Liver Transplantation for Hepatic Metastases from Colorectal Cancer: Current Knowledge and Open Issues
Abstract
:Simple Summary
Abstract
1. Introduction
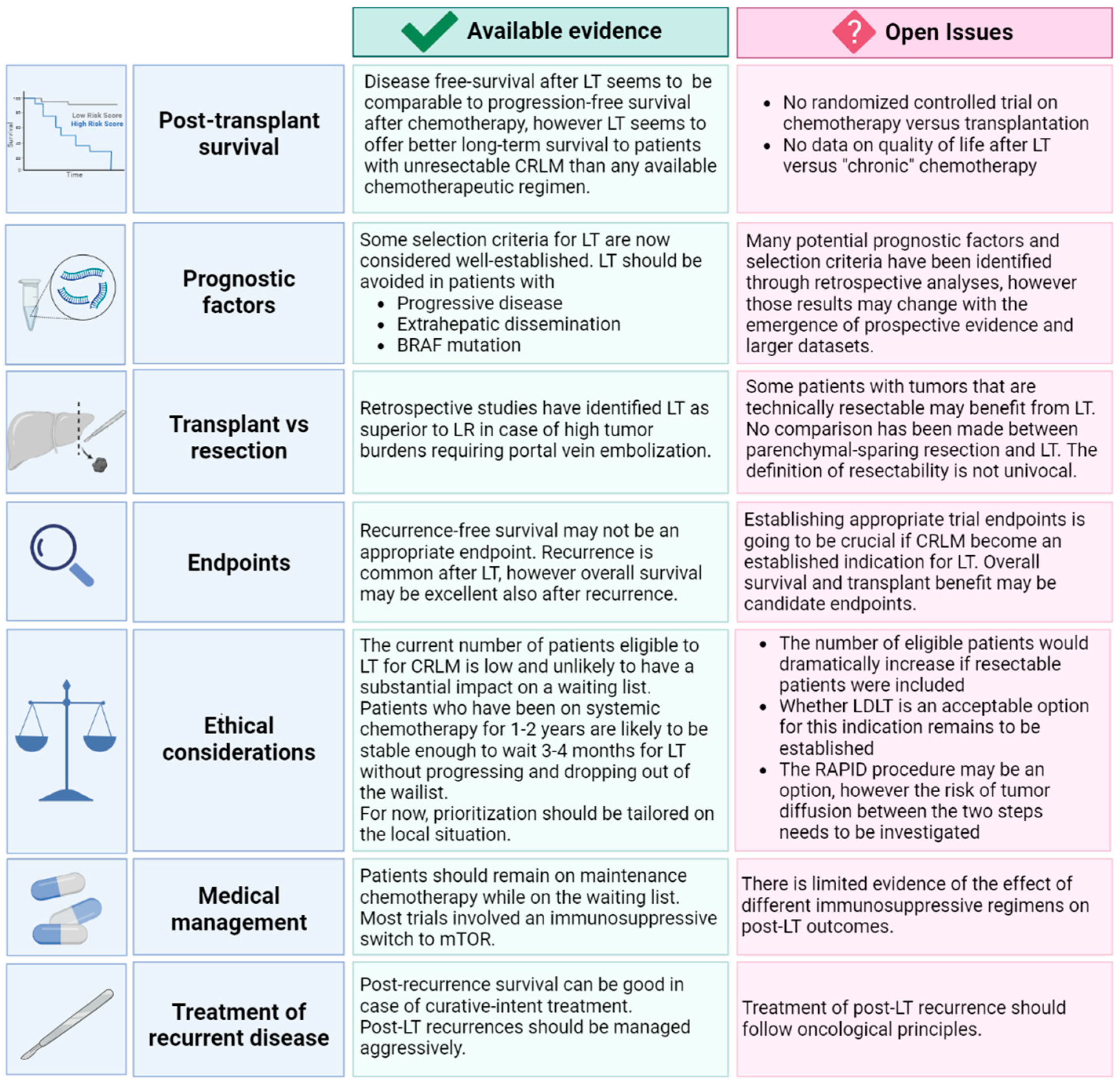
2. Survival after Liver Transplantation
Open Issues in Survival after Liver Transplantation
3. Prognostic Factors
3.1. Factors Associated with the Primary Tumor
3.2. Metabolic Tumor Volume
3.3. Prognostic Scores
3.4. Open Issues in Prognostic Factors
4. Liver Transplant vs. Liver Resection
Open Issues in Liver Transplant vs Liver Resection
5. Endpoints of Liver Transplantation
Open Issues in Endpoints of Liver Tansplant
6. Ethical Considerations
Open Issues in Ethical Considerations
7. Treatment of Recurrent Disease
Open Issues in Treatment of Recurrent Disease
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van der Geest, L.G.M.; Lam-Boer, J.; Koopman, M.; Verhoef, C.; Elferink, M.A.G.; de Wilt, J.H.W. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin. Exp. Metastasis 2015, 32, 457–465. [Google Scholar] [CrossRef]
- Kanas, G.; Taylor, A.; Primrose, J.N.; Langeberg, W.; Kelsh, M.; Mowat, F.; Alexander, D.; Choti, M.; Poston, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin. Epidemiol. 2012, 4, 283–301. [Google Scholar] [CrossRef] [Green Version]
- House, M.G.; Ito, H.; Gönen, M.; Fong, Y.; Allen, P.J.; DeMatteo, R.P.; Brennan, M.F.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Survival after Hepatic Resection for Metastatic Colorectal Cancer: Trends in Outcomes for 1600 Patients during Two Decades at a Single Institution. J. Am. Coll. Surg. 2010, 210, 744–752. [Google Scholar] [CrossRef]
- de Haas, R.J.; Wicherts, D.A.; Andreani, P.; Pascal, G.; Saliba, F.; Ichai, P.; Adam, R.; Castaing, D.; Azoulay, D. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann. Surg. 2011, 253, 1069–1079. [Google Scholar] [CrossRef]
- Gold, J.S.; Are, C.; Kornprat, P.; Jarnagin, W.R.; Gönen, M.; Fong, Y.; DeMatteo, R.P.; Blumgart, L.H.; D’Angelica, M. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: Trends in treatment over time in 440 patients. Ann. Surg. 2008, 247, 109–117. [Google Scholar] [CrossRef]
