Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Study Population
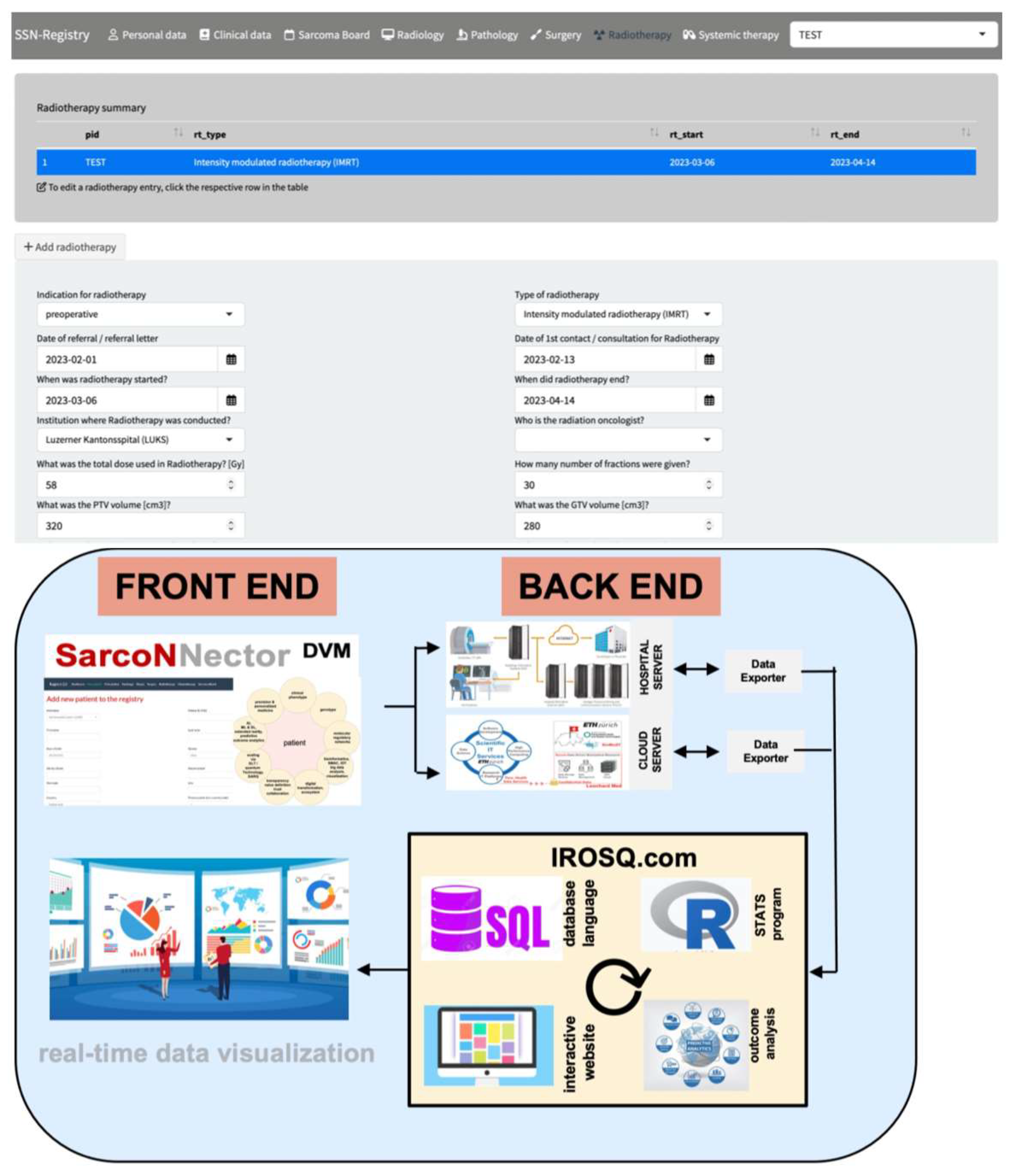
2.3. Sarconnector®
2.4. Statistical Analysis
3. Results
3.1. Basic Data and the Sarconnector®
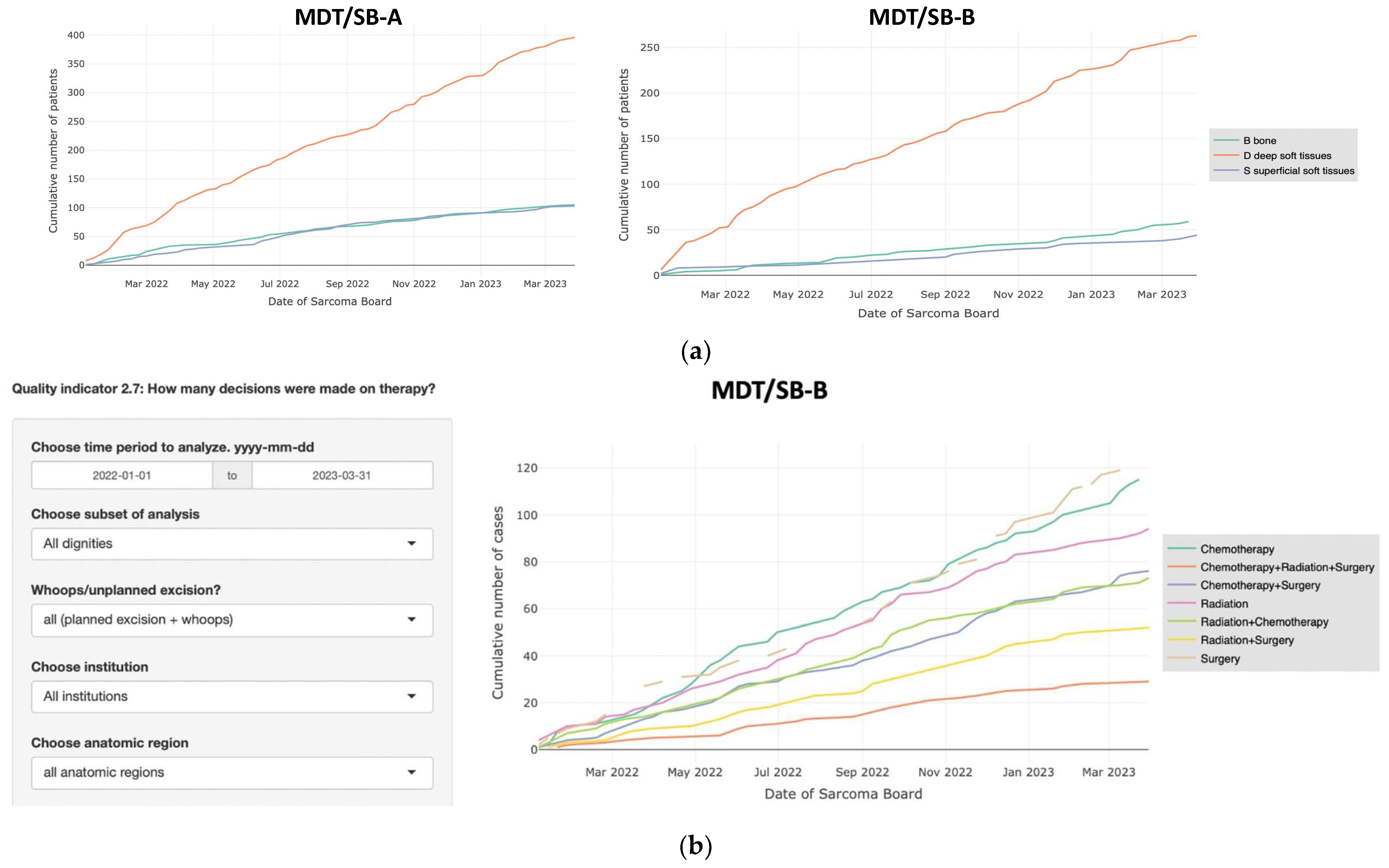
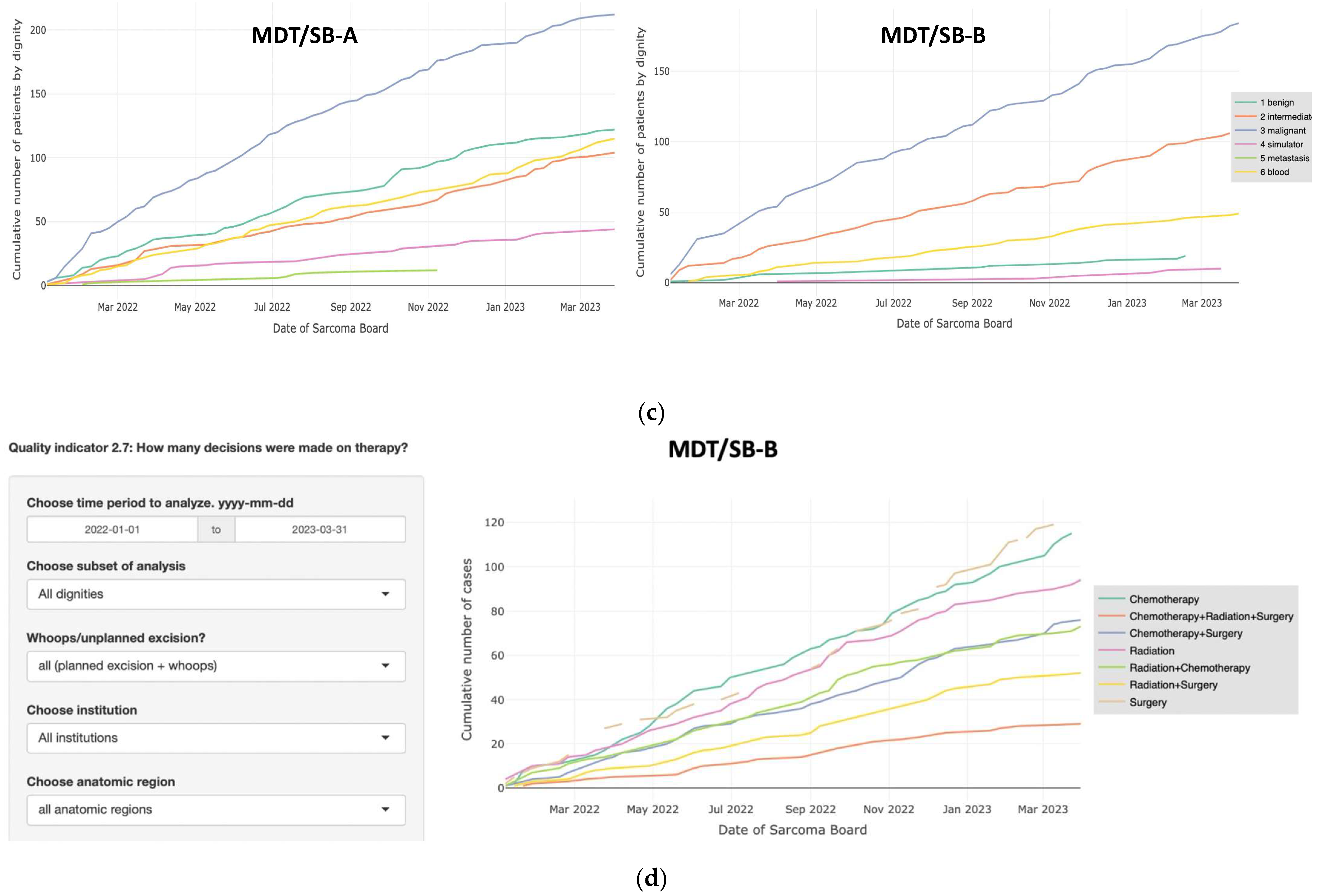
3.2. Comparison of Two MDT/SB
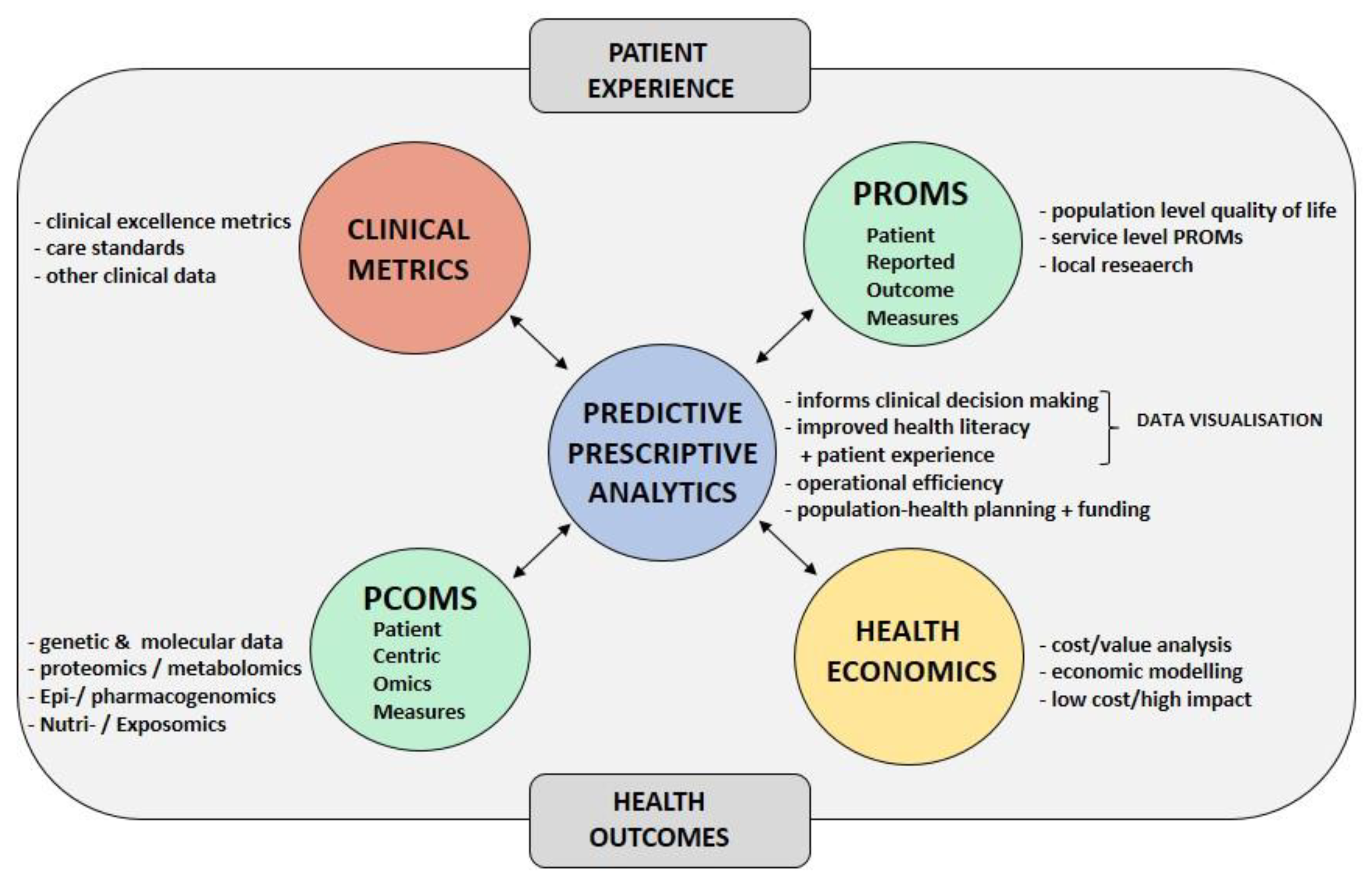
3.3. Interactive Data Analysis and Visualization
3.4. Automated Statistical Analysis and Visualization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blay, J.Y.; Hindi, N.; Bollard, J.; Aguiar, S.; Angel, M.; Araya, B.; Badilla, R.; Bernabeu, D.; Campos, F.; Caro-Sánchez, C.H.S.; et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat. Rev. 2022, 102, 102312. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Palmerini, E.; Bollard, J.; Aguiar, S.; Angel, M.; Araya, B.; Badilla, R.; Bernabeu, D.; Campos, F.; Chs, C.S.; et al. SELNET Clinical practice guidelines for bone sarcoma. Crit. Rev. Oncol. Hematol. 2022, 174, 103685. [Google Scholar] [CrossRef] [PubMed]
- Andritsch, E.; Beishon, M.; Bielack, S.; Bonvalot, S.; Casali, P.; Crul, M.; Delgado Bolton, R.; Donati, D.M.; Douis, H.; Haas, R.; et al. ECCO Essential Requirements for Quality Cancer Care: Soft Tissue Sarcoma in Adults and Bone Sarcoma. A critical review. Crit. Rev. Oncol. Hematol. 2017, 110, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Bonvalot, S.; Gouin, F.; Le Cesne, A.; Penel, N. Criteria for reference centers for sarcomas: Volume but also long-term multidisciplinary organisation. Ann. Oncol. 2019, 30, 2008–2009. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Honore, C.; Stoeckle, E.; Meeus, P.; Jafari, M.; Gouin, F.; Anract, P.; Ferron, G.; Rochwerger, A.; Ropars, M.; et al. Surgery in reference centers improves survival of sarcoma patients: A nationwide study. Ann. Oncol. 2019, 30, 1143–1153. [Google Scholar] [CrossRef]
- Blay, J.Y.; Penel, N.; Gouin, F.; Le Cesne, A.; Toulmonde, M. Improving at a nationwide level the management of patients with sarcomas with an expert network. Ann. Oncol. 2022, 33, 659–661. [Google Scholar] [CrossRef]
- Blay, J.Y.; Soibinet, P.; Penel, N.; Bompas, E.; Duffaud, F.; Stoeckle, E.; Mir, O.; Adam, J.; Chevreau, C.; Bonvalot, S.; et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann. Oncol. 2017, 28, 2852–2859. [Google Scholar] [CrossRef]
- Fayet, Y.; Tetreau, R.; Honore, C.; Le Nail, L.R.; Dalban, C.; Gouin, F.; Causeret, S.; Piperno-Neumann, S.; Mathoulin-Pelissier, S.; Karanian, M.; et al. Determinants of the access to remote specialised services provided by national sarcoma reference centres. BMC Cancer 2021, 21, 631. [Google Scholar] [CrossRef]
