Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
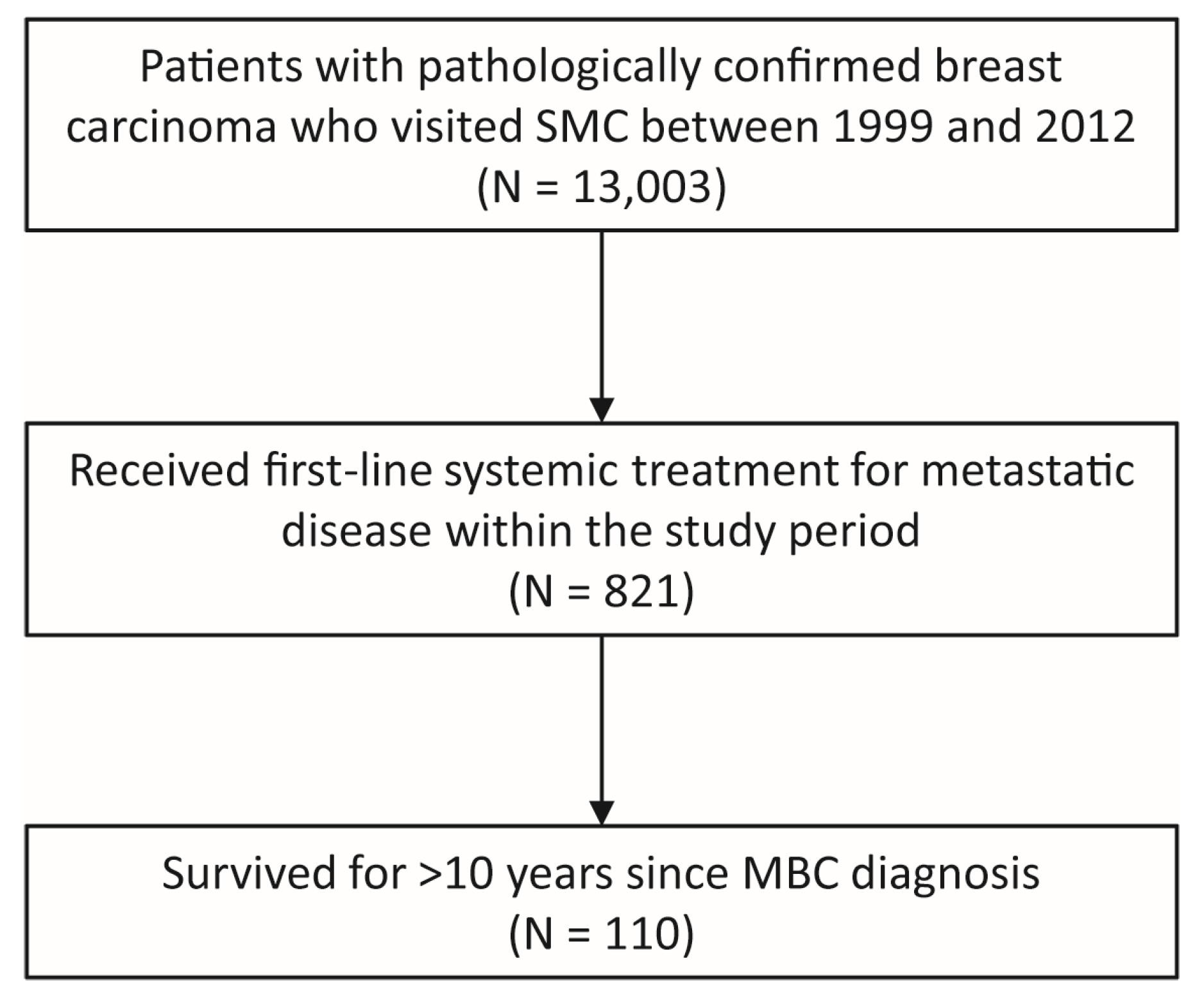
2.1. Eligible Patients and Data Collection
2.2. Pathologic Classification
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
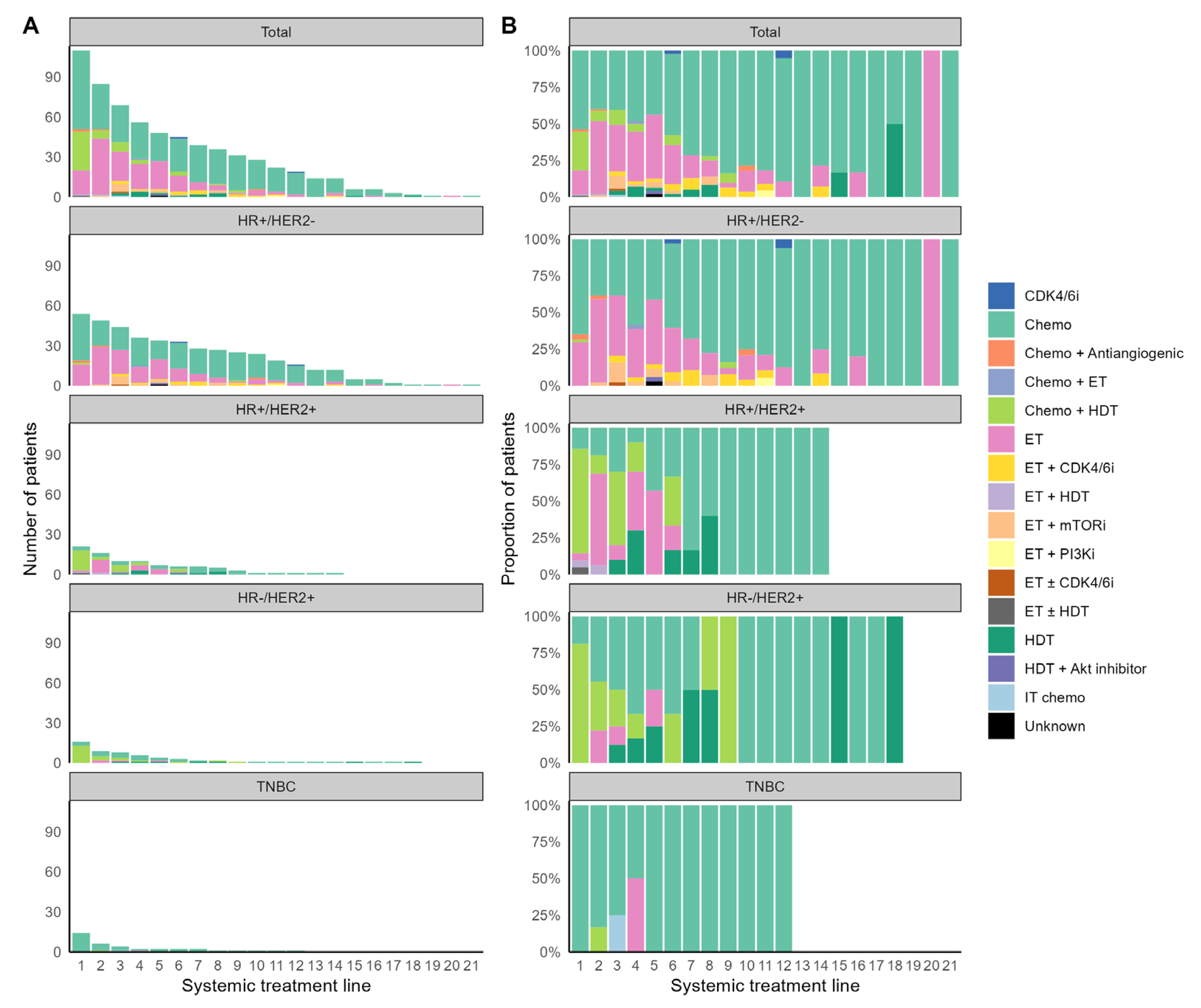
3.2. Systemic Treatment for MBC
3.3. Local Treatment for Metastatic Disease
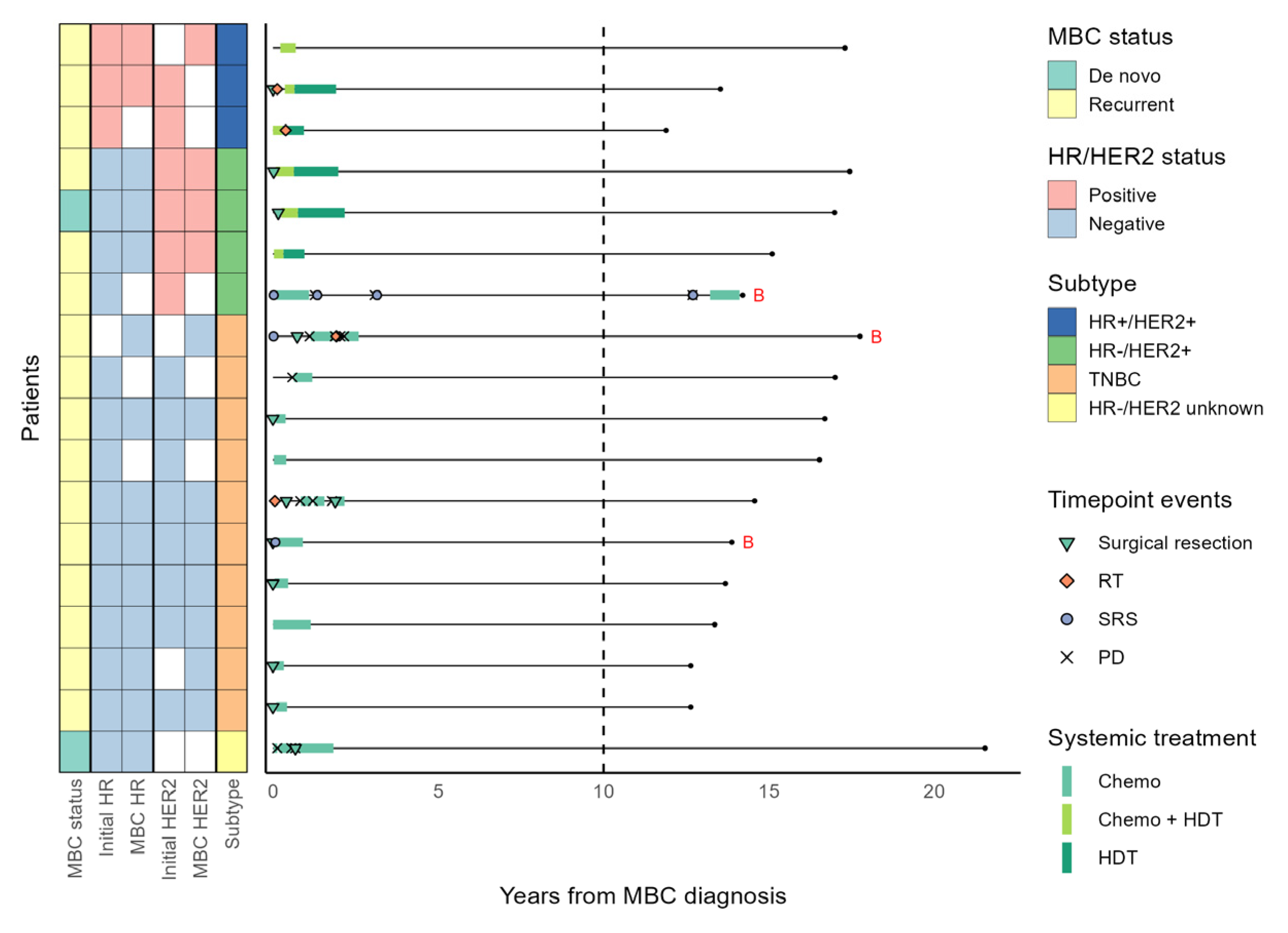
3.4. Patients with a Long Systemic Treatment-Free Interval
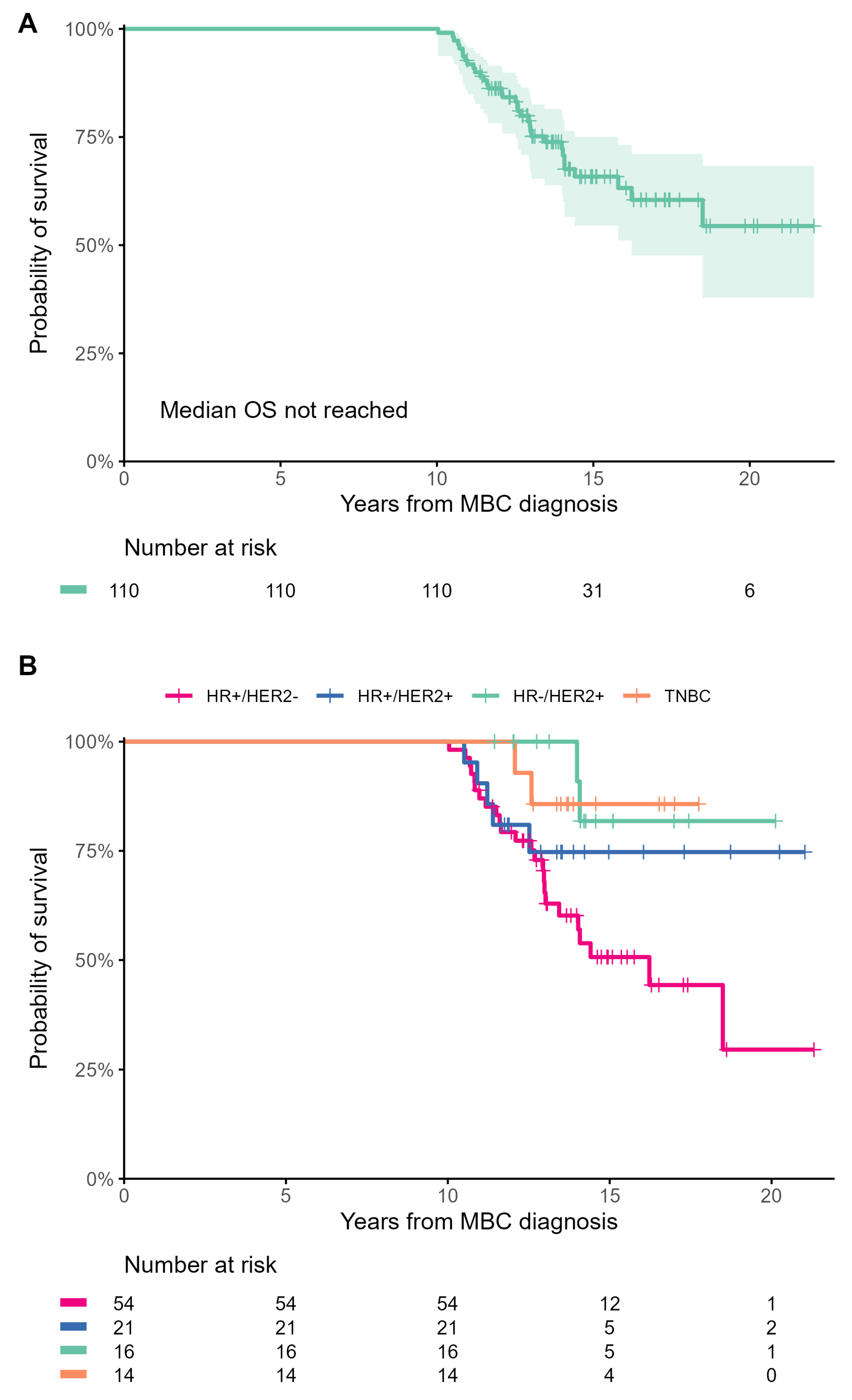
3.5. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Kim, Z.; Park, C.S.; Park, E.H.; Lee, S.B.; Lee, S.K.; Choi, Y.J.; Han, J.; Jung, K.-W.; Kim, H.J.; et al. Breast Cancer Statistics in Korea, 2019. J. Breast Cancer 2023, 26, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Yang, W.; Liu, H. Prognostic significance of molecular subtype, metastatic site and primary tumor surgery for survival in primary metastatic breast cancer: A SEER-based study. Medicine 2021, 100, e26619. [Google Scholar] [CrossRef]
- Eng, L.G.; Dawood, S.; Sopik, V.; Haaland, B.; Tan, P.S.; Bhoo-Pathy, N.; Warner, E.; Iqbal, J.; Narod, S.A.; Dent, R. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res. Treat. 2016, 160, 145–152. [Google Scholar] [CrossRef]
- Greenberg, P.A.; Hortobagyi, G.N.; Smith, T.L.; Ziegler, L.D.; Frye, D.K.; Buzdar, A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 2197–2205. [Google Scholar] [CrossRef]
- Valachis, A.; Carlqvist, P.; Ma, Y.; Szilcz, M.; Freilich, J.; Vertuani, S.; Holm, B.; Lindman, H. Overall survival of patients with metastatic breast cancer in Sweden: A nationwide study. Br. J. Cancer 2022, 127, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Tevaarwerk, A.J.; Gray, R.J.; Schneider, B.P.; Smith, M.L.; Wagner, L.I.; Fetting, J.H.; Davidson, N.; Goldstein, L.J.; Miller, K.D.; Sparano, J.A. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy. Cancer 2013, 119, 1140–1148. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr. Curing Metastatic Breast Cancer. J. Oncol. Pract. 2016, 12, 6–10. [Google Scholar] [CrossRef]