- Torzilli, G.; Viganò, L.; Gatti, A.; Costa, G.; Cimino, M.; Procopio, F.; Donadon, M.; Del Fabbro, D. Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: Impact on surgical practice and oncologic efficacy. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2017, 19, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, E.K.; Adam, R.; Bilchik, A.J.; Jaeck, D.; Vauthey, J.N.; Mahvi, D. Improving Resectability of Hepatic Colorectal Metastases: Expert Consensus Statement. Ann. Surg. Oncol. 2006, 13, 1271–1280. [Google Scholar] [CrossRef]
- Milana, F.; Famularo, S.; Luberto, A.; Rimassa, L.; Scorsetti, M.; Comito, T.; Pressiani, T.; Franzese, C.; Poretti, D.; Di Tommaso, L.; et al. Multidisciplinary Tumor Board in the Management of Patients with Colorectal Liver Metastases: A Single-Center Review of 847 Patients. Cancers 2022, 14, 3952. [Google Scholar] [CrossRef]
- Isoniemi, H.; Uutela, A.; Nordin, A.; Lantto, E.; Kellokumpu, I.; Ovissi, A.; Kosunen, J.; Kallio, R.; Soveri, L.M.; Salminen, T.; et al. Centralized repeated resectability assessment of patients with colorectal liver metastases during first-line treatment: Prospective study. Br. J. Surg. 2021, 108, 817–825. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Sargent, D.; Campbell, M.E.; Morton, R.F.; Fuchs, C.S.; Ramanathan, R.K.; Williamson, S.K.; Findlay, B.P.; Pitot, H.C.; Goldberg, R.M. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J. Clin. Oncol. 2008, 26, 5721–5727. [Google Scholar] [CrossRef]
- Bredt, L.C.; Rachid, A.F. Predictors of recurrence after a first hepatectomy for colorectal cancer liver metastases: A retrospective analysis. World J. Surg. Oncol. 2014, 12, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, T.M.; Scoggins, C.R.; Zorzi, D.; Abdalla, E.K.; Andres, A.; Eng, C.; Curley, S.A.; Loyer, E.M.; Muratore, A.; Mentha, G.; et al. Effect of Surgical Margin Status on Survival and Site of Recurrence After Hepatic Resection for Colorectal Metastases. Ann. Surg. 2005, 241, 715. [Google Scholar] [CrossRef] [PubMed]
- Mühlbacher, F.; Huk, I.; Steininger, R.; Gnant, M.; Götzinger, P.; Wamser, P.; Banhegyi, C.; Piza, F. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant. Proc. 1991, 23 Pt 2, 1567–1568. Available online: https://europepmc.org/article/med/1989293 (accessed on 18 October 2022).
