Y.; Lee, J.H.; Yang, Y. Hypoxia-induced IL-32beta increases glycolysis in breast cancer cells. Cancer Lett. 2015, 356, 800–808. [Google Scholar] [CrossRef]
- Han, J.; Li, J.; Ho, J.C.; Chia, G.S.; Kato, H.; Jha, S.; Yang, H.; Poellinger, L.; Lee, K.L. Hypoxia is a Key Driver of Alternative Splicing in Human Breast Cancer Cells. Sci. Rep. 2017, 7, 4108. [Google Scholar] [CrossRef]
- Abdul-Aziz, A.M.; Shafat, M.S.; Sun, Y.; Marlein, C.R.; Piddock, R.E.; Robinson, S.D.; Edwards, D.R.; Zhou, Z.; Collins, A.; Bowles, K.M.; et al. HIF1alpha drives chemokine factor pro-tumoral signaling pathways in acute myeloid leukemia. Oncogene 2018, 37, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Schioppa, T.; Uranchimeg, B.; Saccani, A.; Biswas, S.K.; Doni, A.; Rapisarda, A.; Bernasconi, S.; Saccani, S.; Nebuloni, M.; Vago, L.; et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J. Exp. Med. 2003, 198, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Packham, G.; Murphy, L.B.; Bateman, A.C.; Conti, J.A.; Fine, D.R.; Johnson, C.D.; Benyon, R.C.; Iredale, J.P. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2004, 10, 7427–7437. [Google Scholar] [CrossRef]
- Baltes, F.; Pfeifer, V.; Silbermann, K.; Caspers, J.; Wantoch von Rekowski, K.; Schlesinger, M.; Bendas, G. beta(1)-Integrin binding to collagen type 1 transmits breast cancer cells into chemoresistance by activating ABC efflux transporters. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118663. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Grunwald, B.; Kruger, A.; Reithmeier, A.; Hahl, T.; Cheng, T.; Feuchtinger, A.; Born, D.; Erkan, M.; Kleeff, J.; et al. Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Lett. 2015, 356, 721–732. [Google Scholar] [CrossRef]
- Duan, W.; Ma, J.; Ma, Q.; Xu, Q.; Lei, J.; Han, L.; Li, X.; Wang, Z.; Wu, Z.; Lv, S.; et al. The Activation of beta1-integrin by Type I Collagen Coupling with the Hedgehog Pathway Promotes the Epithelial-Mesenchymal Transition in Pancreatic Cancer. Curr. Cancer Drug Targets 2014, 14, 446–457. [Google Scholar] [CrossRef]
- Ohlund, D.; Franklin, O.; Lundberg, E.; Lundin, C.; Sund, M. Type IV collagen stimulates pancreatic cancer cell proliferation, migration, and inhibits apoptosis through an autocrine loop. BMC Cancer 2013, 13, 154. [Google Scholar] [CrossRef]
- Menke, A.; Philippi, C.; Vogelmann, R.; Seidel, B.; Lutz, M.P.; Adler, G.; Wedlich, D. Down-regulation of E-cadherin gene expression by collagen type I and type III in pancreatic cancer cell lines. Cancer Res. 2001, 61, 3508–3517. [Google Scholar]
- Siret, C.; Terciolo, C.; Dobric, A.; Habib, M.C.; Germain, S.; Bonnier, R.; Lombardo, D.; Rigot, V.; Andre, F. Interplay between cadherins and alpha2beta1 integrin differentially regulates melanoma cell invasion. Br. J. Cancer 2015, 113, 1445–1453. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Q.; Shen, L.; Liu, Y.; Zhang, X.; Chen, F.; Huang, L. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics 2018, 8, 3781–3796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fredericks, T.; Xiong, G.; Qi, Y.; Rychahou, P.G.; Li, J.D.; Pihlajaniemi, T.; Xu, W.; Xu, R. Membrane associated collagen XIII promotes cancer metastasis and enhances anoikis resistance. Breast Cancer Res. 2018, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Motegi, H.; Kamoshima, Y.; Terasaka, S.; Kobayashi, H.; Houkin, K. Type 1 collagen as a potential niche component for CD133-positive glioblastoma cells. Neuropathology 2014, 34, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Voiles, L.; Lewis, D.E.; Han, L.; Lupov, I.P.; Lin, T.L.; Robertson, M.J.; Petrache, I.; Chang, H.C. Overexpression of type VI collagen in neoplastic lung tissues. Oncol. Rep. 2014, 32, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.S.; Liu, C.C.; Lin, J.H.; Hsu, T.W.; Hsu, J.W.; Li, A.F.; Hung, S.C. Involvement of collagen XVII in pluripotency gene expression and metabolic reprogramming of lung cancer stem cells. J. Biomed. Sci. 2020, 27, 5. [Google Scholar] [CrossRef]
- Chang, H.; Iizasa, T.; Shibuya, K.; Iyoda, A.; Suzuki, M.; Moriya, Y.; Liu, T.L.; Hiwasa, T.; Hiroshima, K.; Fujisawa, T. Increased expression of collagen XVIII and its prognostic value in nonsmall cell lung carcinoma. Cancer 2004, 100, 1665–1672. [Google Scholar] [CrossRef]
- Spivey, K.A.; Banyard, J.; Solis, L.M.; Wistuba, I.I.; Barletta, J.A.; Gandhi, L.; Feldman, H.A.; Rodig, S.J.; Chirieac, L.R.; Zetter, B.R. Collagen XXIII: A potential biomarker for the detection of primary and recurrent non-small cell lung cancer. Cancer Epidemiol. Biomarkers. Prev. 2010, 19, 1362–1372. [Google Scholar] [CrossRef]
- Rada, M.; Nallanthighal, S.; Cha, J.; Ryan, K.; Sage, J.; Eldred, C.; Ullo, M.; Orsulic, S.; Cheon, D.J. Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 2018, 37, 4809–4820. [Google Scholar] [CrossRef]
