Head and Neck Squamous Cell Carcinoma Biopsies Maintained Ex Vivo on a Perfusion Device Show Gene Changes with Time and Clinically Relevant Doses of Irradiation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting Up and Running Perfusion Devices
2.2. RNA Extraction and Nanostring
2.3. Proteome Profiler™ Array
2.4. Statistical Analysis
3. Results
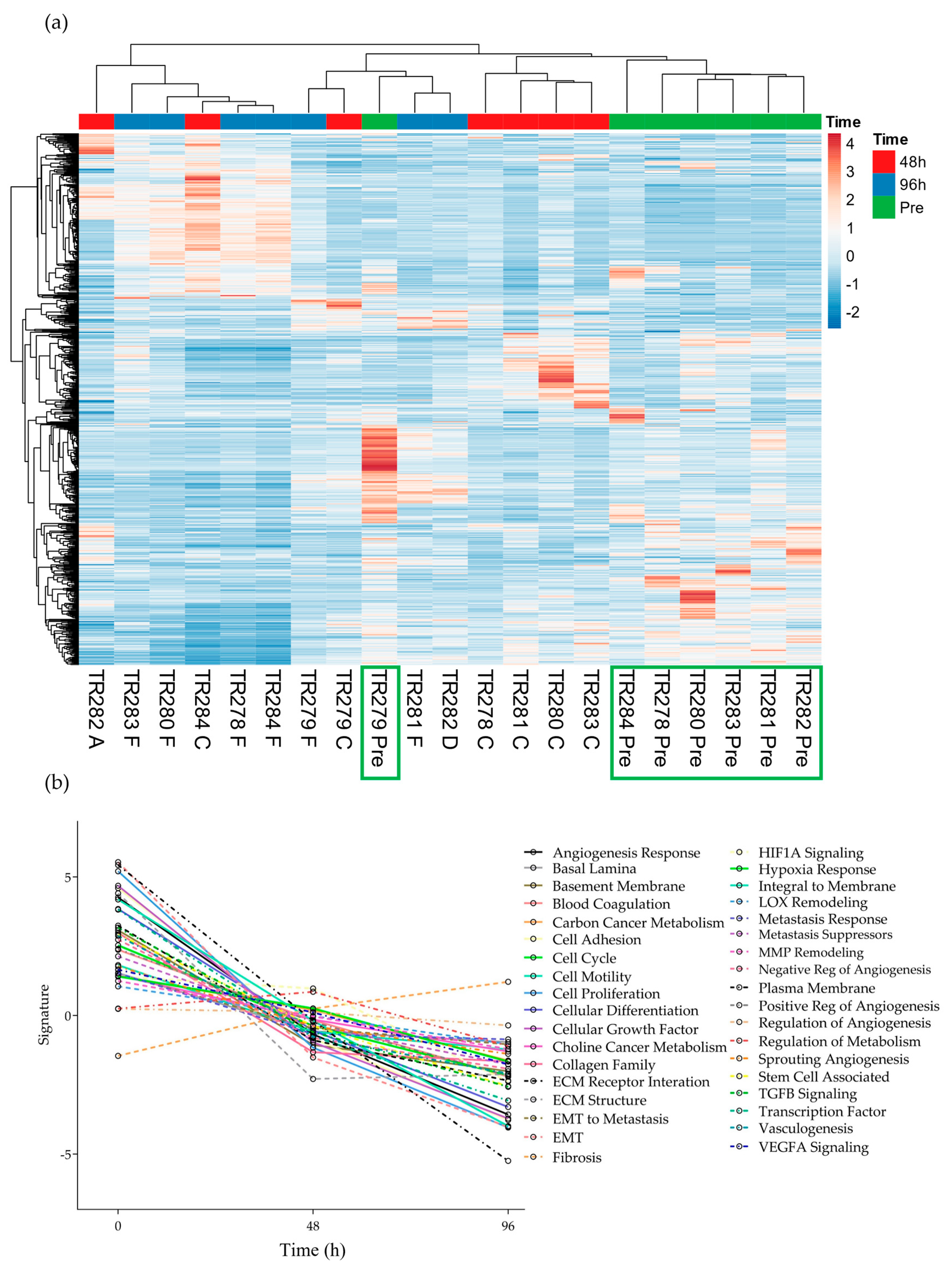
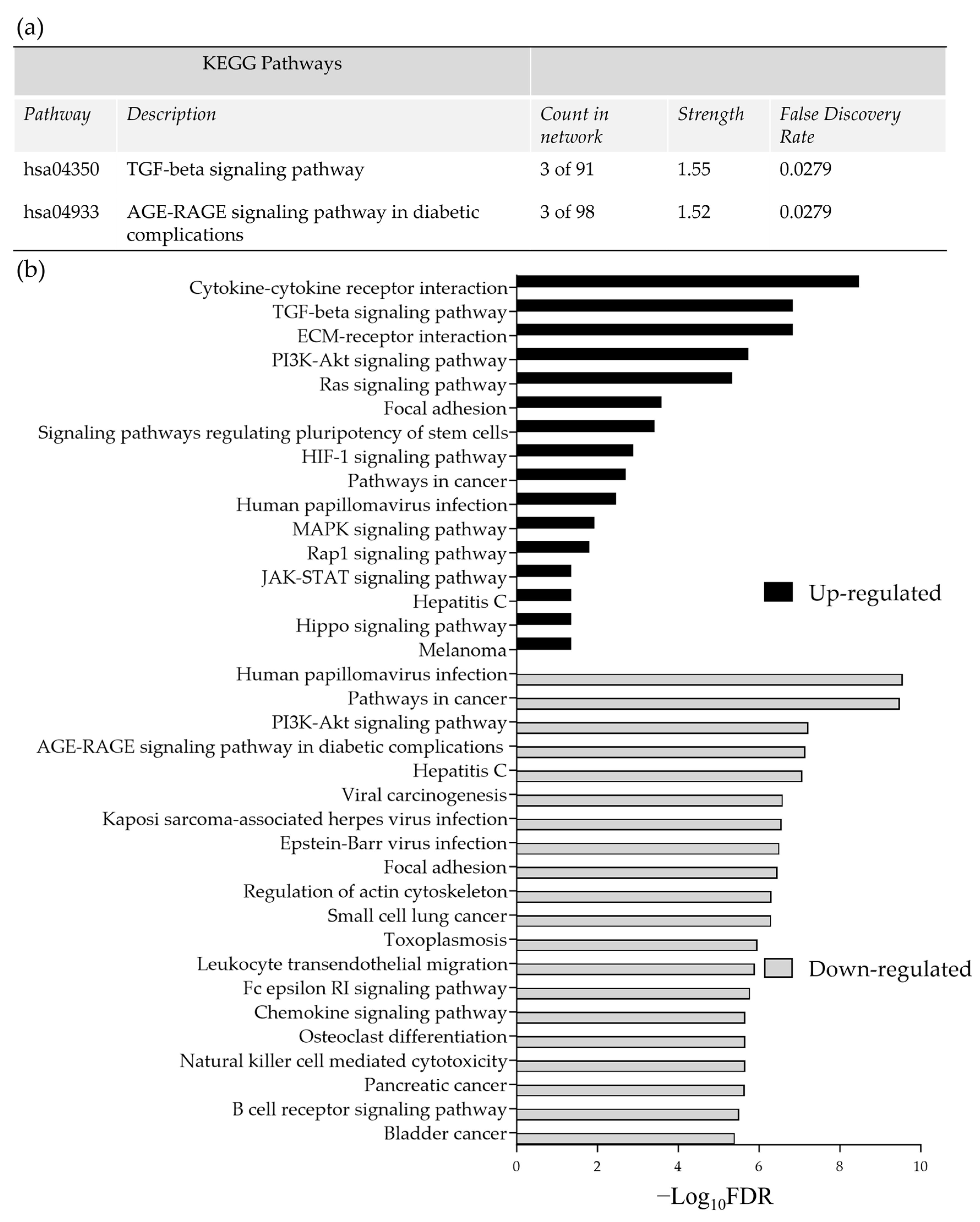
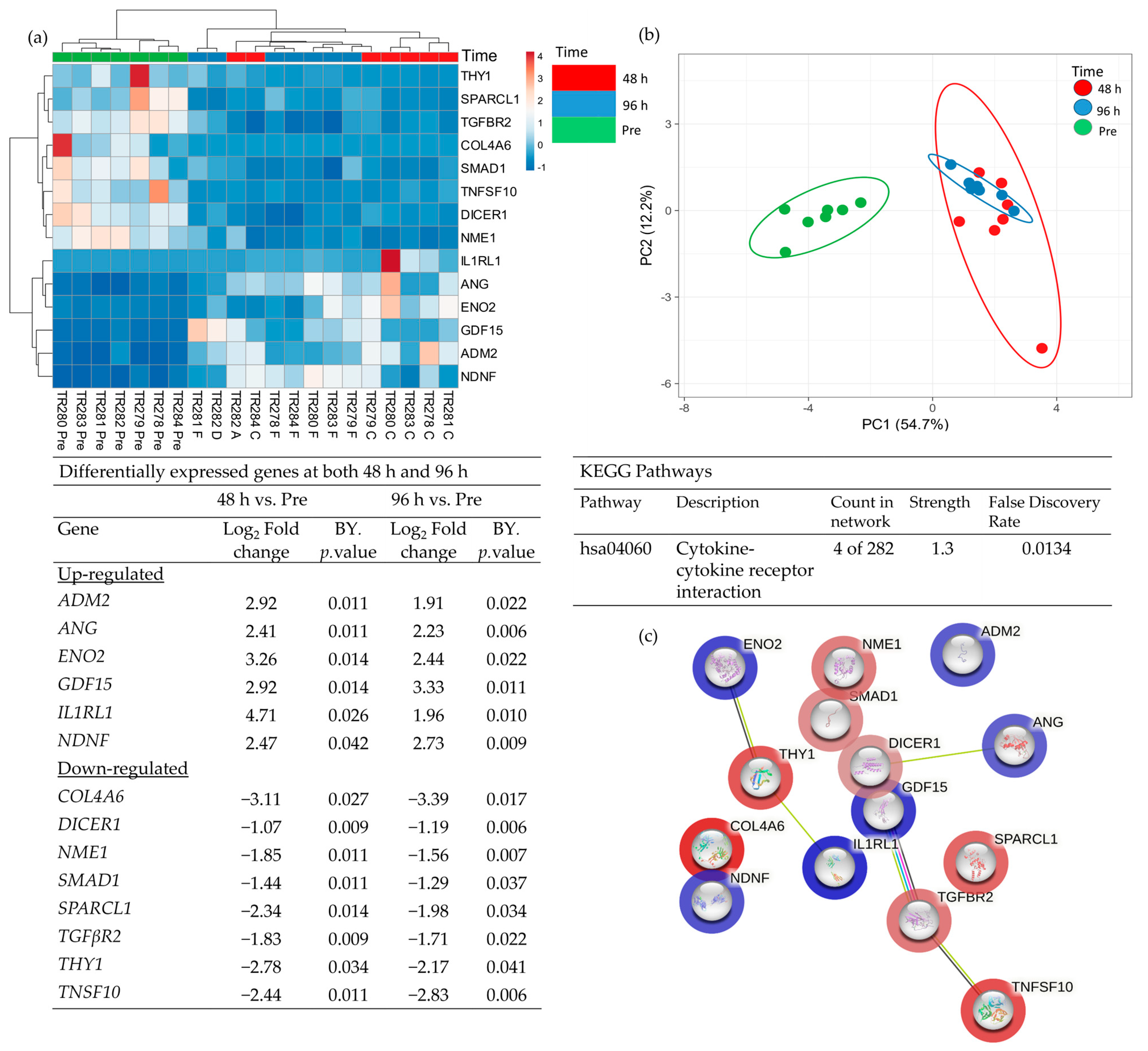