- Moris, D.; Tsilimigras, D.I.; Chakedis, J.; Beal, E.W.; Felekouras, E.; Vernadakis, S.; Schizas, D.; Fung, J.; Pawlik, T.M. Liver transplantation for unresectable colorectal liver metastases: A systematic review. J. Surg. Oncol. 2017, 116, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Hoti, E.; Adam, R. Liver transplantation for primary and metastatic liver cancers. Transpl. Int. 2008, 21, 1107–1117. [Google Scholar] [CrossRef]
- Hagness, M.; Foss, A.; Line, P.-D.; Scholz, T.; Jørgensen, P.F.; Fosby, B.; Boberg, K.M.; Mathisen, Ø.; Gladhaug, I.P.; Egge, T.S.; et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann. Surg. 2013, 257, 800–806. [Google Scholar] [CrossRef]
- Dueland, S.; Syversveen, T.; Solheim, J.M.; Solberg, S.; Grut, H.; Bjørnbeth, B.A.; Hagness, M.; Line, P.-D. Survival following liver transplantation for patients with nonresectable liver-only colorectal metastases. Ann. Surg. 2020, 271, 212–218. [Google Scholar] [CrossRef]
- Dueland, S.; Smedman, T.M.; Røsok, B.; Grut, H.; Syversveen, T.; Jørgensen, L.H.; Line, P. Treatment of relapse and survival outcomes after liver transplantation in patients with colorectal liver metastases. Transpl. Int. 2021, 34, 2205–2213. [Google Scholar] [CrossRef]
- Nadalin, S.; Settmacher, U.; Rauchfuß, F.; Balci, D.; Königsrainer, A.; Line, P.D. RAPID procedure for colorectal cancer liver metastasis. Int. J. Surg. 2020, 82, 93–96. [Google Scholar] [CrossRef]
- Toso, C.; Marques, H.P.; Andres, A.; Sousa, F.C.; Adam, R.; Kalil, A.; Clavien, P.-A.; Furtado, E.; Barroso, E.; Bismuth, H.; et al. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver. Transplant. 2017, 23, 1073–1076. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Alejandro, R.; Ruffolo, L.I.; Sasaki, K.; Tomiyama, K.; Orloff, M.S.; Pineda-Solis, K.; Nair, A.; Errigo, J.; Dokus, M.K.; Cattral, M.; et al. Recipient and Donor Outcomes after Living-Donor Liver Transplant for Unresectable Colorectal Liver Metastases. JAMA Surg. 2022, 157, 524–530. [Google Scholar] [CrossRef]
- Solheim, J.M.; Dueland, S.; Line, P.-D.; Hagness, M. Transplantation for Nonresectable Colorectal Liver Metastases - Long Term follow- up of the First Prospective Pilot Study. Ann. Surg 2022. (Online ahead of print). [Google Scholar] [CrossRef]
- Ravaioli, M.; Brandi, G.; Siniscalchi, A.; Renzulli, M.; Bonatti, C.; Fallani, G.; Prosperi, E.; Serenari, M.; Germinario, G.; Del Gaudio, M.; et al. Heterotopic segmental liver transplantation on splenic vessels after splenectomy with delayed native hepatectomy after graft regeneration: A new technique to enhance liver transplantation. Am. J. Transplant Off. J. Am. Soc. Transplant Am. Soc. Transpl. Surg. 2021, 21, 870–875. [Google Scholar] [CrossRef]
- Dueland, S.; Guren, T.K.; Hagness, M.; Glimelius, B.; Line, P.-D.; Pfeiffer, P.; Foss, A.; Tveit, K.M. Chemotherapy or Liver Transplantation for Nonresectable Liver Metastases from Colorectal Cancer? Ann. Surg. 2015, 261, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Åberg, F. Quality of life after liver transplantation. Best Pract. Res. Clin. Gastroenterol. 2020, 46–47, 101684. [Google Scholar] [CrossRef]
- Duffy, J.P.; Kao, K.; Ko, C.Y.; Farmer, D.G.; McDiarmid, S.V.; Hong, J.C.; Venick, R.S.; Feist, S.; Goldstein, L.; Saab, S.; et al. Long-Term Patient Outcome and Quality of Life After Liver Transplantation: Analysis of 20-Year Survivors. Ann. Surg. 2010, 252, 652–661. [Google Scholar] [CrossRef]
- Lei, J.Y.; Yan, L.N.; Wang, W.T.; Zhu, J.Q.; Li, D.J. Health-Related Quality of Life and Psychological Distress in Patients With Early-Stage Hepatocellular Carcinoma After Hepatic Resection or Transplantation. Transplant. Proc. 2016, 48, 2107–2111. [Google Scholar] [CrossRef]
- Gupta, A.; Eisenhauer, E.A.; Booth, C.M. The Time Toxicity of Cancer Treatment. J. Clin. Oncol. 2022, 40, 1611–1615. [Google Scholar] [CrossRef]
- Gangi, A.; Lu, S.C. Chemotherapy-associated liver injury in colorectal cancer. Ther. Adv. Gastroenterol. 2020, 13, 1756284820924194. [Google Scholar] [CrossRef]
- Fong, Y.; Fortner, J.; Sun, R.L.; Murray, †.; Brennan, F.; Blumgart, L.H. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer Analysis of 1001 Consecutive Cases. Ann. Surg. 1999, 230, 309–318. [Google Scholar] [CrossRef]
