Cardiotoxicity Secondary to Immune Checkpoint Inhibitors in the Elderly: Safety in Real-World Data
Abstract
:Simple Summary
Abstract
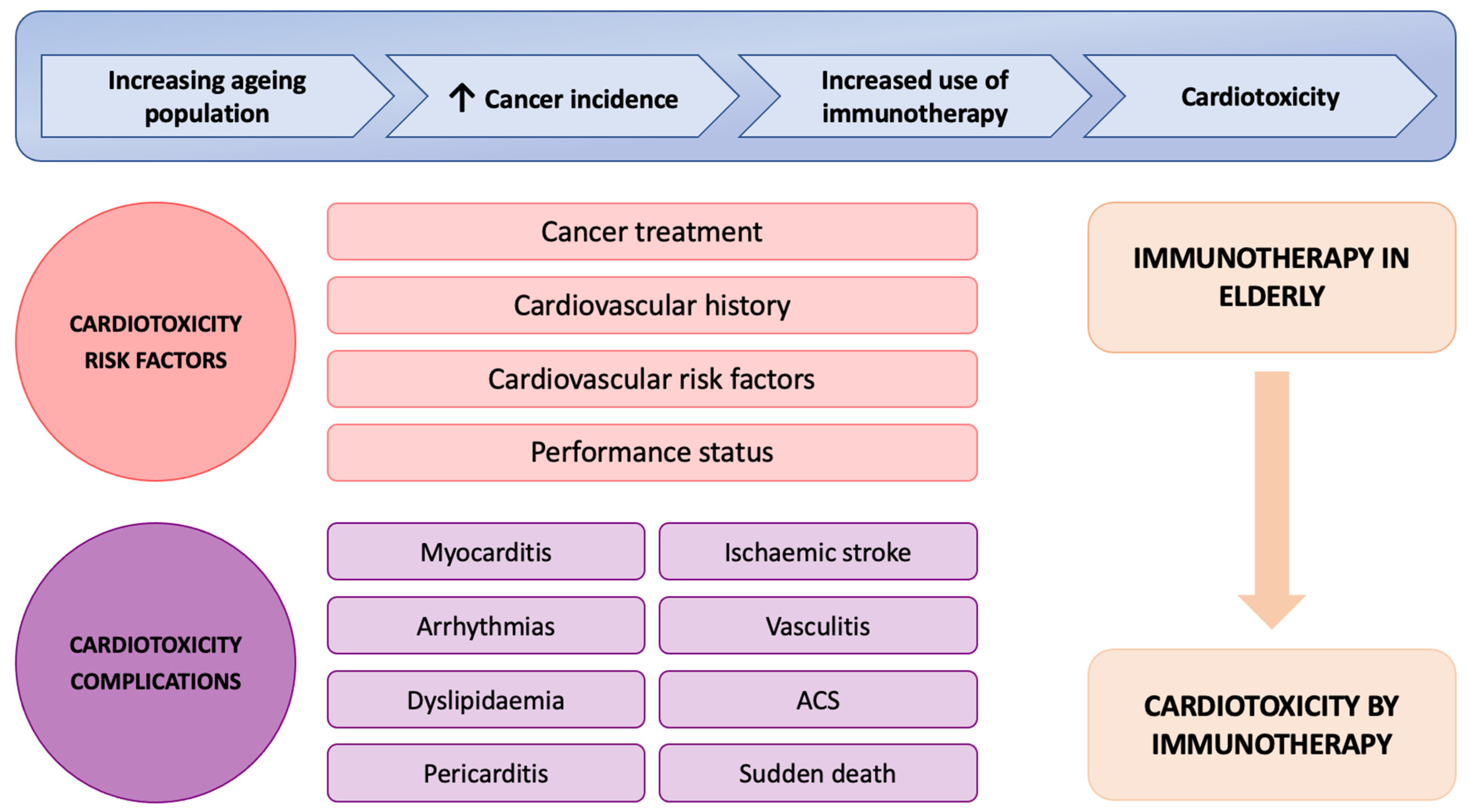
1. Introduction
2. Materials and Methods
2.1. Sources of Data
- -
- Epidemiological: age (years) and sex.
