Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dietary Fibers
2.2. In Vitro Digestion and Fermentation of Dietary Fiber
2.3. Cell Culture
2.4. Growth Inhibition—DAPI Assay
2.5. ROS Formation—DCF Assay
2.6. Protein Expression—Western Blotting
2.7. Statistical Analyses
3. Results
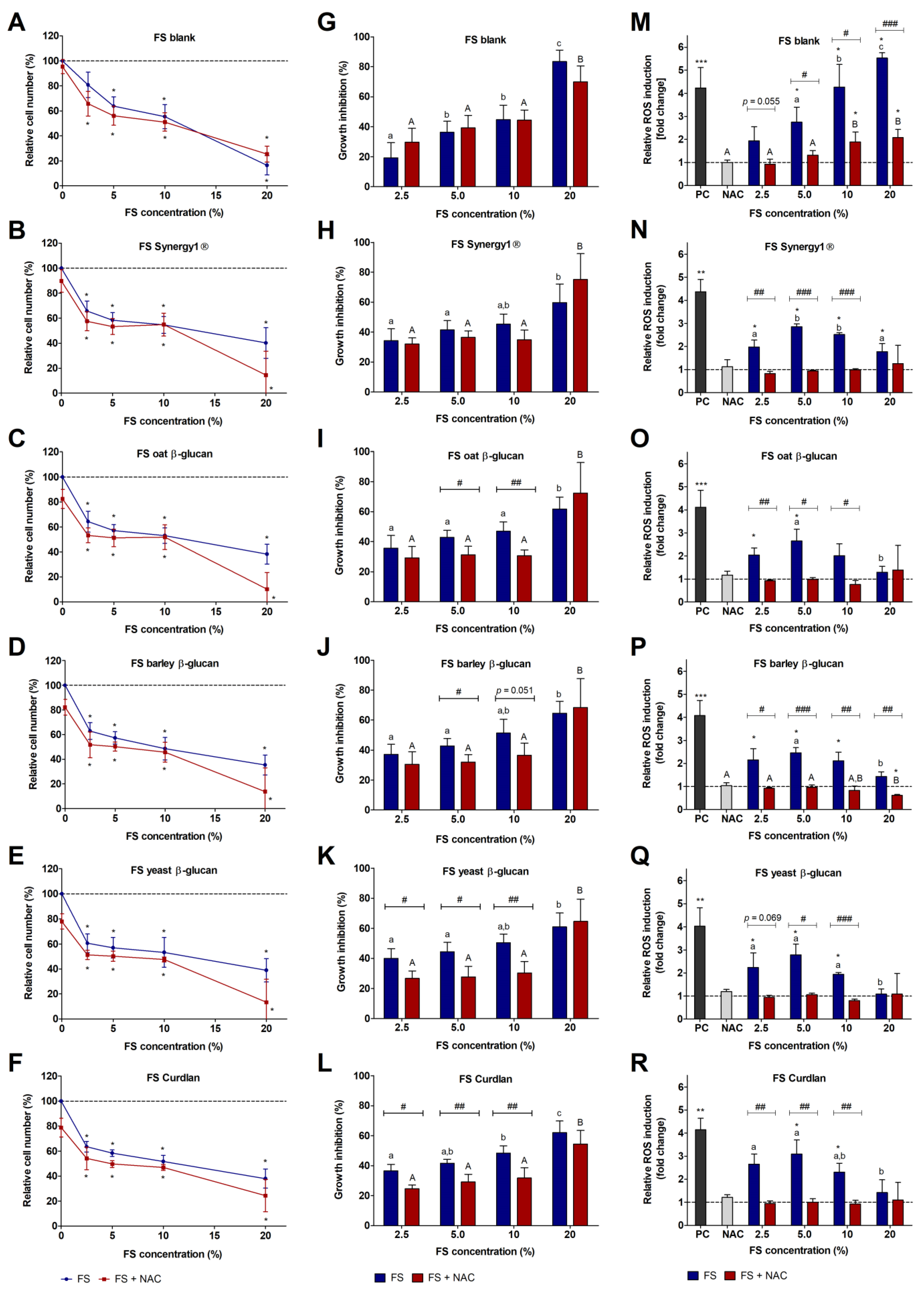
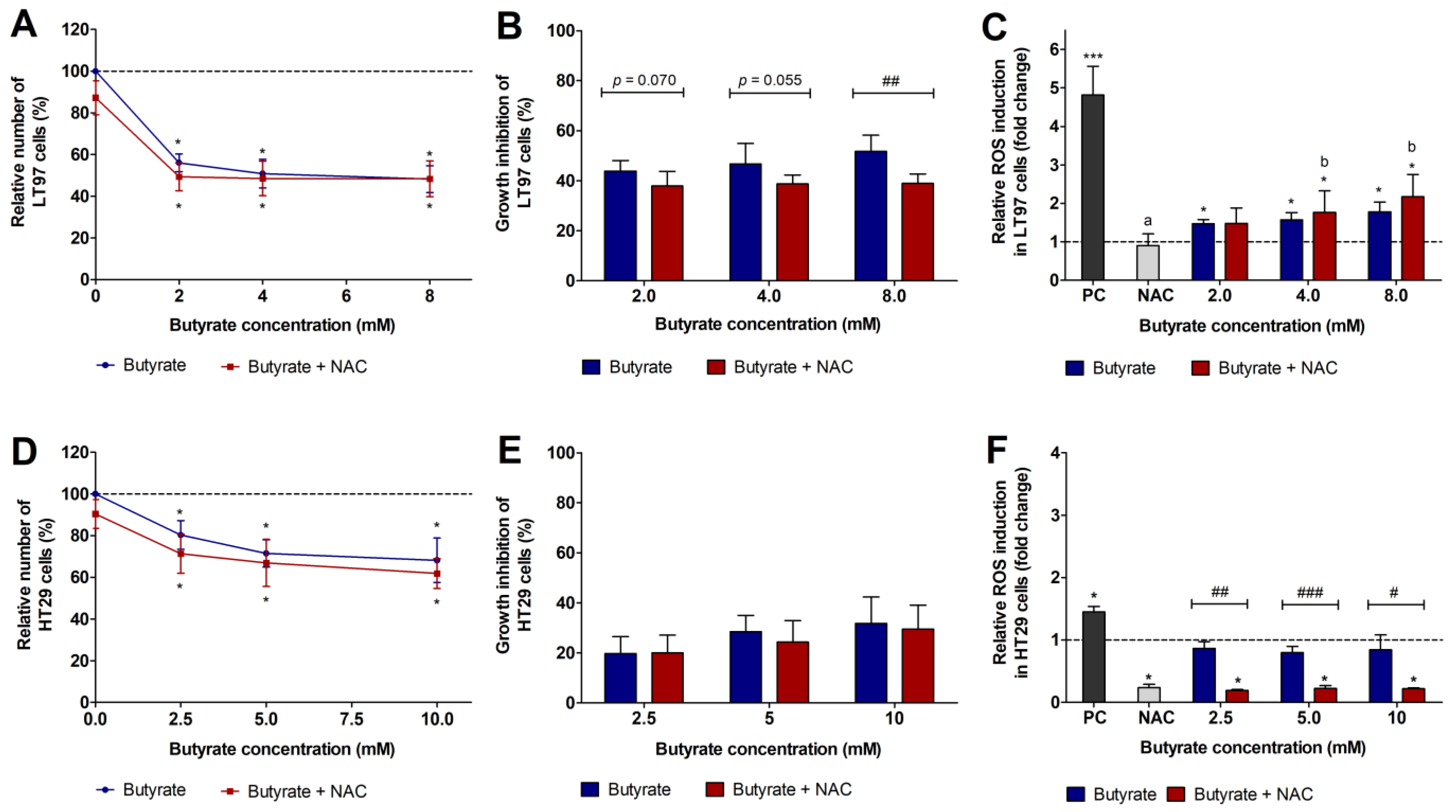
3.1. Impact of FS and Butyrate on LT97 Cell Growth
3.2. Impact of FS and Butyrate on ROS Induction in LT97 Cells
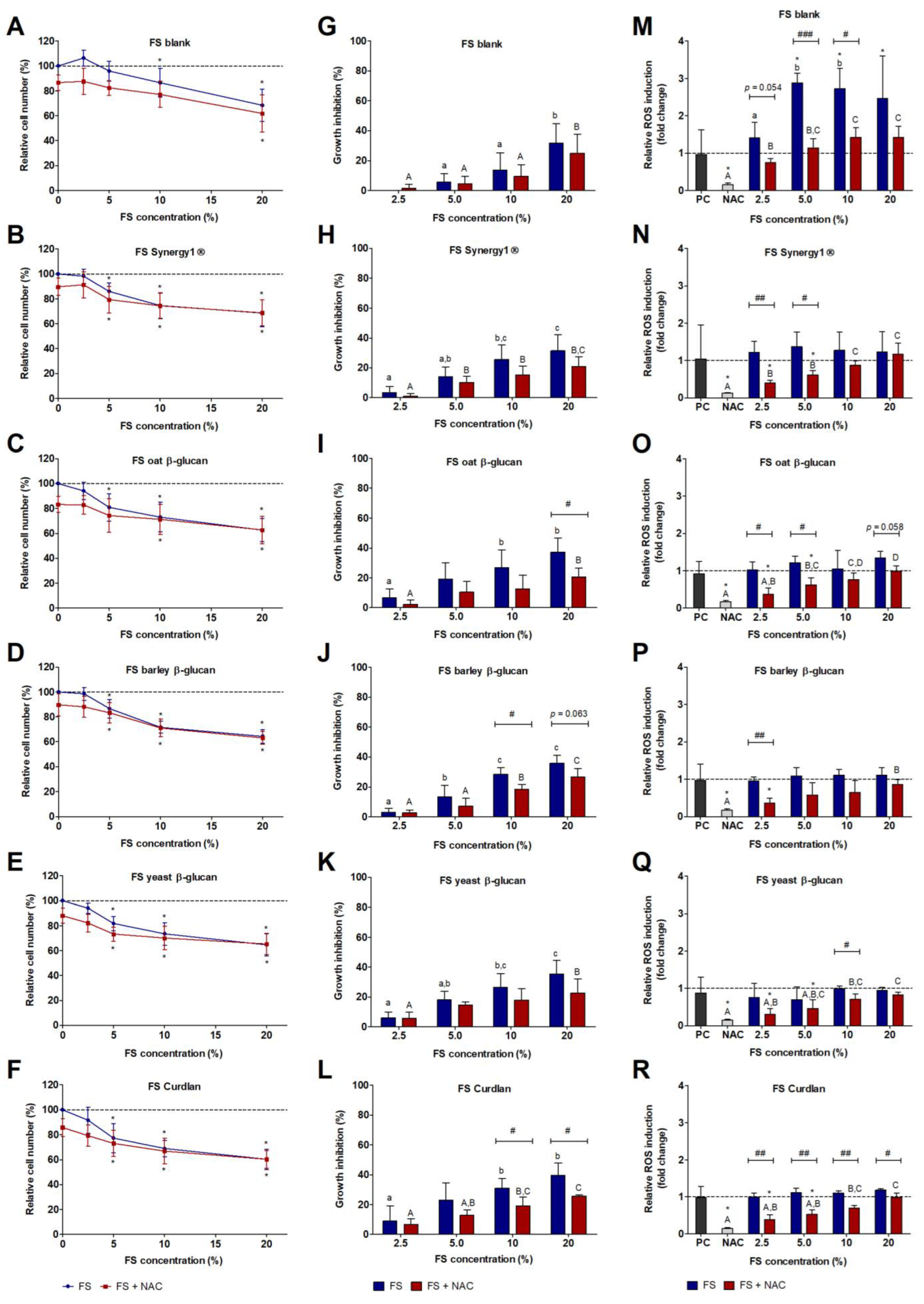
3.3. Impact of FS and Butyrate on HT29 Cell Growth
3.4. Impact of FS and Butyrate on ROS Induction in HT29 cells
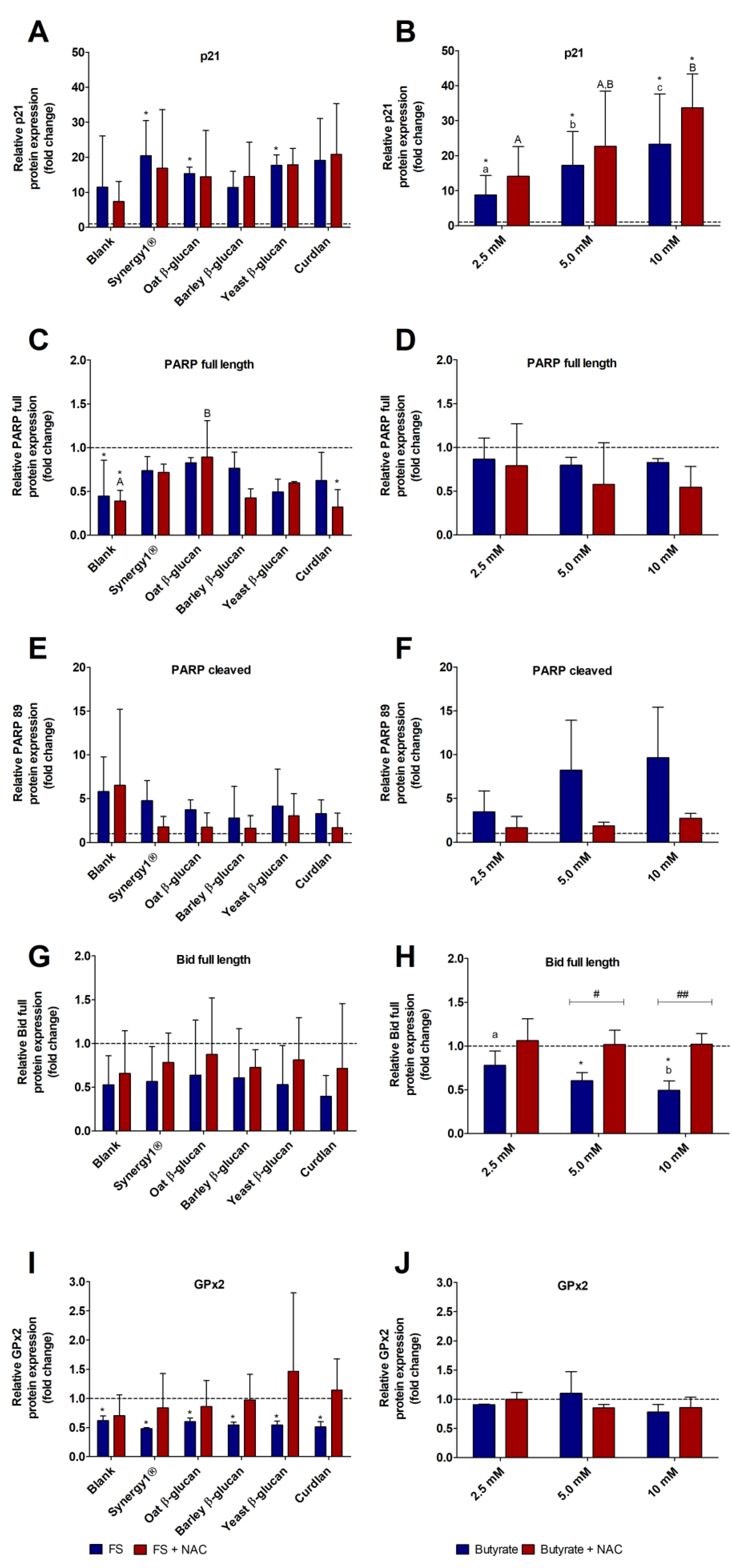
3.5. Impact of FS and Butyrate on the Expression of Cell Cycle-, Apoptosis-, and Antioxidant-Relevant Proteins in LT97 Cells
3.6. Impact of FS and Butyrate on the Expression of Cell Cycle-, Apoptosis-, and Antioxidant-Relevant Proteins in HT29 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, Y.; Xu, P.F. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 479–507. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Aspects Med. 2019, 69, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Durko, L.; Malecka-Panas, E. Lifestyle modifications and colorectal cancer. Curr. Color. Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. Br. Med. J. 2011, 343, d6617. [Google Scholar] [CrossRef]
- Malcomson, F.C. Mechanisms underlying the effects of nutrition, adiposity and physical activity on colorectal cancer risk. Nutr. Bull. 2018, 43, 400–415. [Google Scholar] [CrossRef]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Dietary fibre intake and risks of cancers of the colon and rectum in the european prospective investigation into cancer and nutrition (epic). PLoS One 2012, 7, e39361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.Y.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific opinion on the substantiation of health claims related to beta-glucans from oats and barley and maintenance of normal blood ldl-cholesterol concentrations (id 1236, 1299), increase in satiety leading to a reduction in energy intake (id 851, 852. EFSA J. 2011, 9, 2207–2221. [Google Scholar] [CrossRef] [Green Version]
- Schlörmann, W.; Glei, M. Potential health benefits of beta-glucan from barley und oat. Ernahrungs Umschau 2017, 64, M555–M559. [Google Scholar]
