Immune Checkpoint Inhibitors after Radiation Therapy Improve Overall Survival Rates in Patients with Stage IV Lung Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
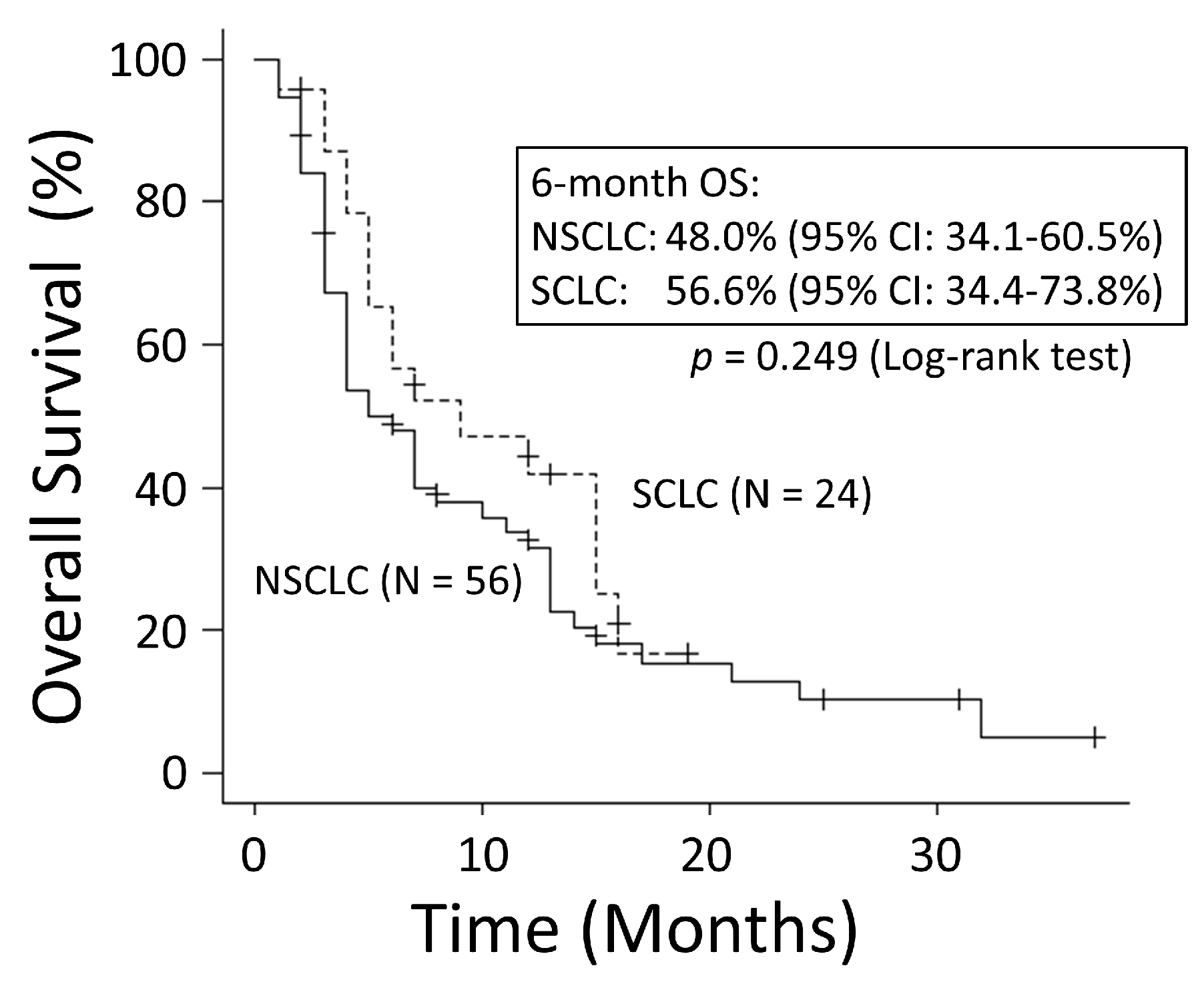
3.2. Overall Survival Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akamatsu, H.; Ninomiya, K.; Kenmotsu, H.; Morise, M.; Daga, H.; Goto, Y.; Kozuki, T.; Miura, S.; Sasaki, T.; Tamiya, A.; et al. The Japanese lung cancer society guideline for non-small cell lung cancer, stage IV. Int. J. Clin. Oncol. 2019, 24, 731–770. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Planchard, D.; Lu, S.; Sun, H.; Yamamoto, N.; Kim, D.W.; Tan, D.S.W.; Yang, J.C.H.; Azrif, M.; Mitsudomi, T.; et al. Pan-Asian adapted clinical practice guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019, 30, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Vansteenkiste, J.; Lee, K.H.; Pentheroudakis, G.; Zhou, C.; Prabhash, K.; Seto, T.; Voon, P.J.; Tan, D.S.W.; Yang, J.C.H.; et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: A KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann. Oncol. 2020, 31, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomized, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Update results from a randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef]
- Shaverdian, N.; Lisberg, A.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the LEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Peulen, H.M.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.N.; de Langen, A.J.; et al. Radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef]
- Fiorica, F.; Belluomini, L.; Stefanelli, A.; Santini, A.; Urbini, B.; Giorgi, C.; Frassoldati, A. Immune checkpoint inhibitor nivolumab and radiotherapy in pretreated lung cancer patients: Efficacy and safety of combination. Am. J. Clin. Oncol. 2018, 41, 1101–1105. [Google Scholar] [CrossRef]
- Öjlert, Å.K.; Nebdal, D.; Lund-Iversen, M.; Åstrøm Ellefsen, R.; Brustugun, O.T.; Gran, J.M.; Halvorsen, A.R.; Helland, Å. Immune checkpoint blockade in the treatment of advanced non-small cell lung cancer-predictors of response and impact of previous radiotherapy. Acta Oncol. 2021, 60, 149–156. [Google Scholar] [CrossRef]
- Hosokawa, S.; Ichihara, E.; Bessho, A.; Harada, D.; Inoue, K.; Shibayama, T.; Kishino, D.; Harita, S.; Ochi, N.; Oda, N.; et al. Impact of previous thoracic radiation therapy on the efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer. Jpn. J. Clin. Oncol. 2021, 51, 279–286. [Google Scholar] [CrossRef]
