Hsa-miR-665 Is a Promising Biomarker in Cancer Prognosis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Signaling Pathways Regulated by miR-665
2.1. MAPK Signaling Pathways
2.2. Wnt/β-Catenin Signaling Pathway
2.3. Regulation of miR-665
3. The Roles of miR-665 in Human Cancers
3.1. Breast Cancers
3.2. Cervical Cancer
3.3. Circulatory Cancers
3.4. Endometrial Cancer
3.5. Gastrointestinal Cancers
3.5.1. Colorectal Cancer
3.5.2. Gastric Cancer
3.5.3. Hepatocellular Carcinoma
3.6. Glioma Cancer
3.7. Lung Cancers
3.8. Neuroblastoma
3.9. Ovarian Cancer
3.10. Retinoblastoma
4. The Effect of miR-665 on Drug Resistance
5. Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwong, G.A.; Ghosh, S.; Gamboa, L.; Patriotis, C.; Srivastava, S.; Bhatia, S.N. Synthetic biomarkers: A twenty-first century path to early cancer detection. Nat. Rev. Cancer 2021, 21, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Chendrimada, T.P.; Cooch, N.; Shiekhattar, R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005, 123, 631–640. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Valinezhad Orang, A.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef] [PubMed]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Kwong, G.A.; von Maltzahn, G.; Murugappan, G.; Abudayyeh, O.; Mo, S.; Papayannopoulos, I.A.; Sverdlov, D.Y.; Liu, S.B.; Warren, A.D.; Popov, Y.; et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat. Biotechnol. 2013, 31, 63–70. [Google Scholar] [CrossRef]
- Aveta, A.; Cilio, S.; Contieri, R.; Spena, G.; Napolitano, L.; Manfredi, C.; Franco, A.; Crocerossa, F.; Cerrato, C.; Ferro, M.; et al. Urinary MicroRNAs as Biomarkers of Urological Cancers: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10846. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Belisario, D.C.; Rebelo, R.; Assaraf, Y.G.; Giovannetti, E.; Kopecka, J.; Vasconcelos, M.H. The role of extracellular vesicles in the transfer of drug resistance competences to cancer cells. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2022, 62, 100833. [Google Scholar] [CrossRef]
- Pavani, K.C.; Meese, T.; Pascottini, O.B.; Guan, X.; Lin, X.; Peelman, L.; Hamacher, J.; Van Nieuwerburgh, F.; Deforce, D.; Boel, A.; et al. Hatching is modulated by microRNA-378a-3p derived from extracellular vesicles secreted by blastocysts. Proc. Natl. Acad. Sci. USA 2022, 119, e2122708119. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, R.; Lupini, L.; Shankaraiah, R.C.; Negrini, M. The Importance of microRNAs in RAS Oncogenic Activation in Human Cancer. Front. Oncol. 2019, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and drug resistance. Cancer Gene Ther. 2010, 17, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-W.; Kim, E.Y.; Jeon, D.; Liu, J.-L.; Kim, H.S.; Choi, J.W.; Ahn, W.S. Differential microRNA expression signatures and cell type-specific association with Taxol resistance in ovarian cancer cells. Drug Des. Devel. Ther. 2014, 8, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Zhao, X.-G.; Hu, J.-Y.; Tang, J.; Yi, W.; Zhang, M.-Y.; Deng, R.; Mai, S.-J.; Weng, N.-Q.; Wang, R.-Q.; Liu, J.; et al. miR-665 expression predicts poor survival and promotes tumor metastasis by targeting NR4A3 in breast cancer. Cell Death Dis. 2019, 10, 479. [Google Scholar] [CrossRef]
- Chen, T.-J.; Zheng, Q.; Gao, F.; Yang, T.; Ren, H.; Li, Y.; Chen, M.-W. MicroRNA-665 facilitates cell proliferation and represses apoptosis through modulating Wnt5a/β-Catenin and Caspase-3 signaling pathways by targeting TRIM8 in LUSC. Cancer Cell Int. 2021, 21, 215. [Google Scholar] [CrossRef]
