Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
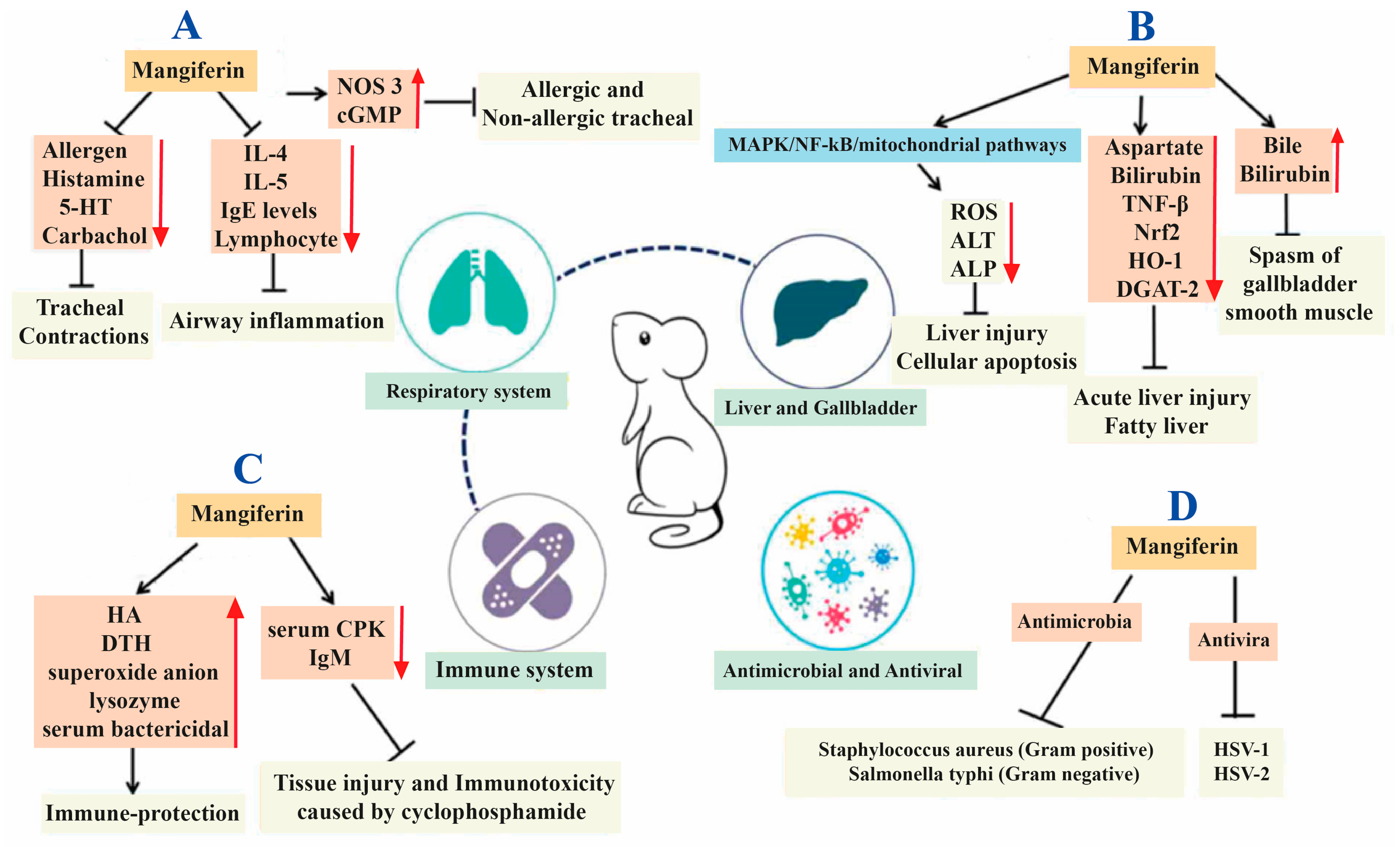
2. Mangiferin
2.1. History and Discovery of Mangiferin
2.2. Source of Mangiferin
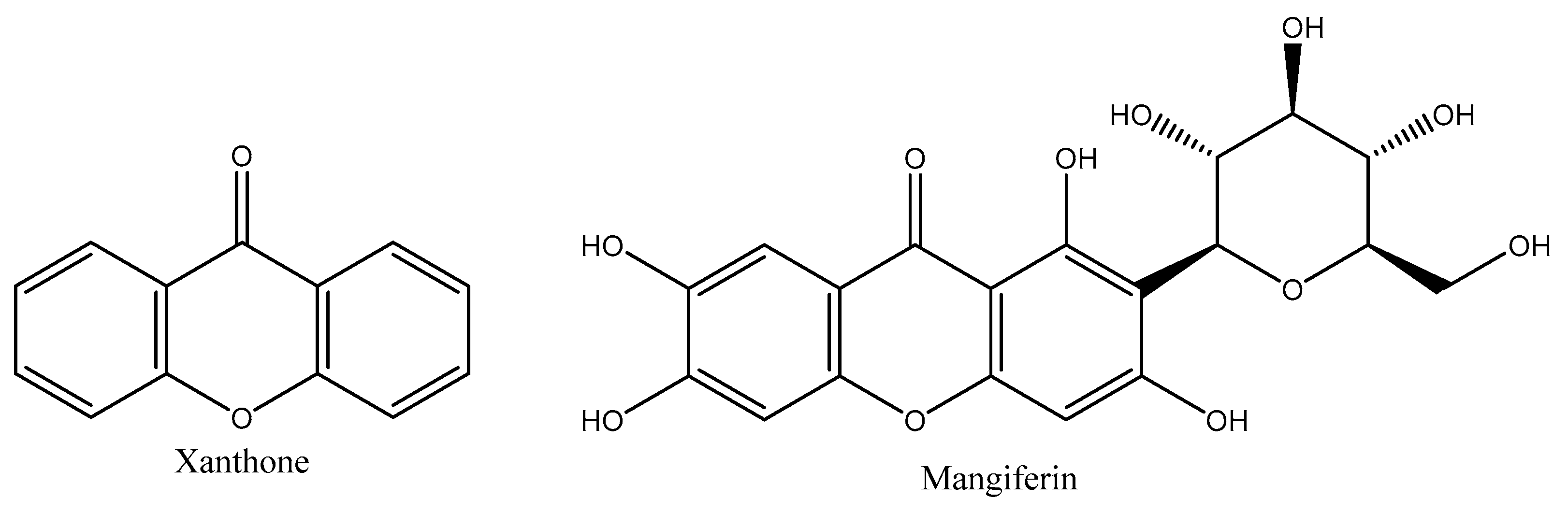
2.3. Extraction, Isolation and Structural Elucidation of Mangiferin
2.4. Structure Elucidation of Mangiferin
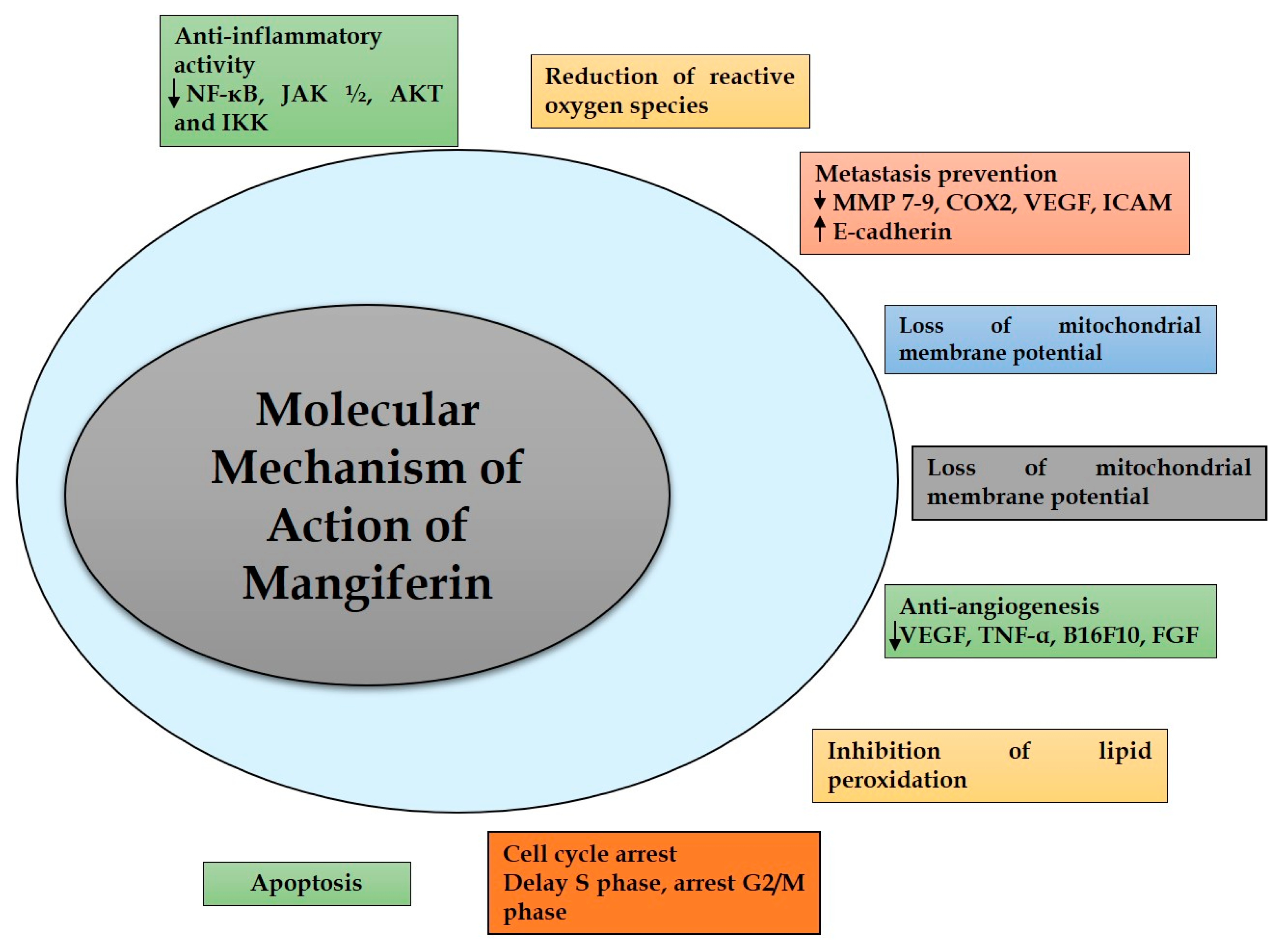
3. Molecular Mechanism of Action of Mangiferin in Cancer
3.1. Inflammation
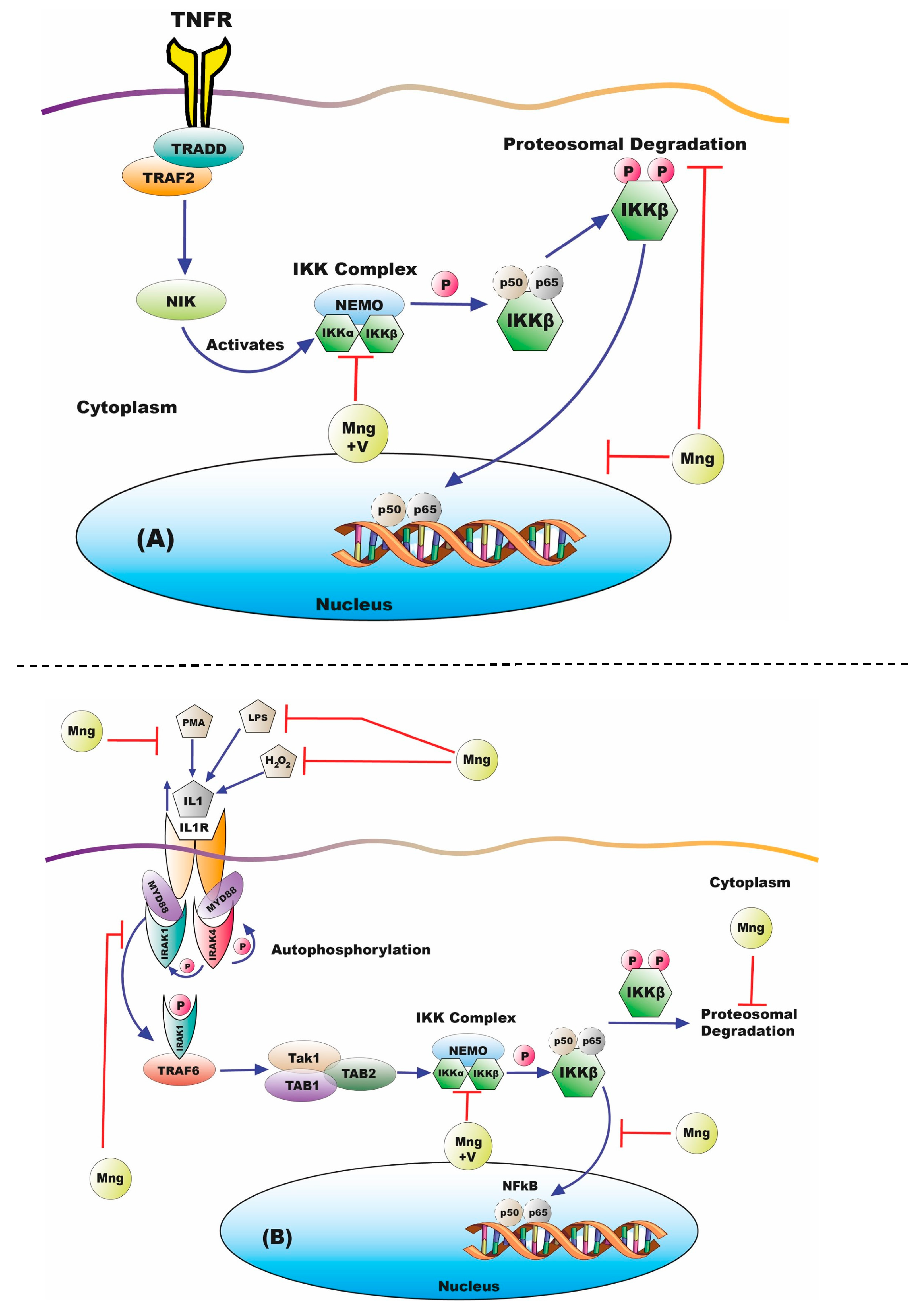
3.2. Nuclear Factor k-Light-Chain-Enhancer of Activated B Cells Activity
3.3. Initial Stimulus for NFκB Activation
3.4. Angiogenesis
3.5. Proliferation/Metastasis
3.6. Apoptosis
3.7. Other Anticancer Pathways
4. Mangiferin: Challenges in the Clinical Translation
4.1. Need for Novel Drug Delivery Systems of Mangiferin
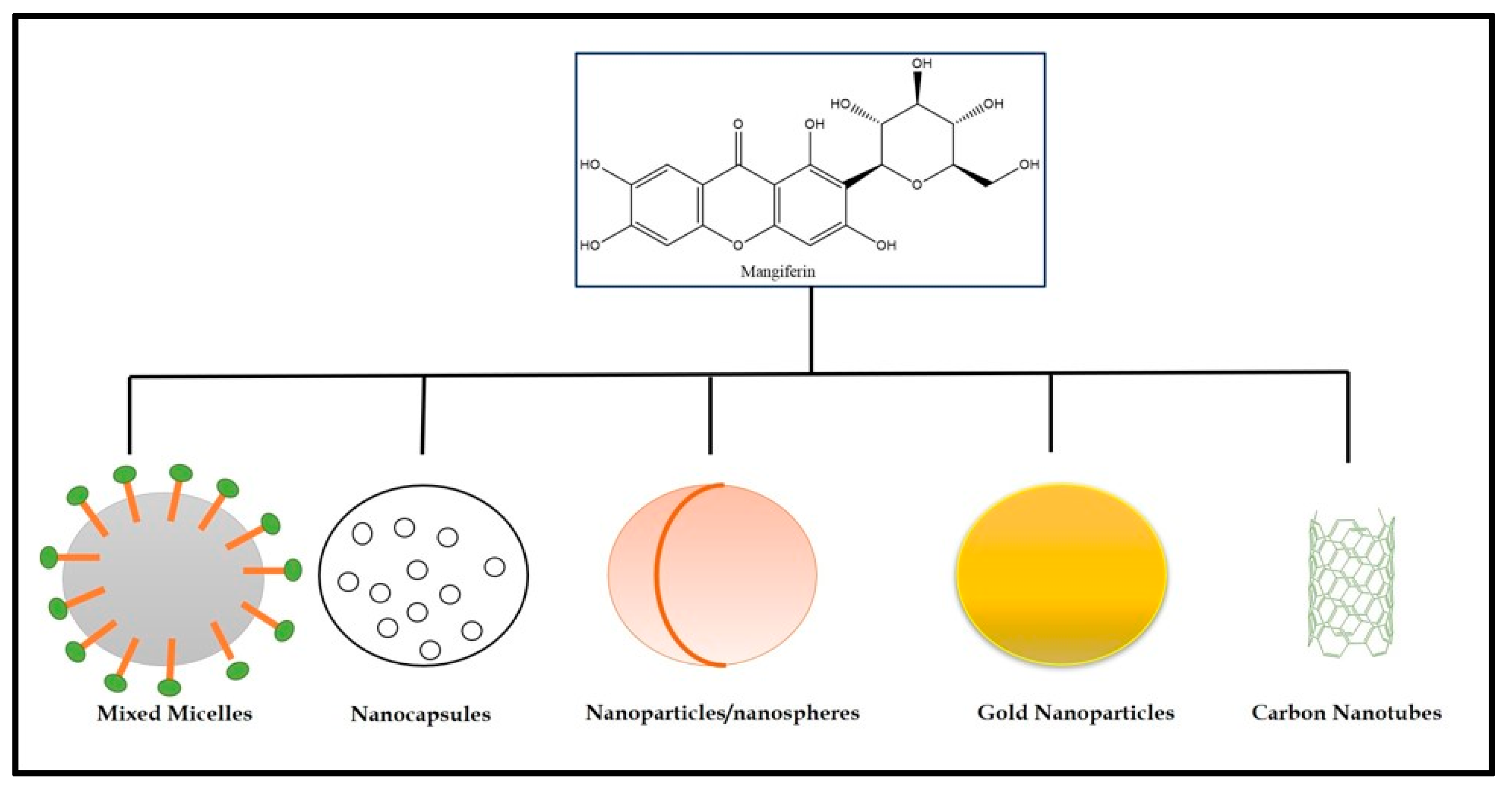
4.2. Nanocarriers of Mangiferin for Cancer Management
4.3. Deep Learning-Based Approaches to Overcoming the Challenges
5. Clinical Trials and Patent Analysis of Mangiferin
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.H.; Bohlin, L.; Borg-Karlson, A.-K.; Göransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-K.; Lu, C.-W.; Chiu, K.-M.; Lee, M.-Y.; Lin, T.-Y.; Wang, S.-J. Mangiferin depresses vesicular glutamate release in synaptosomes from the rat cerebral cortex by decreasing synapsin I phosphorylation. Eur. J. Pharmacol. 2023, 950, 175772. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Perumal, M.; Battino, M.; Kitts, D.D.; Xiao, J.; Ma, H.; Chen, X. Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. Nutr. 2021, 63, 3046–3064. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016, 116, 267–280. [Google Scholar] [CrossRef]