- Domenghino, A.; Walbert, C.; Birrer, D.L.; Puhan, M.A.; Clavien, P.-A.; Heuskel, D.; Man, N.K.; Monteiro, J.; Perellon, J.F.; Pérez Martínez, S.; et al. Consensus recommendations on how to assess the quality of surgical interventions. Nat. Med. 2023, 29, 811–822. [Google Scholar] [CrossRef]
- Sweeney, S.M.; Hamadeh, H.K.; Abrams, N.; Adam, S.J.; Brenner, S.; Connors, D.E.; Davis, G.J.; Fiore, L.D.; Gawel, S.H.; Grossman, R.L.; et al. Case studies for overcoming challenges in using big data in cancer. Cancer Res. 2023, 83, 1175–1190. [Google Scholar] [CrossRef]
- Sweeney, S.M.; Hamadeh, H.K.; Abrams, N.; Adam, S.J.; Brenner, S.; Connors, D.E.; Davis, G.J.; Fiore, L.D.; Gawel, S.H.; Grossman, R.L.; et al. Challenges to using big data in cancer. Cancer Res. 2023, 83, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Rajpurkar, P.; Chen, E.; Banerjee, O.; Topol, E.J. AI in health and medicine. Nat. Med. 2022, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Barkun, J.; Clavien, P.; Pawlik, T.M. The Benefits of Benchmarking-A New Perspective on Measuring Quality in Surgery. JAMA Surg. 2023, 158, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Muller, X.; Staiger, R.D.; Gutschow, C.A.; Vonlanthen, R.; Bueter, M.; Clavien, P.A.; Puhan, M.A. How to Establish Benchmarks for Surgical Outcomes?: A Checklist Based on an International Expert Delphi Consensus. Ann. Surg. 2022, 275, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Staiger, R.D.; Schwandt, H.; Puhan, M.A.; Clavien, P.A. Improving surgical outcomes through benchmarking. Br. J. Surg. 2019, 106, 59–64. [Google Scholar] [CrossRef]
- Wind, A.; Goncalves, F.R.; Marosi, E.; da Pieve, L.; Groza, M.; Asioli, M.; Albini, M.; van Harten, W. Benchmarking Cancer Centers: From Care Pathways to Integrated Practice Units. J. Natl. Compr. Cancer Netw. 2018, 16, 1075–1083. [Google Scholar] [CrossRef]
- Slankamenac, K.; Graf, R.; Barkun, J.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index. Ann. Surg. 2013, 258, 1–7. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Clavien, P.A.; Vetter, D.; Staiger, R.D.; Slankamenac, K.; Mehra, T.; Graf, R.; Puhan, M.A. The Comprehensive Complication Index (CCI(R)): Added Value and Clinical Perspectives 3 Years “Down the Line”. Ann. Surg. 2017, 265, 1045–1050. [Google Scholar] [CrossRef]
- Zilchman, E.; Nicklin, W.; Aggarwal, R.; Bates, D. Health Care 2030: The coming transformatrion. NEJM Catal. 2021, 1, 1–11. [Google Scholar]
- Trutner, Z.D.; Teisberg, E.O.; Bozic, K.J. Value-based Healthcare: Five Strategies to Save Patients, Physicians, and Dollars. Clin. Orthop. Relat. Res. 2022, 480, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E.; Teisberg, E.O. Redefining competition in health care. Harv. Bus. Rev. 2004, 82, 64–76, 136. [Google Scholar] [PubMed]
- Porter, M.E. Value-based health care delivery. Ann. Surg. 2008, 248, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E. A strategy for health care reform--toward a value-based system. N. Engl. J. Med. 2009, 361, 109–112. [Google Scholar] [CrossRef]