3.1. The Effect of Incubation Time on Gene Expression in Ex Vivo HNSCC Tissue Maintained on the Perfusion Device
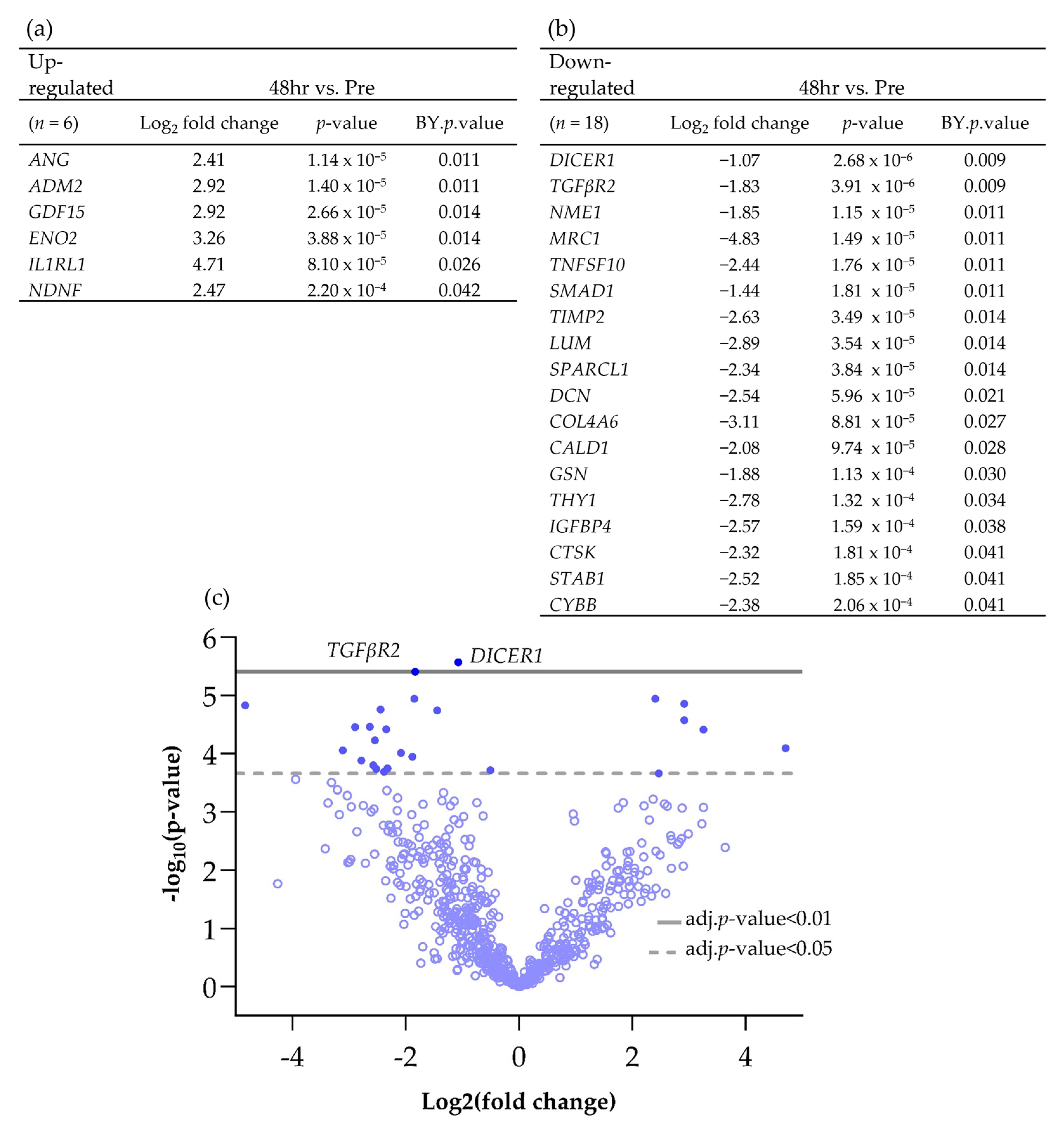
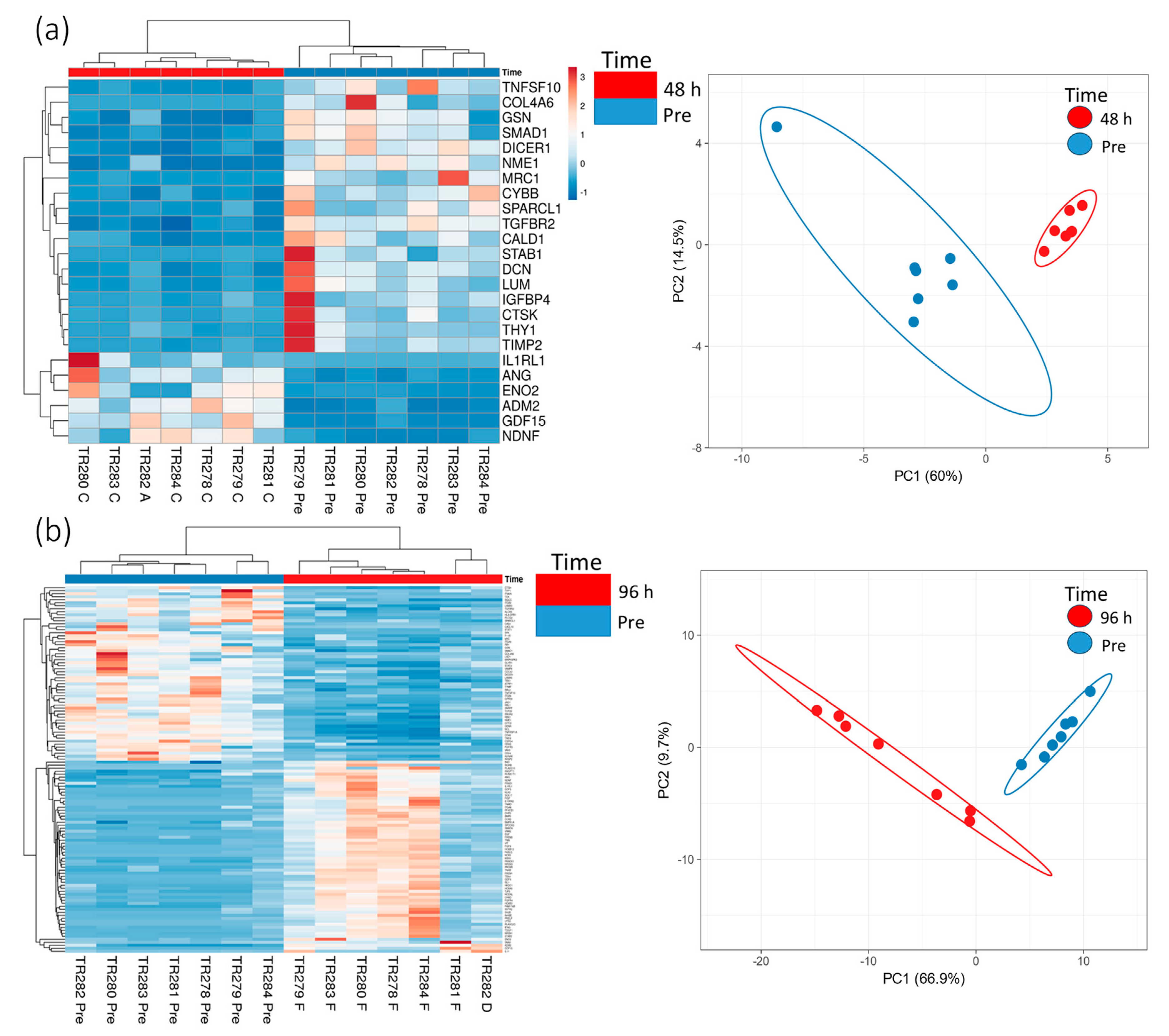
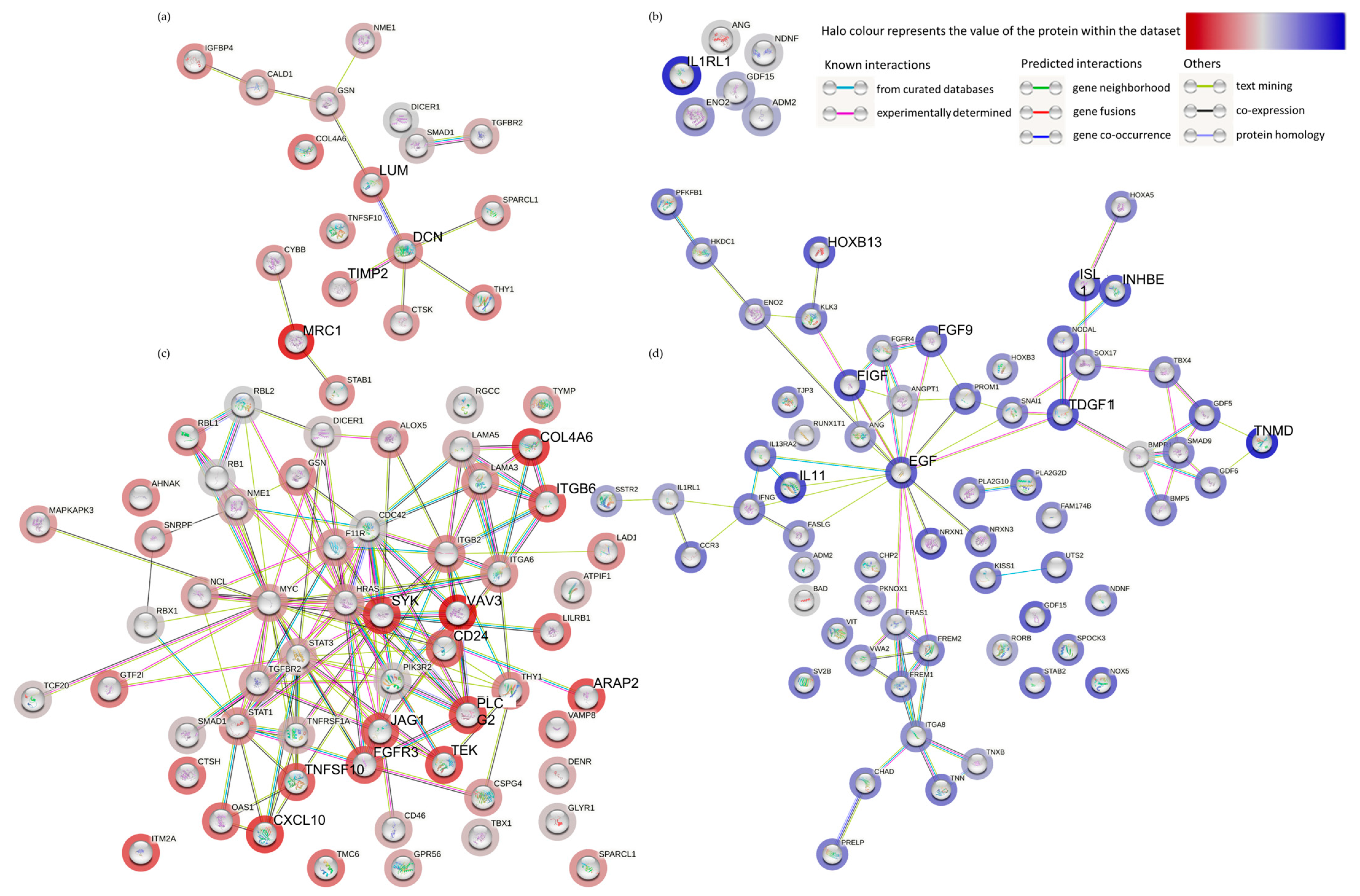
