The Era of Immunotherapy in Hepatocellular Carcinoma: The New Mission and Challenges of Magnetic Resonance Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
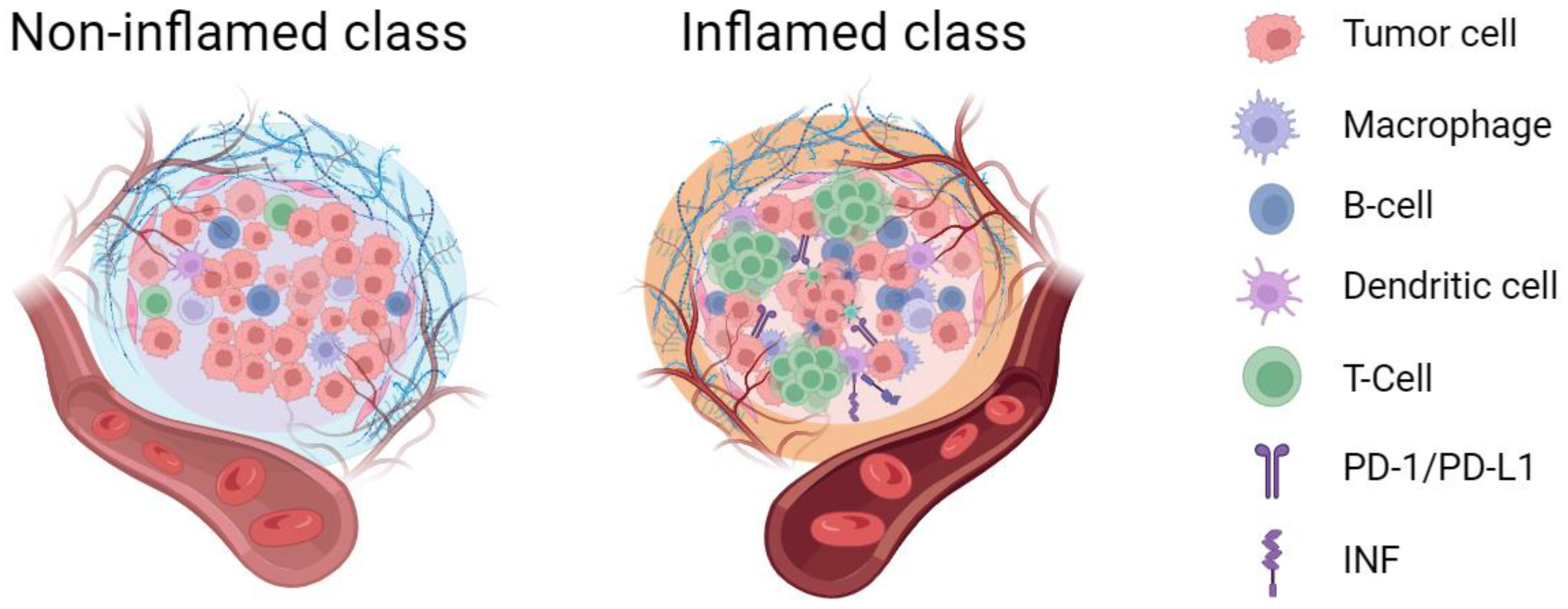
2. The Potential Clinical Significance of Tumor Immune Microenvironment Classification in Hepatocellular Carcinoma
3. The Challenges of Accurately Subtyping the Tumor Immune Microenvironment in Hepatocellular Carcinoma
4. The Current Status of Non-Invasive Evaluation of Tumor Immune Microenvironment Using Magnetic Resonance Imaging
5. Can MRI Accurately Predict the Efficacy of Immunotherapy in Hepatocellular Carcinoma before Treatment?
| Title | Authors | Journal | Date | Conclusions |
|---|---|---|---|---|
| Evaluating the Role of Hepatobiliary Phase of Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Predicting Treatment Impact of Lenvatinib and Atezolizumab plus Bevacizumab on Unresectable Hepatocellular Carcinoma | Sasaki R, Nagata K, Fukushima M et al. [49] | Cancers (Basel) | 2022 | The hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI was useful for predicting the therapeutic effect of atezolizumab plus bevacizumab therapy on unresectable HCC |
| Characteristics and Lenvatinib Treatment Response of Unresectable Hepatocellular Carcinoma with Iso-High Intensity in the Hepatobiliary Phase of EOB-MRI | Kubo A, Suda G, Kimura M et al. [56] | Cancers (Basel) | 2021 | The response to lenvatinib does not differ between HCC with and without iso-high intensity in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI. CTNNB-1 mutations are association with iso-high intensity in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI. |
| Higher Enhancement Intrahepatic Nodules on the Hepatobiliary Phase of Gd-EOB-DTPA-Enhanced MRI as a Poor Responsive Marker of Anti-PD-1/PD-L1 Monotherapy for Unresectable Hepatocellular Carcinoma | Aoki T, Nishida N, Ueshima K et al. [50] | Liver Cancer | 2021 | The intensity of the nodule on the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI is a promising imaging biomarker for predicting unfavorable response with anti-PD-1/PD-L1 monotherapy in patients with HCC |
| Gd-EOB-DTPA-MRI Could Predict WNT/β-Catenin Mutation and Resistance to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma | Kudo M. [48] | Liver Cancer | 2020 | The signal intensity on HBP of Gd-EOB-DTPA-enhanced MRI can be used to noninvasively predict the effect of ICI monotherapy |
6. Prospects of MRI in the Era of Hepatocellular Carcinoma Immunotherapy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Purcell, Y.; Copin, P.; Paulatto, L.; Pommier, R.; Vilgrain, V.; Ronot, M. Hepatocellular carcinoma surveillance: Eastern and Western perspectives. Ultrasonography 2019, 38, 191–199. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and immunotherapy of HCC: Spotlight on innate and innate-like immune cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Jácome, A.A.; Castro, A.C.G.; Vasconcelos, J.P.S.; Silva, M.; Lessa, M.A.O.; Moraes, E.D.; Andrade, A.C.; Lima, F.M.T.; Farias, J.P.F.; Gil, R.A.; et al. Efficacy and Safety Associated with Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: A Meta-analysis. JAMA Netw. Open 2021, 4, e2136128. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abbas, A.R.; de Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.H.; He, A.R.; Ryoo, B.Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.K.; Qin, S.; Tai, D.W.; Lim, H.Y.; Yau, T.; et al. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients with Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Wu, M.; Lau, W.Y.; Zheng, S.; Wang, X.H.; Chen, X.; Fan, J.; Dong, J.; Cai, J.; et al. Chinese Expert Consensus on Immunotherapy for Hepatocellular Carcinoma (2021 Edition). Liver Cancer 2022, 11, 511–526. [Google Scholar] [CrossRef]
- Jing, Y.; Yang, J.; Johnson, D.B.; Moslehi, J.J.; Han, L. Harnessing big data to characterize immune-related adverse events. Nat. Rev. Clin. Oncol. 2022, 19, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of immune microenvironment in hepatocellular carcinoma and its additional impact on histological and molecular classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-Catenin Activation Promotes Immune Escape and Resistance to Anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.K.; Castet, F.; Torres-Martin, M.; Andreu-Oller, C.; Puigvehí, M.; Miho, M.; Radu, P.; Dufour, J.F.; Verslype, C.; Zimpel, C.; et al. Molecular Markers of Response to Anti-PD1 Therapy in Advanced Hepatocellular Carcinoma. Gastroenterology 2023, 164, 72–88.e18. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Cheng, Z.; Liu, Z.; Zhang, L.; Jiang, K.; Geng, H.; Qian, R.; Wang, J.; Huang, X.; et al. Multi-region sequencing with spatial information enables accurate heterogeneity estimation and risk stratification in liver cancer. Genome Med. 2022, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; He, M.; Guo, Y.; Li, H.; Shen, S.; Xie, Y.; Li, X.; Xiao, H.; Fang, L.; Li, D.; et al. The Influence of Immune Heterogeneity on the Effectiveness of Immune Checkpoint Inhibitors in Multifocal Hepatocellular Carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4947–4957. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wang, D.; Qiu, X.; Luo, G.; Wu, T.; Yang, S.; Li, Z.; Zhu, Y.; Wang, S.; Wu, R.; et al. Trajectory and Functional Analysis of PD-1high CD4+CD8+ T Cells in Hepatocellular Carcinoma by Single-Cell Cytometry and Transcriptome Sequencing. Adv. Sci. 2020, 7, 2000224. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Guo, W.; Qiu, X.; Wang, S.; Sui, C.; Lian, Q.; Wu, J.; Shan, Y.; Yang, Z.; Yang, S.; et al. Comprehensive analysis of spatial architecture in primary liver cancer. Sci. Adv. 2021, 7, eabg3750. [Google Scholar] [CrossRef]
