AT-101 Enhances the Antitumor Activity of Lenalidomide in Patients with Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Seahorse Extracellular Flux Assay
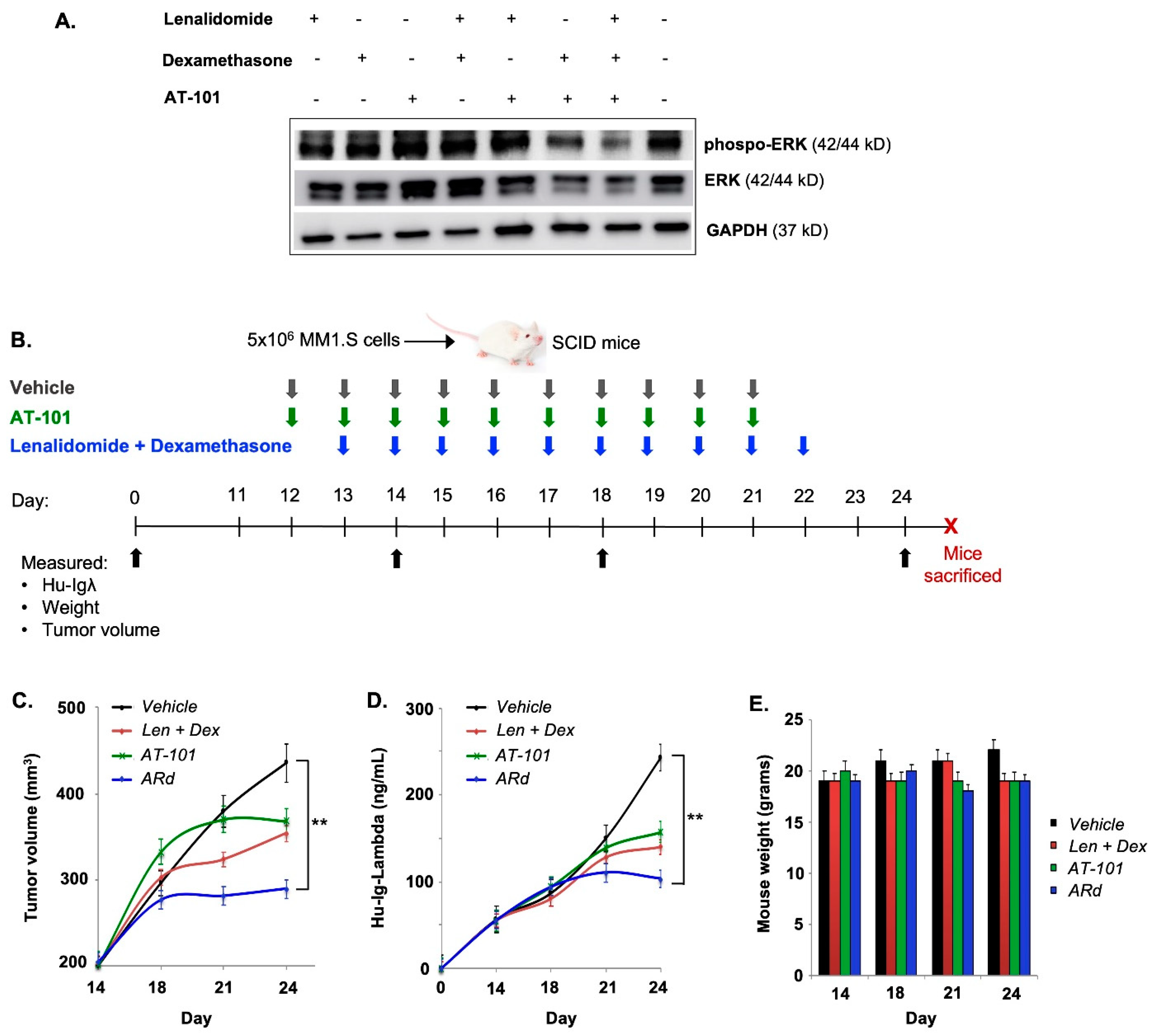
2.2. Animal Studies
3. Results
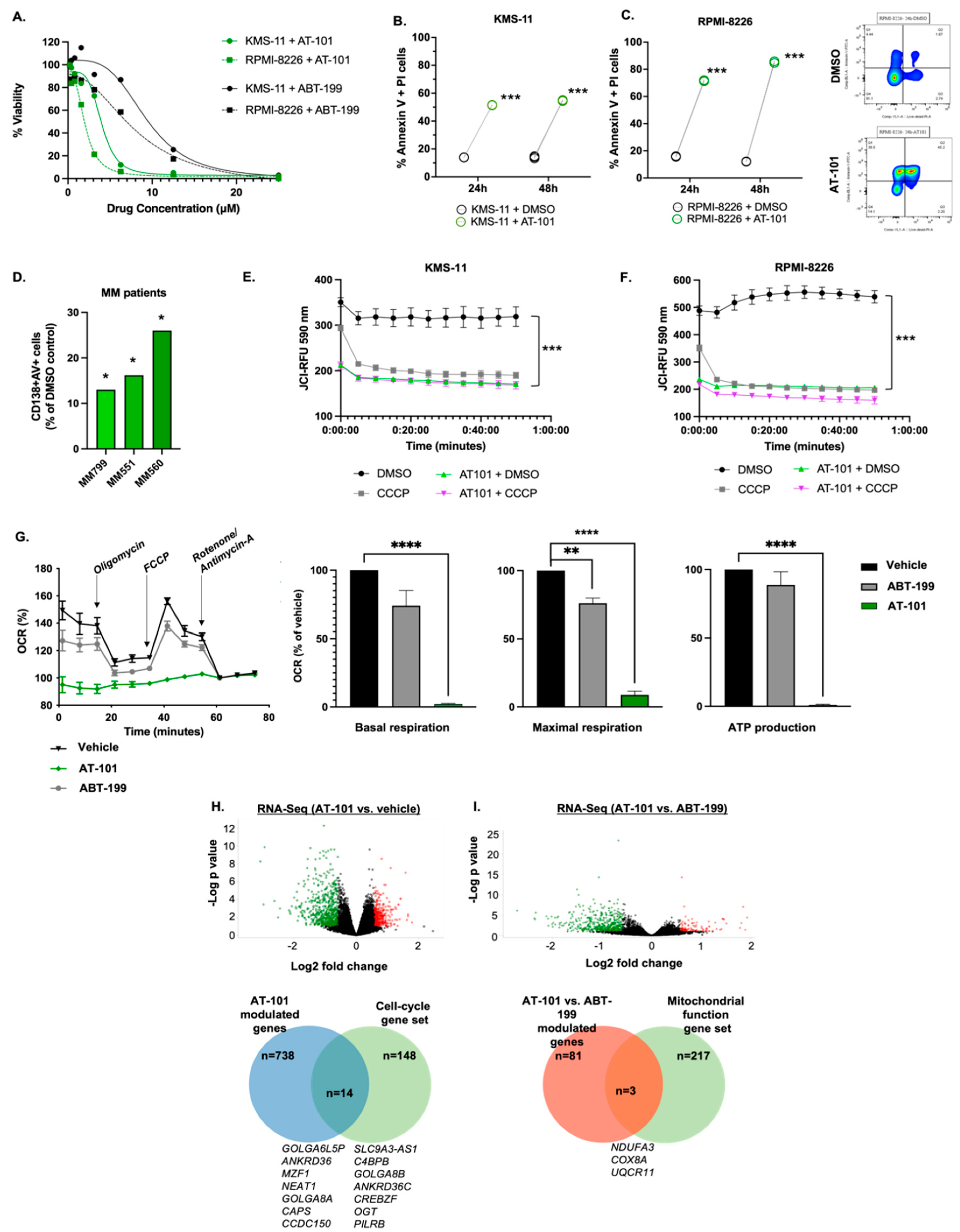
3.1. AT-101 Reduces MM Cell Viability and Leads to Mitochondrial-Mediated Apoptosis
3.2. AT-101 Enhances the Anti-Tumor Activity of Rd in a Xenograft Model of MM
3.3. Clinical Validation: Patient Demographics and Clinical Characteristics
4. Disposition
5. Safety Profile
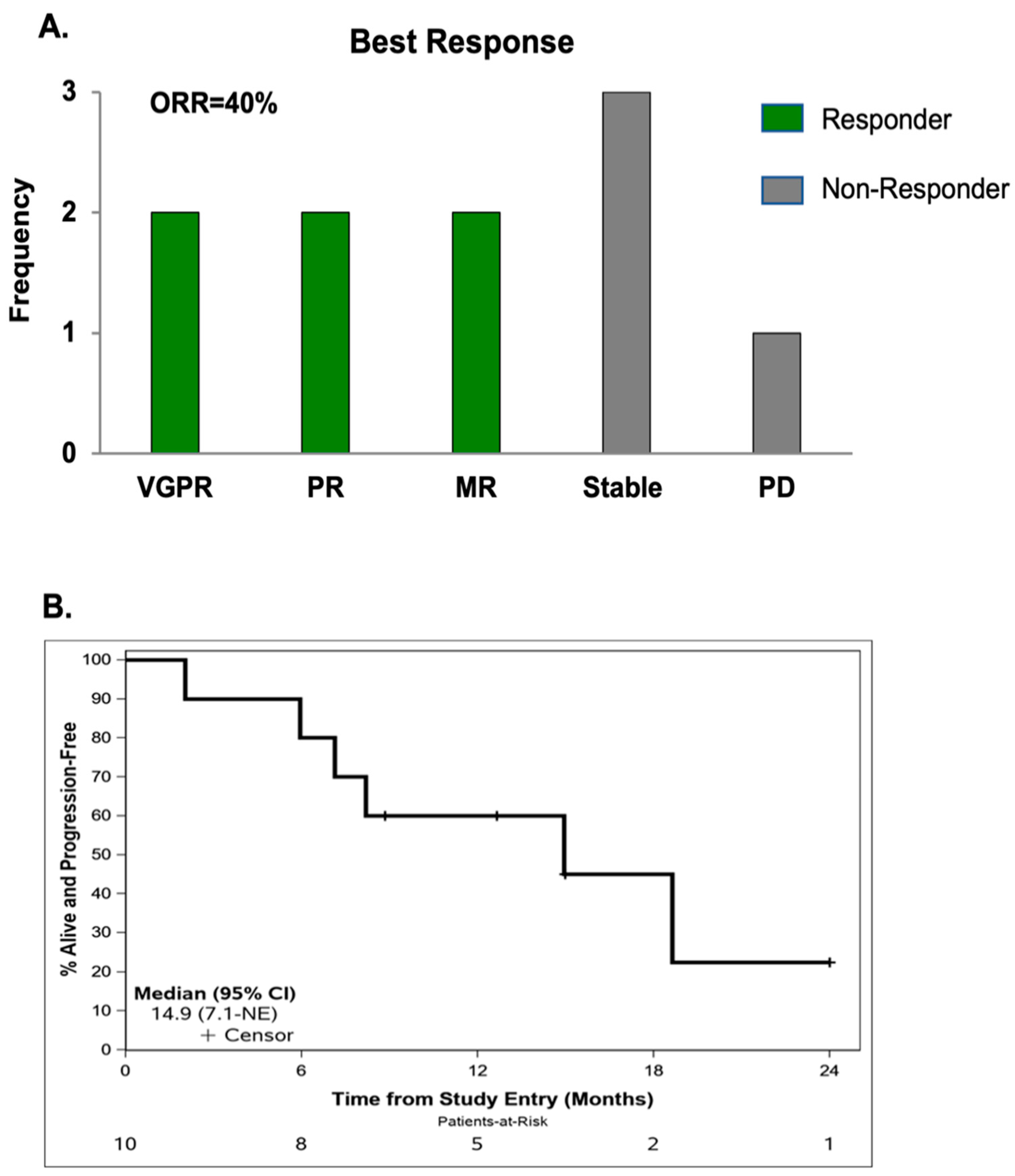
6. Efficacy
7. Correlative Analyses
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.; Garderet, L.; Mahindra, A.; Gahrton, G.; Caers, J.; Sezer, O.; Voorhees, P.; Leleu, X.; Johnsen, H.E.; Streetly, M.; et al. Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia 2016, 30, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Libby, E.; Garcia, D.; Quintana, D.; Fekrazad, M.H.; Bauman, J.; Ebaid, A.; Hromas, R.; Rabinowitz, I.; Wiggins, C. Disease-specific survival for patients with multiple myeloma: Significant improvements over time in all age groups. Leuk Lymphoma 2014, 55, 2850–2857. [Google Scholar] [CrossRef]
- van de Donk, N.W.; Lokhorst, H.M.; Bloem, A.C. Growth factors and antiapoptotic signaling pathways in multiple myeloma. Leukemia 2005, 19, 2177–2185. [Google Scholar] [CrossRef]
- Bodet, L.; Ménoret, E.; Descamps, G.; Pellat-Deceunynck, C.; Bataille, R.; Le Gouill, S.; Moreau, P.; Amiot, M.; Gomez-Bougie, P. BH3-only protein Bik is involved in both apoptosis induction and sensitivity to oxidative stress in multiple myeloma. Br. J. Cancer 2010, 103, 1808–1814. [Google Scholar] [CrossRef]
- Derenne, S.; Monia, B.; Dean, N.M.; Taylor, J.K.; Rapp, M.J.; Harousseau, J.L.; Bataille, R.; Amiot, M. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood 2002, 100, 194–199. [Google Scholar] [CrossRef]