- Tosh, S.M. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur. J. Clin. Nutr. 2013, 67, 310–317. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat beta-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Schlörmann, W.; Bockwoldt, J.A.; Mayr, M.F.; Lorkowski, S.; Dawczynski, C.; Rohn, S.; Ehrmann, M.A.; Glei, M. Fermentation profile, cholesterol-reducing properties and chemopreventive potential of beta-glucans from levilactobacillus brevis and pediococcus claussenii—A comparative study with beta-glucans from different sources. Food Funct. 2021, 12, 10615–10631. [Google Scholar] [CrossRef]
- Chang, M.C.; Tsai, Y.L.; Chen, Y.W.; Chan, C.P.; Huang, C.F.; Lan, W.C.; Lin, C.C.; Lan, W.H.; Jeng, J.H. Butyrate induces reactive oxygen species production and affects cell cycle progression in human gingival fibroblasts. J. Periodontal Res. 2013, 48, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ros-mediated apoptosis by modulating mir-22/sirt-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Salimi, V.; Shahsavari, Z.; Safizadeh, B.; Hosseini, A.; Khademian, N.; Tavakoli-Yaraki, M. Sodium butyrate promotes apoptosis in breast cancer cells through reactive oxygen species (ros) formation and mitochondrial impairment. Lipids Health Dis. 2017, 16, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.F.; Wu, H.S.; Fan, M.J.; Yu, R.K.; Zhang, Y.; Liu, J.X.; Zhou, X.J.; Cai, Y.S.; Huang, S.H.; Hu, Z.H.; et al. Sodium butyrate inhibits migration and induces ampk-mtor pathway-dependent autophagy and ros-mediated apoptosis via the mir-139-5p/bmi-1 axis in human bladder cancer cells. FASEB J. 2020, 34, 4266–4282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Fang, D.; Zhang, H.; Xue, J.; Wangchuk, D.; Du, J.; Jiang, L. Sodium butyrate selectively kills cancer cells and inhibits migration in colorectal cancer by targeting thioredoxin-1. OncoTargets Ther. 2020, 13, 4691–4704. [Google Scholar] [CrossRef]
- Domokos, M.; Jakus, J.; Szeker, K.; Csizinszky, R.; Csiko, G.; Neogrady, Z.; Csordas, A.; Galfi, P. Butyrate-induced cell death and differentiation are associated with distinct patterns of ros in ht29-derived human colon cancer cells. Dig. Dis. Sci. 2010, 55, 920–930. [Google Scholar] [CrossRef]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acids induce apoptosis in colon cancer cells associated with changes to intracellular redox state and glucose metabolism. Chemotherapy 2012, 58, 102–109. [Google Scholar] [CrossRef]