- Lesch, B.; Bräse, S. A short, atom-economical entry to tetrahydroxanthenones. Angew. Chem. Int. Ed. 2004, 43, 115–118. [Google Scholar] [CrossRef]
- Hering, A.; Stefanowicz-Hajduk, J.; Dziomba, S.; Halasa, R.; Krzemieniecki, R.; Sappati, S.; Baginski, M.; Ochocka, J.R. Mangiferin Affects Melanin Synthesis by an Influence on Tyrosinase: Inhibition, Mechanism of Action and Molecular Docking Studies. Antioxidants 2023, 12, 1016. [Google Scholar] [CrossRef]
- Li, S.J.; Jiao, F.W.; Li, W.; Zhang, X.; Yan, W.; Jiao, R.H. Cytotoxic Xanthone Derivatives from the Mangrove-Derived Endophytic Fungus Peniophora incarnata Z4. J. Nat. Prod. 2020, 83, 2976–2982. [Google Scholar] [CrossRef]
- Klein-Júnior, L.C.; Campos, A.; Niero, R.; Corrêa, R.; Heyden, Y.V.; Filho, V.C. Xanthones and Cancer: From Natural Sources to Mechanisms of Action. Chem. Biodivers. 2019, 17, e1900499. [Google Scholar] [CrossRef]
- Lei, L.-Y.; Wang, R.-C.; Pan, Y.-L.; Yue, Z.-G.; Zhou, R.; Xie, P.; Tang, Z.-S. Mangiferin inhibited neuroinflammation through regulating microglial polarization and suppressing NF-κB, NLRP3 pathway. Chin. J. Nat. Med. 2021, 19, 112–119. [Google Scholar] [CrossRef]
- Remali, J.; Sahidin, I.; Aizat, W.M. Xanthone Biosynthetic Pathway in Plants: A Review. Front. Plant Sci. 2022, 13, 809497. [Google Scholar] [CrossRef]
