Zebrafish in Lung Cancer Research
Abstract
:Simple Summary
Abstract
1. Introduction
2. Comparison of Common Cancer Models
2.1. Mice
2.2. Large Mammals
2.3. Chicken Embryo Chorioallantoic Membrane (CAM)
2.4. Drosophila melanogaster
2.5. Zebrafish
2.6. Neoplastic Organoids
3. Expanding Applications of Zebrafish in Biology
4. Zebrafish in LC Research
4.1. Zebrafish in Target Discovery and Validation
4.2. Zebrafish in Studies on the LC Microenvironment
4.3. Zebrafish in Studies on the LC Proliferation and Metastasis
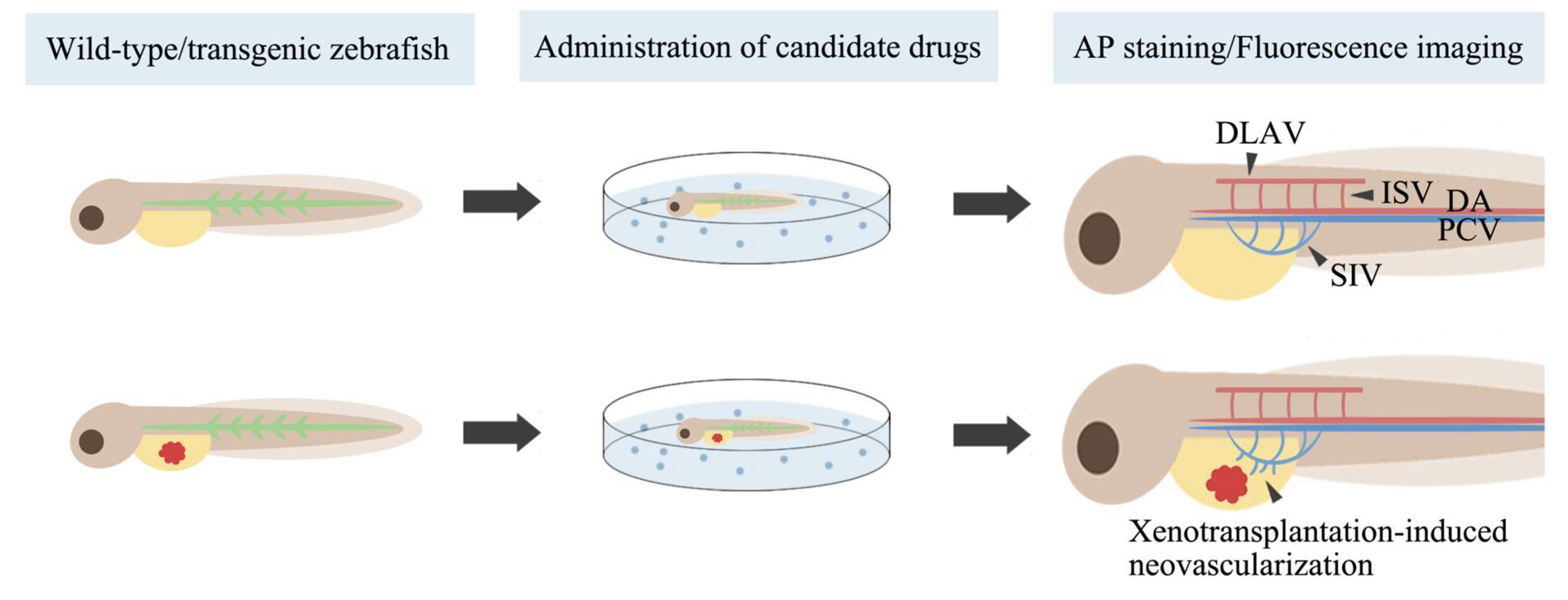
4.4. Zebrafish in Screening for Antiangiogenic Drugs
4.5. Zebrafish in Drug Toxicity Testing
4.6. Zebrafish in Tests of Novel Materials
4.7. Zebrafish in Personalized Medicine
| Zebrafish Line | Transplanted Cells (Number of Cells per Zebrafish) | Transplantation Site and Time | Drug Treatment | Apical Endpoints | Apical Endpoint Measurement Methods | Conclusions | Ref |
|---|---|---|---|---|---|---|---|
| dhx15 knockout Tg(flk1:EGFP) embryo | N/A | N/A | N/A | Angiogenesis, VEGF C gene expression at 4 dpf | Fluorescence imaging, RT-qPCR | dhx15 gene knockdown causes blood and lymphatic vascular defects | [53] |
| dhx33 knockout embryo | N/A | N/A | N/A | Expression levels of genes involved in the cell cycle at 3 dpf | RT-qPCR | dhx33 gene knockdown downregulates critical genes involved in cell cycle control | [54] |
| cxcr7 knockdown embryo | N/A | N/A | N/A | Angiogenesis | Microangiography | The cxcr7 gene plays a key role in angiogenesis during the development | [55] |
| Wild-type embryo | H1299 human lung cancer cells with SOX5 knockdown (800) | Yolk sac at 2 dpf | N/A | Tumor proliferation, metastasis at 3 dpf | Fluorescence imaging | SOX5 gene promotes NSCLC proliferation and metastasis | [56] |
| Tg(fli1:EGFP) embryo | A549 and H460 human lung cancer cells with SOX9 overexpression or SOX9 knockdown (500) | PVS at 2 dpf | N/A | Tumor metastasis at 5 dpf | Fluorescence imaging | SOX9 gene promotes NSCLC metastasis | [57] |
| Wild-type embryo | PC9 human lung cancer cells with UBE2S knockdown | Yolk sac at 3 dpf | N/A | Tumor metastasis at 5 dpf | Fluorescence imaging | UBE2S gene promotes NSCLC metastasis | [58] |
| Wild-type embryo | A549 cells with RSK1 knockdown | Yolk sac at 4 hpf | N/A | Tumor metastasis at 2 dpf | Fluorescence imaging | RSK1 gene inhibits NSCLC metastasis | [59] |
| Tg(fli1:EGFP) embryo | CL1-0 human lung cancer cells with KMT1E knockdown (400) | Yolk sac at 2 dpf | N/A | Tumor metastasis at 5 dpf | Fluorescence imaging | KMT1E gene inhibits NSCLC metastasis | [60] |
| Tg(fli:GFP) embryo | A549 cells with NME2 or vinculin knockdown (50–200) | Pericardium at 3 dpf | N/A | Tumor metastasis at 4 dpf | Fluorescence imaging | NME2-mediated regulation of vinculin favors a signaling pathway that inhibits NSCLC metastasis | [61] |
| Wild-type embryo | Paclitaxel-resistant H23 cells with beclin knockdown (850) | Yolk sac at 2 dpf | Soak in paclitaxel at 2 dpf | Tumor proliferation at 3–4 dpf | Fluorescence imaging | Beclin silencing restores the sensitivity of paclitaxel-resistant NSCLC to paclitaxel | [62] |
| Wild-type embryo | A549 cells with FAM83H-AS1 knockdown (400) | PVS | N/A | Tumor proliferation, metastasis | Fluorescence imaging | Non-coding oncogene FAM83H-AS1 promotes NSCLC proliferation and metastasis | [63] |
| Wild-type and Tg(fli1a:EGFP) embryo | A549 or SPC-A1 cells with knockdown of LINC00152 (400) | PVS at 2 dpf | N/A | Tumor proliferation, metastasis at 6 dpf | Fluorescence imaging | LINC00152 promotes NSCLC proliferation and metastasis | [64] |
| Wild-type embryo | A549 cells with or upregulation of miR-608 (100–200) | Yolk sac | N/A | Tumor apoptosis at 12 hpi | Immunostaining | miR-608 promotes NSCLC apoptosis | [65] |
| Wild-type embryo | A549 cells with downregulation of miR-361-5p (100–200) | Yolk sac | N/A | Tumor apoptosis at 12 hpi | Immunostaining | miR-361-5p inhibits NSCLC apoptosis | [66] |
| Wild-type embryo | A549 cells with downregulation of miR-378 or upregulation of miR-1827 (100) | Yolk sac at 2 dpf | N/A | Tumor metastasis, tumor-induced angiogenesis at 3 dpf | Fluorescence imaging, AP staining | Anti-miR-378 and miR-1827 inhibit NSCLC metastasis and angiogenesis | [67] |
| Wild-type adult zebrafish | A549 cells | Peritoneal cavity | Anti-miR-210-3p LNA was delivered by intratumoral injection | Tumor growth, CCL2 gene expression; monocyte population | Fluorescence imaging, RT-PCR | miR-210-3p impairs monocyte infiltration by inhibiting CCL2 expression and promotes NSCLC growth | [68] |
| Tg(fli1:EGFP) embryo | Murine LLC cells and macrophages (300–500) | PVS at 2 dpf | N/A | Tumor metastasis at 6 dpf | Fluorescence imaging | Tumor-associated macrophages promote NSCLC proliferation and metastasis | [72] |
| Wild-type embryo | A549 cells co-cultured with mesenchymal stem cells on CS-HA membranes (150) | 4 hpf | N/A | Tumor metastasis at 54 hpf | Fluorescence imaging | Co-culture with mesenchymal stem cells on CS-HA membranes promotes NSCLC metastasis | [73] |
| Wild-type, Tg(fli1:EGFP), Tg(fli1:GFP) embryo | A549 cells (50–800), H1299 cells (50–200), H460 (200) | Yolk sac/PVS at 4–48 hpf | Exposure to drugs at 2–4 dpf or cells were pretreated with drugs before transplantation | Tumor proliferation, death at 3–9 dpf | Fluorescence imaging, acridine orange staining | Candidate drugs have tumor-inhibiting effects | [76,77,79,80,81,83,84,85,86,96,97,98,99,100,101,102,103,104] |
| Casper strain of embryo | A549 cells with PAPSS1 knockdown (150–200) | Yolk sac at 48 hpf | Exposure to cisplatin at 60–72 hpf | Tumor proliferation | Fluorescence imaging | PAPSS1 silencing sensitizes NSCLC cells to cisplatin treatment | [87] |
| Wild-type embryo | H2009 human lung cancer cells with knockdown of ACK1 or SRC (200) | Yolk sac at 24–30 hpf | Exposure to bosutinib at 26–32 hpf | Tumor metastasis at 72–78 hpf | Fluorescence imaging | Bosutinib inhibits metastasis via ACK1 in NSCLC with KRAS mutations | [88] |