- Porter, M.E.; Lee, T.H. From Volume to Value in Health Care: The Work Begins. JAMA 2016, 316, 1047–1048. [Google Scholar] [CrossRef]
- Porter, M.E.; Lee, T.H.; Murray, A.C.A. The Value-Based Geography Model of Care. NEJM Catal. 2020, 1. [Google Scholar] [CrossRef]
- Porter, M.E.; Larsson, S.; Lee, T.H. Standardizing Patient Outcomes Measurement. N. Engl. J. Med. 2016, 374, 504–506. [Google Scholar] [CrossRef]
- Porter, M.E.; Lee, T.H. Integrated Practice Units: A Playbook for Health Care Leaders. NEJM Catal. 2021, 2. [Google Scholar] [CrossRef]
- Porter, M.E.; Pabo, E.A.; Lee, T.H. Redesigning primary care: A strategic vision to improve value by organizing around patients’ needs. Health Aff. 2013, 32, 516–525. [Google Scholar] [CrossRef]
- Saesen, R.; Lacombe, D.; Huys, I. Real-world data in oncology: A questionnaire-based analysis of the academic research landscape examining the policies and experiences of the cancer cooperative groups. ESMO Open Cancer Horiz. 2023, 8, 100878. [Google Scholar] [CrossRef] [PubMed]
- Saesen, R.; Van Hemelrijck, M.; Bogaerts, J.; Booth, C.M.; Cornelissen, J.J.; Dekker, A.; Eisenhauer, E.A.; Freitas, A.; Gronchi, A.; Hernán, M.A.; et al. Defining the role of real-world data in cancer clinical research: The position of the European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2023, 186, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.R.; Angrisani, M.; Troxel, W.M.; Gutsche, T.; Ortega, E.; Jain, M.; Boch, A.; Kapteyn, A. American Life in Realtime: A benchmark registry of health data for equitable precision health. Nat. Med. 2023, 29, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Tang, L. FAIR your data. Nat. Methods 2020, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Kerr, D.; Arnold, D.; Blay, J.Y.; Buske, C.; Carrato, A.; Gerritsen, W.; Peeters, M. The Oncology Data Network (ODN): A Collaborative European Data-Sharing Platform to Inform Cancer Care. Oncologist 2020, 25, e1–e4. [Google Scholar] [CrossRef]
- Schwalbe, N.; Wahl, B. Artificial intelligence and the future of global health. Lancet 2020, 395, 1579–1586. [Google Scholar] [CrossRef]
- Birch, J.; Creel, K.A.; Jha, A.K.; Plutynski, A. Clinical decisions using AI must consider patient values. Nat. Med. 2022, 28, 229–232. [Google Scholar] [CrossRef]
- Lehman, C.D.; Wu, S. Stargazing through the lens of AI in clinical oncology. Nat. Cancer 2021, 2, 1265–1267. [Google Scholar] [CrossRef]
- Haug, C.J.; Drazen, J.M. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N. Engl. J. Med. 2023, 388, 1201–1208. [Google Scholar] [CrossRef]