- Berruti, A.; Amoroso, V.; Gallo, F.; Bertaglia, V.; Simoncini, E.; Pedersini, R.; Ferrari, L.; Bottini, A.; Bruzzi, P.; Sormani, M.P. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: A meta-regression of 29 randomized prospective studies. J. Clin. Oncol. 2014, 32, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ichiba, T.; Sakuyama, T.; Arakawa, Y.; Nagasaki, E.; Aiba, K.; Nogi, H.; Kawase, K.; Takeyama, H.; Toriumi, Y. Possible clinical cure of metastatic breast cancer: Lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer 2012, 19, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Güth, U.; Elfgen, C.; Montagna, G.; Schmid, S.M. Long-Term Survival and Cure in Distant Metastatic Breast Cancer. Oncology 2019, 97, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Sytov, A.; Brenin, C.; Millard, T.; Showalter, S.; Dillon, P. Long-Term Non-progression in Metastatic Breast Cancer Beyond 5 Years: Case Series and Review. Curr. Breast Cancer Rep. 2021, 13, 208–215. [Google Scholar] [CrossRef]
- Maltoni, R.; Palleschi, M.; Gallerani, G.; Bravaccini, S.; Cecconetto, L.; Melegari, E.; Altini, M.; Rocca, A. Impressive long-term response with chemo-endocrine therapy in a premenopausal patient with metastatic breast cancer: A case report. Medicine 2020, 99, e20396. [Google Scholar] [CrossRef]
- Steenbruggen, T.G.; Linn, S.C.; Rodenhuis, S.; Sonke, G.S. Ongoing Remission Nineteen Years after High-dose Chemotherapy for Oligometastatic Breast Cancer; What Can We Learn from this Patient? Cureus 2015, 7, e433. [Google Scholar] [CrossRef]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Sachs, M.C.; Brand, A.; Gabriel, E.E. Confidence bands in survival analysis. Br. J. Cancer 2022, 127, 1636–1641. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef] [PubMed]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 2018, 96, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, J.A.; Mayer, M.; Atwood, M.K.; Kaplan, H.G. Differential presentation and survival of de novo and recurrent metastatic breast cancer over time: 1990–2010. Breast Cancer Res. Treat. 2018, 167, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Wang, Y.; Waters, J.; Leung, M.L.; Unruh, A.; Roh, W.; Shi, X.; Chen, K.; Scheet, P.; Vattathil, S.; Liang, H.; et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014, 512, 155–160. [Google Scholar] [CrossRef]
- Shah, S.P.; Roth, A.; Goya, R.; Oloumi, A.; Ha, G.; Zhao, Y.; Turashvili, G.; Ding, J.; Tse, K.; Haffari, G.; et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486, 395–399. [Google Scholar] [CrossRef]
- Weide, R.; Feiten, S.; Friesenhahn, V.; Heymanns, J.; Kleboth, K.; Thomalla, J.; van Roye, C.; Köppler, H. Metastatic breast cancer: Prolongation of survival in routine care is restricted to hormone-receptor- and Her2-positive tumors. SpringerPlus 2014, 3, 535. [Google Scholar] [CrossRef]
- Del Turco, M.R.; Palli, D.; Cariddi, A.; Ciatto, S.; Pacini, P.; Distante, V. Intensive Diagnostic Follow-up After Treatment of Primary Breast Cancer: A Randomized Trial. JAMA 1994, 271, 1593–1597. [Google Scholar] [CrossRef]
- Ghezzi, P.; Magnanini, S.; Rinaldini, M.; Berardi, F.; Di Biagio, G.; Testare, F.; Tavoni, N.; Schittulli, F.; D’Amico, C.; Pedicini, T.; et al. Impact of Follow-up Testing on Survival and Health-Related Quality of Life in Breast Cancer Patients: A Multicenter Randomized Controlled Trial. JAMA 1994, 271, 1587–1592. [Google Scholar] [CrossRef]