- -
- Oncological: tumour (location, stage, histology, and oncological treatment), PDL-1, and driver mutations.
- -
- Type of treatment: type of immunotherapy, number of doses, and line treatment.
- -
- Response: overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and disease control rate (DCR).
- -
- Cardiac: cardiac comorbidity, the treatment of cardiac pathology, and cardiotoxicity (number and types of events).
- -
- Clinical and analytical data and comorbidities: general condition (Eastern Cooperative Oncology Group, ECOG), analytical data (blood count, creatinine, liver function, proteins, and glycemia), body mass index (BMI), general toxicity (grade), and associated cardiovascular factors and comorbidities.
2.2. Cohort Construction
2.2.1. Inclusion Criteria
- -
- Patients who had received ICIs in any line of treatment, both in monotherapy and in different combinations such as with chemotherapy, other ICIs, or tyrosine kinase inhibitors.
- -
- Patients with a diagnosis of a solid tumour in any stage.
- -
- Patients who had all the clinical information necessary for the analysis of the objectives collected.
2.2.2. Exclusion Criteria
- -
- Patients using immunotherapy for haematological tumours.
- -
- The clinical information necessary for the study had not been collected.
2.3. Objectives and Definitions
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of the Sample
3.2. Cardiac Comorbidity of the Sample
3.3. Cardiac Events Post-Immunotherapy (Primary Endpoint)
4. Discussion
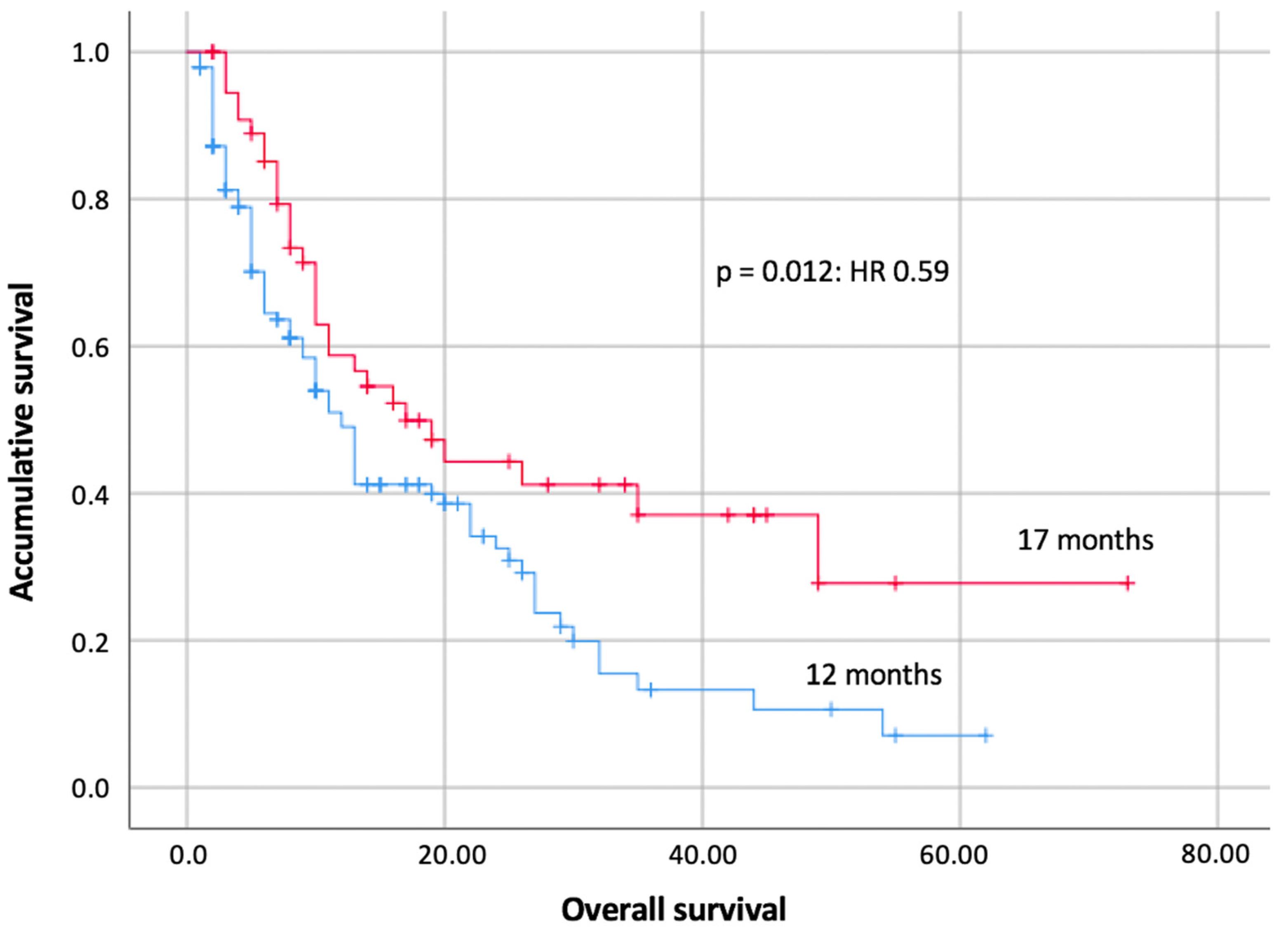
4.1. Submission Times of Cardiotoxicity
4.2. Cardiotoxicity in the Patients of the Sample (Secondary Objectives)
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, T.S.; Singh, N.; Walker, T.D.J.; Newton, C.; Evans, D.G.R.; Crosbie, E.J.; Ryan, N.A.J. Immune checkpoint inhibitors efficacy across solid cancers and the utility of PD-L1 as a biomarker of response: A systematic review and meta-analysis. Front. Med. 2023, 10, 1192762. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Nunno, V.D.; Franceschi, E.; Brandes, A.A. Immunotherapy in elderly patients: Should we stay or should we go? Future Oncol. 2020, 16, 973–974. [Google Scholar] [CrossRef]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef]