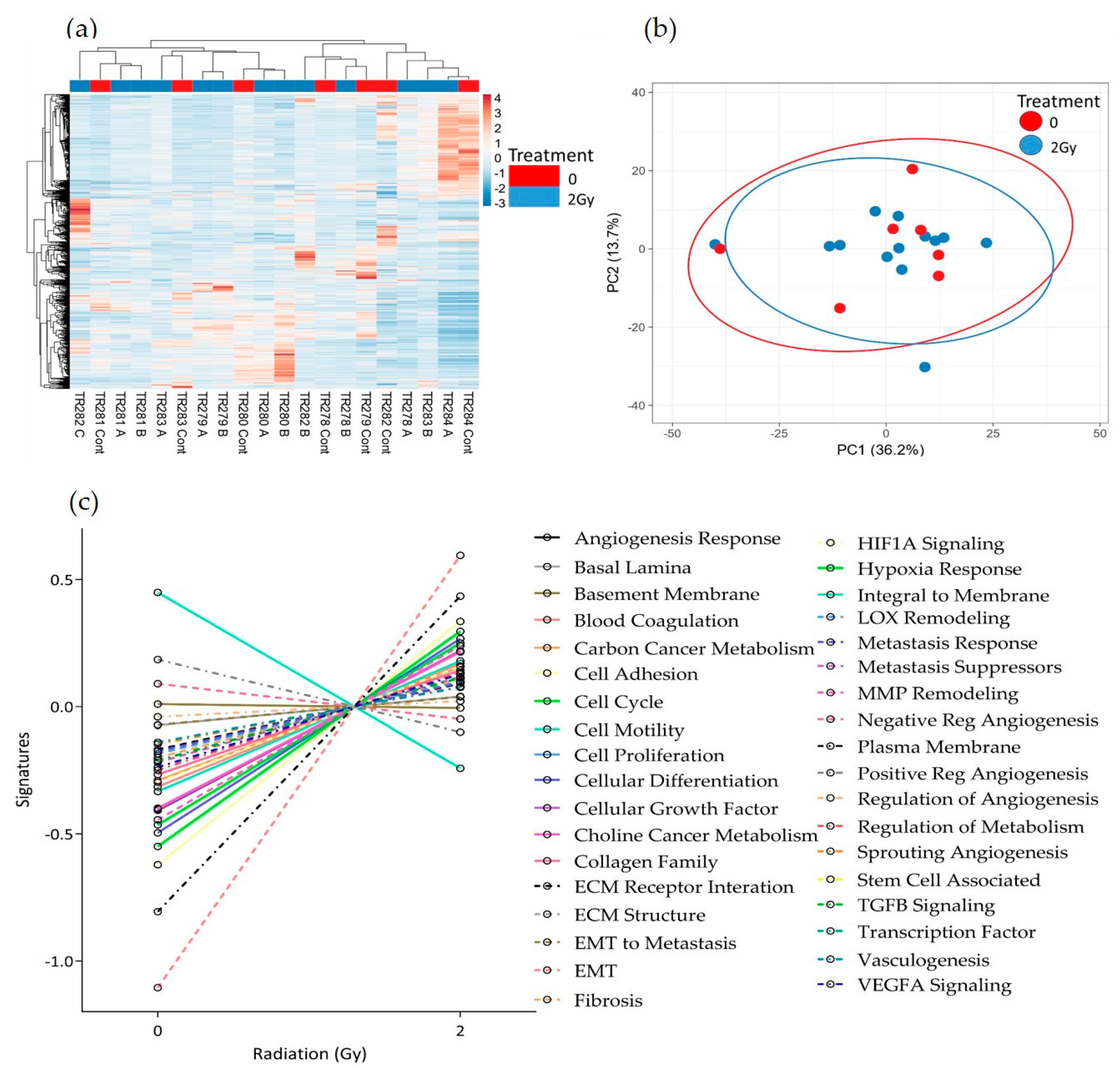
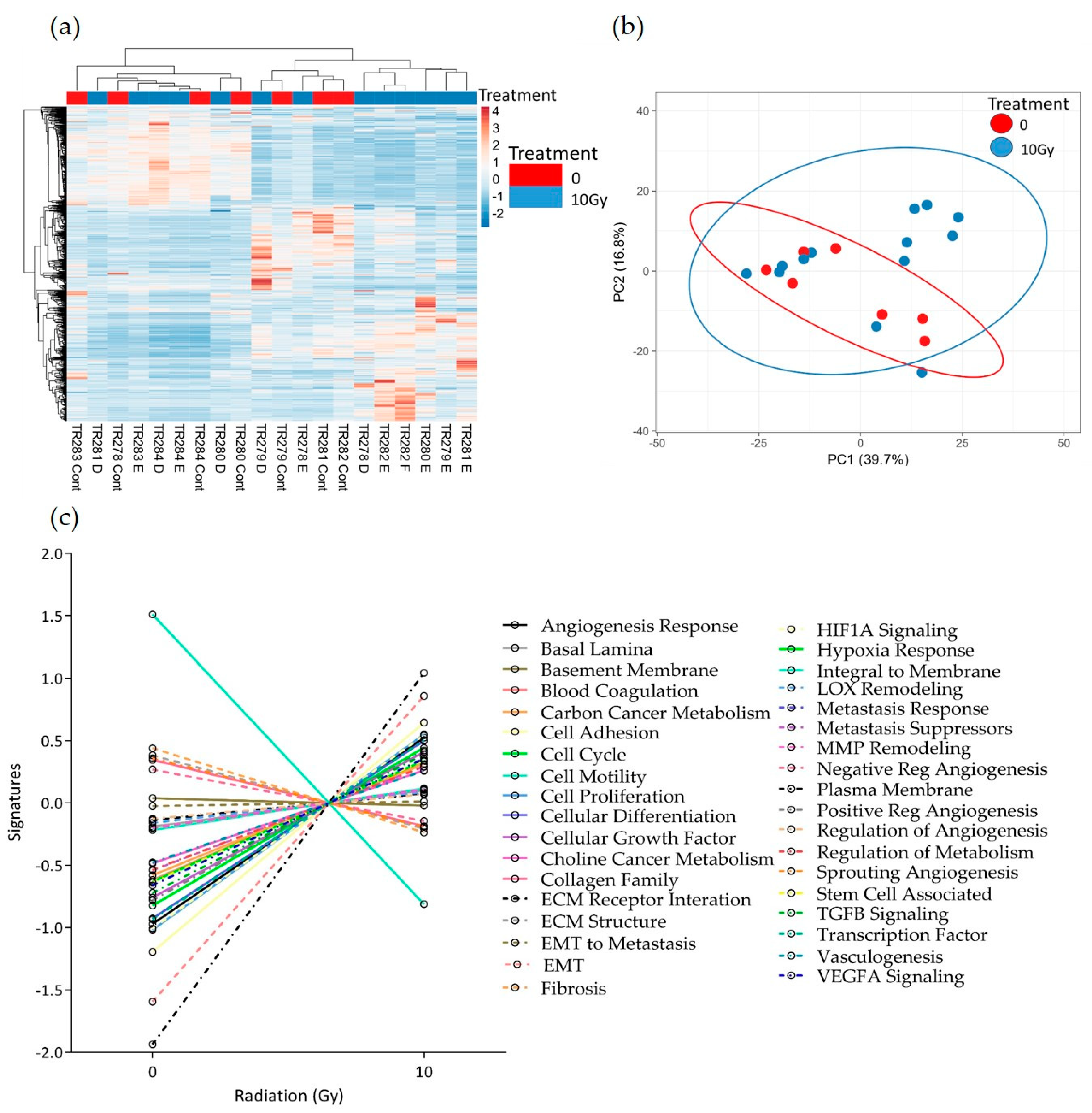
3.2. The Effect of Irradiation on Tissue-on-Chip Gene Expression in Ex Vivo HNSCC Tissue
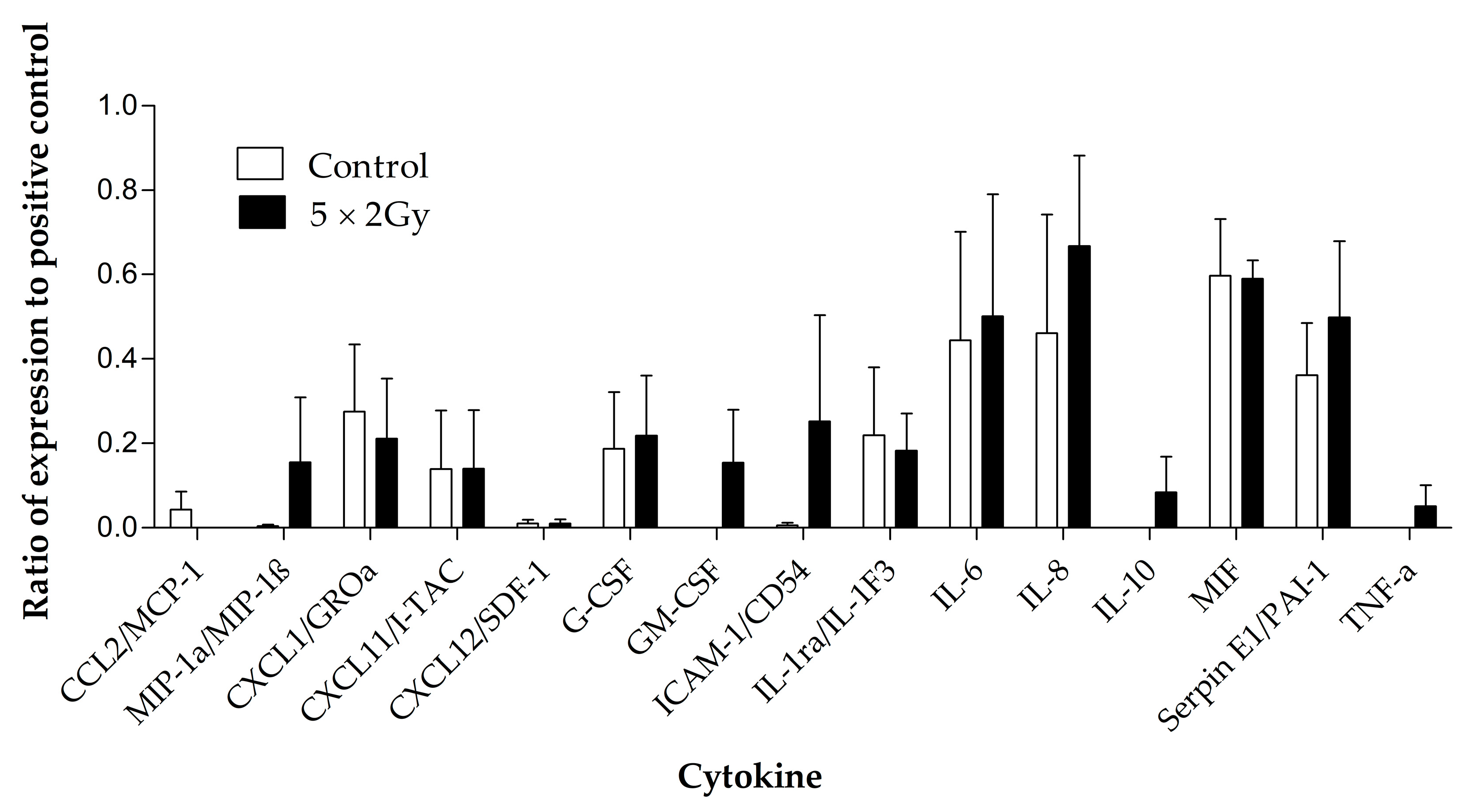
3.3. The Effect of Irradiation on Cytokine Release from HNSCC Tissue Maintained on the Perfusion Device
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Head and Neck Cancers Incidence. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers#heading-Zero (accessed on 29 March 2023).
