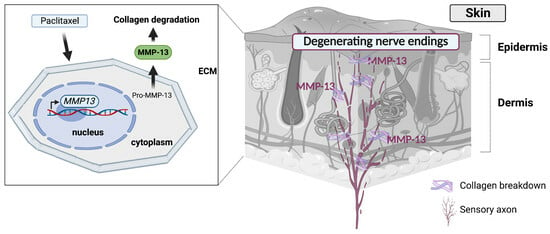
Skin Extracellular Matrix Breakdown Following Paclitaxel Therapy in Patients with Chemotherapy-Induced Peripheral Neuropathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Clinimetric and Biosample Collection
2.2. RNA Sequencing Study
2.3. Immunostaining
2.4. Transmission Electron Microscopy
2.5. Image and Statistical Analyses
3. Results
3.1. Intraepidermal Nerve Fiber Quantifications
3.2. RNA Sequencing
3.3. MMP-13 Expression Is Increased in the Skin of CIPN Patients
3.4. Ultrastructural Analysis of the Skin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, F.; Taglieri, L.; Salerno, G.; Lanza, R.; Scarpa, S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit. Rev. Oncol. Hematol. 2015, 96, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Zolota, V.; Kyriakopoulou, O.; Kalofonos, H.P. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J. BUON 2010, 15, 435–446. [Google Scholar] [PubMed]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef]
- Boehmerle, W.; Huehnchen, P.; Lee, S.L.L.; Harms, C.; Endres, M. TRPV4 inhibition prevents paclitaxel-induced neurotoxicity in preclinical models. Exp. Neurol. 2018, 306, 64–75. [Google Scholar] [CrossRef]
- Li, Y.; Pazyra-Murphy, M.F.; Avizonis, D.; de Sa Tavares Russo, M.; Tang, S.; Chen, C.Y.; Hsueh, Y.P.; Bergholz, J.S.; Jiang, T.; Zhao, J.J.; et al. Sarm1 activation produces cADPR to increase intra-axonal Ca++ and promote axon degeneration in PIPN. J. Cell Biol. 2022, 221, e202106080. [Google Scholar] [CrossRef] [PubMed]
- Pease-Raissi, S.E.; Pazyra-Murphy, M.F.; Li, Y.; Wachter, F.; Fukuda, Y.; Fenstermacher, S.J.; Barclay, L.A.; Bird, G.H.; Walensky, L.D.; Segal, R.A. Paclitaxel Reduces Axonal Bclw to Initiate IP(3)R1-Dependent Axon Degeneration. Neuron 2017, 96, 373–386. [Google Scholar] [CrossRef]
- Bobylev, I.; Joshi, A.R.; Barham, M.; Ritter, C.; Neiss, W.F.; Höke, A.; Lehmann, H.C. Paclitaxel inhibits mRNA transport in axons. Neurobiol. Dis. 2015, 82, 321–331. [Google Scholar] [CrossRef]
- LaPointe, N.E.; Morfini, G.; Brady, S.T.; Feinstein, S.C.; Wilson, L.; Jordan, M.A. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: Implications for chemotherapy-induced peripheral neuropathy. Neurotoxicology 2013, 37, 231–239. [Google Scholar] [CrossRef]
- Theiss, C.; Meller, K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 2000, 299, 213–224. [Google Scholar] [CrossRef]
- André, N.; Carré, M.; Brasseur, G.; Pourroy, B.; Kovacic, H.; Briand, C.; Braguer, D. Paclitaxel targets mitochondria upstream of caspase activation in intact human neuroblastoma cells. FEBS Lett. 2002, 532, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, D.; Hassan, M.; Haikel, Y.; Hengge, U.R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-Jun N-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell. Signal. 2008, 20, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Oka, Y.; Kambe, T.; Koizumi, N.; Abe, K.; Kawakami, K.; Utsunomiya, I.; Taguchi, K. Paclitaxel-induced peripheral neuropathy increases substance P release in rat spinal cord. Eur. J. Pharmacol. 2016, 770, 46–51. [Google Scholar] [CrossRef]
- Darby, L.M.; Meng, H.; Fehrenbacher, J.C. Paclitaxel inhibits the activity and membrane localization of PKCalpha and PKCbetaI/II to elicit a decrease in stimulated calcitonin gene-related peptide release from cultured sensory neurons. Mol. Cell. Neurosci. 2017, 82, 105–117. [Google Scholar] [CrossRef]