- Patel, R.P.; Parikh, R.; Gunturu, K.S.; Tariq, R.Z.; Dani, S.S.; Ganatra, S.; Nohria, A. Cardiotoxicity of Immune Checkpoint Inhibitors. Curr. Oncol. Rep. 2021, 23, 79. [Google Scholar] [CrossRef]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Laenens, D.; Yu, Y.; Santens, B.; Jacobs, J.; Beuselinck, B.; Bechter, O.; Wauters, E.; Staessen, J.; Janssens, S.; Van Aelst, L. Incidence of Cardiovascular Events in Patients Treated with Immune Checkpoint Inhibitors. J. Clin. Oncol. 2022, 40, 3430–3438. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.-L.; Lin, W.-F. Immune checkpoint inhibition mediated with liposomal nanomedicine for cancer therapy. Mil. Med. Res. 2023, 10, 20. [Google Scholar] [CrossRef]
- Rubio-Infante, N.; Ramírez-Flores, Y.A.; Castillo, E.C.; Lozano, O.; García-Rivas, G.; Torre-Amione, G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: A meta-analysis. Eur. J. Heart Fail. 2021, 23, 1739–1747. [Google Scholar] [CrossRef]
- Tan, A.C.; Bagley, S.J.; Wen, P.Y.; Lim, M.; Platten, M.; Colman, H.; Ashley, D.M.; Wick, W.; Chang, S.M.; Galanis, E.; et al. Systematic review of combinations of targeted or im-munotherapy in advanced solid tumors. J Immunother. Cancer 2021, 9, e002459. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Stein-Merlob, A.F.; Rothberg, M.V.; Ribas, A.; Yang, E.H. Cardiotoxicities of novel cancer immunotherapies. Heart 2021, 107, 1694–1703. [Google Scholar] [CrossRef]
- Li, C.; Bhatti, S.A.; Ying, J. Immune Checkpoint Inhibitors—Associated Cardiotoxicity. Cancers 2022, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Ferrajolo, C.; Rafaniello, C.; Danesi, R.; Del Re, M.; Russo, A.; Coscioni, E.; Rossi, F.; Alfano, R.; et al. Immune Checkpoint Inhibitors and Cardi-otoxicity: An Analysis of Spontaneous Reports in Eudravigilance. Drug Saf. 2021, 44, 957–971. [Google Scholar] [CrossRef]
- Schonfeld, S.J.; Tucker, M.A.; Engels, E.A.; Dores, G.M.; Sampson, J.N.; Shiels, M.S.; Chanock, S.J.; Morton, L.M. Immune-Related Adverse Events After Immune Checkpoint Inhibitors for Melanoma Among Older Adults. JAMA Netw. Open 2022, 5, e223461. [Google Scholar] [CrossRef] [PubMed]
- Nebhan, C.A.; Cortellini, A.; Ma, W.; Ganta, T.; Song, H.; Ye, F.; Irlmeier, R.; Dennath, N.; Saeed, A.; Radford, M.; et al. Clinical Outcomes and Toxic Effects of Single-Agent Im-mune Checkpoint Inhibitors Among Patients Aged 80 Years or Older with Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021, 7, 1856–1861. [Google Scholar] [CrossRef]
