Simple Summary
Metastatic colorectal cancer is common and complicated to treat. The first choice of treatment is guided by the genetics of the patients (RAS mutation status) and the location of their primary tumor. However, real-world information on what treatments are actually being used and the survival benefits of these patient-guided treatments in Spain is lacking, so we aimed to determine this. We found that, in general, treatment choice was guided by primary tumor location and mutation status, with mutation status being the more influential characteristic. In addition, non-mutated RAS or left-sided tumors were predictive of longer survival times. We hope the results of this study may serve as a useful benchmark for future studies and assist practicing oncologists in selecting appropriate treatments for their patients.
Abstract
The retrospective, observational RWD-ACROSS study analyzed disease characteristics, systemic treatment, and survival in patients with metastatic colorectal cancer (mCRC) in Spain. In total, 2002 patients were enrolled (mean age 65.3 years; 62.7% male). Overall median overall survival (OS) was 26.72 months, and was longer in patients with left-sided tumors (28.85 vs. 21.04 months (right-sided tumors); p < 0.0001) and in patients receiving first-line anti-epidermal growth factor receptor (EGFR) treatment (31.21 vs. 26.75 (anti-vascular endothelial growth factor (VEGF) treatment) and 24.45 months (chemotherapy); p = 0.002). Overall median progression-free survival (PFS) was 10.72 months and was longer in patients with left-sided tumors (11.24 vs. 9.31 months (right-sided tumors); p < 0.0001), and in patients receiving either first-line anti-EGFR or anti-VEGF (12.13 and 12.00 vs. 8.98 months (chemotherapy); p < 0.001). PFS was longer with anti-VEGF treatment in patients with right-sided tumors and wild-type RAS (11.24 vs. 8.78 (anti-EGFR) and 7.83 months (chemotherapy); p = 0.025). Both anti-EGFR and anti-VEGF produced longer PFS in patients with left-sided tumors and wild-type RAS than chemotherapy alone (12.39 and 13.14 vs. 9.83 months; p = 0.011). In patients with left-sided tumors and mutant RAS, anti-VEGF produced a longer PFS than chemotherapy alone (12.36 vs. 9.34 months; p = 0.001). In Spain, wild-type RAS or left-sided mCRC tumors are predictive of longer survival times.
1. Introduction
Colorectal cancer (CRC) is the most common type of cancer in Spain, with 42,721 new CRC diagnoses estimated for 2023 [1]. Approximately 25% of patients with CRC have metastatic CRC (mCRC) at diagnosis and 20–30% of patients diagnosed with stages I–III develop metastases during follow-up. Overall, approximately 45% of patients with CRC will be treated for metastatic disease at some point in their lives [2].
Clinical trials in the late 1990s established the central role of oxaliplatin- and irinotecan-based regimens in the first-line treatment of mCRC [3,4,5,6]. Subsequent trials demonstrated the ability of adjunctive targeted therapies, such as bevacizumab, cetuximab, and panitumumab, to further improve survival outcomes [7,8,9,10,11]. Since 2010, new cytotoxic agents (e.g., trifluridine/tipiracil (TAS-102) [12]) and targeted therapies (e.g., aflibercept [13], ramucirumab [14], and regorafenib [15]) have expanded the range of available treatment options, particularly in second and later lines of therapy. Additionally, pembrolizumab, an antibody that blocks programmed cell death protein 1 (PD-1) on the surface of lymphocytes, has emerged as a highly effective option for patients with microsatellite instability (MSI)-high tumors with mismatch-repair deficiency [16].
Since 2008, evidence has accumulated showing that mutations in the KRAS (exons 1–4), NRAS (exons 1–4), and BRAF genes are predictive of poor response to anti-epidermal growth factor receptor (EGFR) therapy in patients with mCRC [17]. In patients with wild-type RAS, four studies have directly compared anti-EGFR with anti-vascular endothelial growth factor (VEGF) therapy (both in combination with chemotherapy) as first-line therapy for mCRC (PEAK, FIRE-3, and CALGB/SWOG 80405, as reviewed by Mitchell and Sanoff [18], and the PARADIGM study [19]). A meta-analysis of the first three of these trials found both types of treatments to be associated with prolonged overall survival (OS) and progression-free survival (PFS) and higher overall response rates (ORR) in patients with left-sided versus right-sided primary tumors [20]. Furthermore, the effect of the tumor side on treatment outcomes was larger for anti-EGFR-based treatment than for anti-VEGF-based treatment [20]. In particular, PARADIGM showed a clear efficacy advantage for anti-EGFR in left-sided tumors [19].
As a result of these studies, the choice of systemic first-line treatment for patients with mCRC is now guided by RAS mutation status and primary tumor location [21,22]: cetuximab and panitumumab are preferred for left-sided, wild-type RAS tumors, whereas bevacizumab is preferred for right-sided tumors and RAS-mutated tumors regardless of their location. However, despite the prognostic and predictive importance of RAS mutation status and primary tumor location, treatment is sometimes initiated without this information.
Indeed, real-world data on mCRC are notably lacking in Spain. Population-based registries provide high-level data on mCRC incidence and mortality; however, no national databases provide detailed insights into patient and disease characteristics, treatment choices, and outcomes in oncology. Thus, our knowledge of primary tumor location, RAS mutation status, MSI status, and the type and duration of targeted therapy administered (overall and by the line of therapy) is incomplete. Additionally, we do not know the effectiveness of systemic treatments for mCRC in real-world clinical practice in Spain, nor do we know the optimal treatment sequence to maximize quality-of-life adjusted survival for different patient groups.
To address this, we undertook a retrospective analysis to gather and analyze comprehensive data on disease characteristics, systemic treatment, and survival in patients with mCRC across ten hospitals in Spain.
2. Materials and Methods
RWD-ACROSS was a retrospective, observational study of patients with mCRC conducted across ten specialist oncology centers. Participating centers were selected from eight of the 17 autonomous communities in Spain, covering 71.4% of the population. Collaborating centers were selected in this way to reduce the effect of potential biases resulting from differences in pharmacological approvals between regional and local health authorities, and to lessen the impact of variations in practice between centers.
2.1. Inclusion and Exclusion Criteria
Each collaborating center contributed all patients in their registry, up to a maximum of 250 patients, who received a diagnosis of mCRC during the study period (January 2011–December 2015) and met the inclusion criteria. Patients had to have histologically confirmed mCRC, be aged ≥18 years at the time of diagnosis, and have received ≥1 cycle of chemotherapy for metastatic disease. Previous resection of a primary tumor and subsequent adjuvant treatment was permitted. Additionally, patients needed to have a complete set of medical records for ≥1 year, including and following primary diagnosis of mCRC.
Patients were excluded if they had a synchronous or metachronous malignant tumor diagnosed during the study period, which, in the investigator’s opinion, could have affected their prognosis. Additional exclusion criteria included the administration of any mCRC treatment or resection of metastasis with R0 before January 2011, with or without chemotherapy. Previous adjuvant treatment following primary tumor resection was permitted for patients with metachronous metastatic disease.
2.2. Outcomes
The main objective of the study was to determine OS and PFS for the entire study cohort, and for patients with known prognostic or predictive factors. In the latter analyses, OS and PFS were estimated according to primary tumor location (right-sided vs. left-sided), RAS mutation status (wild-type vs. mutated), therapy type (chemotherapy alone or in combination with an anti-VEGR/anti-EGFR agent), line of therapy, and first-/second-line treatment sequence. Additional objectives were to characterize the patient population in terms of demographic and clinical variables, and to analyze treatment by line of therapy.
All patient data were obtained from medical records and included age, sex, dates of primary tumor and metastatic disease diagnosis, and date of death or last follow-up. Tumor characteristics included location (right-sided or left-sided), the number and location of metastases, RAS and BRAF mutation status, and MSI status. ‘Right-sided’ was defined as primary disease in the caecum, ascending colon, and transverse colon, and ‘left-sided’ included tumors from the splenic flexure to the rectum, inclusive. Data on treatment, including resection of the primary tumor and metastases, were also collected. Where applicable, details of administered chemotherapy and targeted therapies, alongside their dates of administration, were extracted for each of the first three lines of treatment; the initiation dates of any fourth-line treatments were also extracted.