- Benedum, C.M.; Sondhi, A.; Fidyk, E.; Cohen, A.B.; Nemeth, S.; Adamson, B.; Estévez, M.; Bozkurt, S. Replication of Real-World Evidence in Oncology Using Electronic Health Record Data Extracted by Machine Learning. Cancers 2023, 15, 1853. [Google Scholar] [CrossRef]
- Shmatko, A.; Ghaffari Laleh, N.; Gerstung, M.; Kather, J.N. Artificial intelligence in histopathology: Enhancing cancer research and clinical oncology. Nat. Cancer 2022, 3, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Cifci, D.; Veldhuizen, G.P.; Foersch, S.; Kather, J.N. AI in Computational Pathology of Cancer: Improving Diagnostic Workflows and Clinical Outcomes? Annu. Rev. Cancer Biol. 2023, 7, 57–71. [Google Scholar] [CrossRef]
- Boehm, K.M.; Khosravi, P.; Vanguri, R.; Gao, J.; Shah, S.P. Harnessing multimodal data integration to advance precision oncology. Nat. Rev. Cancer 2022, 22, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Theus-Steinmann, C.; Schelling, G.; Heesen, P.; Breitenstein, S.; Scaglioni, M.F.; Fuchs, B. How is the spectrum of sarcoma surgery assessed? Cancers 2023, 15, 1305. [Google Scholar] [CrossRef]
- Frei, A.; Scaglioni, M.; Giovanoli, P.; Breitenstein, S.; Heesen, P.; Fuchs, B. Definition of the Surgical Case Complexity in the Treatment of Soft Tissue Tumors of the Extremities and Trunk. Cancers 2022, 14, 1559. [Google Scholar] [CrossRef]
- Heesen, P.; Studer, G.; Bode, B.; Windegger, H.; Staeheli, B.; Aliu, P.; Martin-Broto, J.; Gronchi, A.; Blay, J.Y.; Le Cesne, A.; et al. Quality of Sarcoma Care: Longitudinal Real-Time Assessment and Evidence Analytics of Quality Indicators. Cancers 2022, 15, 47. [Google Scholar] [CrossRef]
- Mosku, N.; Heesen, P.; Christen, S.; Scaglioni, M.F.; Bode, B.; Studer, G.; Fuchs, B. The Sarcoma-Specific Instrument to Longitudinally Assess Health-Related Outcomes of the Routine Care Cycle. Diagnostics 2023, 13, 1206. [Google Scholar] [CrossRef]
- Mosku, N.; Heesen, P.; Studer, G.; Bode, B.; Spataro, V.; Klass, N.D.; Kern, L.; Scaglioni, M.F.; Fuchs, B. Biopsy Ratio of Suspected to Confirmed Sarcoma Diagnosis. Cancers 2022, 14, 1632. [Google Scholar] [CrossRef]
- Fuchs, B.; Studer, G.; Bode, B.; Wellauer, H.; Frei, A.; Theus, C.; Schupfer, G.; Plock, J.; Windegger, H.; Breitenstein, S.; et al. Development of a value-based healthcare delivery model for sarcoma patients. Swiss Med. Wkly. 2021, 151, w30047. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-WIlk, Kolmogoriv-Smirnov, Lilliefors and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Byrne, J.P.; Haut, E.R. Facts and Fallacy of Benchmark Performance Indicators. Adv. Surg. 2022, 56, 89–109. [Google Scholar] [CrossRef]
- Wind, A.; van Dijk, J.; Nefkens, I.; van Lent, W.; Nagy, P.; Janulionis, E.; Helander, T.; Rocha-Goncalves, F.; van Harten, W. Development of a benchmark tool for cancer centers; results from a pilot exercise. BMC Health Serv. Res. 2018, 18, 764. [Google Scholar] [CrossRef] [PubMed]