- Bhat, A.A.; Younes, S.N.; Raza, S.S.; Zarif, L.; Nisar, S.; Ahmed, I.; Mir, R.; Kumar, S.; Sharawat, S.K.; Hashem, S.; et al. Role of non-coding RNA networks in leukemia progression, metastasis and drug resistance. Mol. Cancer 2020, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, X.; Feng, X.; Fan, X.; Jin, Z. The crosstalk between microRNAs and the Wnt/β-catenin signaling pathway in cancer. Oncotarget 2016, 8, 14089–14106. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK signalling: A master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xiong, T.; Yao, L.; Yuan, J. MicroRNA-665 promotes the proliferation of ovarian cancer cells by targeting SRCIN1. Exp. Ther. Med. 2020, 19, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, O.; Yu, Y.; Tan, S. Paeonol inhibits the malignancy of Apatinib-resistant gastric cancer cells via LINC00665/miR-665/MAPK1 axis. Phytomed. Int. J. Phytother. Phytopharm. 2022, 96, 153903. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-Z.; Zhang, C.-D.; Zhang, C.; Pei, J.-P.; Dai, D.-Q. miR-665 Suppresses the Epithelial-Mesenchymal Transition and Progression of Gastric Cancer by Targeting CRIM1. Cancer Manag. Res. 2020, 12, 3489–3501. [Google Scholar] [CrossRef]
- Esteves, J.V.; Enguita, F.J.; Machado, U.F. MicroRNAs-Mediated Regulation of Skeletal Muscle GLUT4 Expression and Translocation in Insulin Resistance. J. Diabetes Res. 2017, 2017, 7267910. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Deguchi, S.; Katsushima, K.; Hatanaka, A.; Shinjo, K.; Ohka, F.; Wakabayashi, T.; Zong, H.; Natsume, A.; Kondo, Y. Oncogenic effects of evolutionarily conserved noncoding RNA ECONEXIN on gliomagenesis. Oncogene 2017, 36, 4629–4640. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ying, Y.; Xie, H.; Li, J.; Ma, X.; He, L.; Xu, M.; Chen, S.; Shen, H.; Zheng, X.; et al. miR-665 inhibits epithelial-to-mesenchymal transition in bladder cancer via the SMAD3/SNAIL axis. Cell Cycle Georget. Tex 2021, 20, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-W.; Huang, X.; Liu, S.; Lu, Y. EXT1, Regulated by MiR-665, Promotes Cell Apoptosis via ERK1/2 Signaling Pathway in Acute Lymphoblastic Leukemia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 6491–6503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, M.; He, L.; Qi, H.; Shen, J.; Ying, K. Exosomal lncRNA SCIRT/miR-665 Transferring Promotes Lung Cancer Cell Metastasis through the Inhibition of HEYL. J. Oncol. 2021, 2021, 9813773. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jin, H.; Zheng, Y.; Mao, Y.; Fu, Z.; Li, X.; Dong, L. DANCR-mediated microRNA-665 regulates proliferation and metastasis of cervical cancer through the ERK/SMAD pathway. Cancer Sci. 2019, 110, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Pan, A.; He, L.; Wang, J.; Zhou, F.; Lei, Y.; Wang, Y. Long non-coding RNA NHEG1/hsa-miR-665/HMGB1 axis is involved in the regulation of neuroblastoma progression. Bioengineered 2021, 12, 11584–11596. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Zhao, Z.; Feng, M.; Zhang, S. Identification of potential core genes and miRNAs in testicular seminoma via bioinformatics analysis. Mol. Med. Rep. 2019, 20, 4013–4022. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.; Li, B.; Wang, Y.; Wang, B.; Wang, Z.; Wang, M.; Teng, Q. MiR-665 suppresses the progression of diffuse large B cell lymphoma (DLBCL) through targeting LIM and SH3 protein 1 (LASP1). Leuk. Res. 2022, 112, 106769. [Google Scholar] [CrossRef]