The protocol was approved by the Ethics Committee for Drug Research at each participating hospital prior to study commencement (approval number ACR-QUI-2018-01).
2.3. Statistical Analyses
Patients were included in the analysis set if: (i) they met the inclusion criteria; (ii) the date of mCRC diagnosis was known; and (iii) the patient’s status at the date of last contact was known.
All continuous variables were summarized using the following descriptive statistics: number (n; based on the number of recorded values for each parameter), mean, standard deviation (SD), 95% confidence interval (95% CI), median, interquartile range (IQR), maximum and minimum. The number and percentage of observed values (with the denominator being based on the number of recorded values for each parameter) were reported for all categorical variables.
OS was defined as the time from mCRC diagnosis to the date of death (any cause); survival curves were plotted using the Kaplan–Meier method. Patients without an event (e.g., due to loss to follow-up) were censored at the date they were last known to be alive. PFS was also summarized using the Kaplan–Meier method. First-line PFS was measured from the start of first-line treatment to either death (any cause) or the start of second-line treatment. Patients without an event were censored at the date they were last known to be alive and not receiving second-line treatment. Similarly, second- and third-line PFS was measured from the start of second- or third-line treatment, respectively, to death (any cause) or the initiation of third- or fourth-line treatment, respectively. Patients without an event were censored at the date they were last known to be alive and not receiving any subsequent-line treatment.
Univariate analysis was performed to identify factors predictive of OS or PFS. The variables included age (≤70 vs. >70 years), sex, clinical characteristics (primary tumor location; RAS mutation status), number of metastatic sites at diagnosis, and treatment (surgical resection of the primary tumor or metastases; inclusion of biologic therapy in the first-line regimen). The log-rank test was used. Cox regression was used in subsequent multivariate analysis to identify interactions between variables that were significantly associated with OS or PFS. Backward selection was used to identify factors with independent prognostic value. Odds ratios (ORs) and corresponding 95% CIs were calculated.
All statistical analyses were performed using SAS® version 9.4 (SAS, Cary, NC, USA).
3. Results
3.1. Patient and Disease Characteristics
A total of 2024 patients were identified during the screening phase. Of these, 2002 patients received ≥1 line of chemotherapy for mCRC during the study period (Table 1 and CONSORT [Consolidated Standards of Reporting Trials] flow diagram in Figure S1). As several hospitals included fewer than the stipulated maximum of 250 patients, a single hospital was allowed to include additional patients (n = 300).

Table 1.
Patient demographic and tumor characteristics by line of therapy.
Patients were primarily male (n = 1256; 62.7%) and, at the time of first-line therapy, had a mean age of 65.3 years. Most patients had exclusively left-sided primary tumors (n = 1444; 72.1%), almost three quarters of which (n = 1074; 74.4%) were located in the sigmoid colon or rectum (see Table S1 in the online supplement). Although fewer patients had exclusively right-sided primary tumors, the caecum was the third most common location overall, accounting for 10.0% of all primary and 37.5% of all right-sided primary tumors. RAS and BRAF mutation statuses were known in 1675 patients (83.7%) and unknown in 327 patients (16.3%). Among the former group, 821 patients (49.0%) had wild-type RAS and BRAF, 828 (49.4%) had mutated RAS, and 26 (1.6%) had mutated BRAF.
More than half of the patients (n = 1185/2002; 59.2%) had a single metastatic site, most frequently in the liver (n = 754/2002; 37.7%), lungs (n = 164/2002; 8.2%), or peritoneum (n = 152/2002; 7.6%). In the overall study population (n = 2002; i.e., patients with single or multiple metastatic sites), the liver was also the most frequently involved organ (n = 1404; 70.1%; Table 1).
3.2. Treatment
3.2.1. Overall
Systemic treatment for mCRC by line of therapy is shown in Table 2; all patients received chemotherapy as first-line therapy. Patients who received chemotherapy as second- and third-line treatment excluded those who died on first-line treatment and those who underwent surgery for metastasis removal. The proportion of patients who also received a targeted agent was approximately 55% in each line (56.4% in the first and second line, and 54.5% in the third line).

Table 2.
Systemic treatments in first, second and third line.
First-line chemotherapy was with an oxaliplatin-based regimen (e.g., FOLFOX, CAPOX) in 65.7% of patients and with an irinotecan-based regimen (e.g., FOLFIRI) in 20.1% of patients. Additionally, a small percentage of patients received first-line FOLFIRINOX (n = 41; 2.0%). The median duration of first-line chemotherapy was 5.47 months overall, 5.27 months for oxaliplatin-based therapy and 6.19 months for irinotecan-based therapy.
In the second line, most patients received an irinotecan-based regimen (n = 824; 60.1%) whilst, in the third line, 42.8% of patients received an irinotecan-containing regimen, 24.0% received an oxaliplatin-containing regimen, and 26.4% received fluoropyrimidine alone.
Among those receiving targeted therapy, the proportions who received an anti-VEGF agent (i.e., bevacizumab or aflibercept) as a part of first-, second-, and third-line treatment were 62.7%, 68.4%, and 53.0%, respectively (Table 2). Corresponding percentages for anti-EGFR therapy (i.e., cetuximab or panitumumab) were 36.8%, 30.5%, and 39.7%, respectively. Regorafenib was used in 0.5%, 1.0%, and 7.1% of patients in the first, second, and third line, respectively.
Bevacizumab and cetuximab were the most used anti-VEGF and anti-EGFR agents in all lines of therapy, respectively. Aflibercept was mostly used as a second-line treatment, while panitumumab was used in approximately 10% of patients in all three treatment lines (Table 2).
The median duration of treatment with a targeted agent was 5.90 months. Among individual agents, median treatment duration was longest for bevacizumab (6.19 vs. 5.83 months for cetuximab, 5.50 months for aflibercept, 4.83 months for panitumumab, and 3.98 months for regorafenib).
3.2.2. By Tumor Location and RAS Mutation Status
The data in this section refers only to patients with a known RAS mutation status (Table 1). Anti-EGFR agents were infrequently used in patients with mutant RAS (in <5% of patients as first line, and <2.5% of patients as second or third line), regardless of primary tumor location (Table 2).
Right-Sided Disease
Among patients with a right-sided primary tumor and wild-type RAS, anti-EGFR was the most frequently administered targeted therapy (>35% in all three treatment lines; Table 2). In those with right-sided disease and mutated RAS, anti-VEGF agents were administered to approximately half of the patients in first and second treatment lines, and to 35.9% in the third line. At each line of therapy, patients with right-sided tumors and RAS-mutated disease were more likely to receive chemotherapy only (than those with right-side tumors and wild-type RAS). Indeed, chemotherapy alone was the dominant third-line therapeutic strategy in this patient population.
Left-Sided Disease
In patients with a left-sided primary tumor and wild-type RAS, there was a preference for anti-EGFR therapy regardless of treatment line (Table 2). In this setting, anti-VEGF therapy was more likely to be used in second-line treatment. Approximately 30% of patients in all three lines received chemotherapy without a targeted agent.
Among those with left-sided disease and mutated RAS, anti-VEGF agents were used as first-line therapy in 52.3% of patients, as second-line therapy in 48.7%, and as third-line therapy in 43.1%. Conversely, the use of chemotherapy alone increased from 44.5% in the first line to 49.5% in the second and 55.1% in the third line.
3.3. Survival
3.3.1. Overall Survival
In the overall study cohort, median OS following first-line treatment was 26.72 months (95% CI: 25.37–28.07; Table 3, and Figure S2a in the online supplement). Median OS was significantly lower in patients with right-sided primary tumors than in those with left-sided tumors (21.04 vs. 28.85 months, respectively; p < 0.0001). When analyzed by RAS mutation status, median OS was 29.04 months (95% CI: 26.98–31.11) in patients with wild-type RAS and 27.54 months (95% CI: 25.57–29.50) in patients with mutated RAS.

Table 3.
Overall survival and progression-free survival for the study group as a whole, and by primary tumor location and RAS mutation status, following first-line treatment.