- Barcus, C.E.; O’Leary, K.A.; Brockman, J.L.; Rugowski, D.E.; Liu, Y.; Garcia, N.; Yu, M.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 2017, 19, 9. [Google Scholar] [CrossRef]
- Papanicolaou, M.; Parker, A.L.; Yam, M.; Filipe, E.C.; Wu, S.Z.; Chitty, J.L.; Wyllie, K.; Tran, E.; Mok, E.; Nadalini, A.; et al. Temporal profiling of the breast tumour microenvironment reveals collagen XII as a driver of metastasis. Nat. Commun. 2022, 13, 4587. [Google Scholar] [CrossRef]
- Shields, M.A.; Ebine, K.; Sahai, V.; Kumar, K.; Siddiqui, K.; Hwang, R.F.; Grippo, P.J.; Munshi, H.G. Snail cooperates with KrasG12D to promote pancreatic fibrosis. Mol. Cancer Res. 2013, 11, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Newbury, L.J.; Knisely, A.S.; Monia, B.; Hendry, B.M.; Sharpe, C.C. Antisense knockdown of Kras inhibits fibrosis in a rat model of unilateral ureteric obstruction. Am. J. Pathol. 2012, 180, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C.; et al. The Role of Tumor Microenvironment in Chemoresistance: 3D Extracellular Matrices as Accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef]
- Munasinghe, A.; Malik, K.; Mohamedi, F.; Moaraf, S.; Kocher, H.; Jones, L.; Hill, N.J. Fibronectin acts as a molecular switch to determine SPARC function in pancreatic cancer. Cancer Lett. 2020, 477, 88–96. [Google Scholar] [CrossRef]
- Amrutkar, M.; Aasrum, M.; Verbeke, C.S.; Gladhaug, I.P. Secretion of fibronectin by human pancreatic stellate cells promotes chemoresistance to gemcitabine in pancreatic cancer cells. BMC Cancer 2019, 19, 596. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, X.; Lu, W.; Chen, Y.; Wu, X.; Li, M.; Wang, X.A.; Zhang, F.; Jiang, L.; Zhang, Y.; et al. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015, 360, 141–150. [Google Scholar] [CrossRef]
- von Au, A.; Vasel, M.; Kraft, S.; Sens, C.; Hackl, N.; Marx, A.; Stroebel, P.; Hennenlotter, J.; Todenhofer, T.; Stenzl, A.; et al. Circulating fibronectin controls tumor growth. Neoplasia 2013, 15, 925–938. [Google Scholar] [CrossRef]
- Ou, Y.C.; Li, J.R.; Wang, J.D.; Chang, C.Y.; Wu, C.C.; Chen, W.Y.; Kuan, Y.H.; Liao, S.L.; Lu, H.C.; Chen, C.J. Fibronectin Promotes Cell Growth and Migration in Human Renal Cell Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2792. [Google Scholar] [CrossRef]
- You, D.; Jung, S.P.; Jeong, Y.; Bae, S.Y.; Lee, J.E.; Kim, S. Fibronectin expression is upregulated by PI-3K/Akt activation in tamoxifen-resistant breast cancer cells. BMB Rep. 2017, 50, 615–620. [Google Scholar] [CrossRef]
- Maity, G.; Fahreen, S.; Banerji, A.; Roy Choudhury, P.; Sen, T.; Dutta, A.; Chatterjee, A. Fibronectin-integrin mediated signaling in human cervical cancer cells (SiHa). Mol. Cell. Biochem. 2010, 336, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Chakrabarti, J.; Banerji, A.; Das, S.; Chatterjee, A. Culture of human cervical cancer cells, SiHa, in the presence of fibronectin activates MMP-2. J. Cancer Res. Clin. Oncol. 2006, 132, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Oyanagi, J.; Hoshino, D.; Togo, S.; Kumagai, H.; Miyagi, Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci. Rep. 2019, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Horzempa, C.; Jones, D.; McKeown-Longo, P.J. The fibronectin III-1 domain activates a PI3-Kinase/Akt signaling pathway leading to αvβ5 integrin activation and TRAIL resistance in human lung cancer cells. BMC Cancer 2016, 16, 574. [Google Scholar] [CrossRef]
- Moroz, A.; Delella, F.K.; Lacorte, L.M.; Deffune, E.; Felisbino, S.L. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem. Biophys. Res. Commun. 2013, 430, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.B.; Sato, N.; Kohi, S.; Yamaguchi, K. Prognostic impact of hyaluronan and its regulators in pancreatic ductal adenocarcinoma. PLoS ONE 2013, 8, e80765. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.H.; Kultti, A.; Kosma, V.M.; Pirinen, R.; Auvinen, P.; Tammi, M.I. Hyaluronan in human tumors: Pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin. Cancer Biol. 2008, 18, 288–295. [Google Scholar] [CrossRef]
- Peng, C.; Wallwiener, M.; Rudolph, A.; Ćuk, K.; Eilber, U.; Celik, M.; Modugno, C.; Trumpp, A.; Heil, J.; Marmé, F.; et al. Plasma hyaluronic acid level as a prognostic and monitoring marker of metastatic breast cancer. Int. J. Cancer 2016, 138, 2499–2509. [Google Scholar] [CrossRef]
- El-Mezayen, H.A.; Tosonel, S.A.; Darwish, H.; Metwally, F.M. Development of a novel metastatic breast cancer score based on hyaluronic acid metabolism. Med. Oncol. 2013, 30, 404. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Basakran, N.S. CD44 as a potential diagnostic tumor marker. Saudi Med. J. 2015, 36, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Hirata, T.; Hayasaka, H.; Stern, R.; Murai, T.; Miyasaka, M. Tumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragments. J. Biol. Chem. 2006, 281, 5861–5868. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, Y.S.; Choe, J.; Lee, H.; Kim, Y.M.; Jeoung, D. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J. Biol. Chem. 2008, 283, 22513–22528. [Google Scholar] [CrossRef]
