Clinical Characteristics and Treatment Outcomes of Oral Cancers Using Transoral Robotic Surgery in an Endemic Region
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient and Treatment Parameters in Oral Cancer Patients Treated with TORS versus Conventional Open Surgery
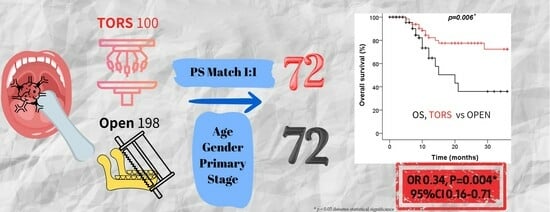
3.2. Comparison of Survival Outcomes in Oral Cancer Patients Treated with TORS versus Conventional Open Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oralhealth (accessed on 18 May 2023).
- International Agency for Research on Cancer. GLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: http://globocan.iarc.fr/ (accessed on 18 May 2023).
- Chou, C.W.; Lin, C.R.; Chung, Y.T.; Tang, C.S. Epidemiology of Oral Cancer in Taiwan: A Population-Based Cancer Registry Study. Cancers 2023, 15, 2175. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.S.; Chen, Y.C.; Chen, T.I.; Lee, Y.K.; Chiang, C.J.; You, S.L.; Hsu, W.L.; Liao, L.J. Incidence trends of oral cavity, oropharyngeal, hypopharyngeal and laryngeal cancers among males in Taiwan, 1980–2019: A population-based cancer registry study. BMC Cancer 2023, 23, 213. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, A.; Gosavi, A.S.; Shirwadkar, U.P.; Wangdale, K.V.; Behera, S.K.; Kurrey, N.K.; Kalia, K.; Mandoli, A. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral. Oncol. 2021, 121, 105451. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Lailler, C.; Testelin, S.; Chauffert, B.; Clatot, F.; Galmiche, A. Contribution of Genomics to the Surgical Management and Study of Oral Cancer. Ann. Surg. Oncol. 2021, 28, 5842–5854. [Google Scholar] [CrossRef]
- Thaler, E.R. History and Acceptance of Transoral Robotic Surgery. Otolaryngol. Clin. N. Am. 2020, 53, 943–948. [Google Scholar] [CrossRef]
- Salmon, K.M.; Ruiz, C.; Cognetti, D.M.; Curry, J.M.; Luginbuhl, A.J.; Bar-Ad, V.; Leiby, B.E. Functional Swallow-Related Outcomes Following Transoral Robotic Surgery for Base of Tongue Carcinoma. Dysphagia 2022, 37, 28–36. [Google Scholar] [CrossRef]
- Rothrie, S.; Fitzgerald, E.; Brady, G.C.; Roe, J.W.G. The role of the speech and language therapist in the rehabilitation of speech, swallowing, voice and trismus in people diagnosed with head and neck cancer. Br. Dent. J. 2022, 233, 801–805. [Google Scholar] [CrossRef]
- Lazarus, C.L.; Ganz, C.; Ru, M.; Miles, B.A.; Kotz, T.; Chai, R.L. Prospective instrumental evaluation of swallowing, tongue function, and QOL measures following transoral robotic surgery alone without adjuvant therapy. Head Neck 2019, 41, 322–328. [Google Scholar] [CrossRef]
- Moore, K.; Ford, P.; Farah, C. Support needs and quality of life in oral cancer: A systematic review. Int. J. Dent. Hyg. 2014, 12, 36–47. [Google Scholar] [CrossRef]
- Genden, E.M.; Desai, S.; Sung, C.K. Transoral robotic surgery for the management of head and neck cancer: A preliminary experience. Head Neck 2009, 31, 283–289. [Google Scholar] [CrossRef]