OS according to first-line treatment, primary tumor location, and RAS mutation status is shown in Table 4. Patients who received first-line treatment with an anti-EGFR agent had a median OS of 31.21 months (95% CI: 27.78–34.63), compared with 26.75 months (95% CI: 24.67–28.83) in those who received an anti-VEGF agent and 24.45 months (95% CI: 22.51–26.40) in those who received chemotherapy alone (p = 0.002 for group comparisons).

Table 4.
Overall survival in patients receiving first-line systemic treatment (n = 1675), according to location of primary tumor, RAS mutation status, and type of systemic treatment.
Choice of first-line therapy did not significantly affect median OS in patients with right-sided disease; the median OS was 22.13, 21.63, and 19.37 months with anti-VEGF, anti-EGFR, and chemotherapy alone, respectively (p = 0.526). In patients with left-sided disease, patients who received first-line treatment with an anti-EGFR agent had a median OS of 34.29 months (95% CI: 31.04–37.54), compared with 28.23 months (95% CI: 25.68–30.77) in those who received an anti-VEGF agent and 26.59 months (95% CI: 24.23–28.94) in those who received chemotherapy alone (p = 0.003 for group comparisons).
Median OS was not significantly affected by choice of first-line therapy in patients with either wild-type or mutated RAS. In patients with wild-type RAS, median OS was 26.62, 30.06, and 29.14 months with anti-VEGF, anti-EGFR, and chemotherapy alone, respectively (p = 0.963). In patients with mutant RAS, median OS was 28.85, 32.19, and 25.34 months, respectively (p = 0.140).
Choice of first-line therapy did not significantly affect median OS in patients with right-sided disease, and either wild-type or mutated RAS. In patients with a right-sided tumor and wild-type RAS, median OS was 18.62, 19.31, and 24.62 months with anti-VEGF, anti-EGFR, and chemotherapy alone, respectively (p = 0.201). In patients with mutant RAS, there was no significant difference in OS between those who received anti-VEGF therapy or chemotherapy alone (23.47 vs. 23.86 months; p = 0.907).
In patients with left-sided disease and wild-type RAS, median OS duration was 28.06, 33.86, and 30.26 months in patients who received first-line anti-VEGF therapy, anti-EGFR therapy, or chemotherapy only, respectively (p = 0.583). In those with mutant RAS, there was no significant difference in OS duration between patients who received anti-VEGF or chemotherapy only (p = 0.182).
Median OS duration following second- and third-line treatment was 14.03 months (95% CI: 13.08–14.98; Figure S2b in the online supplement) and 10.65 months (95% CI: 9.67–11.63; Figure S2c in the online supplement), respectively. Patients (n = 1428) who received second-line treatment had a median OS, from initiation of first-line treatment, of 29.21 months (95% CI: 27.89–29.52). Those who received third-line treatment (n = 725) had a median OS, from initiation of first-line treatment, of 35.14 months (95% CI: 33.42–36.87).
Median duration of OS according to first- and second-line treatment sequence is shown in Table S2 in the online supplement. In this analysis, in general, median OS was longest in patients who received a targeted (biologic) therapy in both treatment lines and shortest in patients who received only chemotherapy in either line.
Among patients who received biologic therapy in both lines, a first-line anti-EGFR agent with a second-line anti-VEGF agent was associated with a longer median OS compared with the reverse (31.93 vs. 25.41 months; Table S2). This pattern was also observed in patients with wild-type RAS regardless of their primary tumor location.
3.3.2. Progression-Free Survival
Overall median PFS after first-line therapy was 10.72 months (95% CI: 10.24–11.19; Figure S3a in the online supplement). Median PFS in patients with wild-type and mutated RAS was 11.24 months (95% CI: 10.53–11.95) and 10.78 months (95% CI: 10.04–11.53), respectively (Table 3). Median PFS was significantly longer in patients with a left-sided primary tumor compared with a right-sided primary tumor (11.24 vs. 9.31 months; p < 0.0001).
Median PFS was approximately 12 months in patients who received an anti-EGFR or an anti-VEGF agent as part of first-line therapy, and lower (8.98 months) in patients who received chemotherapy only (p < 0.001; Table 5).

Table 5.
Progression-free survival in patients receiving first-, second-, or third-line systemic treatment, according to location of primary tumor, RAS mutation status, and type of systemic treatment.
In patients with right-sided tumors, first-line anti-VEGF treatment was associated with a significantly longer median PFS than anti-EGFR therapy or chemotherapy alone (11.44 vs. 10.75 or 7.77 months, respectively; p = 0.002). In contrast, in patients with left-sided tumors, first-line anti-EGFR treatment was associated with a significantly longer median PFS than either anti-VEGF therapy or chemotherapy alone (13.21 vs. 12.39 or 9.50 months; p < 0.0001).
Choice of first-line treatment had no effect on PFS duration in patients with wild-type RAS; however, in patients with mutated RAS, median PFS with first-line treatment with an anti-VEGF agent was 12.36 months (95% CI: 11.33–13.38), compared with 12.03 months (95% CI: 8.34–15.72) in those who received an anti-EGFR agent and 8.98 months (95% CI: 8.37–9.59) in those who received chemotherapy alone (p < 0.0001 for group comparisons).
First-line anti-VEGF treatment was associated with a significantly longer median PFS than either anti-EGFR therapy or chemotherapy alone in patients with right-sided disease and wild-type RAS (p = 0.025). In patients with right-sided disease and mutated RAS, anti-VEGF was associated with a longer median PFS than chemotherapy only but the difference was not statistically significant (p = 0.056). In patients with left-sided disease and wild-type RAS, there was little difference in median PFS between anti-VEGF and anti-EGFR therapies. Both were associated with a longer median PFS than with chemotherapy alone (12.39 and 13.14 months vs. 9.83 months, respectively; p = 0.011). Anti-VEGF therapy was associated with a significantly longer median PFS than chemotherapy alone in those with left-sided disease and mutated RAS (12.36 vs. 9.34 months, respectively; p = 0.001).
Median PFS times for second- and third-line treatment were 7.63 months (95% CI: 7.25–8.02; Figure S3b in the online supplement) and 6.16 months (95% CI: 5.65–6.67; Figure S3c in the online supplement), respectively. Further analysis of PFS following second- and third-line treatment is shown in Table 5.