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.H.; Fischman, V.G.; Wasserman, I.; Siu, J.; Shrime, M.G.; Fagan, J.J.; Koch, W.; Alkire, B.C. Global Burden of Head and Neck Cancer: Economic Consequences, Health, and the Role of Surgery. Otolaryngol. Head. Neck Surg. 2020, 162, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Sun, X.; Chen, Z.; Du, J.; Wu, Y. Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines. Int. J. Pept. Res. Ther. 2022, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef]
- Goel, B.; Tiwari, A.K.; Pandey, R.K.; Singh, A.P.; Kumar, S.; Sinha, A.; Jain, S.K.; Khattri, A. Therapeutic approaches for the treatment of head and neck squamous cell carcinoma-An update on clinical trials. Transl. Oncol. 2022, 21, 101426. [Google Scholar] [CrossRef]
- Corradini, S.; Alongi, F.; Andratschke, N.; Belka, C.; Boldrini, L.; Cellini, F.; Debus, J.; Guckenberger, M.; Horner-Rieber, J.; Lagerwaard, F.J.; et al. MR-guidance in clinical reality: Current treatment challenges and future perspectives. Radiat. Oncol. 2019, 14, 92. [Google Scholar] [CrossRef]
- Machiels, J.P.; Rene Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Hutchinson, M.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef]
- Volman, Y.; Hefetz, R.; Galun, E.; Rachmilewitz, J. DNA damage alters EGFR signaling and reprograms cellular response via Mre-11. Sci. Rep. 2022, 12, 5760. [Google Scholar] [CrossRef]
- Kong, X.; Yu, D.; Wang, Z.; Li, S. Relationship between p53 status and the bioeffect of ionizing radiation (Review). Oncol. Lett. 2021, 22, 661–668. [Google Scholar] [CrossRef]
- You, G.R.; Cheng, A.J.; Lee, L.Y.; Huang, Y.C.; Liu, H.; Chen, Y.J.; Chang, J.T. Prognostic signature associated with radioresistance in head and neck cancer via transcriptomic and bioinformatic analyses. BMC Cancer 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kang, J.W.; Noh, J.K.; Jung, H.R.; Lee, Y.C.; Lee, J.W.; Kong, M.; Eun, Y.G. Gene signature for prediction of radiosensitivity in human papillomavirus-negative head and neck squamous cell carcinoma. Radiat. Oncol. J. 2020, 38, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D cancer models: One step closer to in vitro human studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef] [PubMed]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.; Green, V.L.; Kuvshinova, E.; Kuvshinov, D.; Karsai, L.; Crank, S.T.; Stafford, N.D.; Greenman, J. Maintenance of head and neck tumor on-chip: Gateway to personalized treatment? Future Sci. OA 2017, 3, FSO174. [Google Scholar] [CrossRef]
- Cheah, R.; Srivastava, R.; Stafford, N.D.; Beavis, A.W.; Green, V.; Greenman, J. Measuring the response of human head and neck squamous cell carcinoma to irradiation in a microfluidic model allowing customized therapy. Int. J. Oncol. 2017, 51, 1227–1238. [Google Scholar] [CrossRef]
- Olubajo, F.; Achawal, S.; Greenman, J. Development of a Microfluidic Culture Paradigm for Ex Vivo Maintenance of Human Glioblastoma Tissue: A New Glioblastoma Model? Transl. Oncol. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Foster, H.; Wade, M.; England, J.; Greenman, J.; Green, V. Isolation and characterisation of graves’ disease-specific extracellular vesicles from tissue maintained on a bespoke microfluidic device. Organs-on-a-Chip 2021, 3, 100011. [Google Scholar] [CrossRef]
- Cheah, L.T.; Dou, Y.H.; Seymour, A.M.; Dyer, C.E.; Haswell, S.J.; Wadhawan, J.D.; Greenman, J. Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab. Chip 2010, 10, 2720–2726. [Google Scholar] [CrossRef]
- Hattersley, S.M.; Greenman, J.; Haswell, S.J. Study of ethanol induced toxicity in liver explants using microfluidic devices. Biomed. Microdevices 2011, 13, 1005–1014. [Google Scholar] [CrossRef]
- Carr, S.D.; Green, V.L.; Stafford, N.D.; Greenman, J. Analysis of radiation-induced cell death in head and neck squamous cell carcinoma and rat liver maintained in microfluidic devices. Otolaryngol. Head. Neck Surg. 2014, 150, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.; Dyer, C.; Macfie, J.; Davies, J.; Karsai, L.; Greenman, J.; Jacobsen, M. A microfluidic chip based model for the study of full thickness human intestinal tissue using dual flow. Biomicrofluidics 2016, 10, 064101. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci. Rep. 2019, 9, 6327. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.; Green, V.; Cheah, R.; McKenzie, G.; Karsai, L.; England, J.; Greenman, J. A novel microfluidic device capable of maintaining functional thyroid carcinoma specimens ex vivo provides a new drug screening platform. BMC Cancer 2019, 19, 259. [Google Scholar] [CrossRef]
- Nakajima, T.; Murayama, Y.; Matsuzawa, T.; Koyano, A. Development of a new highly sensitive LiF thermoluminescence dosimeter and its applications. Nucl. Instrum. Methods 1978, 157, 155–162. [Google Scholar] [CrossRef]
- Horowitz, Y.S.; Moscovitch, M. Highlights and pitfalls of 20 years of application of computerised glow curve analysis to thermoluminescence research and dosimetry. Radiat. Prot. Dosimetry 2013, 153, 1–22. [Google Scholar] [CrossRef]
- Moscovitch, M. Personnel Dosimetry Using LiF:Mg, Cu, P. Radiat. Prot. Dosimetry 1999, 85, 49–56. [Google Scholar] [CrossRef]