- Prashad, N. miR-665 targets c-MYC and HDAC8 to inhibit murine neuroblastoma cell growth. Oncotarget 2018, 9, 33186–33201. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.-H.; Lafita-Navarro, M.C.; Zacharias, L.; Borenstein-Auerbach, N.; Kim, M.; Barnes, S.; Kim, J.; Shay, J.; DeBerardinis, R.J.; Conacci-Sorrell, M. Induction of LEF1 by MYC activates the WNT pathway and maintains cell proliferation. Cell Commun. Signal. CCS 2019, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Jiang, M.; Ji, C.; Chen, G.; Zhang, Q.; Liu, P.; Zhang, R.; Ren, X.; Yu, W.; et al. SOCS3 deficiency-dependent autophagy repression promote the survival of early-stage myeloid-derived suppressor cells in breast cancer by activating the Wnt/mTOR pathway. J. Leukoc. Biol. 2023, 113, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, M.; Zhang, R.; Liu, P.; Ye, Y.; Ren, X.; Yu, W.; Yu, J. Abstract 985: SOCS3 deficiency blocked autophagy-dependent myeloid differentiation of early-stage myeloid-derived suppressor cells via the miR-155/C/EBPβ/Wnt axis. Cancer Res. 2020, 80, 985. [Google Scholar] [CrossRef]
- Tang, H.; Long, Q.; Zhuang, K.; Yan, Y.; Han, K.; Guo, H.; Lu, X. miR-665 promotes the progression of gastric adenocarcinoma via elevating FAK activation through targeting SOCS3 and is negatively regulated by lncRNA MEG3. J. Cell. Physiol. 2020, 235, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Albers, P.; Berney, D.M.; Feldman, D.R.; Daugaard, G.; Gilligan, T.; Looijenga, L.H.J. Testicular cancer. Nat. Rev. Dis. Primer 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Sen, R.; Ghosal, S.; Das, S.; Balti, S.; Chakrabarti, J. Competing endogenous RNA: The key to posttranscriptional regulation. Sci. World J. 2014, 2014, 896206. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Chen, L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Long Noncoding RNAs: Molecular Modalities to Organismal Functions. Annu. Rev. Biochem. 2020, 89, 283–308. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.; Wang, Y.; Lin, M.F.; Koegel, A.K.; Kotake, Y.; Grant, G.D.; Horlings, H.M.; Shah, N.; Umbricht, C.; Wang, P.; et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011, 43, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-Y.; Johnson, R.; Stanton, L.W. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012, 31, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, C.; Gao, Y.; Wang, Y.; Ding, Z.; Zhang, Y.; Shen, W.; Zheng, Y.; Wan, Y. A Novel Long Non-coding RNA, MSTRG.51053.2 Regulates Cisplatin Resistance by Sponging the miR-432-5p in Non-small Cell Lung Cancer Cells. Front. Oncol. 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guan, Y.; Sheng, H.; Liu, Y. Crosstalk in competing endogenous RNA network reveals the complex molecular mechanism underlying lung cancer. Oncotarget 2017, 8, 91270–91280. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ni, G.; Mao, L.; Xue, X.; Zhang, J.; Wu, W.; Zhang, S.; Zhao, H.; Ding, L.; Wang, L. LINC00565 promotes proliferation and inhibits apoptosis of gastric cancer by targeting miR-665/AKT3 axis. OncoTargets Ther. 2019, 12, 7865–7875. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Shen, C.; Wu, Z.; Hu, H. Circ_0030586 inhibits cell proliferation and stemness in bladder cancer by inactivating the ERK signaling via miR-665/NR4A3 axis. Acta Histochem. 2021, 123, 151745. [Google Scholar] [CrossRef]