| Wild-type, Tg(fli1:EGFP) embryo | A549, H1975, and H1299 cells (100) | PVS at 2 dpf | N/A | Brain metastases at 6 dpf | Fluorescence imaging, histopathological evaluation | Zebrafish brain metastasis models can discriminate the brain metastasis potential of different NSCLC cells | [89] |
| Wild-type, Tg(fli1:EGFP), Tg(flk1:EGFP) embryo | Drug-resistant PC9 (200–300), HCC827 (50), or A549 cells (200) | Yolk sac at 48 hpf | Exposure to or injection of drugs or cells are pretreated with drugs before transplantation | Tumor proliferation | Fluorescence imaging | Candidate drugs inhibit the proliferation of drug-resistant NSCLC | [90,91,92,93,94,95] |
| Wild-type adult zebrafish | A549 or H460 cells | Sections of gills at multiple sites or muscle region | Administration of drugs orally | Tumor proliferation, metastasis, and angiogenesis | Histopathological evaluation | Candidate drugs inhibit tumor growth, metastasis, and angiogenesis | [105,106,107] |
| Wild-type embryo | N/A | N/A | Exposure to drugs at 1 dpf | Angiogenesis at 1–3 dpf | AP staining | Candidate drugs have or do not have antiangiogenic activity | [110,111,112,113] |
| Tg(fli1:EGFP), Tg(flk:EGFP), Tg(flk1:GFP), Tg(vegfr2:GFP), Tg(kdrl:EGFP;gata1:dsRed) embryo | N/A | N/A | Exposure to drugs/injection of drugs at 4–48 hpf | Angiogenesis at 30–96 hpf | Fluorescence imaging | Candidate drugs have or do not have antiangiogenic activity | [114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132] |
| Tg(flk1:GFP) embryo | H1299 human or murine CL13 cells (600–800) | PVS at 2 dpf | N/A | Tumor-induced angiogenesis at 4 dpf | Fluorescence imaging, AP staining | The zebrafish xenograft model can discriminate the angiogenic activity of different NSCLC cells | [133] |
| Tg(fli1:EGFP), Tg(flk:EGFP) embryo | H1299 human (300) or murine B16-F10 cells (300) | Yolk sac/PVS at 2 dpf | Exposure to drugs at 3 dpf | Tumor-induced angiogenesis at 6 dpf | Fluorescence imaging | Candidate drugs have antiangiogenic activity | [134,135] |
| Wild-type embryo | N/A | N/A | Exposure to drugs at 2 hpf | Embryo survival and morphological changes every 24 h | Microscopic observation | Candidate drugs have low toxicity or are non-toxic at effective concentrations | [136,137,138,139,140,141] |
| Wild-type embryo | N/A | N/A | Exposure to drugs at 6–72 hpf | Cardiotoxicity at 3–5 dpf | Microscopic observation | Candidate drugs do not cause serious cardiac toxicity at effective concentrations | [142] |
| Tg(fabp10a:dsRed;ela3l:EGFP) embryo | N/A | N/A | Exposure to gefitinib and afatinib at 3 dpf | Hepatotoxicity at 6 dpf | Fluorescence imaging, histopathological evaluation, acridine orange and whole oil red O staining, determination of liver-related enzyme activities, RT-PCR | Both gefitinib and afatinib dose-dependently induced hepatotoxicity | [143] |
| Tg(mpx:EGFP) embryo | N/A | N/A | Exposure to onion extracts and doxorubicin at 6 hpf | Myelotoxicity at 72 hpf | Fluorescence imaging | Onion extracts have a strong protective effect against doxorubicin-caused neutropenia | [144] |
| Wild-type adult zebrafish | N/A | N/A | Injection of cisplatin and curcuminoids | Ototoxicity at 48 hpt | Auditory evoked potential measurements | The curcuminoids may prevent cisplatin ototoxicity | [145] |
| Tg(flk1:mCherry), Tg(flk:EGFP) embryo | N/A | N/A | Injection of free drug or drug micelles into circulation | Drug extravasation speed | Fluorescence imaging | The encapsulation of drugs in polymer micelles decreases their extravasation speed | [146,147] |
| Wild-type, Tg(kdrl:GFP) embryo | N/A | N/A | Injection/exposure to fluorescent materials | Trace of fluorescent materials | Fluorescence imaging | Fluorescent materials can be detected and imaged in vivo, which can reveal their traces | [148,149] |
| Wild-type embryo | N/A | N/A | Exposure to BMU-Ru nanosensors with/without BDM at 5 dpf | Imaging of BMU-Ru nanosensors under different conditions | Fluorescence imaging | The process of BMU-Ru nanosensor imaging combined with normoxic and hypoxic conditions is reversible | [150] |
| Tg(fli1a:EGFP) embryo | NSCLC cells from mouse patient-derived xenograft model | PVS at 2 dpf | Exposure to erlotinib or paclitaxel | Tumor proliferation, metastasis | Fluorescence imaging | The zebrafish tumor xenograft model preserves the drug response of tumors and predicts lymph node involvement in patients | [151] |
| Tg(fli1a:EGFP) embryo | Lung carcinoid cells from patients (100–1000) | Sub-peridermal space at 2 dpf | N/A | Tumor metastasis, tumor-induced angiogenesis at 4 dpf | Fluorescence imaging | The zPDX model for lung carcinoid cancer successfully demonstrates proangiogenic and invasive behavior | [152] |
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cáncer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; p. 433. [Google Scholar]
- Osmani, N.; Goetz, J.G. Multiscale Imaging of Metastasis in Zebrafish. Trends Cancer 2019, 5, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Pauli, C.; Hopkins, B.D.; Prandi, D.; Shaw, R.; Fedrizzi, T.; Sboner, A.; Sailer, V.; Augello, M.; Puca, L.; Rosati, R.; et al. Personalized In Vitro and In Vivo Cancer Models to Guide Precision Medicine. Cancer Discov. 2017, 7, 462–477. [Google Scholar] [CrossRef]
- Hutchinson, L.; Kirk, R. High drug attrition rates--where are we going wrong? Nat. Rev. Clin. Oncol. 2011, 8, 189–190. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in translation: Animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Mondal, P.; Bailey, K.L.; Cartwright, S.B.; Band, V.; Carlson, M.A. Large Animal Models of Breast Cancer. Front. Oncol. 2022, 12, 788038. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Adega, F.; Borges, A.; Chaves, R. Cat Mammary Tumors: Genetic Models for the Human Counterpart. Vet. Sci. 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Deycmar, S.; Gomes, B.; Charo, J.; Ceppi, M.; Cline, J.M. Spontaneous, naturally occurring cancers in non-human primates as a translational model for cancer immunotherapy. J. Immunother. Cancer 2023, 11, e005514. [Google Scholar] [CrossRef] [PubMed]