- Dueland, S.; Grut, H.; Syversveen, T.; Hagness, M.; Line, P.D. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am. J. Transplant. 2020, 20, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Grut, H.; Revheim, M.E.; Line, P.D.; Dueland, S. Importance of 18F-FDG PET/CT to select patients with nonresectable colorectal liver metastases for liver transplantation. Nucl. Med. Commun. 2018, 39, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tabbal, M.; Alkhalifa, A.M.; AlQattan, A.S.; AlJawad, M.; Tawfeeq, M.A.; Al Qahtani, M.S. Salvage liver transplantation after resection of colorectal cancer liver metastasis with favorable outcomes: A case report and review of the literature. BMC Gastroenterol. 2021, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Leis, A.; Chandra-Kanthan, S.; Fields, A.; Zaidi, A.; Abbas, T.; Le, D.; Reeder, B.; Pahwa, P. Regional Lymph Nodes Status and Ratio of Metastatic to Examined Lymph Nodes Correlate with Survival in Stage IV Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 2287–2294. [Google Scholar] [CrossRef]
- Pyo, D.H.; Kim, S.H.M.; Ha, S.Y.M.; Yun, S.H.M.; Cho, Y.B.M.; Huh, J.W.M.; Park, Y.A.M.; Shin, J.K.M.; Lee, W.Y.M.; Kim, H.C.M. Revised Nodal Staging Integrating Tumor Deposit Counts with Positive Lymph Nodes in Patients with Stage III Colon Cancer. Ann. Surg. 2021. (Online ahead of print). [Google Scholar] [CrossRef]
- Lanari, J.; Hagness, M.; Sartori, A.; Rosso, E.; Gringeri, E.; Dueland, S.; Cillo, U.; Line, P. Liver transplantation versus liver resection for colorectal liver metastasis: A survival benefit analysis in patients stratified according to tumor burden score. Transpl. Int. 2021, 34, 1722–1732. [Google Scholar] [CrossRef]
- Dueland, S.; Yaqub, S.; Syversveen, T.; Carling, U.; Hagness, M.; Brudvik, K.W.; Line, P.-D. Survival Outcomes after Portal Vein Embolization and Liver Resection Compared with Liver Transplant for Patients with Extensive Colorectal Cancer Liver Metastases. JAMA Surg. 2021, 156, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Procopio, F.; Cimino, M.M.; Donadon, M.; Gatti, A.; Costa, G.; Del Fabbro, D.; Torzilli, G. Is Tumor Detachment from Vascular Structures Equivalent to R0 Resection in Surgery for Colorectal Liver Metastases? An Observational Cohort. Ann. Surg. Oncol. 2016, 23, 1352–1360. [Google Scholar] [CrossRef]
- Procopio, F.; Viganò, L.; Cimino, M.; Donadon, M.; Del Fabbro, D.; Torzilli, G. Does KRAS mutation status impact the risk of local recurrence after R1 vascular resection for colorectal liver metastasis? An observational cohort study. Eur. J. Surg. Oncol. 2020, 46, 818–824. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kopetz, S.; Newhook, T.E.; De Bellis, M.; Chun, Y.S.; Tzeng, C.-W.D.; Aloia, T.A.; Vauthey, J.-N. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin. Cancer Res. 2019, 25, 5843–5851. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yoshino, T. Clinical Utility of Analyzing Circulating Tumor DNA in Patients with Metastatic Colorectal Cancer. Oncologist. 2018, 23, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Kinde, I.; Wang, Y.; Wong, H.; Roebert, J.; Christie, M.; Tacey, M.; Wong, R.; Singh, M.; Karapetis, C.; et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1715–1722. [Google Scholar] [CrossRef]
- Ongaro, E.; Cremolini, C.; Rossini, D.; Corti, F.; Pagani, F.; Morelli, L.; Urbani, L.; Masi, G.; Sposito, C.; Filippi, B.; et al. Clinical and molecular determinants of extrahepatic disease progression in patients with metastatic colorectal cancer with liver-limited metastases deemed initially unresectable. ESMO Open 2019, 4, 496. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Morioka, D.; Conci, S.; Margonis, G.A.; Sawada, Y.; Ruzzenente, A.; Kumamoto, T.; Iacono, C.; Andreatos, N.; Guglielmi, A.; et al. The Tumor Burden Score: A New “metro-ticket” Prognostic Tool for Colorectal Liver Metastases Based on Tumor Size and Number of Tumors. Ann. Surg. 2018, 267, 132–141. [Google Scholar] [CrossRef]