- Gong, J.N.; Khong, T.; Segal, D.; Yao, Y.; Riffkin, C.D.; Garnier, J.M.; Khaw, S.L.; Lessene, G.; Spencer, A.; Herold, M.J.; et al. Hierarchy for targeting prosurvival BCL2 family proteins in multiple myeloma: Pivotal role of MCL1. Blood 2016, 128, 1834–1844. [Google Scholar] [CrossRef]
- Touzeau, C.; Ryan, J.; Guerriero, J.; Moreau, P.; Chonghaile, T.N.; Le Gouill, S.; Richardson, P.; Anderson, K.; Amiot, M.; Letai, A. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia 2016, 30, 761–764. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Miecznikowski, J.; Gaile, D.P.; Wang, D.; Sher, T.; Mulligan, G.; Bryant, B.; Wilding, G.E.; Mashtare, T.; Stein, L.; et al. Bortezomib mitigates adverse prognosis conferred by Bcl-2 overexpression in patients with relapsed/refractory multiple myeloma. Leuk Lymphoma 2012, 53, 1174–1182. [Google Scholar] [CrossRef]
- Wuillème-Toumi, S.; Robillard, N.; Gomez, P.; Moreau, P.; Le Gouill, S.; Avet-Loiseau, H.; Harousseau, J.L.; Amiot, M.; Bataille, R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005, 19, 1248–1252. [Google Scholar] [CrossRef]
- Kawano, Y.; Moschetta, M.; Manier, S.; Glavey, S.; Görgün, G.T.; Roccaro, A.M.; Anderson, K.C.; Ghobrial, I.M. Targeting the bone marrow microenvironment in multiple myeloma. Immunol. Rev. 2015, 263, 160–172. [Google Scholar] [CrossRef]
- Lancman, G.; Richter, J.; Chari, A. Bispecifics, trispecifics, and other novel immune treatments in myeloma. Hematol. Am. Soc. Hematol. Educ. Program. 2020, 2020, 264–271. [Google Scholar] [CrossRef]
- Kumar, S.; Kaufman, J.L.; Gasparetto, C.; Mikhael, J.; Vij, R.; Pegourie, B.; Benboubker, L.; Facon, T.; Amiot, M.; Moreau, P.; et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017, 130, 2401–2409. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Gasparetto, C.J.; Mikhael, J.; Moreau, P.; Touzeau, C.; Vij, R.; Facon, T.; Pegourie, B.; Benboubker, L.; Boise, L.H.; et al. Phase 1 Study of Venetoclax in Combination with Dexamethasone As Targeted Therapy for t(11;14) Relapsed/Refractory Multiple Myeloma. Blood 2017, 130, 3131. [Google Scholar] [CrossRef]