- Pittman, S.K.; Gracias, N.G.; Vasko, M.R.; Fehrenbacher, J.C. Paclitaxel alters the evoked release of calcitonin gene-related peptide from rat sensory neurons in culture. Exp. Neurol. 2014, 253, 146–153. [Google Scholar] [CrossRef]
- Makker, P.G.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Domoto, R.; Nakashima, K.; Yamasoba, D.; Yamanishi, H.; Tsubota, M.; Wake, H.; Nishibori, M.; Kawabata, A. Paclitaxel-induced HMGB1 release from macrophages and its implication for peripheral neuropathy in mice: Evidence for a neuroimmune crosstalk. Neuropharmacology 2018, 141, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, L.; Xie, M.; Li, Y.; Huang, P.; Saunders, T.L.; Fox, D.A.; Rosenquist, R.; Lin, F. Role of Complement in a Rat Model of Paclitaxel-Induced Peripheral Neuropathy. J. Immunol. 2018, 200, 4094–4101. [Google Scholar] [CrossRef]
- Cirrincione, A.M.; Pellegrini, A.D.; Dominy, J.R.; Benjamin, M.E.; Utkina-Sosunova, I.; Lotti, F.; Jergova, S.; Sagen, J.; Rieger, S. Paclitaxel-induced peripheral neuropathy is caused by epidermal ROS and mitochondrial damage through conserved MMP-13 activation. Sci. Rep. 2020, 10, 3970. [Google Scholar] [CrossRef]
- Lisse, T.S.; Middleton, L.J.; Pellegrini, A.D.; Martin, P.B.; Spaulding, E.L.; Lopes, O.; Brochu, E.A.; Carter, E.V.; Waldron, A.; Rieger, S. Paclitaxel-induced epithelial damage and ectopic MMP-13 expression promotes neurotoxicity in zebrafish. Proc. Natl. Acad. Sci. USA 2016, 113, E2189–E2198. [Google Scholar] [CrossRef]
- Bennett, G.J.; Liu, G.K.; Xiao, W.H.; Jin, H.W.; Siau, C. Terminal arbor degeneration–A novel lesion produced by the antineoplastic agent paclitaxel. Eur. J. Neurosci. 2011, 33, 1667–1676. [Google Scholar] [CrossRef]
- Gornstein, E.L.; Schwarz, T.L. Neurotoxic mechanisms of paclitaxel are local to the distal axon and independent of transport defects. Exp. Neurol. 2017, 288, 153–166. [Google Scholar] [CrossRef]
- Ko, M.H.; Hu, M.E.; Hsieh, Y.L.; Lan, C.T.; Tseng, T.J. Peptidergic intraepidermal nerve fibers in the skin contribute to the neuropathic pain in paclitaxel-induced peripheral neuropathy. Neuropeptides 2014, 48, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cadiz Diaz, A.; Cirrincione, A.M.; Schmidt, N.A.; Ugo, M.J.; Amaya Sanchez, M.C.; Reimonn, C.A.; Wuchty, S.; Pellegrini, A.D.; Rude, L.R.K.; Pappalardo, L.G.; et al. Epidermal Eg5 promotes X-ROS dependent paclitaxel neurotoxicity. Biorxiv 2023. [Google Scholar] [CrossRef]
- Dyck, P.J.; Davies, J.L.; Litchy, W.J.; O’Brien, P.C. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997, 49, 229–239. [Google Scholar] [CrossRef]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lantéri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Engelstad, J.K.; Taylor, S.W.; Witt, L.V.; Hoebing, B.J.; Herrmann, D.N.; Dyck, P.J.; Klein, C.J.; Johnson, D.M.; Davies, J.L.; Carter, R.E. Epidermal nerve fibers: Confidence intervals and continuous measures with nerve conduction. Neurology 2012, 79, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef]
- Alberti, P.; Rossi, E.; Cornblath, D.R.; Merkies, I.S.; Postma, T.J.; Frigeni, B.; Bruna, J.; Velasco, R.; Argyriou, A.A.; Kalofonos, H.P.; et al. Physician-assessed and patient-reported outcome measures in chemotherapy-induced sensory peripheral neurotoxicity: Two sides of the same coin. Ann. Oncol. 2014, 25, 257–264. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, G.B.; Corrocher, R. Hepatic fibrosis and its serum markers: A review. Dig. Dis. 1991, 9, 303–316. [Google Scholar] [CrossRef]