3.4. Univariate and Multivariate Analyses
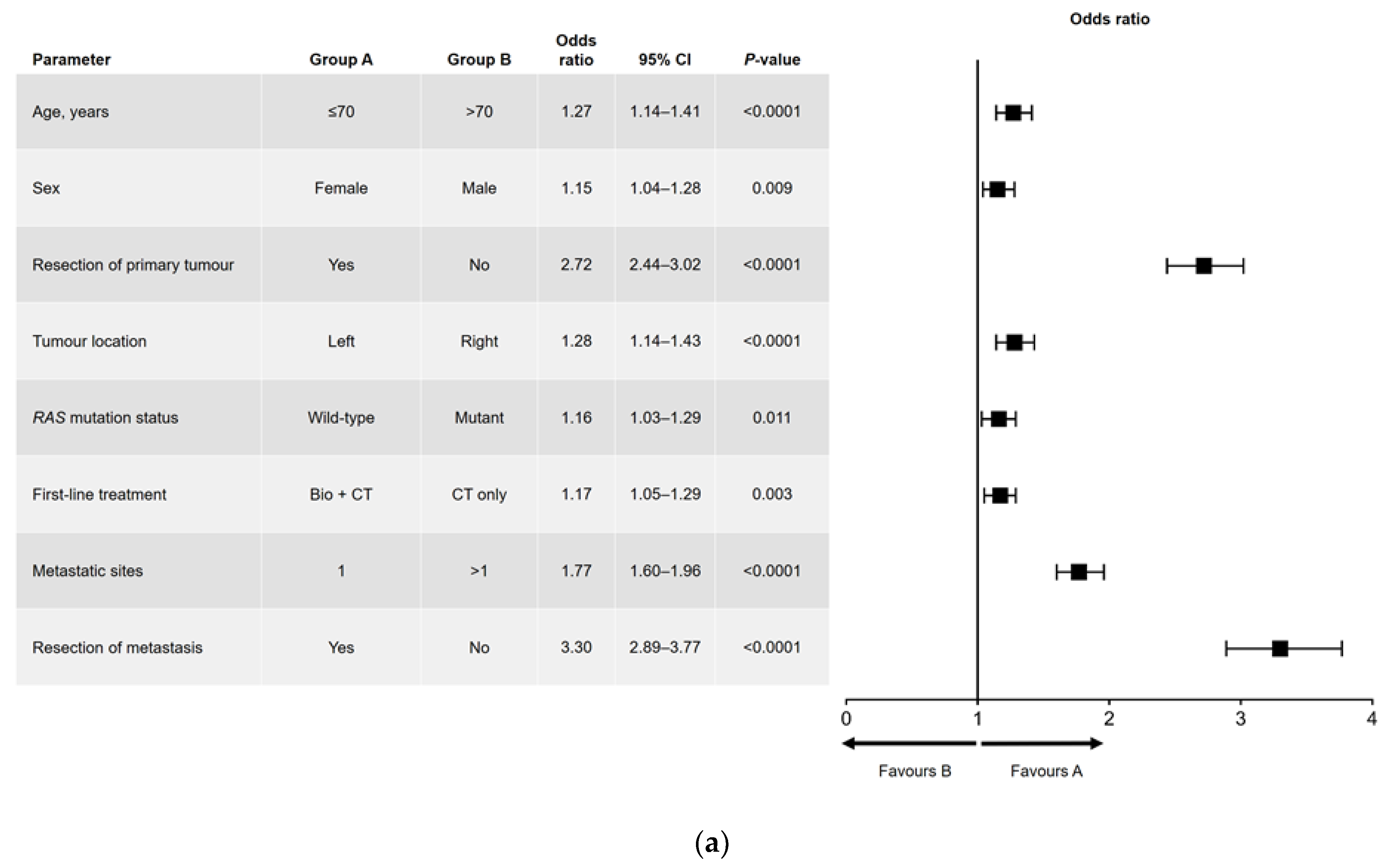
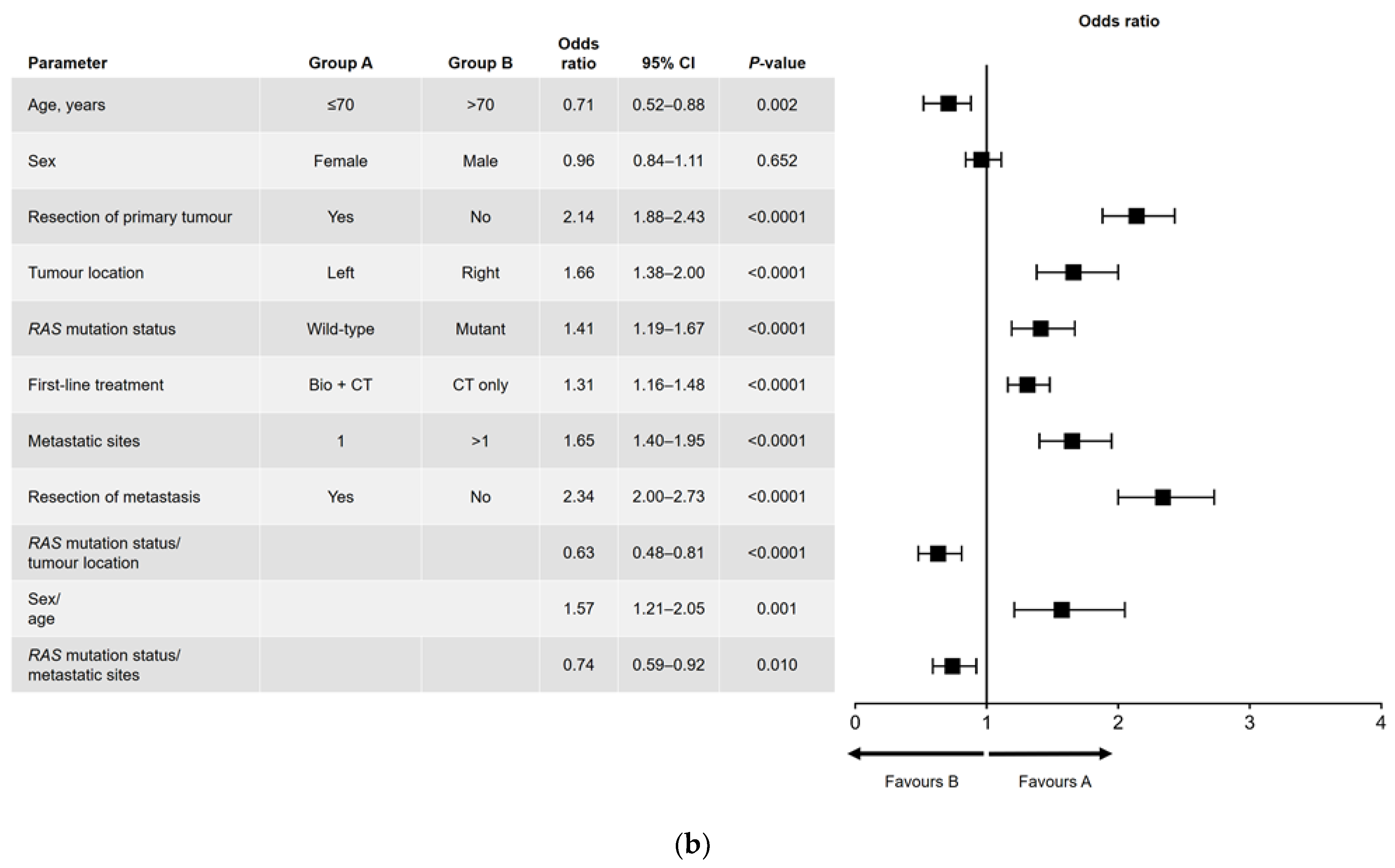
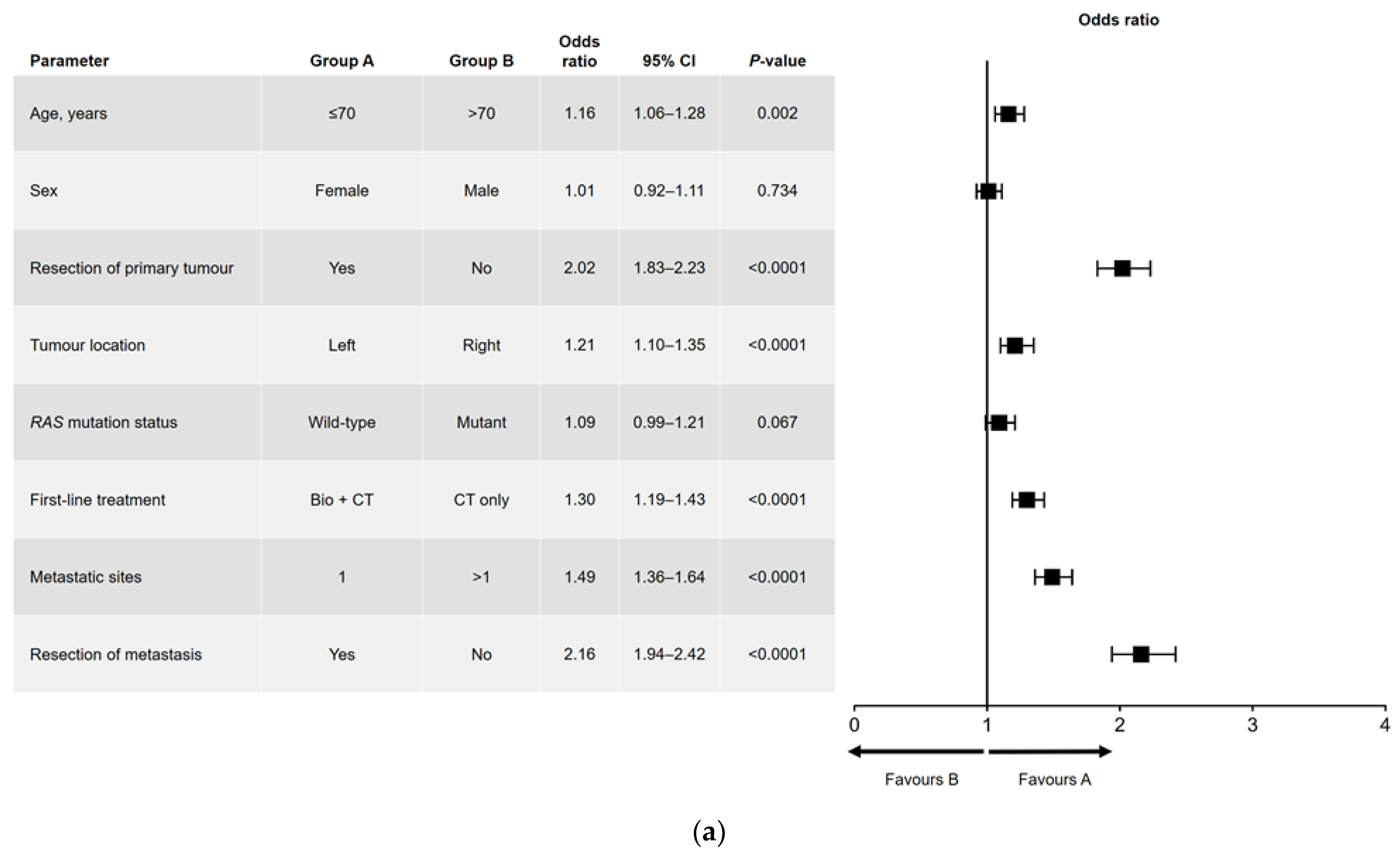
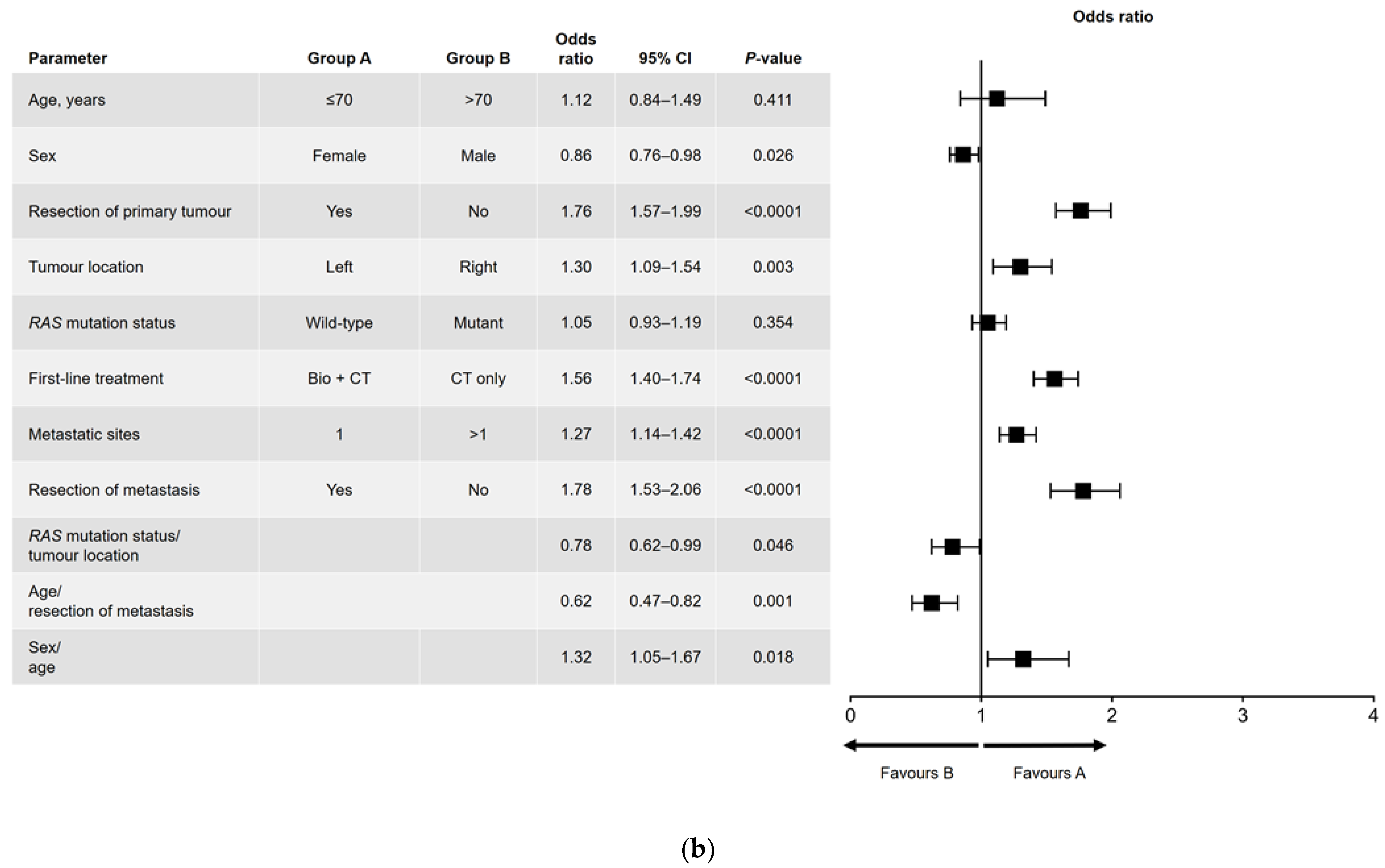
Results of the univariate and multivariate analyses are shown in Figure 1 (OS) and Figure 2 (PFS). In the univariate analysis, a significant association was found for all variables analyzed for OS (Figure 1a) and PFS, except for sex and RAS mutation status (Figure 2a). Among factors with