- Kalyana-Sundaram, S.; Kumar-Sinha, C.; Shankar, S.; Robinson, D.R.; Wu, Y.-M.; Cao, X.; Asangani, I.A.; Kothari, V.; Prensner, J.R.; Lonigro, R.J.; et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell 2012, 149, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Tan, J.; Lee, S.; Kong, L.; Heger, A.; Ponting, C.P. Evidence for conserved post-transcriptional roles of unitary pseudogenes and for frequent bifunctionality of mRNAs. Genome Biol. 2012, 13, R102. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Garcia, V.; Tawil, Y.; Wise, H.M.; Leslie, N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019, 59, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.; Munkácsy, G.; Győrffy, B. Pancancer survival analysis of cancer hallmark genes. Sci. Rep. 2021, 11, 6047. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J. Med. Internet Res. 2021, 23, e27633. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Peng, E.; Xia, F.; Wang, D.; Li, X.; Li, X. CircABCC2 Regulates Hepatocellular Cancer Progression by Decoying MiR-665. J. Cancer 2019, 10, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, T.; Ji, J.; Zhao, F.; Li, C.; Han, X. RHPN1-AS1 promotes cell proliferation and migration via miR-665/Akt3 in ovarian cancer. Cancer Gene Ther. 2021, 28, 33–41. [Google Scholar] [CrossRef]
- Liu, C.; Tang, M.; Zhang, X.; Li, J.; Cao, G. Knockdown of miR-665 Protects Against Cardiomyocyte Ischemia/Reperfusion Injury-Induced ROS Accumulation and Apoptosis Through the Activation of Pak1/Akt Signaling in Myocardial Infarction. Int. Heart J. 2020, 61, 347–354. [Google Scholar] [CrossRef]
- Nygren, M.K.; Tekle, C.; Ingebrigtsen, V.A.; Mäkelä, R.; Krohn, M.; Aure, M.R.; Nunes-Xavier, C.E.; Perälä, M.; Tramm, T.; Alsner, J.; et al. Identifying microRNAs regulating B7-H3 in breast cancer: The clinical impact of microRNA-29c. Br. J. Cancer 2014, 110, 2072–2080. [Google Scholar] [CrossRef]
- Bure, I.; Braun, A.; Kayser, C.; Geddert, H.; Schaefer, I.-M.; Cameron, S.; Ghadimi, M.B.; Ströbel, P.; Werner, M.; Hartmann, A.; et al. The expression of hematopoietic progenitor cell antigen CD34 is regulated by DNA methylation in a site-dependent manner in gastrointestinal stromal tumours. Int. J. Cancer 2017, 141, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Deng, C. Circ_0044556 Promotes the Progression of Colorectal Cancer via the miR-665-Dependent Expression Regulation of Diaphanous Homolog 1. Dig. Dis. Sci. 2021, 67, 4458–4470. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Dong, S.; Liao, H.; Huang, W.; Yuan, X. Circ_0101802 Facilitates Colorectal Cancer Progression Depending on the Regulation of miR-665/DVL3 Signaling. Biochem. Genet. 2022, 60, 2250–2267. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Li, P.; Xie, Y.; Wang, Z.; Wang, R. CircMYBL2 regulates the resistance of cervical cancer cells to paclitaxel via miR-665-dependent regulation of EGFR. Drug Dev. Res. 2021, 82, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wu, J.; Wu, J.; Ji, A.; Qiang, G.; Jiang, Y.; Jiang, C.; Ding, Y. Exosomal miR-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget 2017, 8, 80666–80678. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, D.; Chu, H.; Gong, Z.; Zhang, C.; Gong, B.; Li, Y.; Li, N.; Jiang, L. Screening of differential microRNA expression in gastric signet ring cell carcinoma and gastric adenocarcinoma and target gene prediction. Oncol. Rep. 2015, 33, 2963–2971. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Wu, Y.; Gu, F.; Yan, D.; Yang, L.; Ma, Q.; Fu, C. Circ_0078767 Inhibits the Progression of Non-Small-Cell Lung Cancer by Regulating the GPX3 Expression by Adsorbing miR-665. Int. J. Genom. 2022, 2022, 6361256. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-C.; Pei, L. Dexmedetomidine attenuates propofol-induced apoptosis of neonatal hippocampal astrocytes by inhibiting the Bcl2l1 signalling pathway. Eur. J. Neurosci. 2021, 54, 7775–7789. [Google Scholar] [CrossRef]