- Jenkins, R.G.; Simpson, J.K.; Saini, G.; Bentley, J.H.; Russell, A.M.; Braybrooke, R.; Molyneaux, P.L.; McKeever, T.M.; Wells, A.U.; Flynn, A.; et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: An analysis from the prospective, multicentre PROFILE study. Lancet Respir. Med. 2015, 3, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Juhl, P.; Bay-Jensen, A.C.; Karsdal, M.; Siebuhr, A.S.; Franchimont, N.; Chavez, J. Serum biomarkers of collagen turnover as potential diagnostic tools in diffuse systemic sclerosis: A cross-sectional study. PLoS ONE 2018, 13, e0207324. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.H.; Godskesen, L.E.; Jensen, M.D.; Van Haaften, W.T.; Klinge, L.G.; Olinga, P.; Dijkstra, G.; Kjeldsen, J.; Karsdal, M.A.; Bay-Jensen, A.C.; et al. Fragments of Citrullinated and MMP-degraded Vimentin and MMP-degraded Type III Collagen Are Novel Serological Biomarkers to Differentiate Crohn’s Disease from Ulcerative Colitis. J. Crohns Colitis 2015, 9, 863–872. [Google Scholar] [CrossRef]
- Sand, J.M.; Larsen, L.; Hogaboam, C.; Martinez, F.; Han, M.; Rossel Larsen, M.; Nawrocki, A.; Zheng, Q.; Karsdal, M.A.; Leeming, D.J. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis--validation of two novel biomarker assays. PLoS ONE 2013, 8, e84934. [Google Scholar] [CrossRef]
- Vassiliadis, E.; Veidal, S.S.; Simonsen, H.; Larsen, D.V.; Vainer, B.; Chen, X.; Zheng, Q.; Karsdal, M.A.; Leeming, D.J. Immunological detection of the type V collagen propeptide fragment, PVCP-1230, in connective tissue remodeling associated with liver fibrosis. Biomarkers 2011, 16, 426–433. [Google Scholar] [CrossRef]
- Zannad, F.; Pitt, B. Biomarkers of extracellular matrix turnover. Heart Fail. Clin. 2009, 5, 589–599. [Google Scholar] [CrossRef]
- Zhang, M.; Grote, C.; Wang, J. Epigenetic mechanisms underlying the pathogenesis of osteoarthritis and their clinical relevance. In Prognostic Epigenetics; Academic Press: Cambridge, MA, USA, 2019; Volume 15, pp. 245–268. [Google Scholar]
- Yassin, A.M.; AbuBakr, H.O.; Abdelgalil, A.I.; Khattab, M.S.; El-Behairy, A.M.; Gouda, E.M. COL2A1 and Caspase-3 as Promising Biomarkers for Osteoarthritis Prognosis in an Equus asinus Model. Biomolecules 2020, 10, 354. [Google Scholar] [CrossRef]
- Wu, N.; Jansen, E.D.; Davidson, J.M. Comparison of mouse matrix metalloproteinase 13 expression in free-electron laser and scalpel incisions during wound healing. J. Investig. Dermatol. 2003, 121, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, V.F.; Nancarrow, D.J.; Stark, M.S.; Moser, R.J.; Boyle, G.M.; Aoude, L.G.; Schmidt, C.; Hayward, N.K. Cross-platform array screening identifies COL1A2, THBS1, TNFRSF10D and UCHL1 as genes frequently silenced by methylation in melanoma. PLoS ONE 2011, 6, e26121. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Watabe, K. Immortalized adult rodent Schwann cells as useful tools for the study of peripheral nerve regeneration. Rinsho Shinkeigaku 2013, 53, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Farraj, P.; Coleman, M.P. Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases. Neurotherapeutics 2021, 18, 2200–2221. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Terenghi, G.; Hall, S.M. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia 1997, 20, 333–347. [Google Scholar] [CrossRef]