- Eggemann, H.; Ignatov, T.; Burger, E.; Kantelhardt, E.J.; Fettke, F.; Thomssen, C.; Costa, S.D.; Ignatov, A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr. Relat. Cancer 2015, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.Y.; Kim, H.K.; Lee, J.; Park, H.K.; Kim, Y.S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Sharaf, B.; Abu-Fares, H.; Tamimi, F.; Al-Sawajneh, S.; Salama, O.; Daoud, R.; Alhajahjeh, A.; Al-Lababidi, S.; Abdel-Razeq, H. Differences in Treatment Outcomes Between Patients with HER2-Low versus HER2-Zero, Hormone Receptor-Positive Advanced-Stage Breast Cancer Treated with Ribociclib. Breast Cancer 2023, 15, 541–548. [Google Scholar] [CrossRef]
- Bao, K.K.H.; Sutanto, L.; Tse, S.S.W.; Man Cheung, K.; Chan, J.C.H. The Association of ERBB2-Low Expression With the Efficacy of Cyclin-Dependent Kinase 4/6 Inhibitor in Hormone Receptor–Positive, ERBB2-Negative Metastatic Breast Cancer. JAMA Network Open 2021, 4, e2133132. [Google Scholar] [CrossRef]
- Saner, F.A.M.; Herschtal, A.; Nelson, B.H.; deFazio, A.; Goode, E.L.; Ramus, S.J.; Pandey, A.; Beach, J.A.; Fereday, S.; Berchuck, A.; et al. Going to extremes: Determinants of extraordinary response and survival in patients with cancer. Nat. Rev. Cancer 2019, 19, 339–348. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Takebe, N.; Hinoue, T.; Hoadley, K.A.; Cardenas, M.F.; Hamilton, A.M.; Laird, P.W.; Wang, L.; Johnson, A.; Dewal, N.; et al. Molecular Features of Cancers Exhibiting Exceptional Responses to Treatment. Cancer Cell 2021, 39, 38–53.e37. [Google Scholar] [CrossRef]

| Characteristic | Total (n = 110) | HR+/HER2− (n = 54) | HR+/HER2+ (n = 21) | HR−/HER2+ (n = 16) | TNBC (n = 14) |
|---|---|---|---|---|---|
| Year of MBC diagnosis, n (%) | |||||
| 1999–2002 | 10 (9.1%) | 6 (11.1%) | 1 (4.8%) | 0 (0%) | 0 (0%) |
| 2003–2006 | 33 (30%) | 15 (27.8%) | 6 (28.6%) | 5 (31.3%) | 5 (35.7%) |
| 2007–2010 | 53 (48.2%) | 26 (48.1%) | 10 (47.6%) | 8 (50%) | 9 (64.3%) |
| 2011–2012 | 14 (12.7%) | 7 (13%) | 4 (19%) | 3 (18.8%) | 0 (0%) |
| Age at diagnosis of MBC | |||||