- Song, J.M.; Im, J.; Nho, R.S.; Han, Y.H.; Upadhyaya, P.; Kassie, F. Hyaluronan-CD44/RHAMM interaction-dependent cell proliferation and survival in lung cancer cells. Mol. Carcinog. 2019, 58, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Cannava, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef]
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, C.; Chang, K.; Karnad, A.; Jagirdar, J.; Kumar, A.P.; Freeman, J.W. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 2016, 22, 5592–5604. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013, 13, 476. [Google Scholar] [CrossRef]
- Arnold, J.M.; Gu, F.; Ambati, C.R.; Rasaily, U.; Ramirez-Pena, E.; Joseph, R.; Manikkam, M.; San Martin, R.; Charles, C.; Pan, Y.; et al. UDP-glucose 6-dehydrogenase regulates hyaluronic acid production and promotes breast cancer progression. Oncogene 2020, 39, 3089–3101. [Google Scholar] [CrossRef]
- Wu, M.; Cao, M.; He, Y.; Liu, Y.; Yang, C.; Du, Y.; Wang, W.; Gao, F. A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J. 2015, 29, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Rooney, P.; Kumar, S.; Ponting, J.; Wang, M. The role of hyaluronan in tumour neovascularization (review). Int. J. Cancer 1995, 60, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Qiao, S.P.; Shi, S.L.; Yao, L.F.; Hou, X.L.; Li, C.F.; Lin, F.H.; Guo, K.; Acharya, A.; Chen, X.B.; et al. Modulating Three-Dimensional Microenvironment with Hyaluronan of Different Molecular Weights Alters Breast Cancer Cell Invasion Behavior. ACS Appl. Mater. Interfaces 2017, 9, 9327–9338. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Suh, Y.; Yoo, K.C.; Lee, J.H.; Kim, I.G.; Kim, M.J.; Chang, J.H.; Kang, S.G.; Lee, S.J. Tumor-associated mesenchymal stem-like cells provide extracellular signaling cue for invasiveness of glioblastoma cells. Oncotarget 2017, 8, 1438–1448. [Google Scholar] [CrossRef]
- Junliang, L.; Lili, W.; Xiaolong, L.; Xuguang, L.; Huanwen, W.; Zhiyong, L. High-molecular-weight hyaluronan produced by activated pancreatic stellate cells promotes pancreatic cancer cell migration via paracrine signaling. Biochem. Biophys. Res. Commun. 2019, 515, 493–498. [Google Scholar] [CrossRef]
- Cheng, X.B.; Kohi, S.; Koga, A.; Hirata, K.; Sato, N. Hyaluronan stimulates pancreatic cancer cell motility. Oncotarget 2016, 7, 4829–4840. [Google Scholar] [CrossRef]
- Kay, E.J.; Paterson, K.; Riera-Domingo, C.; Sumpton, D.; Dabritz, J.H.M.; Tardito, S.; Boldrini, C.; Hernandez-Fernaud, J.R.; Athineos, D.; Dhayade, S.; et al. Cancer-associated fibroblasts require proline synthesis by PYCR1 for the deposition of pro-tumorigenic extracellular matrix. Nat. Metab. 2022, 4, 693–710. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, F.; Zhao, F.; Li, G.; Shao, S.; Zhang, X.; Yuan, L.; Feng, Y. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis. 2019, 10, 273. [Google Scholar] [CrossRef]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8, 8633–8647. [Google Scholar] [CrossRef]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wei, W.F.; Wu, H.Z.; Fan, L.S.; Wang, W. Cancer-Associated Fibroblast Heterogeneity: A Factor That Cannot Be Ignored in Immune Microenvironment Remodeling. Front. Immunol. 2021, 12, 671595. [Google Scholar] [CrossRef] [PubMed]
- Pelon, F.; Bourachot, B.; Kieffer, Y.; Magagna, I.; Mermet-Meillon, F.; Bonnet, I.; Costa, A.; Givel, A.M.; Attieh, Y.; Barbazan, J.; et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat. Commun. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479.e10. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef]
- Razidlo, G.L.; Burton, K.M.; McNiven, M.A. Interleukin-6 promotes pancreatic cancer cell migration by rapidly activating the small GTPase CDC42. J. Biol. Chem. 2018, 293, 11143–11153. [Google Scholar] [CrossRef]
- Wang, M.T.; Fer, N.; Galeas, J.; Collisson, E.A.; Kim, S.E.; Sharib, J.; McCormick, F. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat. Commun. 2019, 10, 3055. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Wegner, C.S.; Hauge, A.; Gaustad, J.V.; Andersen, L.M.K.; Simonsen, T.G.; Galappathi, K.; Rofstad, E.K. Dynamic contrast-enhanced MRI of the microenvironment of pancreatic adenocarcinoma xenografts. Acta Oncol. 2017, 56, 1754–1762. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Tang, J.; Qin, S.; Shen, X.; He, S.; Ju, S. CAM-DR: Mechanisms, Roles and Clinical Application in Tumors. Front. Cell Dev. Biol. 2021, 9, 698047. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, H.X.; Wang, M.; Song, X.G.; Cao, J.; Wang, L.; Qiao, J.L.; Lu, X.Y.; Han, Z.X.; Zhu, P.; et al. Stromal cells attenuate the cytotoxicity of imatinib on Philadelphia chromosome-positive leukemia cells by up-regulating the VE-cadherin/beta-catenin signal. Leuk. Res. 2014, 38, 1460–1468. [Google Scholar] [CrossRef]