- Masters, K.-S.; Bräse, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Syeda, K.; Ahmad, A.; Padhye, S.; Sarkar, F.H. Perspectives on medicinal properties of mangiferin. Mini-Rev. Med. Chem. 2012, 12, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, G.A.; Sgroppo, S.C.; Sánchez-Moreno, C.; de Ancos, B. Mango ‘criollo’ by-products as a source of polyphenols with antioxidant capacity. Ultrasound assisted extraction evaluated by response surface methodology and HPLC-ESI-QTOF-MS/MS characterization. Food Chem. 2022, 396, 133738. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.H.; Al-Khayri, J.M. Synergistic Effects of Tragacanth and Anti-ethylene Treatments on Postharvest Quality Maintenance of Mango (Mangifera indica L.). Plants 2023, 12, 1887. [Google Scholar] [CrossRef]
- Shah, K.; Patel, M.; Patel, R.; Parmar, P. Mangifera Indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Harsha, P.; Thotakura, N.; Kumar, M.; Sharma, S.; Mittal, A.; Khurana, R.K.; Singh, B.; Negi, P.; Raza, K. A novel PEGylated carbon nanotube conjugated mangiferin: An explorative nanomedicine for brain cancer cells. J. Drug Deliv. Sci. Technol. 2019, 53, 101186. [Google Scholar] [CrossRef]
- Jyotshna Khare, P.; Shanker, K. Mangiferin: A review of sources and interventions for biological activities. BioFactors 2016, 42, 504–514. [Google Scholar] [CrossRef]
- García-Mahecha, M.; Soto-Valdez, H.; Carvajal-Millan, E.; Madera-Santana, T.J.; Lomelí-Ramírez, M.G.; Colín-Chávez, C. Bioactive Compounds in Extracts from the Agro-Industrial Waste of Mango. Molecules 2023, 28, 458. [Google Scholar] [CrossRef]