- Kalla, D.; Kind, A.; Schnieke, A. Genetically Engineered Pigs to Study Cancer. Int. J. Mol. Sci. 2020, 21, 488. [Google Scholar] [CrossRef]
- Adam, S.J.; Rund, L.A.; Kuzmuk, K.N.; Zachary, J.F.; Schook, L.B.; Counter, C.M. Genetic induction of tumorigenesis in swine. Oncogene 2007, 26, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Mukae, Y.; Kitsuka, T.; Arai, K.; Nakamura, A.; Uchihashi, K.; Toda, S.; Matsubayashi, K.; Oyama, J.I.; Node, K.; et al. Development of an immunodeficient pig model allowing long-term accommodation of artificial human vascular tubes. Nat. Commun. 2019, 10, 2244. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Arantes-Rodrigues, R.; Vasconcelos-Nóbrega, C. Animal models of urinary bladder cancer and their application to novel drug discovery. Expert. Opin. Drug Discov. 2014, 9, 485–503. [Google Scholar] [CrossRef]
- Xu, L.; Fan, Y.; Jiang, X.L.; Yao, Y.G. Molecular evidence on the phylogenetic position of tree shrews. Dongwuxue Yanjiu 2013, 34, 70–76. [Google Scholar] [CrossRef]
- Lu, T.; Peng, H.; Zhong, L.; Wu, P.; He, J.; Deng, Z.; Huang, Y. The Tree Shrew as a Model for Cancer Research. Front. Oncol. 2021, 11, 653236. [Google Scholar] [CrossRef]
- Miebach, L.; Berner, J.; Bekeschus, S. In ovo model in cancer research and tumor immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Munnik, C.; Xaba, M.P.; Malindisa, S.T.; Russell, B.L.; Sooklal, S.A. Drosophila melanogaster: A platform for anticancer drug discovery and personalized therapies. Front. Genet. 2022, 13, 949241. [Google Scholar] [CrossRef]
- Jiang, H.; Kimura, T.; Hai, H.; Yamamura, R.; Sonoshita, M. Drosophila as a toolkit to tackle cancer and its metabolism. Front. Oncol. 2022, 12, 982751. [Google Scholar] [CrossRef] [PubMed]
- Cagan, R.L.; Zon, L.I.; White, R.M. Modeling Cancer with Flies and Fish. Dev. Cell 2019, 49, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, R.; Ooshio, T.; Sonoshita, M. Tiny Drosophila makes giant strides in cancer research. Cancer Sci. 2021, 112, 505–514. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Lv, P.; Ma, D.; Gao, S.; Zhang, Y.; Bae, Y.K.; Liang, G.; Gao, S.; Choi, J.H.; Kim, C.H.; Wang, L.; et al. Generation of foxn1/Casper Mutant Zebrafish for Allograft and Xenograft of Normal and Malignant Cells. Stem Cell Rep. 2020, 15, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Goessling, W.; Sadler, K.C. Zebrafish: An important tool for liver disease research. Gastroenterology 2015, 149, 1361–1377. [Google Scholar] [CrossRef]
- González-Rosa, J.M. Zebrafish Models of Cardiac Disease: From Fortuitous Mutants to Precision Medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef]
- Zizioli, D.; Mione, M.; Varinelli, M.; Malagola, M.; Bernardi, S.; Alghisi, E.; Borsani, G.; Finazzi, D.; Monti, E.; Presta, M.; et al. Zebrafish disease models in hematology: Highlights on biological and translational impact. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiao, D.; Liu, A.; Wu, K. Tumor organoids: Applications in cancer modeling and potentials in precision medicine. J. Hematol. Oncol. 2022, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Rossi, R.; De Angelis, M.L.; Xhelili, E.; Sette, G.; Eramo, A.; De Maria, R.; Cesta Incani, U.; Francescangeli, F.; Zeuner, A. Lung Cancer Organoids: The Rough Path to Personalized Medicine. Cancers 2022, 14, 3703. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Tang, Z.; Chen, Y.; Huang, M.; Liu, H.; Huang, W.; Ye, Q.; Jia, B. Research Progress, Challenges, and Breakthroughs of Organoids as Disease Models. Front. Cell Dev. Biol. 2021, 9, 740574. [Google Scholar] [CrossRef]
- Streisinger, G.; Walker, C.; Dower, N.; Knauber, D.; Singer, F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 1981, 291, 293–296. [Google Scholar] [CrossRef]
- Ochenkowska, K.; Herold, A.; Samarut, É. Zebrafish Is a Powerful Tool for Precision Medicine Approaches to Neurological Disorders. Front. Mol. Neurosci. 2022, 15, 944693. [Google Scholar] [CrossRef]
- Lin, M.H.; Chou, H.C.; Chen, Y.F.; Liu, W.; Lee, C.C.; Liu, L.Y.; Chuang, Y.J. Development of a rapid and economic in vivo electrocardiogram platform for cardiovascular drug assay and electrophysiology research in adult zebrafish. Sci. Rep. 2018, 8, 15986. [Google Scholar] [CrossRef]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Katoch, S.; Patial, V. Zebrafish: An emerging model system to study liver diseases and related drug discovery. J. Appl. Toxicol. 2021, 41, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Noel, N.C.L.; Allison, W.T.; MacDonald, I.M.; Hocking, J.C. Zebrafish and inherited photoreceptor disease: Models and insights. Prog. Retin. Eye Res. 2022, 91, 101096. [Google Scholar] [CrossRef]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Stagaman, K.; Sharpton, T.J.; Guillemin, K. Zebrafish microbiome studies make waves. Lab Anim. 2020, 49, 201–207. [Google Scholar] [CrossRef]
- Kent, M.L.; Wall, E.S.; Sichel, S.; Watral, V.; Stagaman, K.; Sharpton, T.J.; Guillemin, K. Pseudocapillaria tomentosa, Mycoplasma spp., and Intestinal Lesions in Experimentally Infected Zebrafish Danio rerio. Zebrafish 2021, 18, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.G.; Ruetten, M.; Seth-Smith, H.M.; Nufer, L.; Voegtlin, A.; Lehner, A.; Greub, G.; Crosier, P.S.; Neuhauss, S.C.; Vaughan, L. A Zebrafish Model for Chlamydia Infection with the Obligate Intracellular Pathogen Waddlia chondrophila. Front. Microbiol. 2016, 7, 1829. [Google Scholar] [CrossRef]
- Tyrkalska, S.D.; Candel, S.; Pedoto, A.; García-Moreno, D.; Alcaraz-Pérez, F.; Sánchez-Ferrer, Á.; Cayuela, M.L.; Mulero, V. Zebrafish models of COVID-19. FEMS Microbiol. Rev. 2023, 47, fuac042. [Google Scholar] [CrossRef]