- Perret, M.; Bertaut, A.; Niogret, J.; Marilier, S.; Jouanny, P.; Manckoundia, P.; Bengrine-Lefevre, L.; Quipourt, V.; Barben, J. Associated Factors to Efficacy and Tolerance of Immunotherapy in Older Patients with Cancer Aged 70 Years and Over: Impact of Coprescriptions. In Drugs Aging; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–10. [Google Scholar]
- Luciani, A.; Ghidini, A.; Dottorini, L.; Petrelli, F. Safety and Effectiveness of Immune Checkpoint Inhibitors in Older Pa-tients with Cancer: A Systematic Review of 48 Real-World Studies. Drugs Aging 2021, 38, 1055–1065. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Q.; Meng, D.; Li, K.; Zhang, Y. Immune checkpoint inhibitors-related myocarditis in patients with cancer: An analysis of international spontaneous reporting systems. BMC Cancer 2021, 21, 38. [Google Scholar] [CrossRef]
- Kumar, M.; Thangavel, C.; Becker, R.C.; Sadayappan, S. Monoclonal Antibody-Based Immunotherapy and Its Role in the Development of Cardiac Toxicity. Cancers 2020, 13, 86. [Google Scholar] [CrossRef]
- Montisci, A.; Vietri, M.T.; Palmieri, V.; Sala, S.; Donatelli, F.; Napoli, C. Cardiac Toxicity Associated with Cancer Immuno-therapy and Biological Drugs. Cancers 2021, 13, 4797. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, B.; Hussain, F.; Mukherjee, A.; Araujo-Gutierrez, R.; Pingali, S.R.K. Immune Checkpoint Inhibitor-Related Cardiotoxicity. Methodist DeBakey Cardiovasc. J. 2018, 14, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.-A.; Ray, J.C.; Herrmann, J. Precision Cardio-Oncology: A Systems-Based Perspective on Cardiotoxicity of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors. J. Cardiovasc. Transl. Res. 2020, 13, 402–416. [Google Scholar] [CrossRef]
- Michel, L.; Rassaf, T.; Totzeck, M. Cardiotoxicity from immune checkpoint inhibitors. IJC Heart Vasc. 2019, 25, 100420. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, M.; Bonaca, M.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
| Sample | NSCLC (n = 99) | Urothelial (n = 29) | Melanoma (n = 23) | Renal (n = 22) | Miscellany (n = 22) |
|---|---|---|---|---|---|
| Age (range) | 74 (70–86) | 76 (70–88) | 78 (70–93) | 76 (70–87) | 76 (70–84) |
| Sex (M/W) | 86/13 (86.9/14.1%) | 24/5 (82.8/17.2%) | 11/12 (47.8/52.2%) | 15/7 (68.2/31.8%) | 14/8 (63.6/36.4%) |
Type of ICI
| 48 (48.5%) 51 (51.5%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) | 4 (13.8%) 0 (0%) 20 (69%) 0 (0%) 0 (0%) 5 (17.2%) | 0 (0%) 22 (95.7%) 0 (0%) 1 (4.3%) 5 (21.7%) 0 (0%) | 4 (18.2%) 16 (72.8%) 0 (0%) 2 (9.1%) 0 (0%) 0 (0%) | 8 (36.4%) 5 (22.7%) 7 (31.8%) 0 (0%) 0 (0%) 2 (9.1%) |