- Li, S.; Chen, K.; Yang, M.; Hlaing, S.S.; Chen, M.; Gu, P.; Meng, Y.; Yang, H. Radiotherapy improves the outcomes of immunotherapy with Sintilimab in non-small-cell lung cancer: A real-world analysis. Front. Immunol. 2022, 13, 991431. [Google Scholar] [PubMed]
- Su, Z.; Zhang, L.; Xue, S.; Wang, Y.; Ding, R. Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis. Open Life Sci. 2023, 18, 20220559. [Google Scholar] [PubMed]
- Liu, Z.; Xu, T.; Chang, P.; Fu, W.; Wei, J.; Xia, C.; Wang, Q.; Li, M.; Pu, X.; Huang, F.; et al. Efficacy and safety of immune checkpoint inhibitors with or without radiotherapy in metastatic non-small cell lung cancer: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1064227. [Google Scholar]
- Mondini, M.; Levy, A.; Meziani, L.; Milliat, F.; Deutsch, E. Radiotherapy-immunotherapy combinations-perspectives and challenges. Mol. Oncol. 2020, 14, 1529–1537. [Google Scholar]
- Sharabi, A.; Lim, M.; DeWeese, T.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar]
- Liang, Y.; Peng, H. STING-cytosolic DNA sensing: The backbone for an effective tumor radiation therapy. Ann. Transl. Med. 2016, 4, 60. [Google Scholar] [PubMed]
- Popp, I.; Grosu, A.L.; Niedermann, G.; Duda, D.G. Immune modulation by hypofractionated stereotactic radiation therapy: Therapeutic implications. Radiother. Oncol. 2016, 120, 158–194. [Google Scholar]
- Gracia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar]
- Wang, S.J.; Jhawar, S.R.; Rivera-Nunez, Z.; Silk, A.W.; Byun, J.; Miller, E.; Blakaj, D.; Parikh, R.R.; Weiner, J.; Goyal, S. The association of radiation dose-fractionation and immunotherapy use with overall survival in metastatic melanoma patients. Cureus 2020, 12, e8767. [Google Scholar]
- Welsh, J.; Menon, H.; Chen, D.; Verma, V.; Tang, C.; Altan, M.; Hess, K.; de Groot, P.; Nguyen, Q.N.; Varghese, R.; et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J. Immunother. Cancer 2020, 8, e001001. [Google Scholar]
- Luke, J.J.; Lemons, J.M.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [PubMed]
- Fukui, T.; Mitsudomi, T. Mutations in the epidermal growth factor receptor gene and effects of EGFR-tyrosine kinase inhibitors on lung cancers. Gen. Thorac. Cardiovasc. Surg. 2008, 56, 97–103. [Google Scholar] [PubMed]
- Shigematsu, H.; Takahashi, T.; Nomura, M.; Majmudar, K.; Suzuki, M.; Lee, H.; Wistuba, I.I.; Fong, K.M.; Toyooka, S.; Shimizu, N.; et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005, 65, 1642–1646. [Google Scholar] [PubMed]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMAPII). Am. J. Cancer Res. 2015, 5, 2892–2911. [Google Scholar] [PubMed]