- Amygdalos, I.; Müller-Franzes, G.; Bednarsch, J.; Czigany, Z.; Ulmer, T.F.; Bruners, P.; Kuhl, C.; Neumann, U.P.; Truhn, D.; Lang, S.A. Novel machine learning algorithm can identify patients at risk of poor overall survival following curative resection for colorectal liver metastases. J. Hepatobiliary Pancreat Sci. 2022. (Online ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Capussotti, L.; Lapointe, R.; Barroso, E.; Hubert, C.; Giuliante, F.; Ijzermans, J.N.M.; Mirza, D.F.; Elias, D.; Adam, R. Early recurrence after liver resection for colorectal metastases: Risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann. Surg. Oncol. 2014, 21, 1276–1286. [Google Scholar] [CrossRef]
- Mao, R.; Zhao, J.-J.; Bi, X.-Y.; Zhang, Y.-F.; Li, Z.-Y.; Zhou, J.-G.; Wu, X.-L.; Xiao, C.; Zhao, H.; Cai, J.-Q. A postoperative scoring system for post-hepatectomy early recurrence of colorectal liver metastases. Oncotarget. 2017, 8, 102531–102539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Allard, M.-A.; Benitez, C.C.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; Bismuth, H.; Baba, H.; Adam, R. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016, 21, 887–894. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-M.; Zhang, Y.-P.; Wang, C.-W.; Zhang, W.-D.; He, W.; Qiu, J.-L.; Yuan, Y.-C.; Li, B.-K.; Yuan, Y.-F.; Lai, R.-C.; et al. More Liver Metastases Detected Intraoperatively Indicates Worse Prognosis for Colorectal Liver Metastases Patients after Resection Combined with Microwave Ablation. J. Oncol. 2022, 2022, 3819564. [Google Scholar] [CrossRef]
- Donadon, M.; Terrone, A.; Procopio, F.; Cimino, M.; Palmisano, A.; Viganò, L.; Del Fabbro, D.; Di Tommaso, L.; Torzilli, G. Is R1 vascular hepatectomy for hepatocellular carcinoma oncologically adequate? Analysis of 327 consecutive patients. Surgery 2019, 165, 897–904. [Google Scholar] [CrossRef]
- Margonis, G.A.; Sasaki, K.; Gholami, S.; Kim, Y.; Andreatos, N.; Rezaee, N.; Deshwar, A.; Buettner, S.; Allen, P.J.; Kingham, T.P.; et al. Genetic and Morphological Evaluation (GAME) score for patients with colorectal liver metastases. Br. J. Surg. 2018, 105, 1210–1220. [Google Scholar] [CrossRef]
- Osumi, H.; Shinozaki, E.; Yamaguchi, K.; Zembutsu, H. Early change in circulating tumor DNA as a potential predictor of response to chemotherapy in patients with metastatic colorectal cancer. Sci. Rep. 2019, 9, 17358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schadde, E.; Grunhagen, D.J.; Verhoef, C.; Krzywon, L.; Metrakos, P. Limitations in resectability of colorectal liver metastases 2020–A systematic approach for clinicians and patients. Semin. Cancer Biol. 2021, 71, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Huiskens, J.; Bolhuis, K.; Engelbrecht, M.R.; De Jong, K.P.; Kazemier, G.; Liem, M.S.; Verhoef, C.; de Wilt, J.H.; Punt, C.J.; van Gulik, T.M.; et al. Outcomes of Resectability Assessment of the Dutch Colorectal Cancer Group Liver Metastases Expert Panel. J. Am. Coll. Surg. 2019, 229, 523–532.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatavicius, P.; Oberkofler, C.E.; Chapman, W.C.; DeMatteo, R.P.; Clary, B.M.; D’Angelica, M.I.; Tanabe, K.K.; Hong, J.C.; Aloia, T.A.; Pawlik, T.M.; et al. Choices of Therapeutic Strategies for Colorectal Liver Metastases Among Expert Liver Surgeons: A Throw of the Dice? Ann. Surg. 2020, 272, 715–722. [Google Scholar] [CrossRef]
- Adam, R.; De Gramont, A.; Figueras, J.; Guthrie, A.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; et al. The Oncosurgery Approach to Managing Liver Metastases from Colorectal Cancer: A Multidisciplinary International Consensus. Oncologist. 2012, 17, 1225. [Google Scholar] [CrossRef] [Green Version]
- Line, P.D.; Ruffolo, L.I.; Toso, C.; Dueland, S.; Nadalin, S.; Hernandez-Alejandro, R. Liver transplantation for colorectal liver metastases: What do we need to know? Int. J. Surg. 2020, 82, 87–92. [Google Scholar] [CrossRef]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and Endpoints of Clinical Trials in Hepatocellular Carcinoma. JNCI J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef]