- Richter, M.; Jurek, D.; Wrba, F.; Kaserer, K.; Wurzer, G.; Karner-Hanusch, J.; Marian, B. Cells obtained from colorectal microadenomas mirror early premalignant growth patterns in vitro. Eur. J. Cancer 2002, 38, 1937–1945. [Google Scholar] [CrossRef]
- Schaeferhenrich, A.; Beyer-Sehlmeyer, G.; Festag, G.; Kuechler, A.; Haag, N.; Weise, A.; Liehr, T.; Claussen, U.; Marian, B.; Sendt, W.; et al. Human adenoma cells are highly susceptible to the genotoxic action of 4-hydroxy-2-nonenal. Mutat. Res.-Fundam. Mol. Mech. Mutag. 2003, 526, 19–32. [Google Scholar] [CrossRef]
- Schlörmann, W.; Atanasov, J.; Lorkowski, S.; Dawczynski, C.; Glei, M. Study on chemopreventive effects of raw and roasted beta-glucan-rich waxy winter barley using an in vitro human colon digestion model. Food Funct. 2020, 11, 2626–2638. [Google Scholar] [CrossRef]
- Stein, K.; Borowicki, A.; Scharlau, D.; Scheu, K.; Brenner-Weiss, G.; Obst, U.; Hollmann, J.; Lindhauer, M.; Wachter, N.; Glei, M. Modification of an in vitro model simulating the whole digestive process to investigate cellular endpoints of chemoprevention. Br. J. Nutr. 2011, 105, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Harkonen, H.; Fabritius, M.; Poutanen, K. Development of an in vitro enzymic digestion method for removal of starch and protein and assessment of its performance using rye and wheat breads. J. Cereal Sci. 1999, 29, 139–152. [Google Scholar] [CrossRef]
- Barry, J.L.; Hoebler, C.; Macfarlane, G.T.; Macfarlane, S.; Mathers, J.C.; Reed, K.A.; Mortensen, P.B.; Nordgaard, I.; Rowland, I.R.; Rumney, C.J. Estimation of the fermentability of dietary fibre in vitro: A european interlaboratory study. Br. J. Nutr. 1995, 74, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klenow, S.; Pool-Zobel, B.L.; Glei, M. Influence of inorganic and organic iron compounds on parameters of cell growth and survival in human colon cells. Toxicol. In Vitr. 2009, 23, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Hiller, B.; Jahns, F.; Zöger, R.; Hennemeier, I.; Wilhelm, A.; Lindhauer, M.G.; Glei, M. Chemopreventive effects of in vitro digested and fermented bread in human colon cells. Eur. J. Nutr. 2012, 51, 827–839. [Google Scholar] [CrossRef]