- Cornuault, J.K.; Byatt, G.; Paquet, M.E.; De Koninck, P.; Moineau, S. Zebrafish: A big fish in the study of the gut microbiota. Curr. Opin. Biotechnol. 2022, 73, 308–313. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 2022, 82, 333–347. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Hahn, M.E. Gene knockdown by morpholino-modified oligonucleotides in the zebrafish (Danio rerio) model: Applications for developmental toxicology. Methods Mol. Biol. 2012, 889, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Ribera, J.; Portolés, I.; Córdoba-Jover, B.; Rodríguez-Vita, J.; Casals, G.; González-de la Presa, B.; Graupera, M.; Solsona-Vilarrasa, E.; Garcia-Ruiz, C.; Fernández-Checa, J.C.; et al. The loss of DHX15 impairs endothelial energy metabolism, lymphatic drainage and tumor metastasis in mice. Commun. Biol. 2021, 4, 1192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, X.; Fan, C.; You, J.; Liu, Y.; Weber, J.D.; Zhong, H.; Zhang, Y. DHX33 Transcriptionally Controls Genes Involved in the Cell Cycle. Mol. Cell Biol. 2016, 36, 2903–2917. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Luker, K.E.; Summers, B.C.; Berahovich, R.; Bhojani, M.S.; Rehemtulla, A.; Kleer, C.G.; Essner, J.J.; Nasevicius, A.; Luker, G.D.; et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl. Acad. Sci. USA 2007, 104, 15735–15740. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Q.; Wei, F.K.; Xu, X.L.; Ye, S.X.; Song, J.W.; Ding, P.K.; Zhu, J.; Li, H.F.; Luo, X.P.; Gong, H.; et al. SOX9 drives the epithelial-mesenchymal transition in non-small-cell lung cancer through the Wnt/β-catenin pathway. J. Transl. Med. 2019, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, Y.; Xu, H.; Teng, P.; Xie, Q.; Zhang, Y.; Yan, C.; Xu, Y.; Li, C.; Zhou, J.; et al. SOX5 predicts poor prognosis in lung adenocarcinoma and promotes tumor metastasis through epithelial-mesenchymal transition. Oncotarget 2018, 9, 10891–10904. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Lu, H.Y.; Cheng, H.H.; Kuo, Y.C.; Lee, Y.A.; Cheng, C.H. UBE2S activates NF-κB signaling by binding with IκBα and promotes metastasis of lung adenocarcinoma cells. Cell Oncol. 2021, 44, 1325–1338. [Google Scholar] [CrossRef]
- Lara, R.; Mauri, F.A.; Taylor, H.; Derua, R.; Shia, A.; Gray, C.; Nicols, A.; Shiner, R.J.; Schofield, E.; Bates, P.A.; et al. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene 2011, 30, 3513–3521. [Google Scholar] [CrossRef]
- Wu, P.C.; Lu, J.W.; Yang, J.Y.; Lin, I.H.; Ou, D.L.; Lin, Y.H.; Chou, K.H.; Huang, W.F.; Wang, W.P.; Huang, Y.L.; et al. H3K9 histone methyltransferase, KMT1E/SETDB1, cooperates with the SMAD2/3 pathway to suppress lung cancer metastasis. Cancer Res. 2014, 74, 7333–7343. [Google Scholar] [CrossRef]
- Thakur, R.K.; Yadav, V.K.; Kumar, A.; Singh, A.; Pal, K.; Hoeppner, L.; Saha, D.; Purohit, G.; Basundra, R.; Kar, A.; et al. Non-metastatic 2 (NME2)-mediated suppression of lung cancer metastasis involves transcriptional regulation of key cell adhesion factor vinculin. Nucleic Acids Res. 2014, 42, 11589–11600. [Google Scholar] [CrossRef]
- Liu, W.; Lo, Y.L.; Hsu, C.; Wu, Y.T.; Liao, Z.X.; Wu, W.J.; Chen, Y.J.; Kao, C.; Chiu, C.C.; Wang, L.F. CS-PEI/Beclin-siRNA Downregulate Multidrug Resistance Proteins and Increase Paclitaxel Therapeutic Efficacy against NSCLC. Mol. Ther. Nucleic Acids 2019, 17, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, C.; Liu, T.; Ma, Z.; Qiu, M.; Wang, J.; You, Q.; Zheng, X.; Xu, W.; Xia, W.; et al. FAM83H-AS1 is a noncoding oncogenic driver and therapeutic target of lung adenocarcinoma. Clin. Transl. Med. 2021, 11, e316. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Pu, J.; Sun, J.; Tan, B.; Wang, W.; Wang, L.; Cheng, J.; Zuo, Y. Zebrafish xenograft model of human lung cancer for studying the function of LINC00152 in cell proliferation and invasion. Cancer Cell Int. 2020, 20, 376. [Google Scholar] [CrossRef]
- Othman, N.; Nagoor, N.H. miR-608 regulates apoptosis in human lung adenocarcinoma via regulation of AKT2. Int. J. Oncol. 2017, 51, 1757–1764. [Google Scholar] [CrossRef]
- Othman, N.; Nagoor, N.H. Overexpression of miR-361-5p plays an oncogenic role in human lung adenocarcinoma through the regulation of SMAD2. Int. J. Oncol. 2019, 54, 306–314. [Google Scholar] [CrossRef]
- Ho, C.S.; Noor, S.M.; Nagoor, N.H. MiR-378 and MiR-1827 Regulate Tumor Invasion, Migration and Angiogenesis in Human Lung Adenocarcinoma by Targeting RBX1 and CRKL, Respectively. J. Cancer 2018, 9, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Arora, L.; Patra, D.; Roy, S.; Nanda, S.; Singh, N.; Verma, A.K.; Chakraborti, A.; Dasgupta, S.; Pal, D. Hypoxia-induced miR-210-3p expression in lung adenocarcinoma potentiates tumor development by regulating CCL2-mediated monocyte infiltration. Mol. Oncol. 2022. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Sturtzel, C.; Hocking, J.; Kirchberger, S.; Distel, M. Studying the Tumor Microenvironment in Zebrafish. Adv. Exp. Med. Biol. 2021, 1329, 69–92. [Google Scholar] [CrossRef]
- Weiss, J.M.; Lumaquin-Yin, D.; Montal, E.; Suresh, S.; Leonhardt, C.S.; White, R.M. Shifting the focus of zebrafish toward a model of the tumor microenvironment. Elife 2022, 11, e69703. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Z.; Zhang, X.M.; Nakamura, M.; Sun, M.; Hartman, J.; Harris, R.A.; Sun, Y.; Cao, Y. Novel mechanism of macrophage-mediated metastasis revealed in a zebrafish model of tumor development. Cancer Res. 2015, 75, 306–315. [Google Scholar] [CrossRef]
- Han, H.W.; Hsu, S.H. Chitosan-hyaluronan based 3D co-culture platform for studying the crosstalk of lung cancer cells and mesenchymal stem cells. Acta Biomater. 2016, 42, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Veinotte, C.J.; Dellaire, G.; Berman, J.N. Hooking the big one: The potential of zebrafish xenotransplantation to reform cancer drug screening in the genomic era. Dis. Model. Mech. 2014, 7, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.L.; Chou, H.L.; Lee, J.C.; Chen, F.W.; Fong, Y.; Chang, W.C.; Huang, H.W.; Wu, C.Y.; Chang, W.T.; Wang, H.D.; et al. The antiproliferative effect of C2-ceramide on lung cancer cells through apoptosis by inhibiting Akt and NFκB. Cancer Cell Int. 2014, 14, 1. [Google Scholar] [CrossRef]