| Most common histology | ADC 62 (62.6%) | Transitional 27 (93.1%) | Superficial spreading 10 (%) | Clear cell 18 (81.8%) | SCLC 7 (31.8%) |
| Median doses of ICIs | 9 | 6 | 8 | 6 | 6 |
ECOG
| 83 (83.8%) 16 (16.2%) | 25 (86.2%) 4 (13.8%) | 20 (87%) 3 (13%) | 19 (86.4%) 3 (13.6%) | 17 (77.3%) 5 (22.7%) |
| Hypertension | 52 (52.6%) | 15 (51.7%) | 17 (73.9%) | 15 (68.2%) | 13 (59.1%) |
| Dyslipidaemia | 54 (54.5%) | 16 (55.2%) | 11 (47.8%) | 8 (36.4%) | 11 (50%) |
| Type 2 diabetes mellitus | 22 (22.2%) | 8 (28.6%) | 7 (30.4%) | 6 (27.3%) | 3 (13.6%) |
| Cardiac comorbidity | 39 (39.4%) | 9 (31%) | 7 (30.4%) | 6 (27.3%) | 9 (40.9%) |
| Immunotoxicity | Global (n = 195) | NSCLC (n = 99) | Urothelial (n = 29) | Melanoma (n = 23) | Renal (n = 22) | Miscellany (n = 22) |
|---|---|---|---|---|---|---|
Global
| 56 (28.7%) 41 (21%) 15 (7.7%) | 25 (25.3%) 19 (19.2%) 6 (6.1%) | 8 (27.6%) 7 (24.1%) 1 (3.4%) | 11 (47.8%) 5 (21.7%) 6 (26.1%) | 4 (18.2%) 4 (18.2%) 0 (0%) | 8 (36.4%) 6 (27.3%) 2 (9.1%) |
Endocrine
| 14 (7.2%) 14 (7.2%) 0 (0%) | 8 (8.1%) 8 (8.1%) 0 (0%) | 2 (6.9%) 2 (6.9%) 0 (0%) | 1 (4.3%) 1 (4.3%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 3 (13.6%) 3 (13.6%) 0 (0%) |
Gastrointestinal
| 8 (4.1%) 5 (2.6%) 3 (1.5%) | 3 (3%) 1 (1%) 2 (2%) | 2 (6.9%) 2 (6.9%) 0 (0%) | 2 (8.7%) 1 (4.3%) 1 (4.3%) | 0 (0%) 0 (0%) 0 (0%) | 1 (4.5%) 1 (4.5%) 0 (0%) |
Hepatic
| 9 (4.6%) 4 (2.1%) 5 (2.6%) | 4 (4%) 2 (2%) 2 (2%) | 1 (3.4%) 1 (3.4%) 0 (0%) | 2 (8.7%) 0 (0%) 2 (8.7%) | 1 (4.5%) 1 (4.5%) 0 (0%) | 1 (4.5%) 0 (0%) 1 (4.5%) |
Asthenia
| 6 (3.1%) 6 (3.1%) 0 (0%) | 2 (2%) 2 (2%) 0 (0%) | 1 (3.4%) 1 (3.4%) 0 (0%) | 1 (4.3%) 1 (4.3%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 2 (9.1%) 2 (9.1%) 0 (0%) |
Rheumatic
| 4 (2.1%) 4 (2.1%) 0 (0%) | 2 (2%) 2 (2%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 2 (8.7%) 2 (8.7%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) |
Dermal
| 4 (2.1%) 4 (2.1%) 0 (0%) | 2 (2%) 2 (2%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 1 (4.5%) 1 (4.5%) 0 (0%) | 1 (4.5%) 1 (4.5%) 0 (0%) |
Renal
| 7 (3.6%) 4 (2.1%) 3 (1.5%) | 5 (5.1%) 3 (3%) 2 (2%) | 0 (0%) 0 (0%) 0 (0%) | 1 (4.3%) 0 (0%) 1 (4.3%) | 1 (4.5%) 1 (4.5%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) |
Pulmonary
| 5 (2.6%) 2 (1%) 3 (1.5%) | 2 (2%) 1 (1%) 1 (1%) | 1 (3.4%) 0 (0%) 1 (3.4%) | 0 (0%) 0 (0%) 0 (0%) | 1 (4.5%) 1 (4.5%) 0 (0%) | 1 (4.5%) 0 (0%) 1 (4.5%) |
Haematological