- Nolte, E.; Morris, M.; Landon, S.; McKee, M.; Seguin, M.; Butler, J.; Lawler, M. Exploring the link between cancer policies and cancer survival: A comparison of International Cancer Benchmarking Partnership countries. Lancet Oncol. 2022, 23, e502–e514. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Foot, C.; Bomb, M.; Hiom, S.; Coleman, M.; Bryant, H.; Vedsted, P.; Hanson, J.; Richards, M.; Group, I.W. The International Cancer Benchmarking Partnership: An international collaboration to inform cancer policy in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom. Health Policy 2013, 112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.; Maringe, C.; Butler, J.; Brierley, J.D.; Rachet, B.; Coleman, M.P. Comparability of stage data in cancer registries in six countries: Lessons from the International Cancer Benchmarking Partnership. Int. J. Cancer 2013, 132, 676–685. [Google Scholar] [CrossRef]
- Perera, S.K.; Jacob, S.; Sullivan, R.; Barton, M. Evidence-based benchmarks for use of cancer surgery in high-income countries: A population-based analysis. Lancet Oncol. 2021, 22, 173–181. [Google Scholar] [CrossRef]
- Burgess, R.; Lewis, M.; Hill, J.C. Benchmarking community/primary care musculoskeletal services: A narrative review and recommendation. Musculoskelet. Care 2023, 21, 148–158. [Google Scholar] [CrossRef]
- Abbassi, F.; Gero, D.; Muller, X.; Bueno, A.; Figiel, W.; Robin, F.; Laroche, S.; Picard, B.; Shankar, S.; Ivanics, T.; et al. Novel Benchmark Values for Redo Liver Transplantation: Does the Outcome Justify the Effort? Ann. Surg. 2022, 276, 860–867. [Google Scholar] [CrossRef]
- Breuer, E.; Mueller, M.; Doyle, M.B.; Yang, L.; Darwish Murad, S.; Anwar, I.J.; Merani, S.; Limkemann, A.; Jeddou, H.; Kim, S.C.; et al. Liver Transplantation as a New Standard of Care in Patients with Perihilar Cholangiocarcinoma? Results From an International Benchmark Study. Ann. Surg. 2022, 276, 846–853. [Google Scholar] [CrossRef]
- Muller, P.C.; Breuer, E.; Nickel, F.; Zani, S., Jr.; Kauffmann, E.; De Franco, L.; Tschuor, C.; Suno Krohn, P.; Burgdorf, S.K.; Jonas, J.P.; et al. Robotic Distal Pancreatectomy, a Novel Standard of Care? Benchmark Values for Surgical Outcomes from 16 International Expert Centers. Ann. Surg. 2022, 278, 253–259. [Google Scholar] [CrossRef]
- Sanchez-Velazquez, P.; Muller, X.; Malleo, G.; Park, J.S.; Hwang, H.K.; Napoli, N.; Javed, A.A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in Pancreatic Surgery: A Novel Tool for Unbiased Outcome Comparisons. Ann. Surg. 2019, 270, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Gero, D.; Raptis, D.A.; Vleeschouwers, W.; van Veldhuisen, S.L.; Martin, A.S.; Xiao, Y.; Galvao, M.; Giorgi, M.; Benois, M.; Espinoza, F.; et al. Defining Global Benchmarks in Bariatric Surgery: A Retrospective Multicenter Analysis of Minimally Invasive Roux-en-Y Gastric Bypass and Sleeve Gastrectomy. Ann. Surg. 2019, 270, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Kantidakis, G.; Litière, S.; Neven, A.; Vinches, M.; Judson, I.; Blay, J.