- Velbeck, K.J.; Luo, L.Z.; Ramlo, M.J.; Rotunda, J.E. The dose-response of Harshaw TLD-700H. Radiat. Prot. Dosimetry 2006, 119, 255–258. [Google Scholar] [CrossRef]
- Wood, T.J.; Moore, C.S.; Saunderson, J.R.; Beavis, A.W. Validation of a technique for estimating organ doses for kilovoltage cone-beam CT of the prostate using the PCXMC 2.0 patient dose calculator. J. Radiol. Prot. 2015, 35, 153–163. [Google Scholar] [CrossRef]
- Veldman-Jones, M.H.; Brant, R.; Rooney, C.; Geh, C.; Emery, H.; Harbron, C.G.; Wappett, M.; Sharpe, A.; Dymond, M.; Barrett, J.C.; et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015, 75, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, H.; Imtiaz, S.; Noor, T.; Siddiqui, Y.H.; Sajjad, A.; Zia, M. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Anim. Model. Exp. Med. 2021, 4, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Miserocchi, G.; Cocchi, C.; De Vita, A.; Liverani, C.; Spadazzi, C.; Calpona, S.; Di Menna, G.; Bassi, M.; Meccariello, G.; De Luca, G.; et al. Three-dimensional collagen-based scaffold model to study the microenvironment and drug-resistance mechanisms of oropharyngeal squamous cell carcinomas. Cancer Biol. Med. 2021, 18, 502–516. [Google Scholar] [CrossRef]
- Engelmann, L.; Thierauf, J.; Koerich Laureano, N.; Stark, H.J.; Prigge, E.S.; Horn, D.; Freier, K.; Grabe, N.; Rong, C.; Federspil, P.; et al. Organotypic Co-Cultures as a Novel 3D Model for Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2330. [Google Scholar] [CrossRef] [PubMed]
- Mattei, F.; Andreone, S.; Mencattini, A.; De Ninno, A.; Businaro, L.; Martinelli, E.; Schiavoni, G. Oncoimmunology Meets Organs-on-Chip. Front. Mol. Biosci. 2021, 8, 627454. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A. Human tumor xenografts: The good, the bad, and the ugly. Mol. Ther. 2012, 20, 882–884. [Google Scholar] [CrossRef]
- Hum, N.R.; Sebastian, A.; Gilmore, S.F.; He, W.; Martin, K.A.; Hinckley, A.; Dubbin, K.R.; Moya, M.L.; Wheeler, E.K.; Coleman, M.A.; et al. Comparative Molecular Analysis of Cancer Behavior Cultured In Vitro, In Vivo, and Ex Vivo. Cancers 2020, 12, 690. [Google Scholar] [CrossRef]
- Barry, A.; Samuel, S.F.; Hosni, I.; Moursi, A.; Feugere, L.; Sennett, C.J.; Deepak, S.; Achawal, S.; Rajaraman, C.; Iles, A.; et al. Investigating the effects of arginine methylation inhibitors on microdissected brain tumour biopsies maintained in a miniaturised perfusion system. Lab Chip 2023, 23, 2664–2682. [Google Scholar] [CrossRef]
- Peeney, D.; Liu, Y.; Lazaroff, C.; Gurung, S.; Stetler-Stevenson, W.G. Unravelling the distinct biological functions and potential therapeutic applications of TIMP2 in cancer. Carcinogenesis 2022, 43, 405–418. [Google Scholar] [CrossRef]
- Diehl, V.; Huber, L.S.; Trebicka, J.; Wygrecka, M.; Iozzo, R.V.; Schaefer, L. The Role of Decorin and Biglycan Signaling in Tumorigenesis. Front. Oncol. 2021, 11, 801801. [Google Scholar] [CrossRef]
- Liu, M.; Wang, W.; Piao, S.; Shen, Y.; Li, Z.; Ding, W.; Li, J.; Saiyin, W. Relationship of biglycan and decorin expression with clinicopathological features and prognosis in patients with oral squamous cell carcinoma. J. Oral Pathol. Med. 2023, 52, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Dong, Q.; Lu, Y.; Dong, K.; Wang, R.; Liang, Z. Lumican inhibits immune escape and carcinogenic pathways in colorectal adenocarcinoma. Aging 2021, 13, 4388–4408. [Google Scholar] [CrossRef] [PubMed]
- Thariny, E.; Smiline Girija, A.M.; Paramasivam, A.; Vijayashree Priyadharsini, J. Aberrations in SMAD family of genes among HNSCC patients. Bioinformation 2021, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mangone, F.; Walder, F.; Maistro, S.; Pasini, F.; Lehn, C.; Carvalho, M.; Brentani, M.; Snitcovsky, I.; Federico, M. Smad2 and Smad6 as predictors of overall survival in oral squamous cell carcinoma patients. Mol. Cancer 2010, 9, 106–115. [Google Scholar] [CrossRef]
- Zheng, L.; Guan, Z.; Xue, M. TGF-beta Signaling Pathway-Based Model to Predict the Subtype and Prognosis of Head and Neck Squamous Cell Carcinoma. Front. Genet. 2022, 13, 862860. [Google Scholar] [CrossRef]
- Ren, L.; Lou, Y.; Sun, M. The anti-tumor effects of evodiamine on oral squamous cell carcinoma (OSCC) through regulating advanced glycation end products (AGE)/receptor for advanced glycation end products (RAGE) pathway. Bioengineered 2021, 12, 5985–5995. [Google Scholar] [CrossRef]
- El-Far, A.H.; Sroga, G.; Jaouni, S.K.A.; Mousa, S.A. Role and Mechanisms of RAGE-Ligand Complexes and RAGE-Inhibitors in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef]
- Kidacki, M.; Lehman, H.; Warrick, J.; Stairs, D. Signaling Pathways Supporting Tumor Invasion in Head and Neck Squamous Cell Carcinoma. J. Clin. Exp. Pathol. 2015, 5, 227–234. [Google Scholar] [CrossRef]
- Denaro, N.; Solinas, C.; Garrone, O.; Cauchi, C.; Ruatta, F.; Wekking, D.; Abbona, A.; Paccagnella, M.; Merlano, M.C.; Lo Nigro, C. The Role of Cytokinome in the HNSCC Tumor Microenvironment: A Narrative Review and Our Experience. Diagnostics 2022, 12, 2880. [Google Scholar] [CrossRef]