- Moreau, P.; Chanan-Khan, A.; Roberts, A.W.; Agarwal, A.B.; Facon, T.; Kumar, S.; Touzeau, C.; Punnoose, E.A.; Cordero, J.; Munasinghe, W.; et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017, 130, 2392–2400. [Google Scholar] [CrossRef]
- Costa, L.J.; Stadtmauer, E.A.; Morgan, G.J.; Monohan, G.P.; Kovacsovics, T.; Burwick, N.; Jakubowiak, A.J.; Mobasher, M.; Freise, K.; Ross, J.A.; et al. Phase 2 study of venetoclax plus carfilzomib and dexamethasone in patients with relapsed/refractory multiple myeloma. J. Clin. Oncol. 2018, 36, 8004. [Google Scholar] [CrossRef]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Punnoose, E.A.; Leverson, J.D.; Peale, F.; Boghaert, E.R.; Belmont, L.D.; Tan, N.; Young, A.; Mitten, M.; Ingalla, E.; Darbonne, W.C.; et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol. Cancer Ther. 2016, 15, 1132–1144. [Google Scholar] [CrossRef]
- Kline, M.P.; Rajkumar, S.V.; Timm, M.M.; Kimlinger, T.K.; Haug, J.L.; Lust, J.A.; Greipp, P.R.; Kumar, S. R-(-)-gossypol (AT-101) activates programmed cell death in multiple myeloma cells. Exp. Hematol. 2008, 36, 568–576. [Google Scholar] [CrossRef]
- Paulus, A.; Chitta, K.; Akhtar, S.; Personett, D.; Miller, K.C.; Thompson, K.J.; Carr, J.; Kumar, S.; Roy, V.; Ansell, S.M.; et al. AT-101 downregulates BCL2 and MCL1 and potentiates the cytotoxic effects of lenalidomide and dexamethasone in preclinical models of multiple myeloma and Waldenström macroglobulinaemia. Br. J. Haematol. 2014, 164, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Masood, A.; Chitta, K.; Paulus, A.; Khan, A.N.; Sher, T.; Ersing, N.; Miller, K.C.; Manfredi, D.; Ailawadhi, S.; Borrelo, I.; et al. Downregulation of BCL2 by AT-101 enhances the antileukaemic effect of lenalidomide both by an immune dependant and independent manner. Br. J. Haematol. 