- Chou, H.L.; Lin, Y.H.; Liu, W.; Wu, C.Y.; Li, R.N.; Huang, H.W.; Chou, C.H.; Chiou, S.J.; Chiu, C.C. Combination Therapy of Chloroquine and C₂-Ceramide Enhances Cytotoxicity in Lung Cancer H460 and H1299 Cells. Cancers 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.; Miles, M.; Lascuna, P.; Burney, B.; Patel, Z.; Sidoran, K.J.; Sittaramane, V.; Kocerha, J.; Grossie, D.A.; Sessler, J.L.; et al. Dual targeting of the cancer antioxidant network with 1,4-naphthoquinone fused Gold(i) N-heterocyclic carbene complexes. Chem. Sci. 2017, 8, 5918–5929. [Google Scholar] [CrossRef]
- Chang, W.T.; Liu, W.; Chiu, Y.H.; Chen, B.H.; Chuang, S.C.; Chen, Y.C.; Hsu, Y.T.; Lu, M.J.; Chiou, S.J.; Chou, C.K.; et al. A 4-Phenoxyphenol Derivative Exerts Inhibitory Effects on Human Hepatocellular Carcinoma Cells through Regulating Autophagy and Apoptosis Accompanied by Downregulating α-Tubulin Expression. Molecules 2017, 22, 854. [Google Scholar] [CrossRef]
- Liu, W.; Wu, C.Y.; Lu, M.J.; Chuang, Y.J.; Tsai, E.M.; Leu, S.; Lin, I.L.; Ko, C.J.; Chiu, C.C.; Chang, W.T. The Phenoxyphenol Compound 4-HPPP Selectively Induces Antiproliferation Effects and Apoptosis in Human Lung Cancer Cells through Aneupolyploidization and ATR DNA Repair Signaling. Oxid. Med. Cell Longev. 2020, 2020, 5167292. [Google Scholar] [CrossRef]
- Wang, T.; Zou, J.; Wu, Q.; Wang, R.; Yuan, C.L.; Shu, J.; Zhai, B.B.; Huang, X.T.; Liu, N.Z.; Hua, F.Y.; et al. Tanshinone IIA derivatives induced S-phase arrest through stabilizing c-myc G-quadruplex DNA to regulate ROS-mediated PI3K/Akt/mTOR pathway. Eur. J. Pharmacol. 2021, 912, 174586. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Zhang, S.; Liu, H.; Zhao, B.; Du, B.; Wang, W.; Lin, P.; Zhang, Z.; Zhong, Y.; et al. Digoxin Enhances the Anticancer Effect on Non-Small Cell Lung Cancer While Reducing the Cardiotoxicity of Adriamycin. Front. Pharmacol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Liu, H.; Tian, H.; Wang, Y.; Zhang, G.; Lei, Y.; Xue, L.; Zheng, B.; Fan, T.; et al. The therapeutic significance of the novel photodynamic material TPE-IQ-2O in tumors. Aging 2020, 13, 1383–1409. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chou, H.L.; Chen, B.H.; Chang, K.F.; Tseng, C.H.; Fong, Y.; Fu, T.F.; Chang, H.W.; Wu, C.Y.; Tsai, E.M.; et al. BPIQ, a novel synthetic quinoline derivative, inhibits growth and induces mitochondrial apoptosis of lung cancer cells in vitro and in zebrafish xenograft model. BMC Cancer 2015, 15, 962. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Bow, Y.D.; Wang, C.Y.; Chen, Y.C.; Fu, P.R.; Chang, K.F.; Wang, T.W.; Tseng, C.H.; Chen, Y.L.; Chiu, C.C. DFIQ, a Novel Quinoline Derivative, Shows Anticancer Potential by Inducing Apoptosis and Autophagy in NSCLC Cell and In Vivo Zebrafish Xenograft Models. Cancers 2020, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Zhilenkov, A.V.; Kraevaya, O.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.H. Toward Understanding the Antitumor Effects of Water-Soluble Fullerene Derivatives on Lung Cancer Cells: Apoptosis or Autophagy Pathways? J. Med. Chem. 2019, 62, 7111–7125. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Veinotte, C.J.; Melong, N.; Oh, M.H.; Chen, K.; Enfield, K.S.S.; Backstrom, I.; Warburton, C.; Yapp, D.; Berman, J.N.; et al. In Vivo Validation of PAPSS1 (3′-phosphoadenosine 5′-phosphosulfate synthase 1) as a Cisplatin-sensitizing Therapeutic Target. Clin. Cancer Res. 2017, 23, 6555–6566. [Google Scholar] [CrossRef]
- Tan, D.S.; Haaland, B.; Gan, J.M.; Tham, S.C.; Sinha, I.; Tan, E.H.; Lim, K.H.; Takano, A.; Krisna, S.S.; Thu, M.M.; et al. Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol. Cancer 2014, 13, 13. [Google Scholar] [CrossRef]
- Fan, R.Y.; Wu, J.Q.; Liu, Y.Y.; Liu, X.Y.; Qian, S.T.; Li, C.Y.; Wei, P.; Song, Z.; He, M.F. Zebrafish xenograft model for studying mechanism and treatment of non-small cell lung cancer brain metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 371. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Guo, D.W.; Lao, Q.C.; Xu, Y.Q.; Meng, Z.K.; Xia, B.; Yang, H.; Li, C.Q.; Li, P. Sensitization and synergistic anti-cancer effects of Furanodiene identified in zebrafish models. Sci. Rep. 2019, 9, 4541. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Z.; Gu, L.; Li, L.; Gao, Q.; Zhang, X.; Fu, J.; Guo, Y.; Li, Q.; Shen, X.; et al. Ophiopogonin B alleviates cisplatin resistance of lung cancer cells by inducing Caspase-1/GSDMD dependent pyroptosis. J. Cancer 2022, 13, 715–727. [Google Scholar] [CrossRef]
- Pearce, M.C.; Gamble, J.T.; Kopparapu, P.R.; O’Donnell, E.F.; Mueller, M.J.; Jang, H.S.; Greenwood, J.A.; Satterthwait, A.C.; Tanguay, R.L.; Zhang, X.K.; et al. Induction of apoptosis and suppression of tumor growth by Nur77-derived Bcl-2 converting peptide in chemoresistant lung cancer cells. Oncotarget 2018, 9, 26072–26085. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Gamble, J.T.; Pearce, M.C.; Elson, D.J.; Tanguay, R.L.; Kolluri, S.K.; Reich, N.O. Improved in vivo targeting of BCL-2 phenotypic conversion through hollow gold nanoshell delivery. Apoptosis 2019, 24, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Huang, L.T.; Wu, J.Q.; He, M.F.; Zhu, S.H.; Zhan, P.; Lv, T.F.; Song, Y. Zebrafish Xenograft Model of Human Lung Cancer for Evaluating Osimertinib Resistance. Biomed Res. Int. 2019, 2019, 3129748. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, E.S.; Kim, D.; Park, S.H.; Kim, E.J.; Rho, J.; Seo, H.; Kim, M.J.; Yang, W.M.; Ha, I.J.; et al. Cancer cell-specific anticancer effects of Coptis chinensis on gefitinib-resistant lung cancer cells are mediated through the suppression of Mcl-1 and Bcl-2. Int. J. Oncol. 2020, 56, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Li, Y.; Li, Y.; Zhang, H.; Song, Z.; Xu, J.; Guo, Y. A natural xanthone suppresses lung cancer growth and metastasis by targeting STAT3 and FAK signaling pathways. Phytomedicine 2022, 102, 154118. [Google Scholar] [CrossRef] [PubMed]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.; Stivala, L.A. Resveratrol analogue 4,4′-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci. Rep. 2016, 6, 19973. [Google Scholar] [CrossRef]