- Grut, H.; Solberg, S.; Seierstad, T.; Revheim, M.E.; Egge, T.S.; Larsen, S.G.; Line, P.D.; Dueland, S. Growth rates of pulmonary metastases after liver transplantation for unresectable colorectal liver metastases. Br. J. Surg. 2018, 105, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Volk, M.; Cillo, U. Transplant benefit for patients with hepatocellular carcinoma. World J. Gastroenterol. 2013, 19, 9183–9188. [Google Scholar] [CrossRef]
- Line, P.D.; Hagness, M.; Berstad, A.E.; Foss, A.; Dueland, S. A novel concept for partial liver transplantation in nonresectable colorectal liver metastases: The RAPID concept. Ann. Surg. 2015, 262, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Reiling, J.; Butler, N.; Simpson, A.; Hodgkinson, P.; Campbell, C.; Lockwood, D.; Bridle, K.; Santrampurwala, N.; Britton, L.; Crawford, D.; et al. Assessment and Transplantation of Orphan Donor Livers: A Back-to-Base Approach to Normothermic Machine Perfusion. Liver Transplant. 2020, 26, 1618–1628. [Google Scholar] [CrossRef]
- Czigany, Z.; Schöning, W.; Ulmer, T.F.; Bednarsch, J.; Amygdalos, I.; Cramer, T.; Rogiers, X.; Popescu, I.; Botea, F.; Froněk, J.; et al. Hypothermic oxygenated machine perfusion (HOPE) for orthotopic liver transplantation of human liver allografts from extended criteria donors (ECD) in donation after brain death (DBD): A prospective multicentre randomised controlled trial (HOPE ECD-DBD). BMJ Open 2017, 7, e017558. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Kalisvaart, M.M.; O’Rourke, J.; Shetty, S.; Parente, A.; Muller, X.; Isaac, J.; Muellhaupt, B.; Muiesan, P.; Shah, T.; et al. Hypothermic Oxygenated Liver Perfusion (HOPE) Prevents Tumor Recurrence in Liver Transplantation from Donation After Circulatory Death. Ann. Surg. 2020, 272, 759–765. [Google Scholar] [CrossRef]
- la Varga, M.F.-D.; Valle, P.D.P.-D.; Béjar-Serrano, S.; López-Andújar, R.; Berenguer, M.; Prieto, M.; Montalvá, E.; Aguilera, V. Good post-transplant outcomes using liver donors after circulatory death when applying strict selection criteria: A propensity-score matched-cohort study. Ann. Hepatol. 2022, 27, 100724. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ordorica, P.; Palomares, I.; Gastaca, M.; Ventoso, A.; Prieto, M.; Bustamante, F.J.; Salvador, P.; Senosiain, M.; Fernandez, J.R.; Testillanos, M.; et al. Analysis of the liver recovery rate in DCD donors preserved with Normothermic Regional Perfusion: Comparison with the “gold standard” DBD. Transplantation 2021, 105, 165. [Google Scholar] [CrossRef]
- Dueland, S.; Foss, A.; Solheim, J.M.; Hagness, M.; Line, P.D. Survival following liver transplantation for liver-only colorectal metastases compared with hepatocellular carcinoma. Br. J. Surg. 2018, 105, 736–742. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Rela, M.; Eason, J.D.; Line, P.-D.; Fung, J.; Sakamoto, S.; Selzner, N.; Man, N.K.; Ghobrial, R.M.; Sapisochin, G. Liver Transplantation for Colorectal and Neuroendocrine Liver Metastases and Hepatoblastoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1131–1135. [Google Scholar] [CrossRef]
- Earle, C.C.; Landrum, M.B.; Souza, J.M.; Neville, B.A.; Weeks, J.C.; Ayanian, J.Z. Aggressiveness of Cancer Care Near the End of Life: Is It a Quality-of-Care Issue? J. Clin. Oncol. 2008, 26, 3860. [Google Scholar] [CrossRef] [PubMed]


| Clinical Trial Identifier Acronym | Timeline | Country | Interventions | Inclusion & Exclusion Criteria | Expected Enrollment |
|---|---|---|---|---|---|
| Deceased donors, RCT | |||||
| SECAII NCT01479608 | 2011–2027 | Norway | LT vs. resection |
| 25 |
| TRANSMET NCT02597348 | 2015–2027 | France | LT vs. chemotherapy |
| 94 |
| SECAIII NCT03494946 | 2016–2027 | Norway | LT vs. chemotherapy |
| 30 |
| SOULMATE NCT04161092 | 2020–2029 | Sweden | LT vs. best alternative therapy |
| 45 |
| EXCALIBUR1 NCT04898504 | 2021–2026 | Norway | LT vs. HAI vs. standard of care |
| 45 |
| Deceased donors, single arm, matched | |||||
| COLT NCT03803436 | 2019–2024 | Italy | LT vs. chemotherapy |
| 25 |
| MELODIC NCT04870879 | 2020–2025 | Italy | LT vs. chemotherapy |
| 18 |
| Deceased donors, single arm, non-matched | |||||
| SECAI NCT00294827 | 2006–2023 | Norway | LT | 25 | |
| TRASMETIR NCT04616495 | 2021–2028 | Spain | LT |