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Muller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017, 179, 36–49. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Li, Z.; Zhang, H.; Yang, Y.; Qin, H.; Xu, Q.; Zhao, L. Oroxylin A reversed Fibronectin-induced glioma insensitivity to Temozolomide by suppressing IP(3)R1/AKT/beta-catenin pathway. Life Sci. 2020, 260, 118411. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Gao, F.; Xing, L.; Qin, P.; Liang, X.; Zhang, J.; Qiao, X.; Lin, L.; Zhao, Q.; et al. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumour Biol. 2016, 37, 15283–15291. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.M.; Lucas, J.M.; Gomez-Sarosi, L.A.; Coleman, I.; Zhao, S.; Coleman, R.; Nelson, P.S. DNA damage induces GDNF secretion in the tumor microenvironment with paracrine effects promoting prostate cancer treatment resistance. Oncotarget 2015, 6, 2134–2147. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, M.; Boosman, R.J.; Schinkel, A.H.; Huitema, A.D.R.; Beijnen, J.H. Cytochrome P450 3A4, 3A5, and 2C8 expression in breast, prostate, lung, endometrial, and ovarian tumors: Relevance for resistance to taxanes. Cancer Chemother. Pharmacol. 2019, 84, 487–499. [Google Scholar] [CrossRef]
- Mathot, P.; Grandin, M.; Devailly, G.; Souaze, F.; Cahais, V.; Moran, S.; Campone, M.; Herceg, Z.; Esteller, M.; Juin, P.; et al. DNA methylation signal has a major role in the response of human breast cancer cells to the microenvironment. Oncogenesis 2017, 6, e390. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.; Viol, F.; Krause, J.; Fahl, M.; Eggers, C.; Awwad, F.; Schmidt, B.; Benten, D.; Ungefroren, H.; Fraune, C.; et al. Cancer-Associated Fibroblasts Induce Proliferation and Therapeutic Resistance to Everolimus in Neuroendocrine Tumors through STAT3 Activation. Neuroendocrinology 2023, 113, 501–518. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, Z.; Hou, J.; Wu, X.; Yu, Z.; Yao, L.; Pan, T.; Chang, X.; Yu, B.; Li, J.; et al. Calponin 1 increases cancer-associated fibroblasts-mediated matrix stiffness to promote chemoresistance in gastric cancer. Matrix Biol. 2023, 115, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.A.; Chen, Y.F.; Bao, Y.; Mahara, S.; Yatim, S.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Bai, J.; Ren, J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018, 109, 944–955. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Yu, Y.; Cui, Y.; Li, W.; Liu, T.; Liu, F. Activated HIF1alpha of tumor cells promotes chemoresistance development via recruiting GDF15-producing tumor-associated macrophages in gastric cancer. Cancer Immunol. Immunother. 2020, 69, 1973–1987. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Li, D.; Qu, C.; Ning, Z.; Wang, H.; Zang, K.; Zhuang, L.; Chen, L.; Wang, P.; Meng, Z. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am. J. Cancer Res. 2016, 6, 2192–2206. [Google Scholar] [PubMed]
- Goel, H.L.; Sayeed, A.; Breen, M.; Zarif, M.J.; Garlick, D.S.; Leav, I.; Davis, R.J.; Fitzgerald, T.J.; Morrione, A.; Hsieh, C.C.; et al. beta1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J. Cell. Physiol. 2013, 228, 1601–1609. [Google Scholar] [CrossRef]
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011, 71, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zeng, Z.Z.; Fay, K.S.; Veine, D.M.; Staszewski, E.D.; Morgan, M.; Wilder-Romans, K.; Williams, T.M.; Spalding, A.C.; Ben-Josef, E.; et al. Role of α(5)β(1) Integrin Up-regulation in Radiation-Induced Invasion by Human Pancreatic Cancer Cells. Transl. Oncol. 2011, 4, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Clemenson, C.; Martins, I.; Perfettini, J.L.; Deutsch, E. Understanding the functions of tumor stroma in resistance to ionizing radiation: Emerging targets for pharmacological modulation. Drug Resist. Updates 2013, 16, 10–21. [Google Scholar] [CrossRef]
- Ohuchida, K.; Mizumoto, K.; Murakami, M.; Qian, L.W.; Sato, N.; Nagai, E.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004, 64, 3215–3222. [Google Scholar] [CrossRef]
- Shih, C.Y.; Wang, P.T.; Su, W.C.; Teng, H.; Huang, W.L. Nanomedicine-Based Strategies Assisting Photodynamic Therapy for Hypoxic Tumors: State-of-the-Art Approaches and Emerging Trends. Biomedicines 2021, 9, 137. [Google Scholar] [CrossRef]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, R.; de la Guardia, M.; Ahmadi, D.; Yousefi, B. Modulating tumor hypoxia by nanomedicine for effective cancer therapy. J. Cell. Physiol. 2018, 233, 2019–2031. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer Cell Metabolism: Warburg and Beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Gao, X.-Z.; Wang, G.-N.; Zhao, W.-G.; Han, J.; Diao, C.-Y.; Wang, X.-H.; Li, S.-L.; Li, W.-C. Blocking OLFM4/HIF-1α axis alleviates hypoxia-induced invasion, epithelial–mesenchymal transition, and chemotherapy resistance in non-small-cell lung cancer. J. Cell. Physiol. 2019, 234, 15035–15043. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, X.; Zhang, K.; Yin, Y.; Chen, Y.; Zhang, T. Interleukin-6 contributes to chemoresistance in MDA-MB-231 cells via targeting HIF-1α. J. Biochem. Mol. Toxicol. 2018, 32, e22039. [Google Scholar] [CrossRef] [PubMed]