- Zhi, T.X.; Liu, K.Q.; Cai, K.Y.; Zhao, Y.C.; Li, Z.W.; Wang, X.; He, X.H.; Sun, X.Y. Anti-Lung Cancer Activities of 1,2,3-Triazole Curcumin Derivatives via Regulation of the MAPK/NF-κB/STAT3 Signaling Pathways. ChemMedChem 2022, 17, e202100676. [Google Scholar] [CrossRef]
- Schneider, N.F.Z.; Cerella, C.; Lee, J.Y.; Mazumder, A.; Kim, K.R.; de Carvalho, A.; Munkert, J.; Pádua, R.M.; Kreis, W.; Kim, K.W.; et al. Cardiac Glycoside Glucoevatromonoside Induces Cancer Type-Specific Cell Death. Front. Pharmacol. 2018, 9, 70. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, L.; Dai, W.; Yan, B.; Zhang, J.; Yuan, Q.; Zhou, L.; Shan, L.; Efferth, T. Green tea-derived theabrownin suppresses human non-small cell lung carcinoma in xenograft model through activation of not only p53 signaling but also MAPK/JNK signaling pathway. J. Ethnopharmacol. 2022, 291, 115167. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Khan, M.F.; Ahmed, A.F.; Tabassum, S.; Luciano, P.; Chianese, G.; Taglialatela-Scafati, O. Asporychalasin, a bioactive cytochalasan with an unprecedented 6/6/11 skeleton from the Red Sea sediment Aspergillus oryzae. Phytochemistry 2021, 192, 112952. [Google Scholar] [CrossRef]
- Shi, Y.J.; Zheng, H.X.; Hong, Z.P.; Wang, H.B.; Wang, Y.; Li, M.Y.; Li, Z.H. Antitumor effects of different Ganoderma lucidum spore powder in cell- and zebrafish-based bioassays. J. Integr. Med. 2021, 19, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Mazumder, A.; Diederich, M. Preclinical Assessment of the Bioactivity of the Anticancer Coumarin OT48 by Spheroids, Colony Formation Assays, and Zebrafish Xenografts. J. Vis. Exp. 2018, 136, e57490. [Google Scholar] [CrossRef]
- Lee, J.Y.; Talhi, O.; Jang, D.; Cerella, C.; Gaigneaux, A.; Kim, K.W.; Lee, J.W.; Dicato, M.; Bachari, K.; Han, B.W.; et al. Cytostatic hydroxycoumarin OT52 induces ER/Golgi stress and STAT3 inhibition triggering non-canonical cell death and synergy with BH3 mimetics in lung cancer. Cancer Lett. 2018, 416, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.S.; Ahirwar, B. A steroidal derivative from Trigonella foenum graecum L. that induces apoptosis in vitro and in vivo. J. Food Drug Anal. 2019, 27, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, R.; Ceasar, S.A.; Divyarupa, A. Chemosuppressive effect of plumbagin on human non-small lung cancer cell xenotransplanted zebrafish. Indian. J. Cancer 2017, 54, 253–256. [Google Scholar] [CrossRef] [PubMed]
- SM, F.M.; Chitra, K.; Joseph, B.; Sundararajan, R. Gelidiella acerosa inhibits lung cancer proliferation. BMC Complement. Altern. Med. 2018, 18, 104. [Google Scholar] [CrossRef]
- Wilkinson, R.N.; van Eeden, F.J. The zebrafish as a model of vascular development and disease. Prog. Mol. Biol. Transl. Sci. 2014, 124, 93–122. [Google Scholar] [CrossRef]
- Hen, G.; Nicenboim, J.; Mayseless, O.; Asaf, L.; Shin, M.; Busolin, G.; Hofi, R.; Almog, G.; Tiso, N.; Lawson, N.D.; et al. Venous-derived angioblasts generate organ-specific vessels during zebrafish embryonic development. Development 2015, 142, 4266–4278. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Wang, S.F.; Li, Y.; He, Q.X.; Liu, K.C.; Yang, Y.P.; Li, X.L. Isolation of chemical constituents from the aerial parts of Verbascum thapsus and their antiangiogenic and antiproliferative activities. Arch. Pharm. Res. 2011, 34, 703–707. [Google Scholar] [CrossRef]
- Jesubatham, P.D.; VM, B.G.; Viswanathan, S.; Srividya, S. Non-toxic and non teratogenic extract of Thuja orientalis L. inhibited angiogenesis in zebra fish and suppressed the growth of human lung cancer cell line. Biomed. Pharmacother. 2018, 106, 699–706. [Google Scholar] [CrossRef]
- Xie, L.X.; Zhang, H.C.; Wang, H.Y.; Wang, Y.; Wang, F.L.; Sun, J.Y. Two new triterpenoids from Gypsophila oldhamiana. Nat. Prod. Res. 2016, 30, 1068–1074. [Google Scholar] [CrossRef]
- Zhao, Y.L.; He, Q.X.; Li, Y.; Wang, S.F.; Liu, K.C.; Yang, Y.P.; Li, X.L. Chemical constituents of Excoecaria acerifolia and their bioactivities. Molecules 2010, 15, 2178–2186. [Google Scholar] [CrossRef]
- Liu, G.; Kuang, S.; Wu, S.; Jin, W.; Sun, C. A novel polysaccharide from Sargassum integerrimum induces apoptosis in A549 cells and prevents angiogensis in vitro and in vivo. Sci. Rep. 2016, 6, 26722. [Google Scholar] [CrossRef]
- Long, W.; Wang, M.; Luo, X.; Huang, G.; Chen, J. Murrangatin suppresses angiogenesis induced by tumor cell-derived media and inhibits AKT activation in zebrafish and endothelial cells. Drug Des. Dev. Ther. 2018, 12, 3107–3115. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, J.; Lin, Q.; Gong, G.; Sun, H.; Liu, W.; Guo, Q.; Feng, F.; Qu, W. Anti-angiogenic and anticancer effects of baicalein derivatives based on transgenic zebrafish model. Bioorganic Med. Chem. 2018, 26, 4481–4492. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Z.; Wang, L.; Liu, Y.; Stagos, D.; Lin, X.; Liu, M. Marine Bromophenol Bis(2,3,6-Tribromo-4,5-Dihydroxybenzyl)ether Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells and Reduces Vasculogenic Mimicry in Human Lung Cancer A549 Cells. Mar. Drugs 2021, 19, 641. [Google Scholar] [CrossRef]
- Chou, C.K.; Liu, W.; Hong, Y.J.; Dahms, H.U.; Chiu, C.H.; Chang, W.T.; Chien, C.M.; Yen, C.H.; Cheng, Y.B.; Chiu, C.C. Ethyl Acetate Extract of Scindapsus cf. hederaceus Exerts the Inhibitory Bioactivity on Human Non-Small Cell Lung Cancer Cells through Modulating ER Stress. Int. J. Mol. Sci. 2018, 19, 1832. [Google Scholar] [CrossRef]
- Reddy, V.G.; Reddy, T.S.; Jadala, C.; Reddy, M.S.; Sultana, F.; Akunuri, R.; Bhargava, S.K.; Wlodkowic, D.; Srihari, P.; Kamal, A. Pyrazolo-benzothiazole hybrids: Synthesis, anticancer properties and evaluation of antiangiogenic activity using in vitro VEGFR-2 kinase and in vivo transgenic zebrafish model. Eur. J. Med. Chem. 2019, 182, 111609. [Google Scholar] [CrossRef]
- Ganga Reddy, V.; Srinivasa Reddy, T.; Privér, S.H.; Bai, Y.; Mishra, S.; Wlodkowic, D.; Mirzadeh, N.; Bhargava, S. Synthesis of Gold(I) Complexes Containing Cinnamide: In Vitro Evaluation of Anticancer Activity in 2D and 3D Spheroidal Models of Melanoma and In Vivo Angiogenesis. Inorg. Chem. 2019, 58, 5988–5999. [Google Scholar] [CrossRef]