| 30 |
| NCT05185245 | 2021–2030 | Italy | LT |
| 20 |
| NCT05398380 | 2022–2026 | Spain | LT |
| 35 |
| Living donor liver transplantation, single arm | |||||
| Toronto Protocol NCT02864485 | 2016–2023 | Canada | LDLT |
| 20 |
| LIVERT(W)OHEAL NCT03488953 | 2018–2023 | Germany | LDLT |
| 40 |
| NCT05248581 | 2019–2027 | USA | LDLT |
| 25 |
| NCT04874259 | 2022–2026 | Korea | LDLT |
| 20 |
| NCT05175092 | 2022–2030 | USA | LDLT |
| 50 |
| LIVERMORE NCT05186116 | 2022–2032 | Italy | LDLT |
| 25 |
| Resection and Partial Liver Segment 2/3 Transplantation With Delayed Total Hepatectomy | |||||
| NCT02215889 | 2014–2028 | Norway | RAPID |
| 20 |
| RAPID-Padova NCT04865471 | 2020–2025 | Italy | RAPID |
| 18 |
| Currently Accepted | Currently Debated | |
|---|---|---|
| Patient characteristics and setting | Age ≤ 70 | |
| Performance status 0–1 | Salvage transplantation in case of treatment-associated liver failure (e.g., hepatic artery infusion pump, chemotherapy-associated steatohepatitis, elective internal radiation therapy, post-intent-to-resection liver failure with/out untreatable vascular complication) [33] can be considered, although prognosis is worse than elective LT | |
| Primary tumor | Left-sided | Some right-sided primary tumors with demonstrated favorable biology, as well as intraperitoneal rectal tumors, can be discussed as candidate for transplantation |
| No nodal metastases | The impact of primary nodal metastases on prognosis is still to be investigated, as well as if N1 (N1a: 1 lymphnode vs. N1b: 2–3 lymphnodes vs. N1c areas of fat near the lymphnodes but not the lymphnodes themselves) [34,35] vs. N2 lead to different outcomes in the immonosuppressed contest of LT Presence of single, removable N+ at liver hilum at the time of transplant in a patient fulfilling all other transplant requirement may be considered for LT within investigational studies | |
| T stage < 4 | The impact of primary T stage on prognosis is still to be investigated, especially whether T4a (invading the free serosa) vs. T4b (invading other organs/structures) lead to different outcomes in terms of local vs. systemic recurrences | |
| No signet ring cell histology | The impact of vascular/neural/immunotype of primary tumor on outcome is still to be investigated | |
| Disease extent | No extrahepatic metastases | Patients with favorable biology after removal or sustained complete post-chemotherapy response or in case of resectable, limited pulmonary and peritoneal metastases are considered in some protocols of LDLT. Tumor infiltration by CRLM limited to the diaphragm may not be considered as a contraindication to LT. |
| Response to medical and loco-regional therapies | Stable and responding liver-only metastases to ≤2 lines of chemotherapy | Sustained tumor response after >2 lines of chemotherapy can be discussed as candidate for transplantation |
| No current limits in number/size of hepatic metastases as long as the tumor is responding | Currently, no restrictions are applied for size and number of liver metastases, as long as response to chemotherapy is demonstrated. Size of the largest lesion >5.5 cm is associated with worse prognosis [31]. | |
| Low metabolic tumor volume (MTV) | MTV of <70 cm3 measured at 18F-FDG-PET is associated with better patient outcomes [32]. | |
| Hepatic tumor burden | Unresectable disease | A trial testing the benefit of LT versus reseection in case of resectable CRLM is ongoing (SECA II Arm A, see Table 1). “Biologic non-resectable CRLM” can be considered for LT. Biologic non resectability can be inferred in:
|
| Synchronous and metachronous metastases | No current limitation/stratification are applied with respect to the time from primary tumor to CRLM detection | |
| No BRAF mutation | Some specific molecular mutations [40] may be associated with better prognosis and may not be contraindications to transplantation k-RAS mutations are debated and not considered as contraindication in some studies | |
| Molecular characteristics | CEA < 80 ng/mL | Various CEA cutoffs at the time of transplant. No current limitations with respect to CEA level at the time of first referral |