- Goggins, E.; Kakkad, S.; Mironchik, Y.; Jacob, D.; Wildes, F.; Krishnamachary, B.; Bhujwalla, Z.M. Hypoxia Inducible Factors Modify Collagen I Fibers in MDA-MB-231 Triple Negative Breast Cancer Xenografts. Neoplasia 2018, 20, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Myllyharju, J.; Schipani, E. Extracellular matrix genes as hypoxia-inducible targets. Cell Tissue Res. 2010, 339, 19–29. [Google Scholar] [CrossRef]
- Casas, A.; Di Venosa, G.; Hasan, T.; Al, B. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef]
- Kopecka, J.; Salaroglio, I.C.; Perez-Ruiz, E.; Sarmento-Ribeiro, A.B.; Saponara, S.; De Las Rivas, J.; Riganti, C. Hypoxia as a driver of resistance to immunotherapy. Drug Resist. Updates 2021, 59, 100787. [Google Scholar] [CrossRef]
- Hockel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Sundfor, K.; Lyng, H.; Trope, C.G. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br. J. Cancer 2000, 83, 354–359. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S.; et al. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Shannon, A.M.; Bouchier-Hayes, D.J.; Condron, C.M.; Toomey, D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003, 29, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Tsuchida, R.; Malkin, D.; Koren, G.; Baruchel, S.; Yeger, H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008, 26, 1818–1830. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zou, Z. Targeting autophagy to overcome drug resistance: Further developments. J. Hematol. Oncol. 2020, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, N.; Cramer, T. Hypoxia-mediated drug resistance: Novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist. Updates 2011, 14, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Salama, N.N.; Azab, A.K. The role of P-glycoprotein in drug resistance in multiple myeloma. Leuk. Lymphoma 2015, 56, 26–33. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, Z.; Yin, S.; Wang, Q.; Guo, F.; Zhang, Y.; Yu, R.; Liu, Y.; Su, Z. Extending Half Life of H-Ferritin Nanoparticle by Fusing Albumin Binding Domain for Doxorubicin Encapsulation. Biomacromolecules 2018, 19, 773–781. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Chen, H.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.; Xie, J. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef]
- Garcia-Pinel, B.; Porras-Alcala, C.; Ortega-Rodriguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; Lopez-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sheng, D.; Han, Z.; Zhang, L.; Sun, G.; Yang, X.; Wang, X.; Wei, L.; Lu, Y.; Hou, X.; et al. Doxorubicin-liposome combined with clodronate-liposome inhibits hepatocellular carcinoma through the depletion of macrophages and tumor cells. Int. J. Pharm. 2022, 629, 122346. [Google Scholar] [CrossRef] [PubMed]
- Darabi, F.; Saidijam, M.; Nouri, F.; Mahjub, R.; Soleimani, M. Anti-CD44 and EGFR Dual-Targeted Solid Lipid Nanoparticles for Delivery of Doxorubicin to Triple-Negative Breast Cancer Cell Line: Preparation, Statistical Optimization, and In Vitro Characterization. Biomed. Res. Int. 2022, 2022, 6253978. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Babos, G.; Biro, E.; Meiczinger, M.; Feczko, T. Dual Drug Delivery of Sorafenib and Doxorubicin from PLGA and PEG-PLGA Polymeric Nanoparticles. Polymers 2018, 10, 895. [Google Scholar] [CrossRef]
- Zheng, W.; Li, M.; Lin, Y.; Zhan, X. Encapsulation of verapamil and doxorubicin by MPEG-PLA to reverse drug resistance in ovarian cancer. Biomed. Pharmacother. 2018, 108, 565–573. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef]
- Sakthi Devi, R.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Hepokur, C.; Kariper, I.A.; Misir, S.; Ay, E.; Tunoglu, S.; Ersez, M.S.; Zeybek, U.; Kuruca, S.E.; Yaylim, I. Silver nanoparticle/capecitabine for breast cancer cell treatment. Toxicol. Vitro 2019, 61, 104600. [Google Scholar] [CrossRef]
- Shah, S.; Famta, P.; Bagasariya, D.; Charankumar, K.; Sikder, A.; Kashikar, R.; Kotha, A.K.; Chougule, M.B.; Khatri, D.K.; Asthana, A.; et al. Tuning Mesoporous Silica Nanoparticles in Novel Avenues of Cancer Therapy. Mol. Pharm. 2022, 19, 4428–4452. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Aitkulov, A.; Atabaev, T.S.; Blanc, W.; Inglezakis, V.J.; Tosi, D. Green-Synthesized Silver Nanoparticle–Assisted Radiofrequency Ablation for Improved Thermal Treatment Distribution. Nanomaterials 2022, 12, 426. [Google Scholar] [PubMed]