- Stohs, S.; Swaroop, A.; Moriyama, H.; Bagchi, M.; Ahmad, T.; Bagchi, D. A Review on Antioxidant, Anti-Inflammatory and Gastroprotective Abilities of Mango (Magnifera indica) Leaf Extract and Mangiferin. J. Nutr. Health Sci. 2018, 5, 303. [Google Scholar] [CrossRef]
- Namngam, C.; Pinsirodom, P. Antioxidant properties, selected enzyme inhibition capacities, and a cosmetic cream formulation of Thai mango seed kernel extracts. Trop. J. Pharm. Res. 2017, 16, 9. [Google Scholar] [CrossRef]
- Laurindo, L.F.; dos Santos, A.R.d.O.; de Carvalho, A.C.A.; Bechara, M.D.; Guiguer, E.L.; Goulart, R.d.A.; Vargas Sinatora, R.; Araújo, A.C.; Barbalho, S.M. Phytochemicals and Regulation of NF-kB in Inflammatory Bowel Diseases: An Overview of In Vitro and In Vivo Effects. Metabolites 2023, 13, 96. [Google Scholar] [CrossRef]
- Dutta, T.; Das, T.; Gopalakrishnan, A.V.; Saha, S.C.; Ghorai, M.; Nandy, S.; Kumar, M.; Radha; Ghosh, A.; Mukerjee, N.; et al. Mangiferin: The miraculous xanthone with diverse pharmacological properties. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-Ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical characteristics, bioactive compounds and industrial applications of mango kernel and its products: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef]
- Matkowski, A.; Kuś, P.; Góralska, E.; Woźniak, D. Mangiferin—A Bioactive Xanthonoid, Not Only from Mango and Not Just Antioxidant. Mini-Rev. Med. Chem. 2013, 13, 439–455. Available online: https://pubmed.ncbi.nlm.nih.gov/23190031/ (accessed on 15 May 2023).