| 3 (1.5%) 1 (0.5%) 2 (1%) | 0 (0%) 0 (0%) 0 (0%) | 1 (3.4%) 1 (3.4%) 0 (0%) | 2 (8.7%) 0 (0%) 2 (8.7%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) |
Muscular
| 1 (0.5%)0 (0%)1 (0.5%) | 1 (1%) 0 (0%) 1 (1%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) |
| Cluster Sample (n = 195) | Global Cardiotoxicity | Myocarditis | Arrhythmias | Ischaemic | Heart Failure | Cardiomyopathies and Pericardial |
|---|---|---|---|---|---|---|
| Without cardiac comorbidity (n = 121) | 2 (1.65%) | 1 (0.82%) | 1 (0.82%) | 0 (0%) | 0 (0%) | 0 (0%) |
| With cardiac comorbidity (n = 74) | 1 (1.35%) | 1 (1.35%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| All patients (n = 195) | 3 (1.54%) | 2 (1.03%) | 1 (0.51%) | 0 (0%) | 0 (0%) | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toribio-García, I.; Olivares-Hernández, A.; Miramontes-González, J.P.; Posado-Domínguez, L.; Martín García, A.; Eiros Bachiller, R.; Figuero-Pérez, L.; Garijo Martínez, M.; Roldán Ruiz, J.; Bellido Hernández, L.; et al. Cardiotoxicity Secondary to Immune Checkpoint Inhibitors in the Elderly: Safety in Real-World Data. Cancers 2023, 15, 4293. https://doi.org/10.3390/cancers15174293
Toribio-García I, Olivares-Hernández A, Miramontes-González JP, Posado-Domínguez L, Martín García A, Eiros Bachiller R, Figuero-Pérez L, Garijo Martínez M, Roldán Ruiz J, Bellido Hernández L, et al. Cardiotoxicity Secondary to Immune Checkpoint Inhibitors in the Elderly: Safety in Real-World Data. Cancers. 2023; 15(17):4293. https://doi.org/10.3390/cancers15174293
Chicago/Turabian StyleToribio-García, Irene, Alejandro Olivares-Hernández, José Pablo Miramontes-González, Luis Posado-Domínguez, Ana Martín García, Rocío Eiros Bachiller, Luis Figuero-Pérez, María Garijo Martínez, Jonnathan Roldán Ruiz, Lorena Bellido Hernández, and et al. 2023. "Cardiotoxicity Secondary to Immune Checkpoint Inhibitors in the Elderly: Safety in Real-World Data" Cancers 15, no. 17: 4293. https://doi.org/10.3390/cancers15174293
APA StyleToribio-García, I., Olivares-Hernández, A., Miramontes-González, J. P., Posado-Domínguez, L., Martín García, A., Eiros Bachiller, R., Figuero-Pérez, L., Garijo Martínez, M., Roldán Ruiz, J., Bellido Hernández, L., Fonseca-Sánchez, E., Luis Sánchez, P., & del Barco-Morillo, E. (2023). Cardiotoxicity Secondary to Immune Checkpoint Inhibitors in the Elderly: Safety in Real-World Data. Cancers, 15(17), 4293. https://doi.org/10.3390/cancers15174293