- Becker, A.; Leskau, M.; Schlingmann-Molina, B.L.; Hohmeier, S.C.; Alnajjar, S.; Murua Escobar, H.; Ngezahayo, A. Functionalization of gold-nanoparticles by the Clostridium perfringens enterotoxin C-terminus for tumor cell ablation using the gold nanoparticle-mediated laser perforation technique. Sci. Rep. 2018, 8, 14963. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Shi, Z.; Qi, H.; Zhao, M.; Huang, K.; Han, D.; Zhou, J.; Liu, C.; Liu, Y.; Lu, Y.; et al. A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors. Theranostics 2019, 9, 7616–7627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, B.; Yao, S.; Liu, Z.; Li, J. Multifunctional Bi NSs@BSA Nanoplatform Guided by CT Imaging for Effective Photothermal Therapy. Langmuir 2022, 38, 14355–14363. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Nai, J.; Yang, Z.; Zhang, J.; Ma, S.; Zhao, Y.; Li, H.; Li, J.; Yang, Y.; Yang, M.; et al. A novel preparative method for nanoparticle albumin-bound paclitaxel with high drug loading and its evaluation both in vitro and in vivo. PLoS ONE 2021, 16, e0250670. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004, 54, 514–524. [Google Scholar] [CrossRef]
- Gawali, P.; Saraswat, A.; Bhide, S.; Gupta, S.; Patel, K. Human solid tumors and clinical relevance of the enhanced permeation and retention effect: A ‘golden gate’ for nanomedicine in preclinical studies? Nanomedicine 2023, 18, 169–190. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Tredan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug resistance and the solid tumor microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Yeow, Y.L.; Kotamraju, V.R.; Wang, X.; Chopra, M.; Azme, N.; Wu, J.; Schoep, T.D.; Delaney, D.S.; Feindel, K.; Li, J.; et al. Immune-mediated ECM depletion improves tumour perfusion and payload delivery. EMBO Mol. Med. 2019, 11, e10923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wen, Y.; Hu, Y.; Chen, W.; Zheng, X.; Guo, W.; Wang, T.; Qian, Z.; Su, B.L.; He, Q. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 2017, 9, 3637–3645. [Google Scholar] [CrossRef]
- Wu, J.; Song, J.; Yin, X.; Tang, J.; Zhang, J.; Wang, X.; Ji, Y.; Zhao, Y.; Chen, D.; Sheng, J.; et al. Recent Advancements of Nanotechnology-Based Strategies for Overcoming Tumor Microenvironment Hypoxia. Front. Biosci. 2022, 27, 145. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liang, C.; Yi, X.; Zhao, Q.; Cheng, L.; Yang, K.; Liu, Z. Perfluorocarbon-Loaded Hollow Bi2Se3 Nanoparticles for Timely Supply of Oxygen under Near-Infrared Light to Enhance the Radiotherapy of Cancer. Adv. Mater. 2016, 28, 2716–2723. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ai, X.; Cabral, H.; Liu, J.; Huang, Y.; Mi, P. Tumor hypoxia-activated combinatorial nanomedicine triggers systemic antitumor immunity to effectively eradicate advanced breast cancer. Biomaterials 2021, 273, 120847. [Google Scholar] [CrossRef]
- Murakami, T.; Hiroshima, Y.; Miyake, K.; Hwang, H.K.; Kiyuna, T.; DeLong, J.C.; Lwin, T.M.; Matsuyama, R.; Mori, R.; Kumamoto, T.; et al. Color-coded intravital imaging demonstrates a transforming growth factor-β (TGF-β) antagonist selectively targets stromal cells in a human pancreatic-cancer orthotopic mouse model. Cell Cycle 2017, 16, 1008–1014. [Google Scholar] [CrossRef]
- de Visser, K.E.; Kast, W.M. Effects of TGF-beta on the immune system: Implications for cancer immunotherapy. Leukemia 1999, 13, 1188–1199. [Google Scholar] [CrossRef]
- Huber, S.; Schramm, C.; Lehr, H.A.; Mann, A.; Schmitt, S.; Becker, C.; Protschka, M.; Galle, P.R.; Neurath, M.F.; Blessing, M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J. Immunol. 2004, 173, 6526–6531. [Google Scholar] [CrossRef]
- Chaudhury, A.; Hussey, G.S.; Ray, P.S.; Jin, G.; Fox, P.L.; Howe, P.H. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat. Cell Biol. 2010, 12, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhu, Y.; Nilsson, M.; Sundfeldt, K. TGF-β isoforms induce EMT independent migration of ovarian cancer cells. Cancer Cell Int. 2014, 14, 72. [Google Scholar] [CrossRef]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar] [CrossRef]
- Wang, X.; Abraham, S.; McKenzie, J.A.G.; Jeffs, N.; Swire, M.; Tripathi, V.B.; Luhmann, U.F.O.; Lange, C.A.K.; Zhai, Z.; Arthur, H.M.; et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature 2013, 499, 306–311. [Google Scholar] [CrossRef]
- Fang, S.; Pentinmikko, N.; Ilmonen, M.; Salven, P. Dual action of TGF-β induces vascular growth in vivo through recruitment of angiogenic VEGF-producing hematopoietic effector cells. Angiogenesis 2012, 15, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Whitaker-Menezes, D.; Capparelli, C.; Balliet, R.; Lin, Z.; Pestell, R.G.; Howell, A.; Aquila, S.; Andò, S.; Martinez-Outschoorn, U.; et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: Connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle 2012, 11, 3019–3035. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, M.; Wang, J.; Zhou, S.; Liu, Y.; Liu, S.; Huang, Y.; Chen, Y.; Chen, L.; Song, Q.; et al. Sequential delivery of nanoformulated α-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials 2020, 241, 119907. [Google Scholar] [CrossRef]