-Y.; Wardelmann, E.; Stacchiotti, S.; D’Ambrosio, L.; Marréaud, S.; et al. New benchmarks to design clinical trials with advanced or metastatic liposarcoma or synovial sarcoma patients: An EORTC–Soft Tissue and Bone Sarcoma Group (STBSG) meta-analysis based on a literature review for soft-tissue sarcomas. Eur. J. Cancer 2022, 174, 261–276. [Google Scholar] [CrossRef]
- Liu, H.; Stiller, C.A.; Crooks, C.J.; Rous, B.; Bythell, M.; Broggio, J.; Rankin, J.; Nanduri, V.; Lanyon, P.; Card, T.R.; et al. Incidence, prevalence and survival in patients with Langerhans cell histiocytosis: A national registry study from England, 2013–2019. Br. J. Haematol. 2022, 199, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Cerullo, M.; Nussbaum, D.P.; Blazer, D.G., 3rd. Textbook Outcomes Among Patients Undergoing Retroperitoneal Sarcoma Resection. Anticancer Res. 2020, 40, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Boussard, T.; Macklin, P.; Greenspan, E.J.; Gryshuk, A.L.; Stahlberg, E.; Syeda-Mahmood, T.; Shmulevich, I. Digital twins for predictive oncology will be a paradigm shift for precision cancer care. Nat. Med. 2021, 27, 2065–2066. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, L.T.; Rivera, D.R.; Lund, J.L.; Bruno, M.A.; Meyer, A.M. An overview of real-world data sources for oncology and considerations for research. CA Cancer J. Clin. 2022, 72, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Honerlaw, J.P.; Maripuri, M.; Samayamuthu, M.J.; Beaulieu-Jones, B.R.; Baig, H.S.; L’Yi, S.; Ho, Y.-L.; Morris, M.; Panickan, V.A.; et al. Potential pitfalls in the use of real-world data for studying long COVID. Nat. Med. 2023, 29, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A. Time for real-world health data to become routine. Nat. Med. 2023, 29, 1317. [Google Scholar] [CrossRef] [PubMed]
- Luyendijk, M.; Visser, O.; Blommestein, H.M.; de Hingh, I.H.J.T.; Hoebers, F.J.P.; Jager, A.; Sonke, G.S.; de Vries, E.G.E.; Uyl-de Groot, C.A.; Siesling, S. Changes in survival in de novo metastatic cancer in an era of new medicines. JNCI J. Natl. Cancer Inst. 2023, 115, 628–635. [Google Scholar] [CrossRef]
- Jayakrishnan, T.; Aulakh, S.; Baksh, M.; Nguyen, K.; Ailawadhi, M.; Samreen, A.; Parrondo, R.; Sher, T.; Roy, V.; Manochakian, R.; et al. Landmark Cancer Clinical Trials and Real-World Patient Populations: Examining Race and Age Reporting. Cancers 2021, 13, 5770. [Google Scholar] [CrossRef] [PubMed]