- Kanoi, R.; Loachan, P.; Das, S.; Rao, B.S.S. Mangiferin, a naturally occurring polyphenol, mitigates oxidative stress induced premature senescence in human dermal fibroblast cells. Mol. Biol. Rep. 2021, 48, 457–466. [Google Scholar] [CrossRef]
- Viswanadh, E.K.; Rao, B.N. Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Hum. Exp. Toxicol. 2010, 29, 409–418. [Google Scholar] [CrossRef]
- Pardo-Andreu, G.L.; Sánchez-Baldoquín, C.; Ávila-González, R.; Delgado, R.; Naal, Z.; Curti, C. Fe(III) improves antioxidant and cytoprotecting activities of mangiferin. Eur. J. Pharmacol. 2006, 547, 31–36. [Google Scholar] [CrossRef]
- Rodeiro, I.; Gómez-Lechón, M.J.; Perez, G.; Hernandez, I.; Herrera, J.A.; Delgado, R.; Castell, J.V.; Donato, M.T. Mangifera indica L. extract and mangiferin modulate cytochrome P450 and UDP-glucuronosyltransferase enzymes in primary cultures of human hepatocytes. Phytother. Res. 2012, 27, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Nott, P.E.; Roberts, J.C. A synthesis of mangiferin. Phytochem. 1967, 6, 1597–1599. [Google Scholar] [CrossRef]
- da Cruz, J.W.; de Moraes, L.R.; dos Santos, M.H.; da Silva, G.A.; Brigagão, M.R.P.L.; Ellena, J.; Doriguetto, A.C. Crystalline structure of mangiferin, a C-glycosyl-substituted 9H-xanthen-9-one isolated from the stem bark of Mangifera indica. Helvetica Chim. Acta 2008, 91, 144–154. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Astiazaran-Garcia, H.F.; Montiel-Herrera, M.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Wall-Medrano, A. Mango “Ataulfo” Peel Extract Improves Metabolic Dysregulation in Prediabetic Wistar Rats. Life 2022, 12, 532. [Google Scholar] [CrossRef]
- Schieber, A.; Ullrich, W.; Carle, R. Characterization of polyphenols in mango puree concentrate by HPLC with diode array and mass spectrometric detection. Innov. Food Sci. Emerg. Technol. 2000, 1, 161–166. [Google Scholar] [CrossRef]
- Khurana, R.K.; Kaur, R.; Lohan, S.; Singh, K.K.; Singh, B. Mangiferin: A promising anticancer bioactive. Pharm. Pat. Anal. 2016, 5, 169–181. [Google Scholar] [CrossRef]
- Eilstein, J.; Nair, V.; Moore, K.; Pannakal, S.T.; Grégoire, S.; Ekhar, P.; Guy, R.H.; Delgado-Charro, M.B.; Roy, N. Non-destructive, reverse iontophoretic extraction of phytochemicals from Mangifera indica, Centella asiatica, Punica granatum, and Citrus sinensis. Phytochem. Anal. 2023, 34, 408–413. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Nong, C.; He, W.; Fleming, D.; Pan, L.; Huang, H. Capillary electrophoresis analysis of mangiferin extracted from Mangifera indica L. bark and Mangifera persiciformis C.Y. Wu et T.L. Ming leaves. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2005, 826, 226–231. [Google Scholar] [CrossRef]
- Razura-Carmona, F.F.; Pérez-Larios, A.; González-Silva, N.; Herrera-Martínez, M.; Medina-Torres, L.; Sáyago-Ayerdi, S.G.; Sánchez-Burgos, J.A. Mangiferin-loaded polymeric nanoparticles: Optical characterization, effect of anti-topoisomerase I, and cytotoxicity. Cancers 2019, 11, 1965. [Google Scholar] [CrossRef]
- Schieber, A.; Mihalev, K.; Berardini, N.; Mollov, P.; Carle, R. Flavonol Glycosides from Distilled Petals of Rosa damascena Mill. Zeitschrift Für Naturforschung C—Sect. C J. Biosci. 2005, 60, 379–384. [Google Scholar] [CrossRef]
- Alshahrani, S.M.; Thotakura, N.; Sharma, S.; Quadir, S.S.; Chaurawal, N.; Sharma, S.; Chitkara, D.; Raza, K. Influence of Nanocarrier Type on the Drug Delivery Aspects of Docetaxel: Empirical Evidences. J. Pharm. Innov. 2022, 1–12. [Google Scholar] [CrossRef]
- Chaurawal, N.; Misra, C.; Barkat, H.A.; Jatyan, R.; Chitkara, D.; Barkat, A.; Sharma, T.; Singh, B.; Raza, K. Oral sorafenib-loaded microemulsion for breast cancer: Evidences from the in-vitro evaluations and pharmacokinetic studies. Sci. Rep. 2022, 12, 13746. [Google Scholar] [CrossRef]
- Sarkar, A.; Sreenivasan, Y.; Ramesh, G.T.; Manna, S.K. β-d-Glucoside suppresses tumor necrosis factor-induced activation of nuclear transcription factor κB but potentiates apoptosis. J. Biol. Chem. 2004, 279, 33768–33781. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-S.; Jung, K.; Kim, D.-H.; Kim, H.-S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zhou, C.; Hu, H.; Hu, L.; Zhao, M.; Yang, Y.; Chuai, Y.; Ni, J.; Cai, J. Mangiferin aglycone attenuates radiation-induced damage on human intestinal epithelial cells. J. Cell. Biochem. 2012, 113, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Olubiyi, O.O.; van Heerden, F.R. Structural Optimization of Mangiferin Binding to Cancer Molecular Targets: A Guide for Synthetic Derivatization. Curr. Comput. Aided-Drug Des. 2018, 14, 292–301. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Ajila, C.; Aalami, M.; Leelavathi, K.; Rao, U.P. Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innov. Food Sci. Emerg. Technol. 2010, 11, 219–224. [Google Scholar] [CrossRef]
- Pan, L.-L.; Wang, A.-Y.; Huang, Y.-Q.; Luo, Y.; Ling, M. Mangiferin induces apoptosis by regulating Bcl-2 and bax expression in the CNE2 nasopharyngeal carcinoma cell line. Asian Pac. J. Cancer Prev. 2014, 15, 7065–7068. [Google Scholar] [CrossRef]
- Louisa, M.; Soediro, T.M.; Suyatna, F.D. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac. J. Cancer Prev. 2014, 15, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Gold-Smith, F.; Fernandez, A.; Bishop, K. Mangiferin and Cancer: Mechanisms of Action. Nutrients 2016, 8, 396. [Google Scholar] [CrossRef]
- Bhoj, V.G.; Chen, Z.J. Ubiquitylation in innate and adaptive immunity. Nature 2009, 458, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gálvez-Rodríguez, A.; Ferino-Pérez, A.; Rodríguez-Riera, Z.; Guerra, I.R.; Řeha, D.; Minofar, B.; Jáuregui-Haza, U.J. Explaining the interaction of mangiferin with MMP-9 and NF-ƙβ: A computational study. J. Mol. Model. 2022, 28, 266. [Google Scholar] [CrossRef]