- Eng, C.L.; Lawson, M.; Zhu, Q.; Dries, R.; Koulena, N.; Takei, Y.; Yun, J.; Cronin, C.; Karp, C.; Yuan, G.C.; et al. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH+. Nature 2019, 568, 235–239. [Google Scholar] [CrossRef]
- Moncada, R.; Barkley, D.; Wagner, F.; Chiodin, M.; Devlin, J.C.; Baron, M.; Hajdu, C.H.; Simeone, D.M.; Yanai, I. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat. Biotechnol. 2020, 38, 333–342. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, J.; Wang, L.; Tano, V.; Tang, J.; Wang, X.; Wu, J.; Song, J.; Zhao, Y.; Rong, J.; et al. Topological analysis of hepatocellular carcinoma tumour microenvironment based on imaging mass cytometry reveals cellular neighbourhood regulated reversely by macrophages with different ontogeny. Gut 2022, 71, 1176–1191. [Google Scholar] [CrossRef]
- Sun, L.; Gao, F.; Gao, Z.; Ao, L.; Li, N.; Ma, S.; Jia, M.; Li, N.; Lu, P.; Sun, B.; et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J. Immunother. Cancer 2021, 9, e001875. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Y.; Wu, Y.; Wei, H.; Wei, Y.; Zhang, Z.; Duan, T.; Jiang, H.; Song, B. Preoperative prediction of glypican-3 positive expression in solitary hepatocellular carcinoma on gadoxetate-disodium enhanced magnetic resonance imaging. Front. Immunol. 2022, 13, 973153. [Google Scholar] [CrossRef]
- Ueno, A.; Masugi, Y.; Yamazaki, K.; Komuta, M.; Effendi, K.; Tanami, Y.; Tsujikawa, H.; Tanimoto, A.; Okuda, S.; Itano, O.; et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J. Hepatol. 2014, 61, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.G.; Carneiro, B.A.; Mota, J.M.; Costa, R.; Leite, C.A.; Barroso-Sousa, R.; Kaplan, J.B.; Chae, Y.K.; Giles, F.J. Wnt/beta-catenin pathway: Modulating anticancer immune response. J. Hematol. Oncol. 2017, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Mu, L.; Zhou, J.; Tang, W.; Zhang, L.; Xie, S.; Chen, J.; Wang, J. Imaging features of gadoxetic acid-enhanced MR imaging for evaluation of tumor-infiltrating CD8 cells and PD-L1 expression in hepatocellular carcinoma. Cancer Immunol. Immunother. CII 2022, 71, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhou, Q.; Huang, M.; Sun, K.; Wu, T.; Li, X.; Liao, B.; Chen, L.; Liao, J.; Peng, S.; et al. Nomogram development and validation to predict hepatocellular carcinoma tumor behavior by preoperative gadoxetic acid-enhanced MRI. Eur. Radiol. 2021, 31, 8615–8627. [Google Scholar] [CrossRef]
- Jiang, X.; Dudzinski, S.; Beckermann, K.E.; Young, K.; McKinley, E.; McIntyre, J.O.; Rathmell, J.C.; Xu, J.; Gore, J.C. MRI of tumor T cell infiltration in response to checkpoint inhibitor therapy. J. Immunother. Cancer 2020, 8, e000328. [Google Scholar] [CrossRef]
- Zhou, F.; Shang, W.; Yu, X.; Tian, J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med. Res. Rev. 2018, 38, 741–767. [Google Scholar] [CrossRef]