a significant association, metastatic surgery was most strongly associated with a positive outcome, closely followed in prognostic importance by resection of the primary tumor.
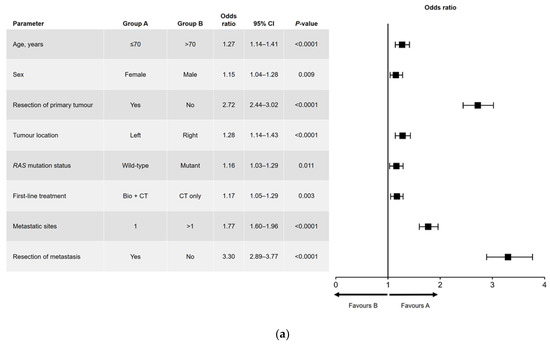
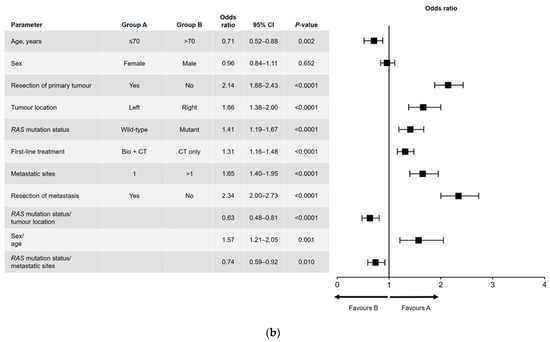
Figure 1.
(a) Univariate and (b) multivariate analyses of overall survival. Bio, biologic therapy; CI, confidence interval; CT, chemotherapy.
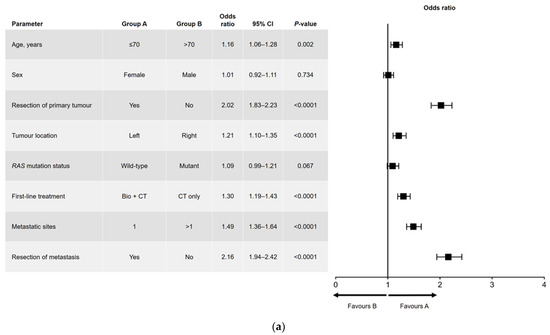
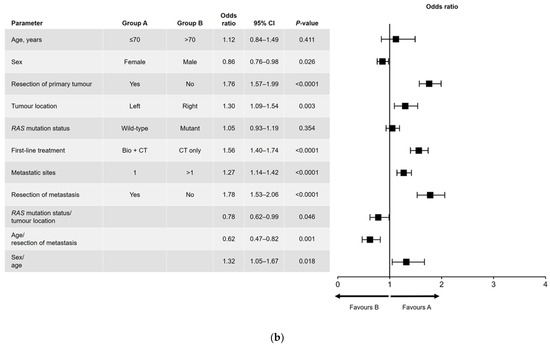
Figure 2.