- Sun, F.; Shaikh, A.S.; Wang, J.; Gao, H.; Yang, Z.; Wang, Z.; Li, Y.; Wang, F.; Tan, H. Higher Anti-angiogenesis Activity, Better Cellular Uptake and Longer Half-life of a Novel Glyco-modified Endostatin by Polysulfated Heparin. Curr. Pharm. Biotechnol. 2018, 19, 996–1004. [Google Scholar] [CrossRef]
- Vogt, A.; McPherson, P.A.; Shen, X.; Balachandran, R.; Zhu, G.; Raccor, B.S.; Nelson, S.G.; Tsang, M.; Day, B.W. High-content analysis of cancer-cell-specific apoptosis and inhibition of in vivo angiogenesis by synthetic (-)-pironetin and analogs. Chem. Biol. Drug Des. 2009, 74, 358–368. [Google Scholar] [CrossRef]
- Di Martile, M.; Gabellini, C.; Desideri, M.; Matraxia, M.; Farini, V.; Valentini, E.; Carradori, S.; Ercolani, C.; Buglioni, S.; Secci, D.; et al. Inhibition of lysine acetyltransferases impairs tumor angiogenesis acting on both endothelial and tumor cells. J. Exp. Clin. Cancer Res. 2020, 39, 103. [Google Scholar] [CrossRef]
- Jin, Y.; Wei, L.; Jiang, Q.; Song, X.; Teng, C.; Fan, C.; Lv, Y.; Liu, Y.; Shen, W.; Li, L.; et al. Comparison of efficacy and toxicity of bevacizumab, endostar and apatinib in transgenic and human lung cancer xenograftzebrafish model. Sci. Rep. 2018, 8, 15837. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ye, H.; Peng, A.; Chen, L. Barbigerone, an isoflavone, inhibits tumor angiogenesis and human non-small-cell lung cancer xenografts growth through VEGFR2 signaling pathways. Cancer Chemother. Pharmacol. 2012, 70, 425–437. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, A.; He, S.; Shao, X.; Nie, C.; Chen, L. Isogambogenic acid inhibits tumour angiogenesis by suppressing Rho GTPases and vascular endothelial growth factor receptor 2 signalling pathway. J. Chemother. 2013, 25, 298–308. [Google Scholar] [CrossRef]
- Buchanan, C.M.; Shih, J.H.; Astin, J.W.; Rewcastle, G.W.; Flanagan, J.U.; Crosier, P.S.; Shepherd, P.R. DMXAA (Vadimezan, ASA404) is a multi-kinase inhibitor targeting VEGFR2 in particular. Clin. Sci. 2012, 122, 449–457. [Google Scholar] [CrossRef]
- Cao, Z.X.; Zheng, R.L.; Lin, H.J.; Luo, S.D.; Zhou, Y.; Xu, Y.Z.; Zeng, X.X.; Wang, Z.; Zhou, L.N.; Mao, Y.Q.; et al. SKLB610: A novel potential inhibitor of vascular endothelial growth factor receptor tyrosine kinases inhibits angiogenesis and tumor growth in vivo. Cell Physiol. Biochem. 2011, 27, 565–574. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, J.; Cao, Z.; Chen, X.; Hu, Y.; Li, L.; Yang, S. Preclinical pharmacodynamic evaluation of drug candidate SKLB-178 in the treatment of non-small cell lung cancer. Oncotarget 2017, 8, 12843–12854. [Google Scholar] [CrossRef]
- Presta, M.; Dell’Era, P.; Mitola, S.; Moroni, E.; Ronca, R.; Rusnati, M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor. Rev. 2005, 16, 159–178. [Google Scholar] [CrossRef]
- Jia, T.; Jacquet, T.; Dalonneau, F.; Coudert, P.; Vaganay, E.; Exbrayat-Héritier, C.; Vollaire, J.; Josserand, V.; Ruggiero, F.; Coll, J.L.; et al. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Hwang, S.J.; Park, H.G.; Park, Y.; Lee, H.J. An α-quaternary chiral latam derivative, YH-304 as a novel broad-spectrum anticancer agent. Int. J. Oncol. 2016, 49, 2480–2486. [Google Scholar] [CrossRef]
- Moshal, K.S.; Ferri-Lagneau, K.F.; Haider, J.; Pardhanani, P.; Leung, T. Discriminating different cancer cells using a zebrafish in vivo assay. Cancers 2011, 3, 4102–4113. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, C.; Liu, D.; Wang, Y.; Wang, H.; Li, T.; Hou, H.; Zhou, N.; Zhu, J.; Lv, H.; et al. Influence and mechanism of lung cavitation development on antiangiogenic therapy. Transl. Lung Cancer Res. 2019, 8, 500–512. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, H.; Meng, N.; Lu, W.; Li, G.; Han, Y.; Dai, X.; Xia, Y.; Song, X.; Yang, S.; et al. YL529, a novel, orally available multikinase inhibitor, potently inhibits angiogenesis and tumour growth in preclinical models. Br. J. Pharmacol. 2013, 169, 1766–1780. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Cai, X.; Chen, S.; Zhang, J.; Li, B.; Chen, W.; Guo, X.; Luo, H.; Chen, J. Cyclometalated Ru(II)-isoquinoline complexes overcome cisplatin resistance of A549/DDP cells by downregulation of Nrf2 via Akt/GSK-3β/Fyn pathway. Bioorg. Chem. 2022, 119, 105516. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Deng, Y.; Wang, T.; Miao, T.; Li, C.; Cai, X.; Liu, Y.; Henri, J.; Chen, L. Ru(II) Complexes Bearing O, O-Chelated Ligands Induced Apoptosis in A549 Cells through the Mitochondrial Apoptotic Pathway. Bioinorg. Chem. Appl. 2020, 2020, 8890950. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Li, N.; Chen, L.; Sun, J. Discovery of novel dihydroartemisinin-cinnamic hybrids inducing lung cancer cells apoptosis via inhibition of Akt/Bad signal pathway. Bioorg. Chem. 2021, 111, 104903. [Google Scholar] [CrossRef]
- Perumal, S.; Gopal Samy, M.V.; Subramanian, D. Selenium nanoparticle synthesis from endangered medicinal herb (Enicostema axillare). Bioprocess. Biosyst. Eng. 2021, 44, 1853–1863. [Google Scholar] [CrossRef]
- Rozalen, M.; Sánchez-Polo, M.; Fernández-Perales, M.; Widmann, T.J.; Rivera-Utrilla, J. Synthesis of controlled-size silver nanoparticles for the administration of methotrexate drug and its activity in colon and lung cancer cells. RSC Adv. 2020, 10, 10646–10660. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Zhang, X.; Sun, Z.; Wang, F.; Cheng, J.; Xie, H.; Yu, B.; Zhou, L. Deoxycholic acid-modified chitooligosaccharide/mPEG-PDLLA mixed micelles loaded with paclitaxel for enhanced antitumor efficacy. Int. J. Pharm. 2014, 475, 60–68. [Google Scholar] [CrossRef]
- Marquez, C.M.D.; Garcia, J.G.; Antonio, J.G.; Jacinto, S.D.; Velarde, M.C. Alangium longiflorum Merr. Leaf Extract Induces Apoptosis in A549 Lung Cancer Cells with Minimal NFκB Transcriptional Activation. Asian Pac. J. Cancer Prev. 2020, 21, 2453–2461. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Zhang, S.R.; Li, C.Y.; Jiang, L.L.; Wei, P.; He, M.F. Mechanism of hepatotoxicity of first-line tyrosine kinase inhibitors: Gefitinib and afatinib. Toxicol. Lett. 2021, 343, 1–10. [Google Scholar] [CrossRef]