- Kautenburger, T.; Beyer-Sehlmeyer, G.; Festag, G.; Haag, N.; Kuhler, S.; Kuchler, A.; Weise, A.; Marian, B.; Peters, W.H.; Liehr, T.; et al. The gut fermentation product butyrate, a chemopreventive agent, suppresses glutathione s-transferase theta (hgstt1) and cell growth more in human colon adenoma (lt97) than tumor (ht29) cells. J. Cancer Res. Clin. Oncol. 2005, 131, 692–700. [Google Scholar] [CrossRef]
- Pelka, J.; Gehrke, H.; Esselen, M.; Turk, M.; Crone, M.; Brase, S.; Muller, T.; Blank, H.; Send, W.; Zibat, V.; et al. Cellular uptake of platinum nanoparticles in human colon carcinoma cells and their impact on cellular redox systems and DNA integrity. Chem. Res. Toxicol. 2009, 22, 649–659. [Google Scholar] [CrossRef]
- Schlörmann, W.; Bockwoldt, J.A.; Hubner, S.M.; Wittwer, E.; Reiners, S.; Lorkowski, S.; Dawczynski, C.; Ehrmann, M.A.; Glei, M. Use of the beta-glucan-producing lactic acid bacteria strains levilactobacillus brevis and pediococcus claussenii for sourdough fermentation-chemical characterization and chemopreventive potential of in situ-enriched wheat and rye sourdoughs and breads. Nutrients 2022, 14, 1510. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Brierley, G.V.; Henderson, S.; Hoffmann, P.; McColl, S.R.; Lockett, T.; Head, R.; Cosgrove, L. Butyrate-induced apoptosis in hct116 colorectal cancer cells includes induction of a cell stress response. J. Proteome Res. 2011, 10, 1860–1869. [Google Scholar] [CrossRef]
- Zhang, J.T.; Yi, M.; Zha, L.Y.; Chen, S.Q.; Li, Z.J.; Li, C.; Gong, M.X.; Deng, H.; Chu, X.W.; Chen, J.H.; et al. Sodium butyrate induces endoplasmic reticulum stress and autophagy in colorectal cells: Implications for apoptosis. PLoS One 2016, 11, e0147218. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. Ros in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ros-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Santoro, M.M. Ros homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeng, J.H.; Kuo, M.Y.P.; Lee, P.H.; Wang, Y.J.; Lee, M.Y.; Lee, J.J.; Lin, B.R.; Tai, T.F.; Chang, M.C. Toxic and metabolic effect of sodium butyrate on sas tongue cancer cells: Role of cell cycle deregulation and redox changes. Toxicology 2006, 223, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.R.; Rowan, A.; Smith, M.E.F.; Kerr, I.B.; Bodmer, W.F.; Gannon, J.V.; Lane, D.P. P53 mutations in colorectal-cancer. Proc. Natl. Acad. Sci. USA 1990, 87, 7555–7559. [Google Scholar] [CrossRef] [Green Version]