- Tully, J.E.; Nolin, J.D.; Guala, A.S.; Hoffman, S.M.; Roberson, E.C.; Lahue, K.G.; van der Velden, J.; Anathy, V.; Blackwell, T.S.; Janssen-Heininger, Y.M.W. Cooperation between classical and alternative NF-κB pathways regulates proinflammatory responses in epithelial cells. Am. J. Respir. Cell Mol. Biol. 2012, 47, 497–508. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Jang, S.-E.; Hyam, S.R.; Han, M.J.; Kim, D.-H. Mangiferin ameliorates colitis by inhibiting IRAK1 phosphorylation in NF-κB and MAPK pathways. Eur. J. Pharmacol. 2014, 740, 652–661. [Google Scholar] [CrossRef]
- Delgado-Hernández, R.; Balmaseda, I.H.; Rodeiro-Guerra, I.; Gonzalez, J.C.R.; De Wever, O.; Logie, E.; Declerck, K.; Pérez-Novo, C.; Berghe, W.V. Anti-angiogenic effects of mangiferin and mechanism of action in metastatic melanoma. Melanoma Res. 2020, 30, 39–51. [Google Scholar] [CrossRef]
- Wee, Z.N.; Yatim, S.M.J.M.; Kohlbauer, V.K.; Feng, M.; Goh, J.Y.; Bao, Y.; Lee, P.L.; Zhang, S.; Wang, P.P.; Lim, E.; et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nat. Commun. 2015, 6, 8746. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewski, R.; Hering, A.; Marszałł, M.; Hajduk, J.S.; Bartoszewska, S.; Kapoor, N.; Kochan, K.; Ochocka, R. Mangiferin has an additive effect on the apoptotic properties of hesperidin in Cyclopia sp. tea extracts. PLoS ONE 2014, 9, e92128. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.K.; Zaidi, A.H.; Gupta, P.; Mokhamatam, R.B.; Raviprakash, N.; Mahali, S.K.; Manna, S.K. A natural xanthone increases catalase activity but decreases NF-kappa B and lipid peroxidation in U-937 and HepG2 cell lines. Eur. J. Pharmacol. 2015, 764, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Márquez, L.; García-Bueno, B.; Madrigal, J.L.M.; Leza, J.C. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr. 2011, 51, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Tsubaki, M.; Sakamoto, K.; Ichimura, E.; Enomoto, A.; Suzuki, Y.; Itoh, T.; Imano, M.; Tanabe, G.; Muraoka, O.; et al. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol. Appl. Pharmacol. 2016, 306, 105–112. [Google Scholar] [CrossRef]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466. [Google Scholar] [CrossRef]
- García-Rivera, D.; Delgado, R.; Bougarne, N.; Haegeman, G.; Berghe, W.V. Gallic acid indanone and mangiferin xanthone are strong determinants of immunosuppressive anti-tumour effects of Mangifera indica L. bark in MDA-MB231 breast cancer cells. Cancer Lett. 2011, 305, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, J.; Yang, B.; Xiang, T.; Yin, X.; Peng, W.; Cheng, W.; Wan, J.; Luo, F.; Li, H.; et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol. Appl. Pharmacol. 2013, 272, 180–190. [Google Scholar] [CrossRef]
- Zou, B.; Wang, H.; Liu, Y.; Qi, P.; Lei, T.; Sun, M.; Wang, Y. Mangiferin induces apoptosis in human ovarian adenocarcinoma OVCAR3 cells via the regulation of Notch3. Oncol. Rep. 2017, 38, 1431–1441. [Google Scholar] [CrossRef]
- Cuccioloni, M.; Bonfili, L.; Mozzicafreddo, M.; Cecarini, V.; Scuri, S.; Cocchioni, M.; Nabissi, M.; Santoni, G.; Eleuteri, A.M.; Angeletti, M. Mangiferin blocks proliferation and induces apoptosis of breast cancer cells via suppression of the mevalonate pathway and by proteasome inhibition. Food Funct. 2016, 7, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wen, G.; Jin, J.; Chen, Y.; Cao, J.; Xu, A. Mangiferin prevents the growth of gastric carcinoma by blocking the PI3K-Akt signalling pathway. Anti-Cancer Drugs 2018, 29, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Ma, H.; Chen, X. Anticancer and anti-inflammatory properties of mangiferin: A review of its molecular mechanisms. Food Chem. Toxicol. 2021, 149, 111997. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Guo, W.; Man, K.; Cheng, C.-S.; Chen, Z.; Feng, Y. Repression of WT1-Mediated LEF1 Transcription by Mangiferin Governs β-Catenin-Independent Wnt Signalling Inactivation in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018, 47, 1819–1834. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.; Rao, B.N.; Mudholkar, K.; Bhuwania, R.; Satish Rao, B.S. Mangiferin, a dietary xanthone protects against mercury-induced toxicity in HepG2 cells. Environ. Toxicol. 2010, 27, 117–127. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.H.; Aung, C.S.; Hewavitharana, A.K.; Wilkinson, A.S.; Pierson, J.-T.; Roberts-Thomson, S.J.; Shaw, P.N.; Monteith, G.R.; Gidley, M.J.; Parat, M.-O. Mango extracts and the mango component mangiferin promote endothelial cell migration. J. Agric. Food Chem. 2010, 58, 5181–5186. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Delgado, R.; Garrido, G. Effects of a Mangifera indica L. stem bark extract and mangiferin on radiation-induced DNA damage in human lymphocytes and lymphoblastoid cells. Cell Prolif. 2014, 47, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Van de Venter, M.; Du Plessis-Stoman, D.; Du Preez, J. Combination Treatment with Oxaliplatin and Mangiferin Causes Increased Apoptosis and Downregulation of NFκB in Cancer Cell Lines. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 177–184. [Google Scholar] [CrossRef]
- Shoji, K.; Tsubaki, M.; Yamazoe, Y.; Satou, T.; Itoh, T.; Kidera, Y.; Tanimori, Y.; Yanae, M.; Matsuda, H.; Taga, A.; et al. Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP expressions and nuclear entry of NF-κB in HL-60 cells. Arch. Pharmacal Res. 2011, 34, 469–475. [Google Scholar] [CrossRef]
- Padma, V.V.; Kalaiselvi, P.; Yuvaraj, R.; Rabeeth, M. Mangiferin induces cell death against rhabdomyosarcoma through sustained oxidative stress. Integr. Med. Res. 2014, 4, 66–75. [Google Scholar] [CrossRef]