- Tong, X.; Yang, Z.; Wang, Q.; Zhang, D. RNF144A-AS1 promotes the development of glioma cells by targeting miR-665/HMGA1 axis. Neurosci. Lett. 2021, 765, 136259. [Google Scholar] [CrossRef]
- Shen, H.; Xu, L.; You, C.; Tang, H.; Wu, H.; Zhang, Y.; Xie, M. miR-665 is downregulated in glioma and inhibits tumor cell proliferation, migration and invasion by targeting high mobility group box 1. Oncol. Lett. 2021, 21, 156. [Google Scholar] [CrossRef]
- Zhang, S.; Long, J.; Hu, Y. Long noncoding RNA LINC00205 enhances the malignant characteristics of retinoblastoma by acting as a molecular sponge of microRNA-665 and consequently increasing HMGB1 expression. Biochem. Biophys. Res. Commun. 2020, 526, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Zhai, Z.; Hao, Z.; Zhang, H.; He, J. CircWHSC1 serves as an oncogene to promote hepatocellular carcinoma progression. Eur. J. Clin. Investig. 2021, 51, e13487. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Y.; Wan, Y.; Zhou, S.; Thapa, S.; Cheng, W. MicroRNA-665 suppresses the growth and migration of ovarian cancer cells by targeting HOXA10. Mol. Med. Rep. 2018, 18, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lei, C.; Shi, P.; Teng, H.; Lu, L.; Guo, H.; Wang, X. LncRNA DCST1-AS1 Promotes Endometrial Cancer Progression by Modulating the MiR-665/HOXB5 and MiR-873-5p/CADM1 Pathways. Front. Oncol. 2021, 11, 714652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jing, B.; Bai, Y.; Zhang, Y.; Yu, H. Circular RNA circTMEM45A Acts as the Sponge of MicroRNA-665 to Promote Hepatocellular Carcinoma Progression. Mol. Ther. Nucleic Acids 2020, 22, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, N.; Kudo, T.; Sato, M.; Kawasaki, Y.; Yonezawa, S.; Takahashi, S.; Miyagi, Y.; Natori, Y.; Sugiyama, A. Reduction of Membrane Protein CRIM1 Decreases E-Cadherin and Increases Claudin-1 and MMPs, Enhancing the Migration and Invasion of Renal Carcinoma Cells. Biol. Pharm. Bull. 2018, 41, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, S.; Qiu, Y.; He, Y.; Chen, B.; Mao, R.; Cui, Y.; Zeng, Z.; Chen, M. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017, 8, e2699. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Yi, A.; Qiao, Y. microRNA-665 is down-regulated in gastric cancer and inhibits proliferation, invasion, and EMT by targeting PPP2R2A. Cell Biochem. Funct. 2020, 38, 409–418. [Google Scholar] [CrossRef]
- Huang, C.; Yue, W.; Li, L.; Li, S.; Gao, C.; Si, L.; Qi, L.; Cheng, C.; Lu, M.; Chen, G.; et al. Circular RNA hsa-circ-000881 suppresses the progression of lung adenocarcinoma in vitro via a miR-665/PRICKLE2 axis. Ann. Transl. Med. 2021, 9, 498. [Google Scholar] [CrossRef]
- Xia, J.; Li, D.; Zhu, X.; Xia, W.; Qi, Z.; Li, G.; Xu, Q. Upregulated miR-665 expression independently predicts poor prognosis of lung cancer and facilitates tumor cell proliferation, migration and invasion. Oncol. Lett. 2020, 19, 3578–3586. [Google Scholar] [CrossRef]
- Dong, C.; Du, Q.; Wang, Z.; Wang, Y.; Wu, S.; Wang, A. MicroRNA-665 suppressed the invasion and metastasis of osteosarcoma by directly inhibiting RAB23. Am. J. Transl. Res. 2016, 8, 4975–4981. [Google Scholar] [PubMed]
- Zhou, B.; Guo, W.; Sun, C.; Zhang, B.; Zheng, F. Linc00462 promotes pancreatic cancer invasiveness through the miR-665/TGFBR1-TGFBR2/SMAD2/3 pathway. Cell Death Dis. 2018, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhou, X.; Chen, Z.; Wang, M.; Zheng, X.; Xie, M. LncRNA BCAR4, targeting to miR-665/STAT3 signaling, maintains cancer stem cells stemness and promotes tumorigenicity in colorectal cancer. Cancer Cell Int. 2019, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, Y.; Li, J.; Zheng, X.; Liang, J.; Cui, G.; Chang, H. LncRNA RPSAP52 promotes cell proliferation and inhibits cell apoptosis via modulating miR-665/STAT3 in gastric cancer. Bioengineered 2022, 13, 8699–8711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Zhao, Y.; Li, D.; Zhang, Q.; Fu, J.; Fan, S. Knockdown of ADORA2A antisense RNA 1 inhibits cell proliferation and enhances imatinib sensitivity in chronic myeloid leukemia. Bioengineered 2022, 13, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.