- Finlay, G.J. Genetics, molecular-biology and colorectal-cancer. Mutat. Res. 1993, 290, 3–12. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, Y.J.; Lee, J.; Shim, D.M.; Park, W.Y.; Seo, S.W. Tp53 alteration determines the combinational cytotoxic effect of doxorubicin and an antioxidant nac. Tumor Biol. 2017, 39, 1010428317700159. [Google Scholar] [CrossRef] [Green Version]
- Lambert, D.W.; Wood, I.S.; Ellis, A.; Shirazi-Beechey, S.P. Molecular changes in the expression of human colonic nutrient transporters during the transition from normality to malignancy. Br. J. Cancer 2002, 86, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Giardina, C.; Inan, M.S. Nonsteroidal anti-inflammatory drugs, short-chain fatty acids, and reactive oxygen metabolism in human colorectal cancer cells. Biochim. Biophys. Acta-Mol. Cell Res. 1998, 1401, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.; Masoumi-Moghaddam, S.; Ehteda, A.; Morris, D.L. Bromelain and n-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: Significance of combination therapy. J. Exp. Clin. Cancer Res. 2014, 33, 92. [Google Scholar]
- Borowicki, A.; Stein, K.; Scharlau, D.; Glei, M. Fermentation supernatants of wheat (Triticum aestivum L.) aleurone beneficially modulate cancer progression in human colon cells. J. Agric. Food Chem. 2010, 58, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Schlörmann, W.; Lamberty, J.; Lorkowski, S.; Ludwig, D.; Mothes, H.; Saupe, C.; Glei, M. Chemopreventive potential of in vitro fermented nuts in lt97 colon adenoma and primary epithelial colon cells. Mol. Carcinog. 2017, 56, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Glei, M.; Zetzmann, S.; Lorkowski, S.; Dawczynski, C.; Schlörmann, W. Chemopreventive effects of raw and roasted oat flakes after in vitro fermentation with human faecal microbiota. Int. J. Food Sci. Nutr. 2021, 72, 57–69. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsanti, L.; Passarelli, V.; Evangelista, V.; Frassanito, A.M.; Gualtieri, P. Chemistry, physico-chemistry and applications linked to biological activities of beta-glucans. Nat. Prod. Rep. 2011, 28, 457–466. [Google Scholar] [CrossRef]