- Wakatsuki, S.; Araki, T.; Sehara-Fujisawa, A. Neuregulin-1/glial growth factor stimulates Schwann cell migration by inducing α5 β1 integrin-ErbB2-focal adhesion kinase complex formation. Genes Cells 2014, 19, 66–77. [Google Scholar] [CrossRef]
- Rani, S.; Kumari, U.; Bhardwaj, S.; Parsad, D.; Sharma, V.L.; Kumar, R. Decreased expression of neuregulin1 in the lesional skin of vitiligo patients. Int. J. Dermatol. 2019, 58, 242–249. [Google Scholar] [CrossRef]
- Yoon, D.; Yoon, D.; Cha, H.J.; Lee, J.S.; Chun, W. Enhancement of wound healing efficiency mediated by artificial dermis functionalized with EGF or NRG1. Biomed. Mater. 2018, 13, 045007. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, H.J.; Kim, J.H.; Kim, T.H.; Seo, C.H.; Sung, Y.K.; Kim, K.H. BMP4-Induced Differentiation of Human Hair Follicle Neural Crest Stem Cells into Precursor Melanocytes from Hair Follicle Bulge. Ann. Dermatol. 2020, 32, 409–416. [Google Scholar] [CrossRef]
- Lin, J.B.; Kang, M.Q.; Huang, L.P.; Zhuo, Y.; Li, X.; Lai, F.C. CHRNA1 promotes the pathogenesis of primary focal hyperhidrosis. Mol. Cell. Neurosci. 2021, 111, 103598. [Google Scholar] [CrossRef]
- Panchal, H.; Wansbury, O.; Parry, S.; Ashworth, A.; Howard, B. Neuregulin3 alters cell fate in the epidermis and mammary gland. BMC Dev. Biol. 2007, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Chateau, Y.; Misery, L. Connections between nerve endings and epidermal cells: Are they synapses? Exp. Dermatol. 2004, 13, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann. N. Y. Acad. Sci. 2013, 1279, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Diotaaz, E. Beyond the AMPA receptor: Diverse roles of SynDIG/PRRT brain-specific transmembrane proteins at excitatory synapses. Curr. Opin. Pharmacol. 2021, 58, 76–82. [Google Scholar] [CrossRef]
- Breiderhoff, T.; Christiansen, G.B.; Pallesen, L.T.; Vaegter, C.; Nykjaer, A.; Holm, M.M.; Glerup, S.; Willnow, T.E. Sortilin-related receptor SORCS3 is a postsynaptic modulator of synaptic depression and fear extinction. PLoS ONE 2013, 8, e75006. [Google Scholar] [CrossRef]
- Willis, D.E.; van Niekerk, E.A.; Sasaki, Y.; Mesngon, M.; Merianda, T.T.; Williams, G.G.; Kendall, M.; Smith, D.S.; Bassell, G.J.; Twiss, J.L. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 2007, 178, 965–980. [Google Scholar] [CrossRef]
- Yoon, B.C.; Zivraj, K.H.; Holt, C.E. Local translation and mRNA trafficking in axon pathfinding. Results Probl. Cell Differ. 2009, 48, 269–288. [Google Scholar] [CrossRef]
- Areti, A.; Yerra, V.G.; Naidu, V.; Kumar, A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014, 2, 289–295. [Google Scholar] [CrossRef]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef]
- Imai, S.; Koyanagi, M.; Azimi, Z.; Nakazato, Y.; Matsumoto, M.; Ogihara, T.; Yonezawa, A.; Omura, T.; Nakagawa, S.; Wakatsuki, S.; et al. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci. Rep. 2017, 7, 5947. [Google Scholar] [CrossRef]
- Pero, M.E.; Meregalli, C.; Qu, X.; Shin, G.J.; Kumar, A.; Shorey, M.; Rolls, M.M.; Tanji, K.; Brannagan, T.H.; Alberti, P.; et al. Pathogenic role of delta 2 tubulin in bortezomib-induced peripheral neuropathy. Proc. Natl. Acad. Sci. USA 2021, 118, e2012685118. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Watkins, S.C.; Gold, M.S. Paclitaxel-induced increase in mitochondrial volume mediates dysregulation of intracellular Ca2+ in putative nociceptive glabrous skin neurons from the rat. Cell Calcium 2017, 62, 16–28. [Google Scholar] [CrossRef] [PubMed]