- Weinstein, G.S.; O’Malley, B.W.; Magnuson, J.S.; Carroll, W.R.; Olsen, K.D.; Daio, L.; Moore, E.J.; Holsinger, F.C. Transoral robotic surgery: A multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 2012, 122, 1701–1707. [Google Scholar] [CrossRef]
- Hackman, T.G.; Patel, S.N.; Deal, A.M.; Neil Hayes, D.; Chera, B.S.; Paul, J.; Knowles, M.; Usenko, D.; Grilley-Olson, J.E.; Weissler, M.C.; et al. Novel induction therapy transoral surgery treatment paradigm with risk-adapted adjuvant therapy for squamous cell carcinoma of the head and neck—Mature clinical and functional outcomes. Oral Oncol. 2020, 110, 104957. [Google Scholar] [CrossRef]
- Olson, B.; Cahill, E.; Imanguli, M. Feasibility and safety of the da Vinci Xi surgical robot for transoral robotic surgery. J. Robot. Surg. 2023, 17, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Iseli, T.A.; Kulbersh, B.D.; Iseli, C.E.; Carroll, W.R.; Rosenthal, E.L.; Magnuson, J.S. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol. Head Neck Surg. 2009, 141, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Delas, B.; Gorphe, P.; Menard, M.; Brasnu, D. Transoral robotic surgery in head and neck cancer. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012, 129, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, K.A.; Holsinger, F.C.; Kupferman, M.E.; Lewin, J.S. Functional outcomes after TORS for oropharyngeal cancer: A systematic review. Eur. Arch. Otorhinolaryngol. 2015, 272, 463–471. [Google Scholar] [CrossRef]
- Ionna, F.; Guida, A.; Califano, L.; Motta, G.; Salzano, G.; Pavone, E.; Aversa, C.; Longo, F.; Villano, S.; Ponzo, L.M.; et al. Transoral robotic surgery in head and neck district: A retrospective study on 67 patients treated in a single center. Infect. Agents Cancer 2020, 15, 40. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Luu, M.; Mallen-St Clair, J.; Mita, A.C.; Scher, K.S.; Lu, D.J.; Shiao, S.L.; Ho, A.S.; Zumsteg, Z.S. Comparison of Survival After Transoral Robotic Surgery vs Nonrobotic Surgery in Patients With Early-Stage Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2020, 6, 1555–1562. [Google Scholar] [CrossRef]
- Roselló, À.; Albuquerque, R.; Roselló-Llabrés, X.; Marí-Roig, A.; Estrugo-Devesa, A.; López-López, J. Transoral robotic surgery vs open surgery in head and neck cancer. A systematic review of the literature. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e599–e607. [Google Scholar] [CrossRef]
- Genden, E.M.; Kotz, T.; Tong, C.C.; Smith, C.; Sikora, A.G.; Teng, M.S.; Packer, S.H.; Lawson, W.L.; Kao, J. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope 2011, 121, 1668–1674. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Ko, Y.C.; Huang, H.L.; Chao, Y.Y.; Tsai, C.C.; Shieh, T.Y.; Lin, L.M. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br. J. Cancer 2003, 88, 366–372. [Google Scholar] [CrossRef] [PubMed]