- Xu, X.; Li, M.; Hu, J.; Chen, Z.; Yu, J.; Dong, Y.; Sun, C.; Han, J. Expression profile analysis identifies a two-gene signature for prediction of head and neck squamous cell carcinoma patient survival. J. Cancer Res. Ther. 2018, 14, 1525–1534. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, B.; Li, M.; Wang, Y.; Yang, H.; Du, K. Identification of potential core genes and pathways predicting pathogenesis in head and neck squamous cell carcinoma. Biosci. Rep. 2021, 41, BSR20204148. [Google Scholar] [CrossRef] [PubMed]
- Marquard, F.E.; Jucker, M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020, 172, 113729. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Xu, Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Blandino, G.; Di Agostino, S. MicroRNAs in head and neck squamous cell carcinoma: A possible challenge as biomarkers, determinants for the choice of therapy and targets for personalized molecular therapies. Transl. Cancer Res. 2021, 10, 3090–3110. [Google Scholar] [CrossRef]
- Chandler, C.; Liu, T.; Buckanovich, R.; Coffman, L.G. The double edge sword of fibrosis in cancer. Transl. Res. 2019, 209, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Michna, A.; Schotz, U.; Selmansberger, M.; Zitzelsberger, H.; Lauber, K.; Unger, K.; Hess, J. Transcriptomic analyses of the radiation response in head and neck squamous cell carcinoma subclones with different radiation sensitivity: Time-course gene expression profiles and gene association networks. Radiat. Oncol. 2016, 11, 94. [Google Scholar] [CrossRef]
- Eke, I.; Aryankalayil, M.J.; Bylicky, M.A.; Makinde, A.Y.; Liotta, L.; Calvert, V.; Petricoin, E.F.; Graves, E.E.; Coleman, C.N. Radiotherapy alters expression of molecular targets in prostate cancer in a fractionation- and time-dependent manner. Sci. Rep. 2022, 12, 3500. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, A.; Kim, Y.; Yang, G.; Ahmed, K.; Caudell, J. Alterations in genetic pathways following radiotherapy for head and neck cancer. Head. Neck 2020, 42, 312–320. [Google Scholar] [CrossRef]
- Millen, R.; De Kort, W.W.B.; Koomen, M.; van Son, G.J.F.; Gobits, R.; Penning de Vries, B.; Begthel, H.; Zandvliet, M.; Doornaert, P.; Raaijmakers, C.P.J.; et al. Patient-derived head and neck cancer organoids allow treatment stratification and serve as a tool for biomarker validation and identification. Med 2023, 4, 290–310.e12. [Google Scholar] [CrossRef]
- Wahba, A.; Lehman, S.L.; Tofilon, P.J. Radiation-induced translational control of gene expression. Translation 2017, 5, e1265703. [Google Scholar] [CrossRef]
- Suzuki, S.; Toyoma, S.; Kawasaki, Y.; Yamada, T. Irradiated fibroblasts increase interleukin-6 expression and induce migration of head and neck squamous cell carcinoma. PLoS ONE 2022, 17, e0262549. [Google Scholar] [CrossRef] [PubMed]


| Tumour Site | Tumour Stage | Age | Gender |
|---|---|---|---|
| Left posterior Tongue | T4N2b | 72 | M |
| FOM | T4N2b | 79 | F |
| FOM, mandible, Tongue | T4aN2c | 60 | F |
| Tongue | T3N0 | 57 | F |
| FOM | T2N0 | 70 | M |
| Retromolar region | T4N0 | 84 | F |
| Lateral Tongue | T2N0 | 60 | F |
| Cytokine | Detection | Cytokine | Detection |
|---|---|---|---|
| CCL1/I-309 | ND | IL-4 | ND |
| CCL2/MCP-1 | C | IL-5 | ND |
| MIP-1α/MIP-1β | B | IL-6 | B |
| CCL5/RANTES | ND | IL-8 | B |
| CD40 Ligand/TNFSF5 | ND | IL-10 | T |
| Complement Component C5/C5a | ND | 1L-12 p70 | ND |
| CXCL1/GROα | B | IL-13 | ND |
| CXCL10/ip-10 | ND | IL-16 | ND |
| CXCL11/I-TAC | B | IL-17A | ND |
| CXCL12/SDF-1 | B | IL-17E | ND |
| G-CSF | B | IL-18/IL-1F4 | ND |
| GM-CSF | T | IL-21 | ND |
| ICAM-1/CD54 | B | IL-27 | ND |
| IFN-γ | ND | IL-32a | ND |
| IL-1α/IL-1F1 | ND | MIF | B |
| IL-1β/IL1-F2 | ND | Serpin E1/PAI-1 | B |
| IL-1ra/IL-1F3 | B | TNF-α | T |
| IL-2 | ND | TREM-1 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Green, V.; Baldwin, L.; England, J.; Marshall, G.; Frost, L.; Moore, C.; Greenman, J. Head and Neck Squamous Cell Carcinoma Biopsies Maintained Ex Vivo on a Perfusion Device Show Gene Changes with Time and Clinically Relevant Doses of Irradiation. Cancers 2023, 15, 4575. https://doi.org/10.3390/cancers15184575
Green V, Baldwin L, England J, Marshall G, Frost L, Moore C, Greenman J. Head and Neck Squamous Cell Carcinoma Biopsies Maintained Ex Vivo on a Perfusion Device Show Gene Changes with Time and Clinically Relevant Doses of Irradiation. Cancers. 2023; 15(18):4575. https://doi.org/10.3390/cancers15184575
Chicago/Turabian StyleGreen, Victoria, Lydia Baldwin, James England, Gayle Marshall, Lucy Frost, Craig Moore, and John Greenman. 2023. "Head and Neck Squamous Cell Carcinoma Biopsies Maintained Ex Vivo on a Perfusion Device Show Gene Changes with Time and Clinically Relevant Doses of Irradiation" Cancers 15, no. 18: 4575. https://doi.org/10.3390/cancers15184575
APA StyleGreen, V., Baldwin, L., England, J., Marshall, G., Frost, L., Moore, C., & Greenman, J. (2023). Head and Neck Squamous Cell Carcinoma Biopsies Maintained Ex Vivo on a Perfusion Device Show Gene Changes with Time and Clinically Relevant Doses of Irradiation. Cancers, 15(18), 4575. https://doi.org/10.3390/cancers15184575