- Bernthal, N.M.; Healey, J.H.; Palmerini, E.; Bauer, S.; Schreuder, H.; Leithner, A.; Martin-Broto, J.; Gouin, F.; Lopez-Bastida, J.; Gelderblom, H.; et al. A prospective real-world study of the diffuse-type tenosynovial giant cell tumor patient journey: A 2-year observational analysis. J. Surg. Oncol. 2022, 126, 1520–1532. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Shachar, C.; Bates, D.W. Digital Inclusion as Health Care-Supporting Health Care Equity with Digital-Infrastructure Initiatives. N. Engl. J. Med. 2022, 386, 1101–1103. [Google Scholar] [CrossRef]
- Blumenthal, D.; James, C.V. A Data Infrastructure for Clinical Trial Diversity. N. Engl. J. Med. 2022, 386, 2355–2356. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D. A Step toward Interoperability of Health IT. N. Engl. J. Med. 2022, 387, 2201–2203. [Google Scholar] [CrossRef]
- Beam, A.L.; Drazen, J.M.; Kohane, I.S.; Leong, T.-Y.; Manrai, A.K.; Rubin, E.J. Artificial Intelligence in Medicine. N. Engl. J. Med. 2023, 388, 1220–1221. [Google Scholar] [CrossRef]
- Collins, R.; Bowman, L.; Landray, M.; Peto, R. The Magic of Randomization versus the Myth of Real-World Evidence. N. Engl. J. Med. 2020, 382, 674–678. [Google Scholar] [CrossRef]
- Concato, J.; Corrigan-Curay, J. Real-World Evidence—Where Are We Now? N. Engl. J. Med. 2022, 386, 1680–1682. [Google Scholar] [CrossRef]
- Merola, D.; Schneeweiss, S.; Sreedhara, S.K.; Zabotka, L.E.; Quinto, K.; Concato, J.; Wang, S.V. Real-World Evidence Prediction of a Phase IV Oncology Trial: Comparative Degarelix vs Leuprolide Safety. JNCI Cancer Spectr. 2022, 6, pkac049. [Google Scholar] [CrossRef]
- Denny, J.C.; Collins, F.S. Precision medicine in 2030-seven ways to transform healthcare. Cell 2021, 184, 1415–1419. [Google Scholar] [CrossRef]
| Overall N = 983 | MDT/SB-A N = 610 | MDT/SB-B N = 373 | p-Value | |
|---|---|---|---|---|
| Female | 452 (46.0%) | 283 (46.4%) | 169 (45.3%) | 0.74 |
| Age at diagnosis | 58.0 (1.0, 95.0) | 60.0 (8.0, 93.0) | 56.0 (1.0, 95.0) | 0.001 |
| Bone tumors Chondrogenic Osteogenic Vascular Others/Unknown Soft-tissue tumors Adipocytic (Myo-)fibroblastic Fibrohistiocytic Muscle tumors Undifferentiated/un- classified Others | 44 (4.5%) 19 (1.9%) 18 (1.8%) 81 (8.2%) 201 (20.5%) 117 (11.9%) 33 (3.4%) 82 (8.3%) 87 (8.9%) 301 (30.6%) | 24 (3.9%) 6 (1.0%) 14 (2.3%) 60 (9.8%) 141 (23.1%) 59 (9.7%) 11 (1.8%) 51 (8.4%) 50 (8.2%) 194 (31.8%) | 20 (5.4%) 13 (3.5%) 4 (1.1%) 21 (5.6%) 60 (16.1%) 58 (15.6%) 22 (5.9%) 31 (8.3%) 37 (9.9%) 107 (28.6%) | <0.001 |
| Primary tumor site Appendicular Axial NA | 558 (56.8%) 367 (37.3%) 58 (5.9%) | 352 (57.7%) 220 (36.1%) 38 (6.1%) | 206 (55.2%) 147 (39.4%) 20 (5.4%) | 0.37 |
| Metastasis at diagnosis | 44 (4.5%) | 26 (4.3%) | 18 (4.8%) | 0.75 |
| OVERALL | MDT-SB/A | MDT-SB/B | p-Value | |
|---|---|---|---|---|
| Total number of patients | 983 | 610 | 373 | <0.001 |
| Total number of presentations | 1556 | 914 | 642 | <0.001 |