- Itoh, S.; Yoshizumi, T.; Kitamura, Y.; Yugawa, K.; Iseda, N.; Shimagaki, T.; Nagao, Y.; Toshima, T.; Harada, N.; Kohashi, K.; et al. Impact of Metabolic Activity in Hepatocellular Carcinoma: Association with Immune Status and Vascular Formation. Hepatol. Commun. 2021, 5, 1278–1289. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: A phase 1/2 trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Q.; Liu, N.; Tao, Y.Y.; Li, L.; Li, Z.M.; Yang, L.; Zhang, X.M. Radiomics models based on multisequence MRI for predicting PD-1/PD-L1 expression in hepatocellular carcinoma. Sci. Rep. 2023, 13, 7710. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhang, Z.; Chen, J.; Liao, M.; Xu, L.; Wu, Z.; Yuan, K.; Song, B.; Zeng, Y. Preoperative Radiomic Approach to Evaluate Tumor-Infiltrating CD8+ T Cells in Hepatocellular Carcinoma Patients Using Contrast-Enhanced Computed Tomography. Ann. Surg. Oncol. 2019, 26, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Chen, S.; Feng, S.; Wei, J.; Liu, F.; Li, B.; Li, X.; Hou, Y.; Gu, D.; Tang, M.; Xiao, H.; et al. Pretreatment prediction of immunoscore in hepatocellular cancer: A radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur. Radiol. 2019, 29, 4177–4187. [Google Scholar] [CrossRef]
- Hectors, S.J.; Lewis, S.; Besa, C.; King, M.J.; Said, D.; Putra, J.; Ward, S.; Higashi, T.; Thung, S.; Yao, S.; et al. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3759–3769. [Google Scholar] [CrossRef]
- Tao, Y.Y.; Shi, Y.; Gong, X.Q.; Li, L.; Li, Z.M.; Yang, L.; Zhang, X.M. Radiomic Analysis Based on Magnetic Resonance Imaging for Predicting PD-L2 Expression in Hepatocellular Carcinoma. Cancers 2023, 15, 365. [Google Scholar] [CrossRef]
- Kudo, M. Gd-EOB-DTPA-MRI Could Predict WNT/β-Catenin Mutation and Resistance to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Liver Cancer 2020, 9, 479–490. [Google Scholar] [CrossRef]
- Sasaki, R.; Nagata, K.; Fukushima, M.; Haraguchi, M.; Miuma, S.; Miyaaki, H.; Soyama, A.; Hidaka, M.; Eguchi, S.; Shigeno, M.; et al. Evaluating the Role of Hepatobiliary Phase of Gadoxetic Acid-Enhanced Magnetic Resonance Imaging in Predicting Treatment Impact of Lenvatinib and Atezolizumab plus Bevacizumab on Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 827. [Google Scholar] [CrossRef]
- Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; et al. Higher Enhancement Intrahepatic Nodules on the Hepatobiliary Phase of Gd-EOB-DTPA-Enhanced MRI as a Poor Responsive Marker of Anti-PD-1/PD-L1 Monotherapy for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 615–628. [Google Scholar] [CrossRef]
- Lin, Z.F.; Qin, L.X.; Chen, J.H. Biomarkers for response to immunotherapy in hepatobiliary malignancies. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2022, 21, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kurebayashi, Y.; Tsujikawa, H.; Sugimoto, K.; Yunaiyama, D.; Araki, Y.; Saito, K.; Takahashi, H.; Kakegawa, T.; Wada, T.; Tomita, Y.; et al. Tumor steatosis and glutamine synthetase expression in patients with advanced hepatocellular carcinoma receiving atezolizumab plus bevacizumab therapy. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Pinyol, R.; Sia, D.; Llovet, J.M. Immune Exclusion-Wnt/CTNNB1 Class Predicts Resistance to Immunotherapies in HCC. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2021–2023. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef]