| Median (range) | 48.5 (26–69) | 47 (35–69) | 48 (26–68) | 54.5 (31–63) | 48.5 (29–67) |
| <50, n (%) | 59 (53.6%) | 30 (55.6%) | 14 (66.7%) | 6 (37.5%) | 7 (50%) |
| 50–64, n (%) | 41 (37.3%) | 20 (37%) | 4 (19%) | 10 (62.5%) | 4 (28.6%) |
| ≥65, n (%) | 10 (9.1%) | 4 (7.4%) | 3 (14.3%) | 0 (0%) | 3 (21.4%) |
| Disease status at MBC diagnosis, n (%) | |||||
| De novo | 20 (18.2%) | 11 (20.4%) | 4 (19.0%) | 4 (25.0%) | 0 (0%) |
| Recurrent a | 90 (81.8%) | 43 (79.6%) | 17 (81.0%) | 12 (75.0%) | 14 (100%) |
| Stage at initial diagnosis of BC, n (%) b | |||||
| I | 20 (18.2%) | 7 (13.0%) | 7 (33.3%) | 2 (12.5%) | 2 (14.3%) |
| II | 34 (30.9%) | 20 (37.0%) | 6 (28.6%) | 4 (25.0%) | 3 (21.4%) |
| III | 21 (19.1%) | 10 (18.5%) | 2 (9.5%) | 5 (31.3%) | 4 (28.6%) |
| IV | 20 (18.2%) | 11 (20.4%) | 4 (19.0%) | 4 (25.0%) | 0 (0%) |
| Unknown | 15 (13.6%) | 6 (11.1%) | 2 (9.5%) | 1 (6.3%) | 5 (35.7%) |
| Prior neoadjuvant or adjuvant treatment, n (%) c | |||||
| Both neoadjuvant and adjuvant chemotherapy | 9 (10.2%) | 3 (7.1%) | 2 (12%) | 1 (8%) | 3 (23.1%) |
| Adjuvant chemotherapy only | 66 (75%) | 34 (81%) | 9 (53%) | 10 (83%) | 9 (69.2%) |
| Adjuvant endocrine therapy | 55 (62.5%) | 32 (76.2%) | 13 (76%) | 5 (42%) | 3 (23.1%) |
| Adjuvant radiation therapy | 52 (59.1%) | 20 (47.6%) | 11 (65%) | 10 (83%) | 10 (76.9%) |
| Disease-free interval (years) | |||||
| Median (range) | 4 (0.2–28.9) | 5.2 (0.5–28.9) | 2.5 (0.2–8.6) | 2.2 (0.9–7.9) | 3.9 (1.1–8.5) |
| <1 year, n (%) | 7 (6.4%) | 2 (3.7%) | 3 (14.3%) | 2 (12.5%) | 0 (0%) |
| 1–2 years, n (%) | 11 (10.0%) | 1 (1.9%) | 4 (19.0%) | 3 (18.8%) | 3 (21.4%) |
| ≥2 years, n (%) | 70 (63.6%) | 39 (72.2%) | 10 (47.6%) | 7 (43.8%) | 10 (71.4%) |
| Did not receive curative breast surgery, n (%) | 22 (20.0%) | 12 (22.2%) | 4 (19.0%) | 4 (25.0%) | 1 (7.1%) |
| Histologic diagnosis, n (%) | |||||