2012, 157, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Advani, P.; Laplant, B.R.; Akhtar, S.; Sher, T.; Rivera, C.E.; Foran, J.M.; Roy, V.; Colon-Otero, G.; Ailawadhi, S.; et al. Phase I/II Clinical Trial of Lenalidomide in Combination with AT101 for the Treatment of Relapsed B-Cell Chronic Lymphocytic Leukemia (B-CLL). Blood 2015, 126, 5299. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Seiller, C.; Maiga, S.; Touzeau, C.; Bellanger, C.; Kervoelen, C.; Descamps, G.; Maillet, L.; Moreau, P.; Pellat-Deceunynck, C.; Gomez-Bougie, P.; et al. Dual targeting of BCL2 and MCL1 rescues myeloma cells resistant to BCL2 and MCL1 inhibitors associated with the formation of BAX/BAK hetero-complexes. Cell Death Dis. 2020, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Bolomsky, A.; Vogler, M.; Köse, M.C.; Heckman, C.A.; Ehx, G.; Ludwig, H.; Caers, J. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 2020, 13, 173. [Google Scholar] [CrossRef]
- Investigator Brochure: AT-101, 9th, ed.; Ascenta Therapeutics, Inc.: Malvern, PA, USA, 2011.
- Zhai, D.; Jin, C.; Satterthwait, A.C.; Reed, J.C. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ. 2006, 13, 1419–1421. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Nikolovska-Coleska, Z.; Yang, C.Y.; Wang, R.; Tang, G.; Guo, J.; Shangary, S.; Qiu, S.; Gao, W.; Yang, D.; et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J. Med. Chem. 2006, 49, 6139–6142. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Goustin, A.S.; Aboukameel, A.; Chen, B.; Banerjee, S.; Wang, G.; Nikolovska-Coleska, Z.; Wang, S.; Al-Katib, A. Preclinical Studies of TW-37, a New Nonpeptidic Small-Molecule Inhibitor of Bcl-2, in Diffuse Large Cell Lymphoma Xenograft Model Reveal Drug Action on Both Bcl-2 and Mcl-1. Clin. Cancer Res. 2007, 13, 2226–2235. [Google Scholar] [CrossRef]