- White, H.N.; Moore, E.J.; Rosenthal, E.L.; Carroll, W.R.; Olsen, K.D.; Desmond, R.A.; Magnuson, J.S. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: One- and 2-year survival analysis. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Philips, R.; Topf, M.C.; Crawley, M.B.; Swendseid, B.; Luginbuhl, A.; Curry, J.; Cognetti, D. Functional and survival outcomes in elderly patients undergoing transoral robotic surgery. Oral Oncol. 2020, 111, 104954. [Google Scholar] [CrossRef] [PubMed]
- Panda, N.K.; Kapoor, A.; Goel, N.; Ghoshal, S.; Singh, V.; Bal, A. Analysis of Outcomes following TORS in a Mixed Cohort of Recurrent and New T1-T2 Oropharyngeal Cancer—A Single Institution Study. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lörincz, B.B.; Knecht, R. TORS for head and neck squamous cell carcinoma (HNSCC): The Hamburg experience. B-ENT 2015, 11 (Suppl. S24), 33–36. [Google Scholar]
- Tirelli, G.; Piccinato, A.; Antonucci, P.; Gatto, A.; Marcuzzo, A.V.; Tofanelli, M. Surgical resection of oral cancer: En-bloc versus discontinuous approach. Eur. Arch. Otorhinolaryngol. 2020, 277, 3127–3135. [Google Scholar] [CrossRef]
- Poon, H.; Li, C.; Gao, W.; Ren, H.; Lim, C.M. Evolution of robotic systems for transoral head and neck surgery. Oral Oncol. 2018, 87, 82–88. [Google Scholar] [CrossRef]
- Tirelli, G.; Boscolo Nata, F.; Piovesana, M.; Quatela, E.; Gardenal, N.; Hayden, R.E. Transoral surgery (TOS) in oropharyngeal cancer: Different tools, a single mini-invasive philosophy. Surg. Oncol. 2018, 27, 643–649. [Google Scholar] [CrossRef]
- Tay, G.; Tan, H.K.; Nguyen, T.K.; Phee, S.J.; Iyer, N.G. Use of the EndoMaster robot-assisted surgical system in transoral robotic surgery: A cadaveric study. Int. J. Med. Robot. 2018, 14, e1930. [Google Scholar] [CrossRef]
- Warner, L.; O’Hara, J.T.; Lin, D.J.; Oozeer, N.; Fox, H.; Meikle, D.; Hamilton, D.; Iqbal, M.S.; Robinson, M.; Paleri, V. Transoral robotic surgery and neck dissection alone for head and neck squamous cell carcinoma: Influence of resection margins on oncological outcomes. Oral Oncol. 2022, 130, 105909. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Thakar, A.; Fernández-Fernández, M.M.; Panda, S.; Sikka, K.; Amit Singh, C.; Kumar, R.; Kakkar, A.; Sharma, A.; Bhasker, S. TransOral UltraSonic surgery (TOUSS) for oral cavity, oropharyngeal and supraglottic malignancy: A prospective study of feasibility, safety, margins, functional and survival outcomes. Oral Oncol. 2022, 124, 105643. [Google Scholar] [CrossRef] [PubMed]
- Tay, G.; Ferrell, J.; Andersen, P. Use of a midline mandibular osteotomy to improve surgical access for transoral robotic resection of the base of tongue in a patient with trismus. Head Neck 2017, 39, E92–E95. [Google Scholar] [CrossRef] [PubMed]
- Iloreta, A.M.; Anderson, K.; Miles, B.A. Mandibular osteotomy for expanded transoral robotic surgery: A novel technique. Laryngoscope 2014, 124, 1836–1842. [Google Scholar] [CrossRef]
- Morisod, B.; Venara-Vulpe, I.I.; Alzuphar, S.; Monnier, Y.; Bongiovanni, M.; Hagmann, P.; Bouchaab, H.; Bourhis, J.; Simon, C. Minimizing adjuvant treatment after transoral robotic surgery through surgical margin revision and exclusion of radiographic extracapsular extension: A Prospective observational cohort study. Head Neck 2017, 39, 965–973. [Google Scholar] [CrossRef]