-M.; Yuan, J.-B.; Li, L.-L.; Wang, C.; Lin, X.-M.; Miao, X.; Shi, Z.-C. MiR-665 inhibits inflammatory response in microglia following spinal cord injury by targeting TREM2. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, X.; Yang, L.; Li, M.; Zhang, Y.; Zhang, J. CircASH2L Promotes Ovarian Cancer Tumorigenesis, Angiogenesis, and Lymphangiogenesis by Regulating the miR-665/VEGFA Axis as a Competing Endogenous RNA. Front. Cell Dev. Biol. 2020, 8, 595585. [Google Scholar] [CrossRef]
- Lin, X.; Huang, C.; Chen, Z.; Wang, H.; Zeng, Y. CircRNA_100876 Is Upregulated in Gastric Cancer (GC) and Promotes the GC Cells’ Growth, Migration and Invasion via miR-665/YAP1 Signaling. Front. Genet. 2020, 11, 546275. [Google Scholar] [CrossRef]
- Li, C.; Qin, F.; Hu, F.; Xu, H.; Sun, G.; Han, G.; Wang, T.; Guo, M. Characterization and selective incorporation of small non-coding RNAs in non-small cell lung cancer extracellular vesicles. Cell Biosci. 2018, 8, 2. [Google Scholar] [CrossRef]
- Liu, W.; Hu, W.; Hou, K.; Zhu, S. Circular RNA Paired-Related Homeobox 1 Promotes Gastric Carcinoma Cell Progression via Regulating MicroRNA-665/YWHAZ Axis. Dig. Dis. Sci. 2021, 66, 3842–3853. [Google Scholar] [CrossRef]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, M.; Xu, F.; Jiang, S. Wnt signaling in breast cancer: Biological mechanisms, challenges and opportunities. Mol. Cancer 2020, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.; Ling, V.; Carreno, B.M. The B7 family of immune-regulatory ligands. Genome Biol. 2005, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Gullick, W.J. c-erbB-4/HER4: Friend or foe? J. Pathol. 2003, 200, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Sundvall, M.; Lundin, M.; Lundin, J.; Tanner, M.; Härkönen, P.; Joensuu, H.; Isola, J.; Elenius, K. Cleavable ErbB4 isoform in estrogen receptor-regulated growth of breast cancer cells. Cancer Res. 2005, 65, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Abrams, S.L.; Fitzgerald, T.L.; Cocco, L.; Martelli, A.M.; Montalto, G.; Cervello, M.; Scalisi, A.; Candido, S.; Libra, M.; et al. Roles of signaling pathways in drug resistance, cancer initiating cells and cancer progression and metastasis. Adv. Biol. Regul. 2015, 57, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, F.; Mesrian Tanha, H.; Mojtabavi Naeini, M.; Ghaedi, K.; Azadeh, M. Tumor-promoting function of single nucleotide polymorphism rs1836724 (C3388T) alters multiple potential legitimate microRNA binding sites at the 3′-untranslated region of ErbB4 in breast cancer. Mol. Med. Rep. 2016, 13, 4494–4498. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-P.; Bian, H.-B.; Wang, X.-Z.; Liu, L.; Wei, D. Association of ERBB4 genetic polymorphism with the risk and prognosis of non-small cell lung cancer in Chinese Han population: A population-based case-control study. Medicine 2021, 100, e25762. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Y.; Zhang, L.; Ma, J.; Xia, Y.; Wang, X. Long noncoding RNAs regulate intrauterine adhesion and cervical cancer development and progression. Semin. Cell Dev. Biol. 2023, in press. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 (Suppl. 4), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G. Metastases go with the flow. Science 2018, 362, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, A.E.; Price, T.T.; Cantelli, G.; Sipkins, D.A. Leukaemia: A model metastatic disease. Nat. Rev. Cancer 2021, 21, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Gascoyne, R.D.; Lunning, M.A.; Cavalli, F. Non-Hodgkin lymphoma. Lancet 2017, 390, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic alterations and their clinical implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.T.; Kousteni, S.; Aifantis, I. Mapping and targeting of the leukemic microenvironment. J. Exp. Med. 2020, 217, e20190589. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, H.; Yang, S.; Su, D. Increased ABCC2 expression predicts cisplatin resistance in non-small cell lung cancer. Cell Biochem. Funct. 2021, 39, 277–286. [Google Scholar] [CrossRef]
- Anaya, J. OncoLnc: Linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput. Sci. 2016, 2, e67. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef]
- Fleming, G.F. Systemic chemotherapy for uterine carcinoma: Metastatic and adjuvant. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Stik, G.; Graf, T. Hoxb5, a Trojan horse to generate T cells. Nat. Immunol. 2018, 19, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ajani, J.A. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet 2004, 364, 1219–1228. [Google Scholar] [CrossRef]
- Chau, I.; Fuchs, C.S.; Ohtsu, A.; Barzi, A.; Liepa, A.M.; Cui, Z.L.; Hsu, Y.; Al-Batran, S.-E. Association of quality of life with disease characteristics and treatment outcomes in patients with advanced gastric cancer: Exploratory analysis of RAINBOW and REGARD phase III trials. Eur. J. Cancer 2019, 107, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Wu, G. Effect of paeonol on proliferation, apoptosis, migration, invasion and glutamine of gastric cancer cells via circSFMBT2/miR-665 axis. Cell. Mol. Biol. 2020, 66, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, Y.; Lin, J.; He, C.; Liao, H.; Li, H.; Zhou, Z.; Wang, J.; Mao, K.; Xiao, Z. Circular RNA circSFMBT2 downregulation by HBx promotes hepatocellular carcinoma metastasis via the miR-665/TIMP3 axis. Mol. Ther. Nucleic Acids 2022, 29, 788–802. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Omar, A.A.A.; El-Awady, R.R.; Hassan, S.M.A.; Eitah, W.M.S.; Ahmed, R.; Khater, A.; Tantawi, O.M.S.; Mohamed, A.A. MiR-155 and MiR-665 Role as Potential Non-invasive Biomarkers for Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. J. Transl. Intern. Med. 2020, 8, 32–40. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, X.; Wang, B.; Cai, Y.; Zheng, L.; Hu, L.; Lu, X.; Xie, S.; Zhang, X.; Liu, H.; et al. LncRNA LIMT (LINC01089) contributes to sorafenib chemoresistance via regulation of miR-665 and epithelial to mesenchymal transition in hepatocellular carcinoma cells. Acta Biochim. Biophys. Sin. 2022, 54, 1–10. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Chen, L.-H.; Hong, M.; Chen, Y.-Y.; Yang, X.-R.; Tang, S.-M.; Yuan, Q.-F.; Chen, W.-W. Serum microRNA profiling and bioinformatics analysis of patients with type 2 diabetes mellitus in a Chinese population. Mol. Med. Rep. 2017, 15, 2143–2153. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.C. Holland-Frei Cancer Medicine, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-1-55009-213-4. [Google Scholar]
- Lancet, T. Lung cancer: Some progress, but still a lot more to do. Lancet 2019, 394, 1880. [Google Scholar] [CrossRef] [PubMed]