| OVERALL | MDT-SB/A | MDT-SB/B | p-value | |
| 1st time presentations | 650 | 416 | 234 | <0.001 |
| Follow-up presentations | 833 | 431 | 402 | <0.001 |
| Dignity: 1st time/fup presentation Benign Intermediate Malignant Simulator Metastasis Blood Others | 650/833 120/70 135/50 186/548 43/20 10/4 53/11 103/30 | 416/431 105/54 61/77 99/256 35/13 10/4 9/1 97/26 | 234/402 15/16 74/3 97/292 8/7 0/0 44/10 6/4 | <0.001/0.17 <0.001/<0.001 <0.001/<0.93 <0.001/<0.001 0.01/0.26 0.02/0.13 <0.01/0.005 <0.01/<0.01 |
| Localization: 1st time/fup presentation Bone Deep soft tissues Superficial soft tissues NA | 650/833 117/141 431/579 95/103 7/10 | 416/431 77/76 269/290 70/62 0/3 | 234/402 40/65 162/289 28/41 4/7 | <0.001/0.17 0.67/0.58 0.26/0.15 0.11/0.07 0.02/0.21 |
| 1st time & intermediate/malignant Bone Deep soft tissues Superficial soft tissues | 321 48 224 49 | 160 20 111 29 | 161 28 113 20 | 0.99 0.27 0.90 0.17 |
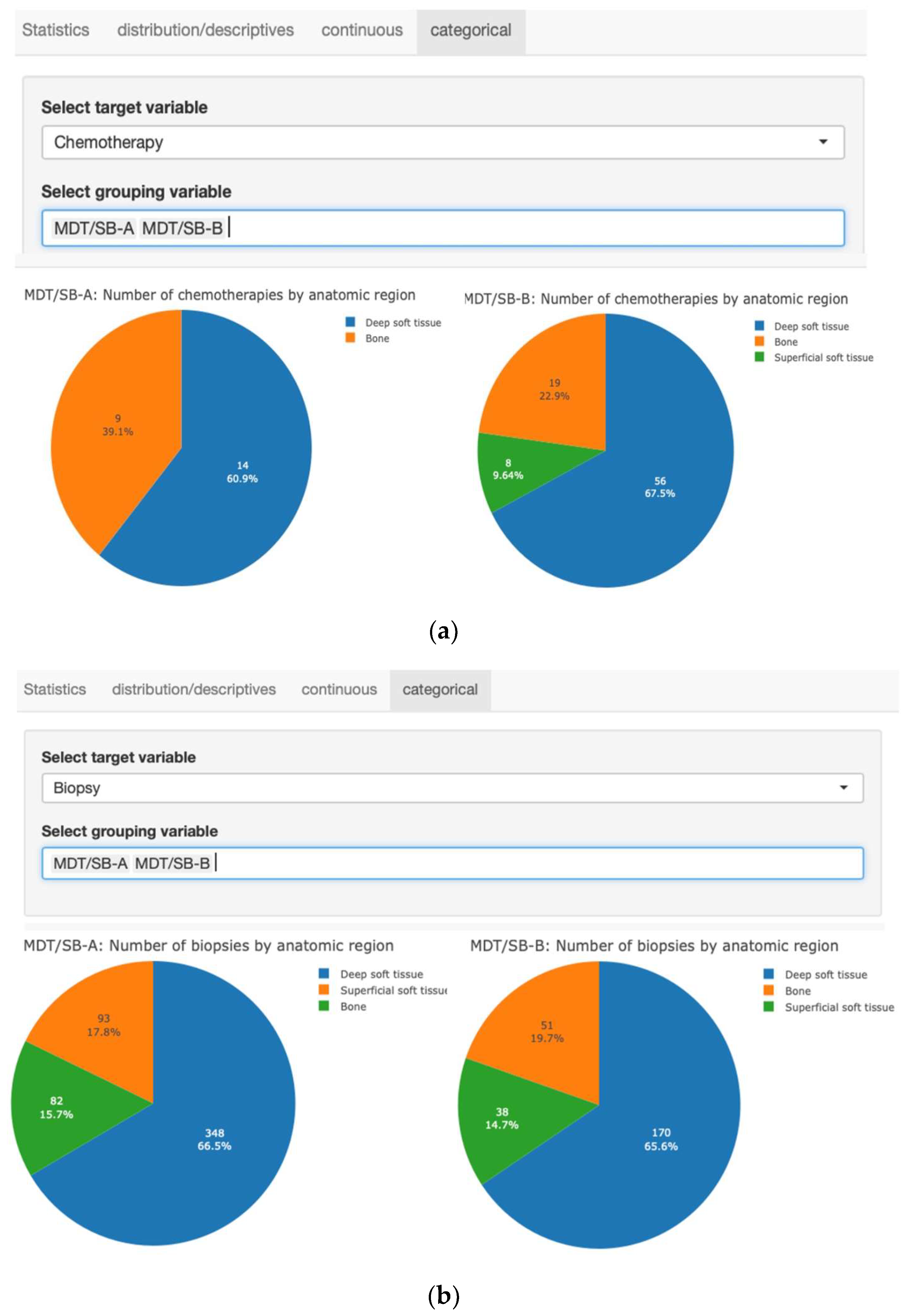
| Total number of biopsies Bone Deep soft tissues Superficial soft tissues | 782 133 518 131 | 523 82 348 93 | 259 51 170 38 | <0.001 0.19 0.81 0.31 |
| Indications for surgery Bone Deep soft tissues Superficial soft tissues | 393 70 259 64 | 244 38 161 45 | 149 32 98 19 | 0.77 0.17 0.99 0.16 |
| Indications for radiotherapy Bone Deep soft tissues Superficial soft tissues | 98 5 73 20 | 46 2 33 11 | 52 3 40 9 | 0.48 0.99 0.65 0.46 |
| Indications for chemotherapy Bone Deep soft tissues Superficial soft tissues | 106 28 70 8 | 23 9 14 0 | 83 19 56 8 | <0.001 0.18 0.62 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs, B.; Schelling, G.; Elyes, M.; Studer, G.; Bode-Lesniewska, B.; Scaglioni, M.F.; Giovanoli, P.; Heesen, P.; on behalf of the SwissSarcomaNetwork. Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes. Cancers 2023, 15, 4395. https://doi.org/10.3390/cancers15174395
Fuchs B, Schelling G, Elyes M, Studer G, Bode-Lesniewska B, Scaglioni MF, Giovanoli P, Heesen P, on behalf of the SwissSarcomaNetwork. Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes. Cancers. 2023; 15(17):4395. https://doi.org/10.3390/cancers15174395
Chicago/Turabian StyleFuchs, Bruno, Georg Schelling, Maria Elyes, Gabriela Studer, Beata Bode-Lesniewska, Mario F. Scaglioni, Pietro Giovanoli, Philip Heesen, and on behalf of the SwissSarcomaNetwork. 2023. "Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes" Cancers 15, no. 17: 4395. https://doi.org/10.3390/cancers15174395
APA StyleFuchs, B., Schelling, G., Elyes, M., Studer, G., Bode-Lesniewska, B., Scaglioni, M. F., Giovanoli, P., Heesen, P., & on behalf of the SwissSarcomaNetwork. (2023). Unlocking the Power of Benchmarking: Real-World-Time Data Analysis for Enhanced Sarcoma Patient Outcomes. Cancers, 15(17), 4395. https://doi.org/10.3390/cancers15174395