- McMillan, J.R.; Akiyama, M.; Shimizu, H. Epidermal basement membrane zone components: Ultrastructural distribution and molecular interactions. J. Dermatol. Sci. 2003, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Liu, B.; Lv, L.; Wang, W.; Gao, D.; Zhang, Q.; Jiang, J.; Chai, M.; Yun, Z.; et al. Time-Resolved Extracellular Matrix Atlas of the Developing Human Skin Dermis. Front. Cell. Dev. Biol. 2021, 9, 783456. [Google Scholar] [CrossRef]
- Solomonov, I.; Zehorai, E.; Talmi-Frank, D.; Wolf, S.G.; Shainskaya, A.; Zhuravlev, A.; Kartvelishvily, E.; Visse, R.; Levin, Y.; Kampf, N.; et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc. Natl. Acad. Sci. USA 2016, 113, 10884–10889. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, T.; Hughes, R.O.; Engber, T.; Devraj, R.; Brearley, A.; Danker, K.; Young, K.; Kopatz, J.; Hermann, M.; Berthemy, A.; et al. Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy. Brain J. Neurol. 2021, 144, 3226–3238. [Google Scholar] [CrossRef]
- Kanda, K.; Fujimoto, K.; Mochizuki, R.; Ishida, K.; Lee, B. Development and validation of the comprehensive assessment scale for chemotherapy-induced peripheral neuropathy in survivors of cancer. BMC Cancer 2019, 19, 904. [Google Scholar] [CrossRef]




| Study ID | Treatment | Sex | Age at Consent | Date of Enrollment | Weeks form Neuropathy to Consent | NIS-LL | CIPN20 Score (Total: 19–76) | CIPN20 Score (Sensory: 9–36) | CIPN20 Score (Sensory: 3–12) | IENF |
|---|---|---|---|---|---|---|---|---|---|---|
| 001 | Pctx | F | 70 | 4/17/18 | 35 | 2 | 31 | 22 | 3 | 8.9 |
| 002 | Pctx | F | 70 | 6/7/18 | 31.43 | 12 | 34 | 22 | 4 | 5.3 |
| 003 | Pctx | F | 60 | 9/18/18 | 4.86 | 4 | 25 | 16 | 3 | 9.6 |
| 005 | CTRL | F | 70 | 9/21/21 | n/a | 0 | 19 | 9 | 3 | 14.9 |
| 006 | CTRL | F | 65 | 10/5/21 | n/a | 0 | 19 | 9 | 3 | 7.6 |
| 007 | CTRL | F | 64 | 11/16/21 | n/a | 0 | 19 | 9 | 3 | 7.6 |
| Average (Pctx vs. CTRL) | 67/66 | 23.76333333 | 6 | 30/19 | 20/9 | 3.3/3.0 | 7.9/10.3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staff, N.P.; Hrstka, S.C.; Dasari, S.; Capobianco, E.; Rieger, S. Skin Extracellular Matrix Breakdown Following Paclitaxel Therapy in Patients with Chemotherapy-Induced Peripheral Neuropathy. Cancers 2023, 15, 4191. https://doi.org/10.3390/cancers15164191
Staff NP, Hrstka SC, Dasari S, Capobianco E, Rieger S. Skin Extracellular Matrix Breakdown Following Paclitaxel Therapy in Patients with Chemotherapy-Induced Peripheral Neuropathy. Cancers. 2023; 15(16):4191. https://doi.org/10.3390/cancers15164191
Chicago/Turabian StyleStaff, Nathan P., Sybil C. Hrstka, Surendra Dasari, Enrico Capobianco, and Sandra Rieger. 2023. "Skin Extracellular Matrix Breakdown Following Paclitaxel Therapy in Patients with Chemotherapy-Induced Peripheral Neuropathy" Cancers 15, no. 16: 4191. https://doi.org/10.3390/cancers15164191
APA StyleStaff, N. P., Hrstka, S. C., Dasari, S., Capobianco, E., & Rieger, S. (2023). Skin Extracellular Matrix Breakdown Following Paclitaxel Therapy in Patients with Chemotherapy-Induced Peripheral Neuropathy. Cancers, 15(16), 4191. https://doi.org/10.3390/cancers15164191