| Age (Years) | Median (Range) | 70 (43–88) |
|---|---|---|
| Sex | Male/female | 69 (86.3%)/11 (13.8%) |
| ECOG performance status | 0 | 15 (18.8%) |
| 1 | 40 (50.0%) | |
| 2 | 13 (16.3%) | |
| 3 | 9 (11.3%) | |
| 4 | 3 (3.8%) | |
| BED (α/β = 10) (Gy) | Median (range) | 39.0 (27.3–72.0) |
| Irradiated site | Bone | 44 (55.0%) |
| Brain | 21 (26.3%) | |
| Mediastinum | 7 (8.8%) | |
| Cervical lymph node | 4 (5.0%) | |
| Others | 4 (5.0%) | |
| Other metastases | Yes/no | 64 (80.0%)/16 (20.0%) |
| ICI use | Yes/no | 30 (37.5%)/50 (62.5%) |
| Follow-up period (months) | Median (range) | 6 (1–37) |
| ICI (+) (N = 30) | ICI (−) (N = 50) | ||
|---|---|---|---|
| Age (years) | Median (range) | 70 (43–86) | 71 (48–88) |
| Sex | Male/female | 27 (90.0%)/3 (10.0%) | 43 (86.0%)/7 (14.0%) |
| ECOG performance status | 0 | 8 (26.7%) | 7 (14.0%) |
| 1 | 16 (53.3%) | 24 (48.0%) | |
| 2 | 4 (13.3%) | 9 (18.0%) | |
| 3 | 1 (3.3%) | 8 (16.0%) | |
| 4 | 1 (3.3%) | 2 (4.0%) | |
| Histology | Adenocarcinoma | 19 (63.3%) | 12 (24.0%) |
| Squamous carcinoma | 7 (23.3%) | 12 (24.0%) | |
| Small cell carcinoma | 3 (10.0%) | 18 (36.0%) | |
| Others | 1 (3.3%) | 8 (16.0%) | |
| PD-L1 expression | ≥25% | 12 (40.0%) | 2 (4.0%) |
| <25%, 1%≥ | 6 (20.0%) | 5 (10.0) | |
| <1% or unknown | 12 (40.0%) | 43 (86.0%) | |
| BED (α/β = 10) (Gy) | Median (range) | 39.0 (28.0–72.0) | 39.0 (27.3–48.0) |
| Irradiated site | Bone | 16 (53.3%) | 28 (56.0%) |
| Brain | 6 (20.0%) | 15 (30.0%) | |
| Mediastinum | 3 (10.0%) | 4 (8.0%) | |
| Cervical lymph node | 3 (10.0%) | 1 (2.0%) | |
| Others | 2 (6.7%) | 2 (4.0%) | |
| Other metastases | Yes/no | 24 (80.0%)/6 (20.0%) | 40 (80.0%)/10 (20.0%) |
| Chemotherapy | Yes/no | 30 (100%)/0 (0%) | 35 (70.0%)/15 (30.0%) |
| ICIs after RT (N = 19) | ICIs before RT (N = 10) | ||
|---|---|---|---|
| Age (years) | Median (range) | 71 (43–79) | 66 (46–86) |
| Sex | Male/female | 19 (100%)/0 (0%) | 7 (70.0%)/3 (30.0%) |
| ECOG performance status | 0 | 5 (26.3%) | 2 (20.0%) |
| 1 | 12 (63.2%) | 4 (40.0%) | |
| 2 | 1 (5.3%) | 3 (30.0%) | |
| 3 | 0 (0%) | 1 (10.0%) | |
| 4 | 1 (5.3%) | 0 (0%) | |
| Smoking status | Current smoker | 1 (5.3%) | 3 (30.0%) |
| Past smoker | 4 (21.1%) | 1 (10.0%) | |
| Never smoked | 14 (73.7%) | 6 (60.0%) | |
| Histology | Adenocarcinoma | 10 (52.6%) | 8 (80.0%) |
| Squamous carcinoma | 6 (31.6%) | 1 (10.0%) | |
| Small cell carcinoma | 2 (10.5%) | 1 (10.0%) | |