- Kavitha, M.; Manivasagam, T.; Essa, M.M.; Tamilselvam, K.; Selvakumar, G.P.; Karthikeyan, S.; Thenmozhi, J.A.; Subash, S. Mangiferin antagonizes rotenone: Induced apoptosis through attenuating mitochondrial dysfunction and oxidative stress in SK-N-SH neuroblastoma cells. Neurochem. Res. 2014, 39, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Alkholifi, F.K.; Alam, A.; Foudah, A.I.; Yusufoglu, H.S. Phospholipid-Based Topical Nano-Hydrogel of Mangiferin: Enhanced Topical Delivery and Improved Dermatokinetics. Gels 2023, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Hou, J.; Ma, J.; Yu, B.; Ren, J.; Jin, W.; Wu, J.; Zheng, D.; Fan, K. Mangiferin loaded magnetic PCEC microspheres: Preparation, characterization and antitumor activity studies in vitro. Arch. Pharmacal Res. 2014, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Gaspar, B.L.; Welsby, G.; Katare, O.P.; Singh, K.K.; Singh, B. Improving the biopharmaceutical attributes of mangiferin using vitamin E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv. Transl. Res. 2018, 8, 617–632. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.R.R.; Feitosa, J.P.; Ricardo, N.M.; Trevisan, M.T.S.; de Paula, H.C.B.; Ulrich, C.M.; Owen, R.W. Spray-drying encapsulation of mangiferin using natural polymers. Food Hydrocoll. 2013, 33, 10–18. [Google Scholar] [CrossRef]
- Chaurawal, N.; Raza, K. Models Used for Pharmacokinetic Evaluation of Nanoparticulate Drug Delivery Systems (NPDDS). In Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems; Springer: Cham, Switzerland, 2022; pp. 53–67. [Google Scholar] [CrossRef]
- Caro, C.; Pozo, D. Polysaccharide Colloids as Smart Vehicles in Cancer Therapy. Curr. Pharm. Des. 2015, 21, 4822–4836. [Google Scholar] [CrossRef]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef]
- Chaurawal, N.; Misra, C.; Raza, K. Lipid-based Nanocarriers Loaded with Taxanes for the Management of Breast Cancer: Promises and Challenges. Curr. Drug Targets 2022, 23, 544–558. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, P.; Rajawat, J.S.; Malik, R.; Sharma, G.; Modgil, A. Nanotechnology: Revolutionizing the Science of Drug Delivery. Curr. Pharm. Des. 2019, 24, 5086–5107. [Google Scholar] [CrossRef]
- Manjappa, A.S.; Kumbhar, P.S.; Patil, A.B.; Disouza, J.I.; Patravale, V.B. Polymeric Mixed Micelles: Improving the Anticancer Efficacy of Single-Copolymer Micelles. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 1–58. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; Araújo, R.S.; Mosqueira, V.C.F. PEGylated and Functionalized Polylactide-Based Nanocapsules: An Overview; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef] [PubMed]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Gold Nanoparticles Synthesized Using Extracts of Cyclopia intermedia, Commonly Known as Honeybush, Amplify the Cytotoxic Effects of Doxorubicin. Nanomaterials 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shao, W.; Lu, X.; Gao, C.; Fang, L.; Yang, X.; Zhu, P. Synthesis, characterization, and in vitro anti-tumor activity studies of the hyaluronic acid-mangiferin-methotrexate nanodrug targeted delivery system. Int. J. Biol. Macromol. 2023, 239, 124208. [Google Scholar] [CrossRef]
- Al-Yasiri, A.Y.; Khoobchandani, M.; Cutler, C.S.; Watkinson, L.; Carmack, T.; Smith, C.J.; Kuchuk, M.; Loyalka, S.K.; Lugão, A.B.; Katti, K.V. Mangiferin functionalized radioactive gold nanoparticles (MGF-198AuNPs) in prostate tumor therapy: Green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans. 2017, 46, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Khoobchandani, M.; Khan, A.; Katti, K.K.; Thipe, V.C.; Yasiri, A.; MohanDoss, D.K.D.; Nicholl, M.B.; Lugao, A.B.; Hans, C.P.; Katti, K.V. Green nanotechnology of MG-AuNPs for immunomodulatory intervention in prostate cancer therapy. Sci. Rep. 2021, 11, 16797. [Google Scholar] [CrossRef] [PubMed]
- Razura Carmona, F.F.; Herrera, M.; Manuel, Z.G.V.; Guadalupe, S.A.S.; Larios, A.P.; Sanchez-Burgos, J. Characterization of functionalized PLGA nanoparticles loaded with mangiferin and lupeol, and their effect on BEAS-2B and HepG-2 cell lines. Anti-Cancer Agents Med. Chem. 2023, 10, 1174–1183. [Google Scholar]
- Zhou, Q.; Hou, K.; Fu, Z. Transferrin-Modified Mangiferin-Loaded SLNs: Preparation, Characterization, and Application in A549 Lung Cancer Cell. Drug Des. Dev. Ther. 2022, 16, 1767–1778. [Google Scholar] [CrossRef]
- Morozkina, S.N.; Vu, T.H.N.; Generalova, Y.E.; Snetkov, P.P.; Uspenskaya, M.V. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems—A novel research direction. Biomolecules 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Kousar, S.; Raj, E.R.; Shweta Bala, E.; Tech, M. A cnn-lbp image modeling and classification scheme for mango leaf disease detection. Int. J. Technol. Res. Eng. 2021, 8, 34–38. [Google Scholar]
- Kullu, J.; Dutta, A.; Constales, D.; Chaudhuri, S.; Dutta, D. Experimental and modeling studies on microwave-assisted extraction of mangiferin from Curcuma amada. 3 Biotech 2013, 4, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Song, S.; Lan, X.; Li, Y.; Yuan, X.; Yang, J.; Li, M.; Cao, T.; Zhang, J. Comprehensive Profiling of Mangiferin Metabolites In Vivo and In Vitro Based on the “Drug Metabolite Clusters” Analytical Strategy. ACS Omega 2022, 8, 9934–9946. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, A.; Rembiałkowska, N.; Rorbach-Dolata, A.; Garbiec, A.; Ślusarczyk, S.; Dobosz, A.; Długosz, A.; Marchewka, Z.; Matkowski, A.; Saczko, J. Anemarrhenae asphodeloides rhizoma extract enriched in mangiferin protects PC12 cells against a neurotoxic agent-3-nitropropionic acid. Int. J. Mol. Sci. 2020, 21, 2510. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, R.; Hering, A.; Stefanowicz-Hajduk, J.; Cal, K.; Barańska, H. The effect of mangiferin on skin: Penetration, permeation and inhibition of ECM enzymes. PLoS ONE 2017, 12, e0181542. [Google Scholar] [CrossRef] [PubMed]
- Home. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 1 October 2022).