(a) Univariate and (b) multivariate analyses of progression-free survival. Bio, biologic therapy; CI, confidence interval; CT, chemotherapy.
In the multivariate analysis of OS (Figure 1b), no significant difference in outcome was found between men and women. The main interactions between variables were for sex and age (better OS for women aged ≤70 years), RAS mutation status and primary tumor location (better OS in patients with left-sided, RAS wild-type tumors), and RAS mutation status and number of metastatic sites at diagnosis (better OS in patients with RAS wild-type disease and a single metastatic site).
In the multivariate analysis of PFS (Figure 2b), age and RAS mutation status were not independently prognostic. The interactions that were significant for PFS were sex and age (i.e., better PFS in women aged ≤70 years), age and metastatic surgery (i.e., better PFS in patients aged ≤70 years who had undergone metastatic resection), and RAS mutation status and primary tumor location (i.e., better PFS in patients with a left-sided primary tumor and wild-type RAS).
4. Discussion
In this paper, we present detailed findings of RWD-ACROSS, the first multicenter study of real-world survival outcomes following first-, second-, and third-line systemic treatment for mCRC in Spain. We analyzed data from over 2000 patients diagnosed with mCRC between 2011 and 2015. The male:female ratio was approximately 3:2 and the mean age was 65.3 years. There was a predominance of left-sided tumors (approximately 3:1) and an equal balance between RAS wild-type and mutated tumors. Most patients had a single metastatic site, mainly in the liver; nearly two-thirds had undergone resection of the primary tumor, whereas only one in four had received a metastasectomy. Median OS and PFS were 26.72 and 10.72 months, respectively. As expected, median OS times were longer in patients with either left-sided or wild-type RAS tumors.
As per the inclusion criteria, all patients received fluoropyrimidine-based chemotherapy. Since 2004, when Tournigand and colleagues published the seminal GERCOR study results [23], patients have been treated with oxaliplatin- or irinotecan-based chemotherapy as first-line treatment, and for second-line treatment switched to the drug not administered in the first line, as illustrated in the following three studies. A US study of patients with mCRC treated between 2004 and 2011 found oxaliplatin was administered in the first line to 60.7% of patients and irinotecan to 11.2% of patients [24]. A Spanish study of patients with mCRC treated between 2016 and 2017 reported that 66.2% of patients received oxaliplatin-based chemotherapy and 18.7% received irinotecan-based chemotherapy as first-line treatment, followed respectively by 21.5% and 48.9% as second-line treatment [25]. Finally, the randomized CALGB 80,405 study, where the choice of first-line chemotherapy was not prespecified, found that 73.4% of patients received oxaliplatin-based chemotherapy and 26.6% received irinotecan-based chemotherapy as first-line treatment [26]. In our study, 65.7% of patients received oxaliplatin-based therapy and 20.1% received irinotecan-based therapy in the first line, and 27.3% and 60.1% in the second line, respectively, indicating our results are in line with standard practice in Western countries.
Approximately 43.0% of patients in our study did not receive a targeted agent in either the first or second treatment line. The proportions of patients receiving biologic drugs (the anti-VEGF bevacizumab, or anti-EGFR agents cetuximab and panitumumab) as first-line treatment of mCRC have varied subsequent to approval of the first biologic in 2004, in part due to restrictions introduced in 2007 regarding the use of anti-EGFR agents in patients with mutant RAS and due to the outcomes of comparative studies of these biologics. In the above-mentioned 2004–2011 US study, biologics were added to the first-line regimen in 55% of patients (51% anti-VEGF and 4% anti-EGFR) [24]. When the analysis was limited to patients treated from 2009 onwards, the percentage of patients treated with an anti-EGFR agent rose to 34%. Similarly, in the abovementioned 2016–2017 Spanish study, biologics were added to chemotherapy as first-line treatment for 61.4% of patients (mainly anti-VEGF (38.8% of patients) and anti-EGFR agents (22%)) [25]. Another European study, by Kafatos and colleagues, of first-line treatment in 2018 of patients with mCRC for whom RAS and BRAF status was known, reported that 81.0% of patients with wild-type RAS tumors received a biologic agent (anti-EGFR + chemotherapy 62.6% of patients; anti-VEGF + chemotherapy 18.4% of patients), whereas 19.0% received chemotherapy alone [27]. A biologic agent was received by 61.9% of patients with mutated RAS tumors [27]. In addition to intercountry variation, Kafatos and colleagues’ study reflects prescribing decisions made in 2018, several years after our RWD-ACROSS study was conducted. In our study, 56.4% of patients received a biologic agent (anti-VEGF 62.1% or anti-EGFR 36.8%) as first-line treatment. Given that our study included patients diagnosed between 2011 and 2015, our results reflect an increase in the use of biologics in the first line of treatment. However, our results suggest we are still far from offering biologic treatment to 100% of patients as recommended by European clinical guidelines [2]. Although we did not specifically investigate the reasons for initial and subsequent treatment choices, our data raise the possibility that biologic agents may have been under-utilized in Spain during the study period. It should be noted, however, that our study population showed a broad range of clinical characteristics, and included patients with Eastern Cooperative Oncology Group performance status (ECOG-PS) scores of 2–3 who were ineligible for biologic combination therapy and received only fluoropyrimidine monotherapy, which may help explain our lower-than-expected use of biologics.
Anti-VEGF agents were used more frequently than anti-EGFR agents, regardless of treatment line. However, anti-EGFR use was more common in patients with wild-type RAS but rare in patients with mutated RAS. Because wild-type and mutant RAS occurred with approximately equal frequency, the net effect was greater use of anti-VEGF agents overall. The most commonly used anti-VEGF and anti-EGFR agents were bevacizumab and cetuximab, respectively, although variations were seen in the use of targeted agents across treatment lines. Bevacizumab use decreased at each successive treatment line, while aflibercept was most commonly used in the second line and regorafenib was almost exclusively used in the third line.
Subsequent to 2017 when the aforementioned meta-analysis by Arnold and colleagues was published [20], the results of which were later confirmed by the PARADIGM study [19], patients with a wild-type RAS mCRC tumor have generally been treated with a combination of chemotherapy and an anti-EGFR agent. Analysis of the Kafatos et al. study, which was conducted prior to the PARADIGM study, shows that the use of anti-EGFR treatment for this population group did not exceed 63% [27]. In our study 45% of patients with a primary tumor (in the left colon) that was wild-type for RAS received anti-EGFR combination therapy; this rate probably reflects our patient enrolment period (up to 2015), which was 1 year prior to publication of the Arnold et al. meta-analysis and 7 years prior to the PARADIGM study. Our results showed that primary tumor location had less influence than RAS mutation status on the choice of targeted therapy. Regardless of tumor location, approximately half of the patients with mutated RAS received chemotherapy only; the remaining half received chemotherapy plus anti-VEGF in the first and second treatment lines. In the third line, however, chemotherapy only was the dominant strategy in RAS-mutated patients irrespective of tumor-sidedness.
Meta-analyses of randomized controlled trials have shown that biologic agents significantly improve survival outcomes when added to standard chemotherapy regimens in mCRC [28,29]. Consistent with this, we found that median OS and PFS were significantly longer in patients who received first-line treatment with a biologic agent than in those who received chemotherapy only. First-line treatment with anti-EGFR therapy was associated with a median OS of 31.21 months and median PFS of 12.00 months, while corresponding values for first-line anti-VEGF therapy were 26.75 and 12.13 months, respectively. In contrast, patients who received chemotherapy only had a median OS of 24.45 months and a median PFS of 8.98 months.