- Beyer-Sehlmeyer, G.; Glei, M.; Hartmann, E.; Hughes, R.; Persin, C.; Bohm, V.; Rowland, I.; Schubert, R.; Jahreis, G.; Pool-Zobel, B.L. Butyrate is only one of several growth inhibitors produced during gut flora-mediated fermentation of dietary fibre sources. Br. J. Nutr. 2003, 90, 1057–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, H.; Claycombe, K.J.; Reindl, K.M. Butyrate and deoxycholic acid play common and distinct roles in hct116 human colon cell proliferation. J. Nutr. Biochem. 2015, 26, 1022–1028. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary bile acids and short chain fatty acids in the colon: A focus on colonic microbiome, cell proliferation, inflammation, and cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef] [Green Version]
- Katona, B.W.; Anant, S.; Covey, D.F.; Stenson, W.F. Characterization of enantiomeric bile acid-induced apoptosis in colon cancer cell lines. J. Biol. Chem. 2009, 284, 3354–3364. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Zhang, S.A.; Zhou, W.J.; Hu, D.; Xu, H.C.; Ji, G. Secondary bile acids and tumorigenesis in colorectal cancer. Front. Oncol. 2022, 12, 813745. [Google Scholar] [CrossRef]
- Zhu, C.P.; Hu, W.; Wu, H.; Hu, X. No evident dose-response relationship between cellular ros level and its cytotoxicity—A paradoxical issue in ros-based cancer therapy. Sci. Rep. 2014, 4, 5029. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Vrzackova, N.; Ruml, T.; Zelenka, J. Postbiotics, metabolic signaling, and cancer. Molecules 2021, 26, 1528. [Google Scholar] [CrossRef] [PubMed]
- Mermelshtein, A.; Gerson, A.; Walfisch, S.; Delgado, B.; Shechter-Maor, G.; Delgado, J.; Fich, A.; Gheber, L. Expression of d-type cyclins in colon cancer and in cell lines from colon carcinomas. Br. J. Cancer 2005, 93, 338–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlörmann, W.; Naumann, S.; Renner, C.; Glei, M. Influence of mirna-106b and mirna-135a on butyrate-regulated expression of p21 and cyclin d2 in human colon adenoma cells. Genes Nutr. 2015, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Siavoshian, S.; Segain, J.P.; Kornprobst, M.; Bonnet, C.; Cherbut, C.; Galmiche, J.P.; Blottiere, H.M. Butyrate and trichostatin a effects on the proliferation/differentiation of human intestinal epithelial cells: Induction of cyclin d3 and p21 expression. Gut 2000, 46, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Tan, E.M.; Fleming, S.E. Sodium butyrate inhibits cell growth and stimulates p21waf1/cip1 protein in human colonic adenocarcinoma cells independently of p53 status. Nutr. Cancer 2003, 46, 202–211. [Google Scholar] [CrossRef]