| Others | 1 (5.3%) | 0 (0%) | |
| PD-L1 expression | ≥25% | 7 (36.8%) | 5 (50.0%) |
| <25%, 1% ≥ | 3 (15.8%) | 2 (20.0) | |
| <1% or unknown | 9 (47.4%) | 3 (30.0%) | |
| BED (α/β = 10) (Gy) | Median (range) | 39.0 (28.0–72.0) | 39.0 (28.0–43.8) |
| Irradiated site | Bone | 13 (68.4%) | 3 (30.0%) |
| Brain | 4 (21.1%) | 2 (20.0%) | |
| Mediastinum | 2 (10.5%) | 1 (10.0%) | |
| Cervical lymph node | 0 (0%) | 2 (20.0%) | |
| Others | 0 (0%) | 2 (20.0%) | |
| Period between RT and ICI therapy * | Median (range) | 1 (0–13) | 4 (0–25) |
| Duration of ICI therapy | Median (range) | 4 (1–13) | 4 (1–20) |
| Other metastases | Yes/no | 13 (68.4%)/6 (31.6%) | 10 (100%)/0 (0%) |
| Chemotherapy | Yes/no | 19 (100%)/0 (0%) | 10 (100%)/0 (0%) |
| No. of systemic therapy regimens | 5 | 1 (5.3%) | 0 (0%) |
| 4 | 1 (5.3%) | 1 (10.0%) | |
| 3 | 2 (10.5%) | 5 (50.0%) | |
| 2 | 12 (63.2%) | 2 (20.0%) | |
| 1 | 2 (10.5%) | 2 (20.0%) | |
| 0 | 1 (5.3%) | 0 (0%) |
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age (years) | <70 vs. ≥70 | 1.321 (0.7978–2.186) | 0.280 | ||
| Pathology | NSCLC vs. SCLC | 0.7261 (0.4084–1.291) | 0.276 | ||
| BED (Gy) | ≤39 vs. >39 | 1.133 (0.5896–2.179) | 0.707 | ||
| Dose per fraction (Gy) | ≤3 vs. >3 | 0.9796 (0.4809–1.996) | 0.955 | ||
| Other metastases | Yes vs. no | 1.704 (0.8595–3.378) | 0.127 | ||
| ECOG performance status | 0–1 vs. 2–4 | 2.587 (1.475–4.537) | <0.001 | 1.8560 (1.055–3.265) | 0.032 |
| ICI use after RT | Yes vs. no | 0.2121 (0.09915–0.4538) | <0.001 | 0.2438 (0.112–0.531) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Ueda, K.; Karita, M.; Ono, T.; Manabe, Y.; Kajima, M.; Fujimoto, K.; Yuasa, Y.; Shiinoki, T. Immune Checkpoint Inhibitors after Radiation Therapy Improve Overall Survival Rates in Patients with Stage IV Lung Cancer. Cancers 2023, 15, 4260. https://doi.org/10.3390/cancers15174260
Tanaka H, Ueda K, Karita M, Ono T, Manabe Y, Kajima M, Fujimoto K, Yuasa Y, Shiinoki T. Immune Checkpoint Inhibitors after Radiation Therapy Improve Overall Survival Rates in Patients with Stage IV Lung Cancer. Cancers. 2023; 15(17):4260. https://doi.org/10.3390/cancers15174260
Chicago/Turabian StyleTanaka, Hidekazu, Kazushi Ueda, Masako Karita, Taiki Ono, Yuki Manabe, Miki Kajima, Koya Fujimoto, Yuki Yuasa, and Takehiro Shiinoki. 2023. "Immune Checkpoint Inhibitors after Radiation Therapy Improve Overall Survival Rates in Patients with Stage IV Lung Cancer" Cancers 15, no. 17: 4260. https://doi.org/10.3390/cancers15174260