- Wei, J.; Kitada, S.; Rega, M.F.; Stebbins, J.L.; Zhai, D.; Cellitti, J.; Yuan, H.; Emdadi, A.; Dahl, R.; Zhang, Z.; et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J. Med. Chem. 2009, 52, 4511–4523. [Google Scholar] [CrossRef]
- Azmi, A.S.; Mohammad, R.M. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J. Cell Physiol. 2009, 218, 13–21. [Google Scholar] [CrossRef]
- Mallick, D.J.; Eastman, A. AT101 [(-)-Gossypol] Selectively Inhibits MCL1 and Sensitizes Carcinoma to BH3 Mimetics by Inducing and Stabilizing NOXA. Cancers 2020, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Kyle, R.A.; Rajkumar, S.V.; Stewart, A.K.; Weber, D.; Richardson, P. Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008, 22, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Touzeau, C.; Dousset, C.; Le Gouill, S.; Sampath, D.; Leverson, J.D.; Souers, A.J.; Maïga, S.; Béné, M.C.; Moreau, P.; Pellat-Deceunynck, C.; et al. The Bcl-2 specific BH3 mimetic ABT-199: A promising targeted therapy for t(11;14) multiple myeloma. Leukemia 2014, 28, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Gasparetto, C.; Schjesvold, F.H.; Moreau, P.; Touzeau, C.; Facon, T.; Boise, L.H.; Jiang, Y.; Yang, X.; Dunbar, F.; et al. Targeting BCL-2 with venetoclax and dexamethasone in patients with relapsed/refractory t(11;14) multiple myeloma. Am. J. Hematol. 2021, 96, 418–427. [Google Scholar] [CrossRef]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Bodet, L.; Gomez-Bougie, P.; Touzeau, C.; Dousset, C.; Descamps, G.; Maïga, S.; Avet-Loiseau, H.; Bataille, R.; Moreau, P.; Le Gouill, S.; et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood 2011, 118, 3901–3910. [Google Scholar] [CrossRef]
- Weber, D.M.; Chen, C.; Niesvizky, R.; Wang, M.; Belch, A.; Stadtmauer, E.A.; Siegel, D.; Borrello, I.; Rajkumar, S.V.; Chanan-Khan, A.A.; et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N. Engl. J. Med. 2007, 357, 2133–2142. [Google Scholar] [CrossRef]