- Quadri, F.; Telang, M.; Mandhare, A. Therapeutic and cosmetic applications of mangiferin: An updated patent review (patents published after 2013). Expert Opin. Ther. Pat. 2019, 29, 463–479. [Google Scholar] [CrossRef]


| Plant Parts | Chemical Constituents | Therapeutic Applications |
|---|---|---|
| Fruits | Carotenes, xanthophyll esters, tocopherols and mangiferin | Prevent heat stroke, inflammation, prostate cancer, colon cancer, breast cancer and liver cancer |
| Stem bark | Mangifera indicasterol, manghopana, mangifera indica coumarin, mangifera indicaleanone and terpenoidal saponin indicoside A and B | Diabetes, anemia, menorrhagia, scabies, syphilis and cutaneous infections |
| Leaves | Catechin, alanine, mangiferin, tetracyclic triterpenoids, shikimic acid, protocatechuic acid and glycine | Scalds and dysentery |
| S. No. | Biochemical Markers | Targets | References |
|---|---|---|---|
| 1. | Anti-inflammatory Potent inhibitor of CXCR4, ICAM1, NF-κB and XIAP | Nf-κB, AKT, IKK, STAT3, JAK1/2, IκB-α and MAPK | [66] |
| 2. | ROS: Scavenge ROS present in cancer cells and inhibit xanthine oxidase | ROS | [73,74] |
| 3. | Metastasis Inhibits activation of β-catenin and prevents the breast cancer | COX-2, VEGF, E-cadherin, ICAM and MMP7–9 | [75] |
| 4. | Antiangiogenesis Inhibit the growth of some tumors | TN-α, B16F10, FGF and VEGF | [68,76] |
| 5. | Apoptosis Induces apoptosis by inhibiting NF-κB activation | Bcl-xL, Caspase 9, 7, 3, Bcl2 and XIAP | [77,78,79] |
| 6. | Mitochondrial membrane potential | Induces loss of mitochondrial membrane potential and activates apoptotic proteins. | [80] |
| S. No. | Formulations | Excipients | Techniques | Outcomes |
|---|---|---|---|---|
| 1. | Mangiferin emulsion | Copolymer of ethylene vinyl acetate, vinyl acetate and toluene | Solvent evaporation technique | Increased antioxidant activity, increased tensile strength and increased mangiferin clearance. |
| 2. | Mangiferin hydrogel | Polyvinyl alcohol, gelatin and chitosan | Sol-Gel technique | Controlled release of mangiferin from the matrix |
| 3. | Mangiferin nanoemulsion | Hyaluronic acid, glycerin, water, lipoid S75 and trasncutol | Nanoemulsion technique | The average size of 296 nm, improved permeability and appropriate anti-inflammatory effect |
| 4. | Mangiferin microparticles | Cellulose acetate phthalate | Supercritical antisolvent technique | Controlled release of mangiferin |
| 5. | Mangiferin mixed micelles | Pluronic F127, vitamin E TPGS and pluronic P123 | Thin-film hydration technique | Spherical morphology of micelles, high solubility and sustained release in the intestinal environment |
| 6. | Mangiferin nanoparticles | Chitosan | Spray-drying technique | Accurate nano-size with cr(IV) removal pH-dependent release |
| S. No. | Compound Name | Application | Mechanism | Patent Number |
|---|---|---|---|---|
| 1. | Norathyriol, tetraacetate | Prostate cancer | 5α-reductase inhibitor | CN 104013611A |
| 2. | Acetylated aglycone derivative I | Prostate cancer | 5α-reductase inhibitor | CN 104013611A |
| 3. | 3-O-methyl-mangiferin acetate | Tumor | NA | CN 103755692A |
| 4. | Mangiferin berberine salt | Anticancer | AMPK activator | WO 2010145192 |
| 5. | 3-O-methyl-mangiferin benzoate | Tumor | NA | CN 103755693A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, M.; Khan, A.; Batiha, G.E.-S.; Akhtar, M.F.; Saleem, A.; Ajiboye, B.O.; Kamal, M.; Ali, A.; Alotaibi, N.M.; Aaghaz, S.; et al. Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy. Cancers 2023, 15, 4194. https://doi.org/10.3390/cancers15164194
Sarfraz M, Khan A, Batiha GE-S, Akhtar MF, Saleem A, Ajiboye BO, Kamal M, Ali A, Alotaibi NM, Aaghaz S, et al. Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy. Cancers. 2023; 15(16):4194. https://doi.org/10.3390/cancers15164194
Chicago/Turabian StyleSarfraz, Muhammad, Abida Khan, Gaber El-Saber Batiha, Muhammad Furqan Akhtar, Ammara Saleem, Basiru Olaitan Ajiboye, Mehnaz Kamal, Abuzer Ali, Nawaf M. Alotaibi, Shams Aaghaz, and et al. 2023. "Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy" Cancers 15, no. 16: 4194. https://doi.org/10.3390/cancers15164194
APA StyleSarfraz, M., Khan, A., Batiha, G. E.-S., Akhtar, M. F., Saleem, A., Ajiboye, B. O., Kamal, M., Ali, A., Alotaibi, N. M., Aaghaz, S., Siddique, M. I., & Imran, M. (2023). Nanotechnology-Based Drug Delivery Approaches of Mangiferin: Promises, Reality and Challenges in Cancer Chemotherapy. Cancers, 15(16), 4194. https://doi.org/10.3390/cancers15164194