| Biomarkers | Circulating cancer byproducts (liquid biopsy) | ctDNA monitoring is increasingly utilized for decision making in CRC patients [41,42] |
| At least 1 year between resection of the primary and transplant | At least 2 years between resection of the primary and transplant | |
| Timing | At least 1 year between resection of the primary and transplant | At least 2 years between resection of the primary and transplant |
| Factor | Evidence | Rational for Transplantation |
| Potential transplant benefit | ||
| Tumor bunder score (TBS) > 9 [44] Increasing number and size of metastases [45] | A TBS > 9 has been associated with reduced OS Increasing number (HR 1.3, 1.1–1.6) and diameter (HR 1.1, 1–1.2) associated with reduced OS. | Recurrence in the presence of these factor is likely to be due to microscopic, undetectable disease left behind during resection. The complete hepatectomy performed during LT may reduce this recurrence risk by eliminating all intrahepatic disease. |
| Need for intraoperative ablation [46] | Associated with early recurrence, OR 1.6 (1.1–2.5) | |
| Surgical margin 0 mm [46] | Associated with early recurrence, OR 1.5 (1–2.2) | |
| R1 resection [47] † | Associated with early recurrence, HR 2.2 (1.2–4.2) | |
| Need for portal vein embolization [37,48] | Associated with reduced OS, HR 1.48 (1.09–1.98) In patients with high tumor load, median OS 19.2 months (95% CI, 0.0–39.5 months) after PVE vs. 40.5 months (95%CI, 26.3–54.7 months) after LT (p = 0.007) | |
| Initially unresectable disease [47] | Associated with early recurrence (HR 1.9, 1.02–3.7) | |
| More metastases detected intraoperatively [49] | Associated with reduced OS (HR 3.19, 1.28–7.97) | |
| Need for preoperative chemotherapy [45] | Associated with reduced OS, HR 1.7 (1.2–2.5) | |
| Transplant benefit unlikely | ||
| More than 1 preoperative chemotherapy line [48] | Associated with early recurrence, RR 1.6 (1.1–2.4) | Recurrence in the presence of these factors is likely to be due to aggressive biological characteristics, thus a liver transplant is unlikely to change the prognosis |
| Progression during last-line chemotherapy [48] | Associated with early recurrence, RR 2.18 (1.11–4.47) | |
| Higher CEA levels [47] | CEA > 30 ng/m associated with early recurrence, HR 2.3 (1.2–4.7) | |
| Higher CA 19-9 [48] levels | CA 19-9 levels > 60 U/mL associated with early recurrence, RR 2.21 (1.44–3.43).CA 19-9 levels > 100 U/mL associated with reduces OS, HR 1.86 (1.37–2.48) | |
| Primary tumor T stage > 2 [45,46] | Associated with reduced OS, HR 1.4 (1.1–2) Associated with early recurrence, OR 2.6 (1.4–4.8) | |
| Right-sided primary tumor [45] | Associated with reduced OS, HR 1.5 (1–2.1) | |
| Primary tumor lymphovascular invasion [47] | Associated with early recurrence, HR 2.5, 1.3–4.8 | |
| Nodal positive primary [48] | Associated with reduced OS, HR 1.46 (1.13–1.89) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maspero, M.; Sposito, C.; Virdis, M.; Citterio, D.; Pietrantonio, F.; Bhoori, S.; Belli, F.; Mazzaferro, V. Liver Transplantation for Hepatic Metastases from Colorectal Cancer: Current Knowledge and Open Issues. Cancers 2023, 15, 345. https://doi.org/10.3390/cancers15020345
Maspero M, Sposito C, Virdis M, Citterio D, Pietrantonio F, Bhoori S, Belli F, Mazzaferro V. Liver Transplantation for Hepatic Metastases from Colorectal Cancer: Current Knowledge and Open Issues. Cancers. 2023; 15(2):345. https://doi.org/10.3390/cancers15020345
Chicago/Turabian StyleMaspero, Marianna, Carlo Sposito, Matteo Virdis, Davide Citterio, Filippo Pietrantonio, Sherrie Bhoori, Filiberto Belli, and Vincenzo Mazzaferro. 2023. "Liver Transplantation for Hepatic Metastases from Colorectal Cancer: Current Knowledge and Open Issues" Cancers 15, no. 2: 345. https://doi.org/10.3390/cancers15020345
APA StyleMaspero, M., Sposito, C., Virdis, M., Citterio, D., Pietrantonio, F., Bhoori, S., Belli, F., & Mazzaferro, V. (2023). Liver Transplantation for Hepatic Metastases from Colorectal Cancer: Current Knowledge and Open Issues. Cancers, 15(2), 345. https://doi.org/10.3390/cancers15020345