- Cramer, J.D.; Ferris, R.L.; Kim, S.; Duvvuri, U. Primary surgery for human papillomavirus-associated oropharyngeal cancer: Survival outcomes with or without adjuvant treatment. Oral Oncol. 2018, 87, 170–176. [Google Scholar] [CrossRef]
- Bozec, A.; Culié, D.; Poissonnet, G.; Dassonville, O. Current role of primary surgical treatment in patients with head and neck squamous cell carcinoma. Curr. Opin. Oncol. 2019, 31, 138–145. [Google Scholar] [CrossRef]
- Dabas, S.; Gupta, K.; Ranjan, R.; Sharma, A.K.; Shukla, H.; Dinesh, A. Oncological outcome following de-intensification of treatment for stage I and II HPV negative oropharyngeal cancers with transoral robotic surgery (TORS): A prospective trial. Oral Oncol. 2017, 69, 80–83. [Google Scholar] [CrossRef]
- Brody, R.M.; Shimunov, D.; Cohen, R.B.; Lin, A.; Lukens, J.N.; Hartner, L.; Aggarwal, C.; Duvvuri, U.; Montone, K.T.; Jalaly, J.B.; et al. A benchmark for oncologic outcomes and model for lethal recurrence risk after transoral robotic resection of HPV-related oropharyngeal cancers. Oral Oncol. 2022, 127, 105798. [Google Scholar] [CrossRef]



| Characteristic | Open (n = 72) | TORS (n = 72) | Total (n = 144) | p-Value |
|---|---|---|---|---|
| Age, mean ± SD | 58.6 ± 11.0 | 57.1 ± 11.5 | 57.8 ± 11.3 | 0.418 |
| Gender, n (%) | ||||
| Male | 69 (95.8%) | 67 (93.1%) | 136 (94.4%) | 0.467 |
| Female | 3 (4.2%) | 5 (6.9%) | 8 (5.6%) | |
| Primary site (ICD 10), n (%) | ||||
| Tongue, dorsal (C020) | 0 (0%) | 1 (1.4%) | 1 (0.7%) | 0.775 |
| Tongue, border (C021) | 23 (31.9%) | 17 (23.6%) | 40 (27.8%) | |
| Tongue, ventral (C022) | 2 (2.8%) | 4 (5.6%) | 6 (4.2%) | |
| Gum, upper (C030) | 2 (2.8%) | 2 (2.8%) | 4 (2.8%) | |
| Gum, lower (C031) | 10 (13.9%) | 8 (11.1%) | 18 (12.5%) | |
| Buccal (C060) | 31 (43.1%) | 34 (47.2%) | 65 (45.1%) | |
| Retromolar area (C062) | 4 (5.6%) | 5 (6.9%) | 9 (6.3%) | |
| Overlapping sites of mouth (C068) | 0 (0%) | 1 (1.4%) | 1 (0.7%) | |
| Clinical T-stage, n (%) | ||||
| T1 | 14 (19.4%) | 14 (19.4%) | 28 (19.4%) | 0.755 |
| T2 | 24 (33.3%) | 23 (31.9%) | 47 (32.6%) | |
| T3 | 6 (8.3%) | 10 (13.9%) | 16 (11.1%) | |
| T4 | 28 (38.9%) | 25 (34.7%) | 53 (36.8%) | |
| Clinical N-stage, n (%) | ||||
| N0 | 40 (55.6%) | 33 (45.8%) | 73 (50.7%) | 0.335 |
| N1 | 10 (13.9%) | 10 (13.9%) | 20 (13.9%) | |
| N2 | 20 (27.8%) | 25 (24.7%) | 45 (31.3%) | |
| N3 | 2 (2.8%) | 1 (1.4%) | 3 (2.1%) | |
| Nx | 0 (0%) | 3 (4.2%) | 3 (2.1%) | |
| Clinical disease stage, n (%) | ||||
| I | 10 (13.9%) | 11 (15.3%) | 21 (14.6%) | 0.987 |
| II | 13 (18.1%) | 14 (19.4%) | 27 (18.8%) | |
| III | 9 (12.5%) | 9 (12.5%) | 18 (12.5%) | |
| IV | 40 (55.6%) | 38 (52.8%) | 78 (54.2%) | |
| Margin status, n (%) | ||||
| Free | 60 (81.9%) | 70 (97.2%) | 130 (89.6%) | 0.011 * |
| Invasion | 12 (16.7%) | 2 (2.8%) | 12 (9.7%) | |
| Unkown | 1 (1.4%) | 0 (0%) | 1 (0.7%) |
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| Progression-Free Survival | Overall Survival | Progression-Free Survival | Overall Survival | |||||