- Panagi, M.; Voutouri, C.; Mpekris, F.; Papageorgis, P.; Martin, M.R.; Martin, J.D.; Demetriou, P.; Pierides, C.; Polydorou, C.; Stylianou, A.; et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 2020, 10, 1910–1922. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, J.; Zuo, T.; Wu, G.; Xu, Y.; Yang, Y.; Yang, J.; Shen, Q. Chemo-Photothermal Combination Cancer Therapy with ROS Scavenging, Extracellular Matrix Depletion, and Tumor Immune Activation by Telmisartan and Diselenide-Paclitaxel Prodrug Loaded Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 31292–31308. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, V.; Ahmadi, A.; Malakotikhah, F.; Chaleshtari, M.G.; Baghi Moornani, M.; Masjedi, A.; Sojoodi, M.; Atyabi, F.; Nikkhoo, A.; Rostami, N.; et al. Silencing of p68 and STAT3 synergistically diminishes cancer progression. Life Sci. 2020, 249, 117499. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, L.; Huang, Y.; Wang, J.; Feng, J.; Xu, M.; Chen, Y.; Song, Q.; Jiang, G.; Gu, X.; et al. Sequential Targeting TGF-beta Signaling and KRAS Mutation Increases Therapeutic Efficacy in Pancreatic Cancer. Small 2019, 15, e1900631. [Google Scholar] [CrossRef]
- Sarper, M.; Cortes, E.; Lieberthal, T.J.; Del Río Hernández, A. ATRA modulates mechanical activation of TGF-β by pancreatic stellate cells. Sci. Rep. 2016, 6, 27639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, C.; Qian, P.; Lu, X.; Sun, B.; Zhang, X.; Wang, L.; Gao, X.; Li, H.; Chen, Z.; et al. Gd-metallofullerenol nanomaterial as non-toxic breast cancer stem cell-specific inhibitor. Nat. Commun. 2015, 6, 5988. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Niyaz, M.; Khan, M.S.; Mudassar, S. Hedgehog Signaling: An Achilles’ Heel in Cancer. Transl. Oncol. 2019, 12, 1334–1344. [Google Scholar] [CrossRef]
- Mpekris, F.; Papageorgis, P.; Polydorou, C.; Voutouri, C.; Kalli, M.; Pirentis, A.P.; Stylianopoulos, T. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Control. Release 2017, 261, 105–112. [Google Scholar] [CrossRef]
- Guo, X.L.; Kang, X.X.; Wang, Y.Q.; Zhang, X.J.; Li, C.J.; Liu, Y.; Du, L.B. Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater. 2019, 84, 367–377. [Google Scholar] [CrossRef]
- Ko, A.H.; LoConte, N.; Tempero, M.A.; Walker, E.J.; Kate Kelley, R.; Lewis, S.; Chang, W.C.; Kantoff, E.; Vannier, M.W.; Catenacci, D.V.; et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas 2016, 45, 370–375. [Google Scholar] [CrossRef]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef]
- Singha, N.C.; Nekoroski, T.; Zhao, C.; Symons, R.; Jiang, P.; Frost, G.I.; Huang, Z.; Shepard, H.M. Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol. Cancer Ther. 2015, 14, 523–532. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.Y.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients with Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2020, 38, 3185–3194. [Google Scholar] [CrossRef]
- Hakim, N.; Patel, R.; Devoe, C.; Saif, M.W. Why HALO 301 Failed and Implications for Treatment of Pancreatic Cancer. Pancreas 2019, 3, e1–e4. [Google Scholar] [CrossRef]
- Ward, J.P.; Shum, M.; Bertino, E.; Lin, V.; Kuruvadi, V.; Heineman, T. Efficacy and safety of pegvorhyaluronidase alfa (PEGPH20; PVHA) and pembrolizumab (pembro) combination therapy in patients (Pts) with stage III/IV non-small cell lung cancer (NSCLC). Ann. Oncol. 2019, 30, ix171. [Google Scholar] [CrossRef]
- Morosi, L.; Meroni, M.; Ubezio, P.; Fuso Nerini, I.; Minoli, L.; Porcu, L.; Panini, N.; Colombo, M.; Blouw, B.; Kang, D.W.; et al. PEGylated recombinant human hyaluronidase (PEGPH20) pre-treatment improves intra-tumour distribution and efficacy of paclitaxel in preclinical models. J. Exp. Clin. Cancer Res. 2021, 40, 286. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Sadhukha, T.; Kim, H.; Khanna, V.; Koniar, B.; Panyam, J. Fibrinolytic Enzyme Cotherapy Improves Tumor Perfusion and Therapeutic Efficacy of Anticancer Nanomedicine. Cancer Res. 2017, 77, 1465–1475. [Google Scholar] [CrossRef]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, S.; Ling, Y.; Meng, G.; Yang, Y.; Zhang, W. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J. Control. Release 2015, 220, 529–544. [Google Scholar] [CrossRef]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef]
- Elahi-Gedwillo, K.Y.; Carlson, M.; Zettervall, J.; Provenzano, P.P. Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2019, 79, 372–386. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.; Liu, H.; Liu, X.; Situ, A.; Wu, B.; Ji, Z.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef]