We also examined the effect of treatment sequencing on survival. Patients who received biologic therapy in both the first and second lines of treatment had numerically longer median OS than those who received chemotherapy only in both lines (31.74 vs. 25.54 months, respectively) or only one line of biologic therapy. The optimal order for using anti-VEGF and anti-EGFR agents in patients with wild-type RAS is unknown. A previous study of 490 patients found no significant difference in median OS between patients who received an anti-VEGF agent in the first line and an anti-EGFR agent in the second line, compared with the reverse sequence (31.4 vs. 31.8 months, respectively) [30]. In contrast, we observed numerically longer median OS in patients who received anti-EGFR first and anti-VEGF second (31.93 months), compared with the reverse sequence (25.41 months). Neither study can definitively describe the optimal order of biologic therapy use, although the 2017 meta-analysis by Arnold and colleagues [20] suggested that the best therapeutic strategy in patients with wild-type RAS with a left-sided tumor would be to start with a combination of chemotherapy plus anti-EGFR therapy followed by second-line chemotherapy plus an anti-VEGF agent. Further studies investigating this are warranted.
In patients with mutated RAS, longer median OS was observed when anti-VEGF treatment was used in both the first and second treatment lines than when chemotherapy alone was used in both. Interestingly, however, in the same subset of patients, there was little evidence from individual treatment lines that anti-VEGF therapy was associated with significant improvements in OS or PFS, compared with chemotherapy alone. Anti-VEGF therapy significantly improved PFS, relative to chemotherapy only, in first-line treatment, but not in the second or third lines; in addition, no OS benefit was observed in the first line.
Regarding primary tumor location, median OS and PFS were consistently longer in patients with left-sided versus right-sided primary disease, regardless of treatment type.
In the univariate analyses, we found that younger age (≤70 years), left-sided disease, a solitary metastatic site, and the inclusion of a biologic agent in first-line therapy were all significantly associated with improved OS and PFS. Additionally, wild-type RAS mutational status and female sex were found to be predictive of OS but not PFS. In comparison, surgical resection—of either the primary tumor or metastases—was more strongly predictive of both OS and PFS. A favorable association between metastasectomy and prolonged OS in mCRC has been described previously [31]. It is unclear whether this effect is due to the surgery itself or due to factors associated with surgical candidacy.
In the multivariate analyses, the only interaction that was significantly associated with both OS and PFS was between age and sex, with women aged ≤70 years having favorable outcomes.
The key strength of our study lies in its depth and breadth. To the best of our knowledge, no other real-world studies of mCRC conducted since the introduction of targeted therapy describe the interactions between patient and tumor characteristics, systemic treatment patterns, and survival outcomes. We have also not identified any studies in which survival outcomes were simultaneously analyzed by treatment line, primary tumor location, RAS mutational status, treatment type (targeted + chemotherapy vs. chemotherapy alone), and treatment sequence. Many real-world studies of systemic treatment for mCRC have investigated outcomes with specific drugs in a single line of therapy or in a specific, defined patient population [32,33,34,35,36,37,38,39,40,41,42]. Relative to these studies, our work has a broader scope and, thus, greater potential utility, providing survival outcomes for the more comprehensive range of patients and scenarios that oncologists are likely to encounter daily. Additionally, by including centers in different autonomous regions, we reduced the impact of local and regional variations in treatment availability and practice. Therefore, our findings are both representative of and relevant to clinical practice in Spain. Finally, the large sample size ( > 2000 patients) allows a degree of confidence in the results overall and in those of the primary subgroup analyses. However, stratification by two or more variables inevitably reduces sample size, which should be considered when interpreting the findings.
In general, the findings from retrospective observational studies should be interpreted with caution because of potential confounding factors and continually evolving clinical practice. Our study only included patients treated at Spanish hospitals between 2011 and 2015. Thus, while the data may be helpful for benchmarking purposes, their applicability to other settings and healthcare systems is likely to be limited.
5. Conclusions
The RWD-ACROSS study provides detailed real-world data on patient and tumor characteristics, treatment patterns, and survival among patients receiving systemic treatment for mCRC in Spain. Its findings may serve as a useful benchmark for future studies of real-world outcomes in mCRC and assist practicing oncologists in selecting appropriate treatments for their patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15184603/s1, Table S1: Specific primary tumor locations; Table S2: Median overall survival (OS) according to the sequence of first- and second-line therapies in 1428 patients who received second-line therapy; Figure S1: CONSORT flow chart describing mCRC patients screened in the RWD-ACROSS study by line of therapy and mutation status; Figure S2: Kaplan–Meier estimate of overall survival probability following (a) first-line, (b) second-line, and (c) third-line systemic treatment; Figures S3: Kaplan–Meier estimate of progression-free survival probability following (a) first-line, (b) second-line, and (c) third-line systemic treatment.
Author Contributions
Conceptualization: C.P., J.C.O. and L.C.; Methodology: C.P. and L.C.; Investigation: C.P. and L.C.; Formal analysis: C.P., J.C.O. and L.C.; Writing—original draft preparation: C.P.; Writing—review and editing: all authors. All authors approved the final version for publication and agree to be accountable for the work presented. All authors have read and agreed to the published version of the manuscript.
Funding
This research: editorial assistance for the preparation of this article and APC for the publication of this article was funded by Merck, S.L., Spain, an affiliate of Merck KGaA, Darmstadt, Germany.
Institutional Review Board Statement
The protocol was approved by the Ethics Committee for Drug Research at each participating hospital prior to study commencement (approval number ACR-QUI-2018-01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated/analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to acknowledge the editorial assistance of Richard Crampton and Arneak Kooner of Springer Healthcare Communications in the preparation of the manuscript, and Kate Palmer of Springer Healthcare Communications for assistance with post-submission revisions. This assistance was funded by Merck Serono, Spain.
Conflicts of Interest
Medical writing assistance for the preparation of this article was funded by Merck, S.L., Spain. The sponsor was not involved in the writing of the manuscript or in the decision to publish the results. The authors received no honoraria related to the development of this publication. C.P. reports consultancies from Merck, Roche, Amgen, and MSD; paid expert testimony from Merck, Amgen, and Servier; and grants/other funding from Merck Genzyme. A.F.M. reports payments/honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events from Servier, Lilly, Sanofi, AstraZeneca, and MSD; and participation on a data safety monitoring board or advisory board for MSD, BMS, and Bayer. V.A.O. reports paid expert testimony for Amgen, Hoffmann-La Roche, Merck-Serono, Servier, Sanofi, Genzyme, Ipsen Pharma, Novartis, and Adacap; and travel, accommodation, and expenses from Hoffmann-La Roche, Merck-Serono, Novartis, Ipsen Pharma, and Sanofi Genzyme. I.M.D., E.A.M., N.R.S., E.T., D.C.L., R.M.R.A., E.F., J.C.O., and L.C. have no conflicts of interest or financial disclosures to report.
References
- Red Española de Registros de Cáncer (REDECAN). Estimaciones de la Incidencia del Cáncer en España. 2023. Available online: https://redecan.org/storage/documents/02d62122-9adb-4d35-b6d0-551435dbe4ae.pdf (accessed on 19 January 2023). (In Spanish).