| Invasive ductal carcinoma | 96 (87.3%) | 46 (85.2%) | 19 (90.5%) | 14 (87.5%) | 13 (92.9%) |
| Invasive lobular carcinoma | 1 (0.9%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Invasive micropapillary carcinoma | 3 (2.7%) | 0 (0%) | 1 (4.8%) | 1 (6.3%) | 1 (7.1%) |
| Invasive cribriform carcinoma | 2 (1.8%) | 1 (1.9%) | 1 (4.8%) | 0 (0%) | 0 (0%) |
| Invasive apocrine carcinoma | 1 (0.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Invasive tubulolobular carcinoma | 1 (0.9%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mucinous carcinoma | 2 (1.8%) | 2 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Signet ring cell carcinoma | 1 (0.9%) | 0 (0%) | 0 (0%) | 1 (6.3%) | 0 (0%) |
| Adenocarcinoma d | 2 (1.8%) | 2 (3.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Unknown | 1 (0.9%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| ER Allred score e | |||||
| 0–2 | 35 (31.8%) | 1 (1.9%) f | 2 (9.5%) g | 16 (100%) | 14 (100%) |
| 3–5 | 15 (13.6%) | 7 (13.0%) | 7 (33.3%) | 0 (0%) | 0 (0%) |
| 6–8 | 58 (52.7%) | 45 (83.3%) | 12 (57.1%) | 0 (0%) | 0 (0%) |
| Unknown | 2 (1.8%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PR Allred score e | |||||
| 0–2 | 54 (49.1%) | 12 (22.2%) | 8 (38.1%) | 16 (100%) | 14 (100%) |
| 3–5 | 15 (13.6%) | 9 (16.7%) | 6 (28.6%) | 0 (0%) | 0 (0%) |
| 6–8 | 39 (35.5%) | 32 (59.3%) | 7 (33.3%) | 0 (0%) | 0 (0%) |
| Unknown | 2 (1.8%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HER2 IHC score e | |||||
| 0 | 45 (40.9%) | 33 (61.1%) | 0 (0%) | 0 (0%) | 12 (85.7%) |
| 1 | 15 (13.6%) | 13 (24.1%) | 1 (4.8%) h | 0 (0%) | 1 (7.1%) |
| 2 | 18 (16.4%) | 4 (7.4%) | 6 (28.6%) | 5 (31.3%) | 1 (7.1%) |
| 3 | 25 (22.7%) | 0 (0%) | 14 (66.7%) | 11 (68.8%) | 0 (0%) |
| Unknown | 7 (6.4%) | 4 (7.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Number of metastatic organs at MBC diagnosis, n (%) | |||||
| 1 | 78 (70.9%) | 35 (64.8%) | 17 (81.0%) | 11 (68.8%) | 10 (71.4%) |