| Variable | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Gender | ||||||||
| Female (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Male | 1.23 (0.45–3.36) | 0.691 | 0.81 (0.19–3.37) | 0.767 | 1.47 (0.51–4.23) | 0.477 | 0.89 (0.19–4.14) | 0.880 |
| Age | 1.00 (0.98–1.02) | 0.701 | 1.01 (0.98–1.04) | 0.450 | 1.00 (0.98–1.02) | 0.872 | 1.02 (0.98–1.05) | 0.315 |
| Clinical disease stage | ||||||||
| I and II (ref.) | 1.00 | 1.00 | ||||||
| III and IV | 1.06 (0.65–1.72) | 0.816 | 1.85 (0.8–4.29) | 0.151 | ||||
| Surgical approach | ||||||||
| Open (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| TORS | 0.92 (0.59–1.45) | 0.722 | 0.38 (0.18–0.79) | 0.009 * | 0.92 (0.57–1.47) | 0.722 | 0.35 (0.16–0.77) | 0.009 * |
| Margin status | ||||||||
| Free (ref.) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Invasion | 2.08 (1.09–3.97) | 0.026 * | 3.05 (1.31–7.11) | 0.010 * | 1.99 (1.02–3.90) | 0.044 * | 1.13 (0.41–3.15) | 0.812 |
| Unkown | 0.00 (0.00–9.28) | 0.967 | 0.00 (0.00–0.00) | 0.986 | 0.00 (0.00–2.46) | 0.970 | 0.00 (0.00–0.00) | 0.982 |
| CCRT | ||||||||
| Yes vs. No | 2.05 (1.29–3.26) | 0.002 * | 1.91 (0.92–3.98) | 0.082 | 1.57 (0.80–3.08) | 0.192 | ||
| RT | ||||||||
| Yes vs. No | 2.13 (1) | 0.005 * | 3.82 (1.34–10.92) | 0.012 * | 1.49 (0.69–3.25) | 0.315 | 2.96 (0.93–9.43) | 0.066 |
| Chemotherapy | ||||||||
| Yes vs. No | 1.89 (1.04–3.43) | 0.036 * | 4.32 (1.03–18.08) | 0.045 * | 1.09 (0.50–2.38) | 0.836 | 1.34 (0.27–6.67) | 0.721 |
| Cetuximab | ||||||||
| Yes vs. No | 1.45 (0.89–2.36) | 0.140 | 2.62 (1.31–5.26) | 0.007 * | 2.27 (1.07–4.84) | 0.034 * | ||
| Nivolumab/ Pembrolizumab | ||||||||
| Yes vs. No | 1.79 (0.97–3.31) | 0.065 | 3.23 (1.49–6.98) | 0.003 * | 2.23 (0.93–5.36) | 0.072 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Chen, C.-H.; Hsieh, T.-L.; Chang, K.-H.; Huang, J.-Y.; Lin, F.C.-F.; Tsai, S.C.-S. Clinical Characteristics and Treatment Outcomes of Oral Cancers Using Transoral Robotic Surgery in an Endemic Region. Cancers 2023, 15, 4896. https://doi.org/10.3390/cancers15194896
Chang C-C, Chen C-H, Hsieh T-L, Chang K-H, Huang J-Y, Lin FC-F, Tsai SC-S. Clinical Characteristics and Treatment Outcomes of Oral Cancers Using Transoral Robotic Surgery in an Endemic Region. Cancers. 2023; 15(19):4896. https://doi.org/10.3390/cancers15194896
Chicago/Turabian StyleChang, Chia-Chun, Chung-Hsiung Chen, Tsai-Ling Hsieh, Kuang-Hsi Chang, Jing-Yang Huang, Frank Cheau-Feng Lin, and Stella Chin-Shaw Tsai. 2023. "Clinical Characteristics and Treatment Outcomes of Oral Cancers Using Transoral Robotic Surgery in an Endemic Region" Cancers 15, no. 19: 4896. https://doi.org/10.3390/cancers15194896
APA StyleChang, C. -C., Chen, C. -H., Hsieh, T. -L., Chang, K. -H., Huang, J. -Y., Lin, F. C. -F., & Tsai, S. C. -S. (2023). Clinical Characteristics and Treatment Outcomes of Oral Cancers Using Transoral Robotic Surgery in an Endemic Region. Cancers, 15(19), 4896. https://doi.org/10.3390/cancers15194896