- Coradini, D.; Pellizzaro, C.; Marimpietri, D.; Abolafio, G.; Daidone, M.G. Sodium butyrate modulates cell cycle-related proteins in ht29 human colonic adenocarcinoma cells. Cell Prolif. 2000, 33, 139–146. [Google Scholar] [CrossRef]
- Inoue, T.; Kato, K.; Kato, H.; Asanoma, K.; Kuboyama, A.; Ueoka, Y.; Yamaguchi, S.; Ohgami, T.; Wake, N. Level of reactive oxygen species induced by p21(waf(1)/cip(1)) is critical for the determination of cell fate. Cancer Sci. 2009, 100, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Macip, S.; Igarashi, M.; Fang, L.; Chen, A.; Pan, Z.Q.; Lee, S.W.; Aaronson, S.A. Inhibition of p21-mediated ros accumulation can rescue p21-induced senescence. EMBO J. 2002, 21, 2180–2188. [Google Scholar] [CrossRef]
- Lim, Y.; Dorstyn, L.; Kumar, S. The p53-caspase-2 axis in the cell cycle and DNA damage response. Exp. Mol. Med. 2021, 53, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.L.; Zhao, Q.L.; Yu, D.Y.; Hassan, M.A.; Kondo, T. Enhancement of sodium butyrate-induced cell death by hyperthermia in hct 116 human colorectal cancer cells. Anticancer Res. 2008, 28, 1693–1700. [Google Scholar] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Vigneswara, V.; Ahmed, Z. The role of caspase-2 in regulating cell fate. Cells 2020, 9, 1259. [Google Scholar] [CrossRef]
- Fung, K.Y.C.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.H.; Kim, H.S.; Kim, S.U.; Noh, E.J.; Lee, J.S.; Choi, K.S. Sodium butyrate sensitizes human glioma cells to trail-mediated apoptosis through inhibition of cdc2 and the subsequent downregulation of survivin and xiap. Oncogene 2005, 24, 6877–6889. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, D.; Lee, A.; Wong, T.; Marian, B.; Chiaro, C.; Rainey, C.; Bordonaro, M. Modulation of wnt activity and cell physiology by butyrate in lt97 microadenoma cells. J. Cancer 2014, 5, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Lazarova, D.L.; Bordonaro, M.; Carbone, R.; Sartorelli, A.C. Linear relationship between wnt activity levels and apoptosis in colorectal carcinoma cells exposed to butyrate. Int. J. Cancer 2004, 110, 523–531. [Google Scholar] [CrossRef]