| ≥2 | 32 (29.1%) | 19 (35.2%) | 4 (19.0%) | 5 (31.3%) | 4 (28.6%) |
| Metastatic sites at MBC diagnosis, n (%) | |||||
| Lung | 51 (46.4%) | 33 (61.1%) | 6 (28.6%) | 3 (18.8%) | 8 (57.1%) |
| Distant lymph node | 41 (37.3%) | 16 (29.6%) | 10 (47.6%) | 9 (56.3%) | 5 (35.7%) |
| Bone | 33 (30.0%) | 18 (33.3%) | 6 (28.6%) | 4 (25.0%) | 2 (14.3%) |
| Liver | 15 (13.6%) | 10 (18.5%) | 1 (4.8%) | 3 (18.8%) | 1 (7.1%) |
| Brain | 4 (3.6%) | 1 (1.9%) | 0 (0%) | 1 (6.3%) | 2 (14.3%) |
| Others | 13 (11.8%) | 8 (14.8%) | 2 (9.5%) | 2 (12.5%) | 1 (7.1%) |
| Visceral i | 66 (60.0%) | 39 (72.2%) | 8 (38.1%) | 8 (50.0%) | 10 (71.4%) |
| Local Treatment, n (%) | Anytime | Within a Year of MBC Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR+/HER2− | HR+/HER2+ | HR−/HER2+ | TNBC | HR+/HER2− | HR+/HER2+ | HR−/HER2+ | TNBC | |
| Any | 33 (61.1%) | 17 (81%) | 12 (75%) | 11 (78.6%) | 18 (33.3%) | 11 (52.4%) | 6 (37.5%) | 10 (71.4%) |
| Surgical resection | 25 (46.3%) | 10 (47.6%) | 9 (56.2%) | 10 (71.4%) | 15 (27.8%) | 7 (33.3%) | 3 (18.8%) | 9 (64.3%) |
| Radiation therapy | 20 (37%) | 12 (57.1%) | 4 (25%) | 5 (35.7%) | 6 (11.1%) | 7 (33.3%) | 2 (12.5%) | 3 (21.4%) |
| Stereotactic radiosurgery | 8 (14.8%) | 2 (9.5%) | 2 (12.5%) | 2 (14.3%) | 1 (1.9%) | 0 (0%) | 1 (6.2%) | 2 (14.3%) |
| Radiofrequency ablation | 0 (0%) | 0 (0%) | 1 (6.2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6.2%) | 0 (0%) |
| Variable | Systemic Treatment Duration < 2 Years (n = 18) | Systemic Treatment Duration ≥ 2 Years (n = 92) | p-Value |
|---|---|---|---|
| Year at MBC diagnosis, n (%) | 0.71 | ||
| 1999–2002 | 1 (5.6%) | 9 (9.8%) | |
| 2003–2006 | 7 (38.9%) | 26 (28.3%) | |
| 2007–2010 | 9 (50.0%) | 44 (47.8%) | |
| 2011–2012 | 1 (5.6%) | 13 (14.1%) | |
| Disease status at MBC diagnosis, n (%) | 0.519 | ||
| De novo | 2 (11.1%) | 18 (19.6%) | |