- Ning, Y.; Riggins, R.B.; Mulla, J.E.; Chung, H.; Zwart, A.; Clarke, R. IFNgamma restores breast cancer sensitivity to fulvestrant by regulating STAT1, IFN regulatory factor 1, NF-kappaB, BCL2 family members, and signaling to caspase-dependent apoptosis. Mol. Cancer Ther. 2010, 9, 1274–1285. [Google Scholar] [CrossRef]
- Paulus, A.; Manna, A.; Akhtar, S.; Paulus, S.M.; Sharma, M.; Coignet, M.V.; Jiang, L.; Roy, V.; Witzig, T.E.; Ansell, S.M.; et al. Targeting CD38 with daratumumab is lethal to Waldenstrom macroglobulinaemia cells. Br. J. Haematol. 2018, 183, 196–211. [Google Scholar] [CrossRef]
- Paulus, A.; Akhtar, S.; Caulfield, T.R.; Samuel, K.; Yousaf, H.; Bashir, Y.; Paulus, S.M.; Tran, D.; Hudec, R.; Cogen, D.; et al. Coinhibition of the deubiquitinating enzymes, USP14 and UCHL5, with VLX1570 is lethal to ibrutinib- or bortezomib-resistant Waldenstrom macroglobulinemia tumor cells. Blood Cancer J. 2016, 6, e492. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Akhtar, S.; Yousaf, H.; Manna, A.; Paulus, S.M.; Bashir, Y.; Caulfield, T.R.; Kuranz-Blake, M.; Chitta, K.; Wang, X.; et al. Waldenstrom macroglobulinemia cells devoid of BTKC481S or CXCR4WHIM-like mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J. 2017, 7, e565. [Google Scholar] [CrossRef] [PubMed]
- Kalari, K.R.; A Nair, A.; Bhavsar, J.D.; O’Brien, D.R.; I Davila, J.; A Bockol, M.; Nie, J.; Tang, X.; Baheti, S.; Doughty, J.B.; et al. MAP-RSeq: Mayo Analysis Pipeline for RNA sequencing. BMC Bioinform. 2014, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Davila, J.I.; Baheti, S.; Bhagwate, A.V.; Wang, X.; Kocher, J.-P.A.; Slager, S.L.; Feldman, A.L.; Novak, A.J.; Cerhan, J.R.; et al. RVboost: RNA-seq variants prioritization using a boosting method. Bioinformatics 2014, 30, 3414–3416. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Anders, S.; Reyes, A.; Huber, W. Detecting differential usage of exons from RNA-Seq data. Nat. Précéd. 2012, 1–11. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 4 April 2022).