- Mandal, M.; Olson, D.J.; Sharma, T.; Vadlamudi, R.K.; Kumar, R. Butyric acid induces apoptosis by up-regulating bax expression via stimulation of the c-jun n-terminal kinase/activation protein-1 pathway in human colon cancer cells. Gastroenterology 2001, 120, 71–78. [Google Scholar] [CrossRef]
- Pajak, B.; Orzechowski, A.; Gajkowska, B. Molecular basis of sodium butyrate-dependent proapoptotic activity in cancer cells. Adv. Med. Sci. 2007, 52, 83–88. [Google Scholar]
- Wang, L.; Luo, H.S.; Xia, H. Sodium butyrate induces human colon carcinoma ht-29 cell apoptosis through a mitochondrial pathway. J. Int. Med. Res. 2009, 37, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Bao, Y.L.; Wu, Y.; Yu, C.L.; Huang, Y.X.; Sun, Y.; Zheng, L.H.; Li, Y.X. Butyrate induces cell apoptosis through activation of jnk map kinase pathway in human colon cancer rko cells. Chem. Biol. Interact. 2010, 185, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Emmink, B.L.; Laoukili, J.; Kipp, A.P.; Koster, J.; Govaert, K.M.; Fatrai, S.; Verheem, A.; Steller, E.J.A.; Brigelius-Flohe, R.; Jimenez, C.R.; et al. Gpx2 suppression of h2o2 stress links the formation of differentiated tumor mass to metastatic capacity in colorectal cancer. Cancer Res. 2014, 74, 6717–6730. [Google Scholar] [CrossRef] [Green Version]
- Trachootham, D.; Lu, W.Q.; Ogasawara, M.A.; Valle, N.R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlörmann, W.; Horlebein, C.; Hübner, S.M.; Wittwer, E.; Glei, M. Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells. Cancers 2023, 15, 440. https://doi.org/10.3390/cancers15020440
Schlörmann W, Horlebein C, Hübner SM, Wittwer E, Glei M. Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells. Cancers. 2023; 15(2):440. https://doi.org/10.3390/cancers15020440
Chicago/Turabian StyleSchlörmann, Wiebke, Christoph Horlebein, Sabine M. Hübner, Elisa Wittwer, and Michael Glei. 2023. "Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells" Cancers 15, no. 2: 440. https://doi.org/10.3390/cancers15020440
APA StyleSchlörmann, W., Horlebein, C., Hübner, S. M., Wittwer, E., & Glei, M. (2023). Potential Role of ROS in Butyrate- and Dietary Fiber-Mediated Growth Inhibition and Modulation of Cell Cycle-, Apoptosis- and Antioxidant-Relevant Proteins in LT97 Colon Adenoma and HT29 Colon Carcinoma Cells. Cancers, 15(2), 440. https://doi.org/10.3390/cancers15020440