- Aleksandar, P.; Dragana, M.; Nebojša, J.; Biljana, N.; Nataša, S.; Branka, V.; Jelena, K.V. Wild edible onions—Allium flavum and Allium carinatum—Successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491. [Google Scholar] [CrossRef]
- Monroe, J.D.; Hodzic, D.; Millay, M.H.; Patty, B.G.; Smith, M.E. Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin. Molecules 2019, 24, 3889. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xie, Y.; Wu, Q.; Wang, Y.; Deng, S.; Xiong, D.; Liu, L.; Xiang, M.; Qian, Z.; Wei, Y. Improving anti-tumor activity with polymeric micelles entrapping paclitaxel in pulmonary carcinoma. Nanoscale 2012, 4, 6004–6017. [Google Scholar] [CrossRef]
- Gou, M.; Shi, H.; Guo, G.; Men, K.; Zhang, J.; Zheng, L.; Li, Z.; Luo, F.; Qian, Z.; Zhao, X.; et al. Improving anticancer activity and reducing systemic toxicity of doxorubicin by self-assembled polymeric micelles. Nanotechnology 2011, 22, 095102. [Google Scholar] [CrossRef]
- Askes, S.H.C.; Bossert, N.; Bussmann, J.; Talens, V.S.; Meijer, M.S.; Kieltyka, R.E.; Kros, A.; Bonnet, S.; Heinrich, D. Dynamics of dual-fluorescent polymersomes with durable integrity in living cancer cells and zebrafish embryos. Biomaterials 2018, 168, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Song, Y.; Zhu, C.; Wang, J.; Wang, K.; Guo, Z. Detecting and delivering platinum anticancer drugs using fluorescent maghemite nanoparticles. Chem. Commun. 2013, 49, 2786–2788. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Z.; Jin, J.; Liu, Y.; Chen, C.; Tang, Z. Optimizing Energy Transfer in Nanostructures Enables In Vivo Cancer Lesion Tracking via Near-Infrared Excited Hypoxia Imaging. Adv. Mater. 2020, 32, e1907718. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Vildevall, M.; Rodriguez, G.V.; Tandiono, D.; Vamvakaris, I.; Evangelou, G.; Lolas, G.; Syrigos, K.N.; Villanueva, A.; Wick, M.; et al. Zebrafish patient-derived xenograft models predict lymph node involvement and treatment outcome in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 58. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Gaudenzi, G.; Dicitore, A.; Cantone, M.C.; Plebani, A.; Saronni, D.; Zappavigna, S.; Caraglia, M.; Candeo, A.; Bassi, A.; et al. Modeling Lung Carcinoids with Zebrafish Tumor Xenograft. Int. J. Mol. Sci. 2022, 23, 8126. [Google Scholar] [CrossRef] [PubMed]
- Cadiz, L.; Jonz, M.G. A comparative perspective on lung and gill regeneration. J. Exp. Biol. 2020, 223, jeb226076. [Google Scholar] [CrossRef] [PubMed]

| Mutation Models | Transgenic Models | Xenograft Models | Drug Screening | ||
|---|---|---|---|---|---|
| Mice | ++++ | ++++ | ++++ | +++ | |
| Advantages | Well-established technology Diverse tumor types | Well-established technology | Possibility of orthotopic tissue transplantation Suitable for expansion of primary tumor samples | Relatively high conservation of genes and proteins that have homologs in humans | |
| Disadvantages | Difficulty in phenotypic identification | Long development cycle | Additional immunosuppression Poor metastatic potential | Relatively high cost Long test cycle (several months) Ethical issues | |
| Large mammals | + | ++ | N/A | + | |
| Advantages | Dogs and primates High conservation of genes and proteins that have homologs in humans Usually exposed to environmental risk factors similar to those affecting humans | Pigs High conservation of genes and proteins that have homologs in humans | High conservation of genes and proteins that have homologs in humans Easy clinical translation of experimental data | ||
| Disadvantages | Only spontaneous models are available Large individual differences | Lack of suitable technology | High cost Long test cycle (months to years) Ethical issues | ||
| Chicken chorioallantoic membrane | N/A | N/A | ++ | +++ | |
| Advantages | Naturally immunodeficient Simple operation | Low costShort test cycle Easy to image No ethical issues | |||
| Disadvantages | Low conservation of genes and proteins that have homologs in humans Lack of tumor microenvironment | Low conservation of genes and proteins that have homologs in humans Unsuitable for immune therapy | |||
| Drosophila melanogaster | N/A | +++ | N/A | +++ | |
| Advantages | Low genetic redundancy Well-established technology | Low cost Short test cycle | |||
| Disadvantages | Low conservation of genes and proteins that have homologs in humans | Low conservation of genes and proteins that have homologs in humans Unsuitable for immune and anti-vascular therapy | |||
| Zebrafish | +++ | ++++ | +++ | ++++ | |
| Advantages | Well-established technology Diverse tumor types Convenient phenotypic identification | Well-established technology | Simple operation Naturally immunodeficient (embryo) | Medium conservation of genes and proteins that have homologs in humans Low cost Short test cycle Transparent body, easy to image Administration by dissolving | |
| Disadvantages | Genetic redundancy | Relatively low conservation of genes and proteins that have homologs in humans | Impossibility of orthotopic transplantation (breast, lung, and prostate tumors) Lack of tumor microenvironment | Unsuitable for immune therapy | |
| Neoplastic organoids | N/A | N/A | N/A | ++++ | |
| Advantages | Low cost Short test cycle No ethical issues | ||||
| Disadvantages | Technology requires optimization Unsuitable for anti-vascular therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Hua, X.; Xu, K.; Song, Y.; Lv, T. Zebrafish in Lung Cancer Research. Cancers 2023, 15, 4721. https://doi.org/10.3390/cancers15194721
Wu X, Hua X, Xu K, Song Y, Lv T. Zebrafish in Lung Cancer Research. Cancers. 2023; 15(19):4721. https://doi.org/10.3390/cancers15194721
Chicago/Turabian StyleWu, Xiaodi, Xin Hua, Ke Xu, Yong Song, and Tangfeng Lv. 2023. "Zebrafish in Lung Cancer Research" Cancers 15, no. 19: 4721. https://doi.org/10.3390/cancers15194721