- Manna, A.; Kellett, T.; Aulakh, S.; Lewis-Tuffin, L.J.; Dutta, N.; Knutson, K.; Chini, E.; Pinilla-Ibarz, J.; Lamanna, N.; Manochakian, R.; et al. Targeting CD38 is lethal to Breg-like chronic lymphocytic leukemia cells and Tregs, but restores CD8+ T-cell responses. Blood Adv. 2020, 4, 2143–2157. [Google Scholar] [CrossRef] [PubMed]


| Sex | |
|---|---|
| Female | 4 (40%) |
| Male | 6 (60%) |
| Age, median (range), y | 69 (56–74) |
| Time from diagnosis to trial enrollment, median (range), y | 4 (0–7) |
| ISS stage | |
| I | 1 (10%) |
| II/III | 7 (70%) |
| Unknown | 2 (20%) |
| ECOG PS | |
| 0 | 6 (60%) |
| 1 | 4 (40%) |
| ≥2 | 0 |
| Cytogenetic Abnormalities | |
| High Risk Cytogenetics | 8 (80%) |
| Del(17p) | 4 (40%) |
| 1q dup | 4 (40%) |
| T(11;14) | 1 (10%) |
| Induction Regimen | |
| VRD | 8 (80%) |
| RD | 1 (10%) |
| CyBorD | 1 (10%) |
| Autologous Stem Cell Transplant | 7 (70%) |
| Lines of Prior Therapy, median (range) | 2 (1–2) |
| Lenalidomide Refractory | 3 (30%) |
| Bortezomib Refractory | 2 (20%) |
| Daratumumab Refractory | (30%) |
| AE | Any Grade | Grade 3/4 |
|---|---|---|
| Any AE | 10 (100%) | 8 (80%) |
| Hematologic Events | ||
| Thrombocytopenia | 9 (90%) | 2 (20%) |
| Low White Blood Cell Count | 8 (80%) | 3 (30%) |
| Anemia | 9 (90%) | 3 (30%) |
| Neutropenia | 9 (90%) | 5 (50%) |
| Gastrointestinal Events | ||
| Diarrhea | 5 (50%) | 0 |
| Constipation | 3 (30%) | 0 |
| Nausea | 3 (30%) | 0 |
| Vomiting | 1 (10%) | 0 |
| All other AEs | ||
| Fatigue | 10 (100%) | 0 |
| Back Pain | 1 (10%) | 1 (10%) |
| Peripheral Neuropathy | 6 (60%) | 0 |
| Hyperglycemia | 3 (30%) | 0 |
| Hyperkalemia | 2 (20%) | 0 |
| Hypokalemia | 6 (60%) | 1 (10%) |
| Hyponatremia | 4 (40%) | 0 |
| Atrial Flutter | 1 (10%) | 1 (10%) |
| Febrile Neutropenia | 1 (10%) | 1 (10%) |
| Increased Creatinine | 2 (20%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailawadhi, S.; Parrondo, R.D.; Dutta, N.; Han, B.; Ciccio, G.; Cherukuri, Y.; Alegria, V.R.; LaPlant, B.R.; Roy, V.; Sher, T.; et al. AT-101 Enhances the Antitumor Activity of Lenalidomide in Patients with Multiple Myeloma. Cancers 2023, 15, 477. https://doi.org/10.3390/cancers15020477
Ailawadhi S, Parrondo RD, Dutta N, Han B, Ciccio G, Cherukuri Y, Alegria VR, LaPlant BR, Roy V, Sher T, et al. AT-101 Enhances the Antitumor Activity of Lenalidomide in Patients with Multiple Myeloma. Cancers. 2023; 15(2):477. https://doi.org/10.3390/cancers15020477
Chicago/Turabian StyleAilawadhi, Sikander, Ricardo D. Parrondo, Navnita Dutta, Bing Han, Gina Ciccio, Yesesri Cherukuri, Victoria R. Alegria, Betsy R. LaPlant, Vivek Roy, Taimur Sher, and et al. 2023. "AT-101 Enhances the Antitumor Activity of Lenalidomide in Patients with Multiple Myeloma" Cancers 15, no. 2: 477. https://doi.org/10.3390/cancers15020477
APA StyleAilawadhi, S., Parrondo, R. D., Dutta, N., Han, B., Ciccio, G., Cherukuri, Y., Alegria, V. R., LaPlant, B. R., Roy, V., Sher, T., Edwards, B., Lanier, S., Manna, A., Heslop, K., Caulfield, T., Maldosevic, E., Storz, P., Manochakian, R., Asmann, Y., ... Paulus, A. (2023). AT-101 Enhances the Antitumor Activity of Lenalidomide in Patients with Multiple Myeloma. Cancers, 15(2), 477. https://doi.org/10.3390/cancers15020477