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and Fluorouracil with or without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Rougier, P.; Van Cutsem, E.; Bajetta, E.; Niederle, N.; Possinger, K.; Labianca, R.; Navarro, M.; Morant, R.; Bleiberg, H.; Wils, J.; et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 1998, 352, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. Irinotecan Study Group N. Engl. J. Med. 2000, 343, 905–914. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Donea, S.; Ludwig, H.; Schuch, G.; Stroh, C.; et al. Fluorouracil, Leucovorin, and Oxaliplatin with and without Cetuximab in the First-Line Treatment of Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 663–671. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Randomized, Phase III Trial of Panitumumab with Infusional Fluorouracil, Leucovorin, and Oxaliplatin (FOLFOX4) Versus FOLFOX4 Alone as First-Line Treatment in Patients with Previously Untreated Metastatic Colorectal Cancer: The PRIME Study. J. Clin. Oncol. 2010, 28, 4697–4705. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Saltz, L.B.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy as First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Hitre, E.; Zaluski, J.; Chien, C.-R.C.; Makhson, A.; D’Haens, G.; Pintér, T.; Lim, R.; Bodoky, G.; et al. Cetuximab and Chemotherapy as Initial Treatment for Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 1408–1417. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated with an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Köhne, C.-H.; Láng, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Mitchell, A.P.; Sanoff, H.K. Anti-EGFR and Anti-VEGF Agents in First-Line Therapy for Advanced Colorectal Cancer. Curr. Color. Cancer Rep. 2017, 13, 257–263. [Google Scholar] [CrossRef]
- Yoshino, T.; Watanabe, J.; Shitara, K.; Yasui, H.; Ohori, H.; Shiozawa, M.; Yamazaki, K.; Oki, E.; Sato, T.; Naitoh, T.; et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J. Clin. Oncol. 2022, 40, LBA1. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.-Y.; Peeters, M.; Lenz, H.-J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.-P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; van Krieken, J.H.; Aderka, D.; Aguilar, E.A.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; André, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI Followed by FOLFOX6 or the Reverse Sequence in Advanced Colorectal Cancer: A Randomized GERCOR Study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Abrams, T.A.; Meyer, G.; Schrag, D.; Meyerhardt, J.A.; Moloney, J.; Fuchs, C.S. Chemotherapy Usage Patterns in a US-Wide Cohort of Patients With Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2014, 106, djt371. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; Polo, E.; Camps, C.; Carrato, A.; Díaz-Rubio, E.; Guillem, V.; López, R.; Antón, A. Treatment patterns for metastatic colorectal cancer in Spain. Clin. Transl. Oncol. 2020, 22, 1455–1462. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A randomized clinical trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Kafatos, G.; Banks, V.; Burdon, P.; Neasham, D.; Lowe, K.A.; Anger, C.; Manuguid, F.; Trojan, J. Impact of biomarkers and primary tumor location on the metastatic colorectal cancer first-line treatment landscape in five European countries. Futur. Oncol. 2021, 17, 1495–1505. [Google Scholar] [CrossRef]
- Kumachev, A.; Yan, M.; Berry, S.; Ko, Y.-J.; Martinez, M.C.R.; Shah, K.; Chan, K.K.W. A Systematic Review and Network Meta-Analysis of Biologic Agents in the First Line Setting for Advanced Colorectal Cancer. PLoS ONE 2015, 10, e0140187. [Google Scholar] [CrossRef]
- Segelov, E.; Chan, D.; Shapiro, J.; Price, T.J.; Karapetis, C.S.; Tebbutt, N.C.; Pavlakis, N. The role of biological therapy in metastatic colorectal cancer after first-line treatment: A meta-analysis of randomised trials. Br. J. Cancer 2014, 111, 1122–1131. [Google Scholar] [CrossRef]
- Buchler, T.; Chloupkova, R.; Poprach, A.; Fiala, O.; Kiss, I.; Kopeckova, K.; Dušek, L.; Veskrnova, V.; Slavicek, L.; Kohoutek, M.; et al. Sequential therapy with bevacizumab and EGFR inhibitors for metastatic colorectal carcinoma: A national registry-based analysis. Cancer Manag. Res. 2019, 11, 359–368. [Google Scholar] [CrossRef]
- Oweira, H.; Mehrabi, A.; Reissfelder, C.; Abdel-Rahman, O. A Real-World, Population-Based Analysis of the Outcomes of Colorectal Cancer Patients with Isolated Synchronous Liver or Lung Metastases Treated with Metastasectomy. World J. Surg. 2020, 44, 1604–1611. [Google Scholar] [CrossRef]
- Aggarwal, H.; Sheffield, K.M.; Li, L.; Lenis, D.; Sorg, R.; Barzi, A.; Miksad, R. Primary tumor location and survival in colorectal cancer: A retrospective cohort study. World J. Gastrointest. Oncol. 2020, 12, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.H.; Hong, Y.S.; Lee, J.S.; Lee, K.-W.; Han, H.S.; Kim, S.Y.; Kim, H.K.; Kim, J.W.; Eun, C.K.; Kim, T.W.; et al. Effectiveness of Combining Bevacizumab with First-Line Chemotherapy Regimens for Metastatic Colorectal Cancer in Real-World Practice. Clin. Color. Cancer 2021, 20, 101–112.e106. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Fakih, M.; García-Alfonso, P.; Linke, Z.; Casado, A.R.; Marques, E.P.; Picard, P.; Celanovic, M.; Cartwright, T. Safety and Effectiveness of Aflibercept + Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) for the Treatment of Patients with Metastatic Colorectal Cancer (mCRC) in Current Clinical Practice: OZONE Study. Cancers 2020, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.; Barni, S.; Tagliabue, G.; Ricci, P.; Mazzucco, W.; Tumino, R.; Caputo, A.; Corrao, G. Effectiveness of First-Line Bevacizumab in Metastatic Colorectal Cancer: The Observational Cohort Study GRETA. Oncologist 2019, 24, 358–365. [Google Scholar] [CrossRef]
- Franchi, M.; Garau, D.; Kirchmayer, U.; Di Martino, M.; Romero, M.; De Carlo, I.; Scondotto, S.; Corrao, G. Effectiveness and Costs Associated to Adding Cetuximab or Bevacizumab to Chemotherapy as Initial Treatment in Metastatic Colorectal Cancer: Results from the Observational FABIO Project. Cancers 2020, 12, 839. [Google Scholar] [CrossRef]
- Khakoo, S.; Chau, I.; Pedley, I.; Ellis, R.; Steward, W.; Harrison, M.; Baijal, S.; Tahir, S.; Ross, P.; Raouf, S.; et al. ACORN: Observational Study of Bevacizumab in Combination with First-Line Chemotherapy for Treatment of Metastatic Colorectal Cancer in the UK. Clin. Color. Cancer 2019, 18, 280–291.e285. [Google Scholar] [CrossRef]
- Parikh, R.C.; Du, X.L.; Morgan, R.O.; Lairson, D.R. Patterns of Treatment Sequences in Chemotherapy and Targeted Biologics for Metastatic Colorectal Cancer: Findings from a Large Community-Based Cohort of Elderly Patients. Drugs—Real World Outcomes 2016, 3, 69–82. [Google Scholar] [CrossRef]
- Parisi, A.; Cortellini, A.; Cannita, K.; Venditti, O.; Camarda, F.; Calegari, M.A.; Salvatore, L.; Tortora, G.; Rossini, D.; Germani, M.M.; et al. Evaluation of Second-Line Anti-VEGF after First-Line Anti-EGFR Based Therapy in RAS Wild-Type Metastatic Colorectal Cancer: The Multicenter “SLAVE” Study. Cancers 2020, 12, 1259. [Google Scholar] [CrossRef]
- Pinto, C.; Di Fabio, F.; Rosati, G.; Lolli, I.R.; Ruggeri, E.M.; Ciuffreda, L.; Ferrari, D.; Re, G.L.; Rosti, G.; Tralongo, P.; et al. Observational study on quality of life, safety, and effectiveness of first-line cetuximab plus chemotherapy in KRAS wild-type metastatic colorectal cancer patients: The ObservEr Study. Cancer Med. 2016, 5, 3272–3281. [Google Scholar] [CrossRef]
- Shankaran, V.; Mummy, D.; Koepl, L.; Bansal, A.; Mirick, D.K.; Yu, E.; Morlock, R.; Ogale, S.; Ramsey, S.D. Survival and Lifetime Costs Associated with First-Line Bevacizumab Use in Older Patients with Metastatic Colorectal Cancer. Oncol. 2014, 19, 892–899. [Google Scholar] [CrossRef]
- Yamazaki, K.; Yuki, S.; Oki, E.; Sano, F.; Makishima, M.; Aoki, K.; Hamano, T.; Yamanaka, T. Real-World Evidence on Second-Line Treatment of Metastatic Colorectal Cancer Using Fluoropyrimidine, Irinotecan, and Angiogenesis Inhibitor. Clin. Color. Cancer 2021, 20, e173–e184. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).