- Kubo, A.; Suda, G.; Kimura, M.; Maehara, O.; Tokuchi, Y.; Kitagataya, T.; Ohara, M.; Yamada, R.; Shigesawa, T.; Suzuki, K.; et al. Characteristics and Lenvatinib Treatment Response of Unresectable Hepatocellular Carcinoma with Iso-High Intensity in the Hepatobiliary Phase of EOB-MRI. Cancers 2021, 13, 3633. [Google Scholar] [CrossRef]
- Liu, F.; Qin, L.; Liao, Z.; Song, J.; Yuan, C.; Liu, Y.; Wang, Y.; Xu, H.; Zhang, Q.; Pei, Y.; et al. Microenvironment characterization and multi-omics signatures related to prognosis and immunotherapy response of hepatocellular carcinoma. Exp. Hematol. Oncol. 2020, 9, 10. [Google Scholar] [CrossRef]
- Murai, H.; Kodama, T.; Maesaka, K.; Tange, S.; Motooka, D.; Suzuki, Y.; Shigematsu, Y.; Inamura, K.; Mise, Y.; Saiura, A.; et al. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology 2023, 77, 77–91. [Google Scholar] [CrossRef]
- Liu, Y.; Xun, Z.; Ma, K.; Liang, S.; Li, X.; Zhou, S.; Sun, L.; Liu, Y.; Du, Y.; Guo, X.; et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J. Hepatol. 2023, 78, 770–782. [Google Scholar] [CrossRef]
- Calderaro, J.; Seraphin, T.P.; Luedde, T.; Simon, T.G. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1348–1361. [Google Scholar] [CrossRef]
- Chen, B.; Garmire, L.; Calvisi, D.F.; Chua, M.S.; Kelley, R.K.; Chen, X. Harnessing big ‘omics’ data and AI for drug discovery in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 238–251. [Google Scholar] [CrossRef]
- Rajpurkar, P.; Lungren, M.P. The Current and Future State of AI Interpretation of Medical Images. N. Engl. J. Med. 2023, 388, 1981–1990. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, C.; Sheng, L.; Jiang, H.; Song, B. The Era of Immunotherapy in Hepatocellular Carcinoma: The New Mission and Challenges of Magnetic Resonance Imaging. Cancers 2023, 15, 4677. https://doi.org/10.3390/cancers15194677
Chen Y, Yang C, Sheng L, Jiang H, Song B. The Era of Immunotherapy in Hepatocellular Carcinoma: The New Mission and Challenges of Magnetic Resonance Imaging. Cancers. 2023; 15(19):4677. https://doi.org/10.3390/cancers15194677
Chicago/Turabian StyleChen, Yidi, Chongtu Yang, Liuji Sheng, Hanyu Jiang, and Bin Song. 2023. "The Era of Immunotherapy in Hepatocellular Carcinoma: The New Mission and Challenges of Magnetic Resonance Imaging" Cancers 15, no. 19: 4677. https://doi.org/10.3390/cancers15194677
APA StyleChen, Y., Yang, C., Sheng, L., Jiang, H., & Song, B. (2023). The Era of Immunotherapy in Hepatocellular Carcinoma: The New Mission and Challenges of Magnetic Resonance Imaging. Cancers, 15(19), 4677. https://doi.org/10.3390/cancers15194677