| Recurrent | 16 (88.9%) | 74 (80.4%) | |
| Disease-free interval (years), n (%) | 0.054 | ||
| <1 year | 0 (0%) | 7 (7.6%) | |
| 1–2 years | 5 (27.8%) | 6 (6.5%) | |
| ≥2 year | 11 (61.1%) | 59 (64.1%) | |
| Did not receive curative breast surgery | 2 (11.1%) | 20 (21.7%) | |
| Molecular subtype | <0.001 | ||
| HR+/HER2− | 0 (0%) | 54 (58.7%) | |
| HR+/HER2+ | 3 (16.7%) | 18 (19.6%) | |
| HR−/HER2+ | 4 (22.2%) | 12 (13.0%) | |
| TNBC | 10 (55.6%) | 4 (4.3%) | |
| HR+/HER2 unknown | 0 (0%) | 4 (4.3%) | |
| HR−/HER2 unknown | 1 (5.6%) | 0 (0%) | |
| Number of metastatic organs at MBC diagnosis, n (%) | 0.777 | ||
| 1 | 12 (66.7%) | 66 (71.7%) | |
| ≥2 | 6 (33.3%) | 26 (28.3%) | |
| Curative local treatment within a year of MBC diagnosis | 0.002 | ||
| Done | 12 (66.7%) | 25 (27.2%) | |
| Not done | 6 (33.3%) | 67 (72.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Kim, J.-Y.; Oh, J.M.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Park, W.; Park, Y.H.; Ahn, J.S.; Im, Y.-H. Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer. Cancers 2023, 15, 4720. https://doi.org/10.3390/cancers15194720
Shin J, Kim J-Y, Oh JM, Lee JE, Kim SW, Nam SJ, Park W, Park YH, Ahn JS, Im Y-H. Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer. Cancers. 2023; 15(19):4720. https://doi.org/10.3390/cancers15194720
Chicago/Turabian StyleShin, Junghoon, Ji-Yeon Kim, Jung Min Oh, Jeong Eon Lee, Seok Won Kim, Seok Jin Nam, Won Park, Yeon Hee Park, Jin Seok Ahn, and Young-Hyuck Im. 2023. "Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer" Cancers 15, no. 19: 4720. https://doi.org/10.3390/cancers15194720
APA StyleShin, J., Kim, J.-Y., Oh, J. M., Lee, J. E., Kim, S. W., Nam, S. J., Park, W., Park, Y. H., Ahn, J. S., & Im, Y.-H. (2023). Comprehensive Clinical Characterization of Decade-Long Survivors of Metastatic Breast Cancer. Cancers, 15(19), 4720. https://doi.org/10.3390/cancers15194720