- Mardhian, D.F.; Storm, G.; Bansal, R.; Prakash, J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Control. Release 2018, 290, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karimnia, V.; Stanley, M.E.; Fitzgerald, C.T.; Rizvi, I.; Slack, F.J.; Celli, J.P. Photodynamic Stromal Depletion Enhances Therapeutic Nanoparticle Delivery in 3D Pancreatic Ductal Adenocarcinoma Tumor Models. Photochem. Photobiol. 2023, 99, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.G.; Zhou, G.; Yang, P.; Liu, Y.; Sun, B.; Huynh, T.; Meng, H.; Zhao, L.; Xing, G.; Chen, C.; et al. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proc. Natl. Acad. Sci. USA 2012, 109, 15431–15436. [Google Scholar] [CrossRef]
- Ouahoud, S.; Voorneveld, P.W.; van der Burg, L.R.A.; de Jonge-Muller, E.S.M.; Schoonderwoerd, M.J.A.; Paauwe, M.; de Vos, T.; de Wit, S.; van Pelt, G.W.; Mesker, W.E.; et al. Bidirectional tumor/stroma crosstalk promotes metastasis in mesenchymal colorectal cancer. Oncogene 2020, 39, 2453–2466. [Google Scholar] [CrossRef]
- Guo, L.; Shi, D.; Meng, D.; Shang, M.; Sun, X.; Zhou, X.; Liu, X.; Zhao, Y.; Li, J. New FH peptide-modified ultrasonic nanobubbles for delivery of doxorubicin to cancer-associated fibroblasts. Nanomedicine 2019, 14, 2957–2971. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Z.; Sun, J.; Song, Q.; He, B.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomedicine 2016, 12, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, O.R.; Choi, Y.S.; Lee, H.; Park, K.; Lee, C.T.; Kang, K.W.; Jeong, S. Selection and characterization of tenascin C targeting peptide. Mol. Cells 2012, 33, 71–77. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Li, J.; Tang, X.; Wang, X.; Cun, X.; Xu, S.; Liu, Y.; Li, M.; Zhang, Z.; et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J. Control. Release 2020, 321, 564–575. [Google Scholar] [CrossRef]
- Hou, L.; Chen, D.; Hao, L.; Tian, C.; Yan, Y.; Zhu, L.; Zhang, H.; Zhang, Y.; Zhang, Z. Transformable nanoparticles triggered by cancer-associated fibroblasts for improving drug permeability and efficacy in desmoplastic tumors. Nanoscale 2019, 11, 20030–20044. [Google Scholar] [CrossRef] [PubMed]
- Cun, X.; Chen, J.; Li, M.; He, X.; Tang, X.; Guo, R.; Deng, M.; Li, M.; Zhang, Z.; He, Q. Tumor-Associated Fibroblast-Targeted Regulation and Deep Tumor Delivery of Chemotherapeutic Drugs with a Multifunctional Size-Switchable Nanoparticle. ACS Appl. Mater. Interfaces 2019, 11, 39545–39559. [Google Scholar] [CrossRef]
- Lang, J.; Zhao, X.; Qi, Y.; Zhang, Y.; Han, X.; Ding, Y.; Guan, J.; Ji, T.; Zhao, Y.; Nie, G. Reshaping Prostate Tumor Microenvironment To Suppress Metastasis via Cancer-Associated Fibroblast Inactivation with Peptide-Assembly-Based Nanosystem. ACS Nano 2019, 13, 12357–12371. [Google Scholar] [CrossRef] [PubMed]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Gore, J.; Korc, M. Pancreatic cancer stroma: Friend or foe? Cancer Cell 2014, 25, 711–712. [Google Scholar] [CrossRef] [PubMed]
- Izumi, D.; Ishimoto, T.; Miyake, K.; Sugihara, H.; Eto, K.; Sawayama, H.; Yasuda, T.; Kiyozumi, Y.; Kaida, T.; Kurashige, J.; et al. CXCL12/CXCR4 activation by cancer-associated fibroblasts promotes integrin β1 clustering and invasiveness in gastric cancer. Int. J. Cancer 2016, 138, 1207–1219. [Google Scholar] [CrossRef]
- Wei, L.; Ye, H.; Li, G.; Lu, Y.; Zhou, Q.; Zheng, S.; Lin, Q.; Liu, Y.; Li, Z.; Chen, R. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 2018, 9, 1065. [Google Scholar] [CrossRef]
- Aronovich, A.; Moyal, L.; Gorovitz, B.; Amitay-Laish, I.; Naveh, H.P.; Forer, Y.; Maron, L.; Knaneh, J.; Ad-El, D.; Yaacobi, D.; et al. Cancer-Associated Fibroblasts in Mycosis Fungoides Promote Tumor Cell Migration and Drug Resistance through CXCL12/CXCR4. J. Investig. Dermatol. 2021, 141, 619–627.e2. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.N.; Rao, G.; Dey, A.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticle Transforms Activated Cancer-Associated Fibroblasts to Quiescence. ACS Appl. Mater. Interfaces 2019, 11, 26060–26068. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Lee, J.J.; Perera, R.M.; Wang, H.; Wu, D.C.; Liu, X.S.; Han, S.; Fitamant, J.; Jones, P.D.; Ghanta, K.S.; Kawano, S.; et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl. Acad. Sci. USA 2014, 111, E3091–E3100. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Astakhova, A.A.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. High and Low Molecular Weight Hyaluronic Acid Differentially Influences Oxylipins Synthesis in Course of Neuroinflammation. Int. J. Mol. Sci. 2019, 20, 3894. [Google Scholar] [CrossRef] [PubMed]
- Lavie, D.; Ben-Shmuel, A.; Erez, N.; Scherz-Shouval, R. Cancer-associated fibroblasts in the single-cell era. Nat. Cancer 2022, 3, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Karpisheh, V.; Ahmadi, M.; Abbaszadeh-Goudarzi, K.; Mohammadpour Saray, M.; Barshidi, A.; Mohammadi, H.; Yousefi, M.; Jadidi-Niaragh, F. The role of Th17 cells in the pathogenesis and treatment of breast cancer. Cancer Cell Int. 2022, 22, 108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).