- Frese, K.K.; Simpson, K.L.; Dive, C. Small cell lung cancer enters the era of precision medicine. Cancer Cell 2021, 39, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, L.; Xu, Z.; Cui, Y. MiR-665 Promotes the Biological Behavior of Small Cell Lung Cancer by Targeting LLGL1. Zhongguo Fei Ai Za Zhi Chin. J. Lung Cancer 2020, 23, 223–232. [Google Scholar] [CrossRef]
- Woodard, G.A.; Jones, K.D.; Jablons, D.M. Lung Cancer Staging and Prognosis. Cancer Treat. Res. 2016, 170, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.E.; Gerson, J.; Schnaufer, L. Spontaneous regression of neuroblastoma. Natl. Cancer Inst. Monogr. 1976, 44, 49–54. [Google Scholar] [PubMed]
- Qiu, B.; Matthay, K.K. Advancing therapy for neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L. HMGB1-induced autophagy in Schwann cells promotes neuroblastoma proliferation. Int. J. Clin. Exp. Pathol. 2015, 8, 504–510. [Google Scholar]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Vergote, I.; Gonzalez-Martin, A.; Lorusso, D.; Gourley, C.; Mirza, M.R.; Kurtz, J.-E.; Okamoto, A.; Moore, K.; Kridelka, F.; McNeish, I.; et al. Clinical research in ovarian cancer: Consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022, 23, e374–e384. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.; Zhao, Y.; Yin, L.; Fu, M.; Zhang, X.; Jiang, P. The functional roles of long noncoding RNA DANCR in Human Cancers. J. Cancer 2020, 11, 6970–6981. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br. J. Ophthalmol. 2009, 93, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Duan, G.; Du, B. Downregulation of MIAT reduces the proliferation and migratory and invasive abilities of retinoblastoma cells by sponging miR-665 and regulating LASP1. Exp. Ther. Med. 2021, 22, 1342. [Google Scholar] [CrossRef] [PubMed]
- Slotta-Huspenina, J.; Drecoll, E.; Feith, M.; Habermehl, D.; Combs, S.; Weichert, W.; Bettstetter, M.; Becker, K.; Langer, R. MicroRNA expression profiling for the prediction of resistance to neoadjuvant radiochemotherapy in squamous cell carcinoma of the esophagus. J. Transl. Med. 2018, 16, 109. [Google Scholar] [CrossRef]
- Zhao, W.-X.; Tang, Y.-L.; Wang, W.-H.; Bao, M.-W. Up-regulation of circ_0000353 impedes the proliferation and metastasis of non-small cell lung cancer cells via adsorbing miR-411-5p and increasing forkhead box O1. Cancer Biomark. Sect. Dis. Markers 2020, 29, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-C.; Liang, Z.-D.; Pei, L. Propofol-induced rno-miR-665 targets BCL2L1 and influences apoptosis in rodent developing hippocampal astrocytes. Neurotoxicology 2015, 51, 87–95. [Google Scholar] [CrossRef]
- Huang, T.; Chen, Y.; Zeng, Y.; Xu, C.; Huang, J.; Hu, W.; Chen, X.; Fu, H. Long non-coding RNA PSMA3-AS1 promotes glioma progression through modulating the miR-411-3p/HOXA10 pathway. BMC Cancer 2021, 21, 844. [Google Scholar] [CrossRef]
- Lánczky, A.; Nagy, Á.; Bottai, G.; Munkácsy, G.; Szabó, A.; Santarpia, L.; Győrffy, B. miRpower: A web-tool to validate survival-associated miRNAs utilizing expression data from 2178 breast cancer patients. Breast Cancer Res. Treat. 2016, 160, 439–446. [Google Scholar] [CrossRef]
- Sharifi, Z.; Talkhabi, M.; Taleahmad, S. Identification of potential microRNA diagnostic panels and uncovering regulatory mechanisms in breast cancer pathogenesis. Sci. Rep. 2022, 12, 20135. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zahra Bokhari, S.E.; Fan, X.X.; Malik, S.I. The role of exosomes derived miRNAs in cancer. JPMA J. Pak. Med. Assoc. 2021, 71, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic Nanoparticles for RNA-Based Cancer Treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
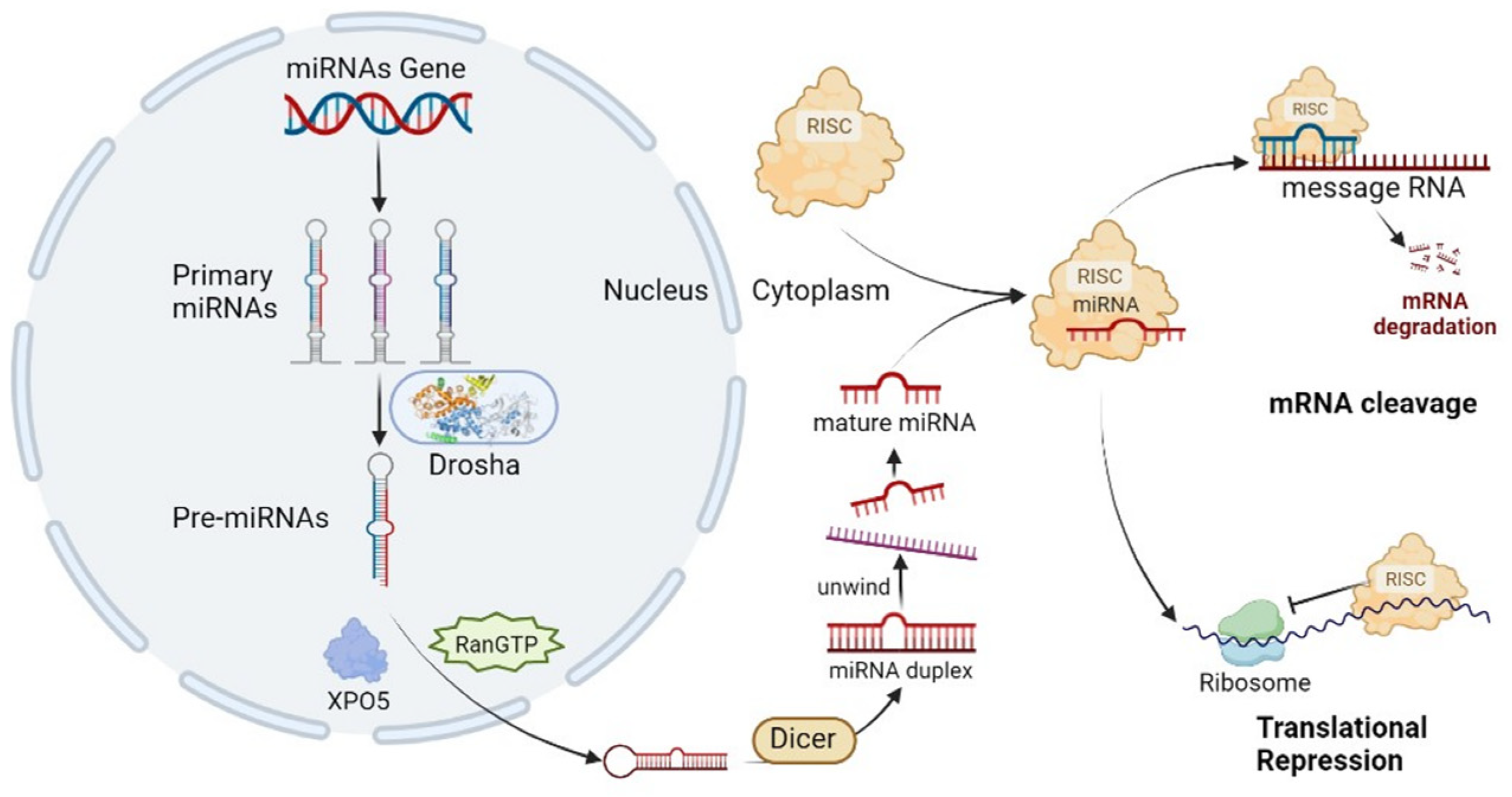
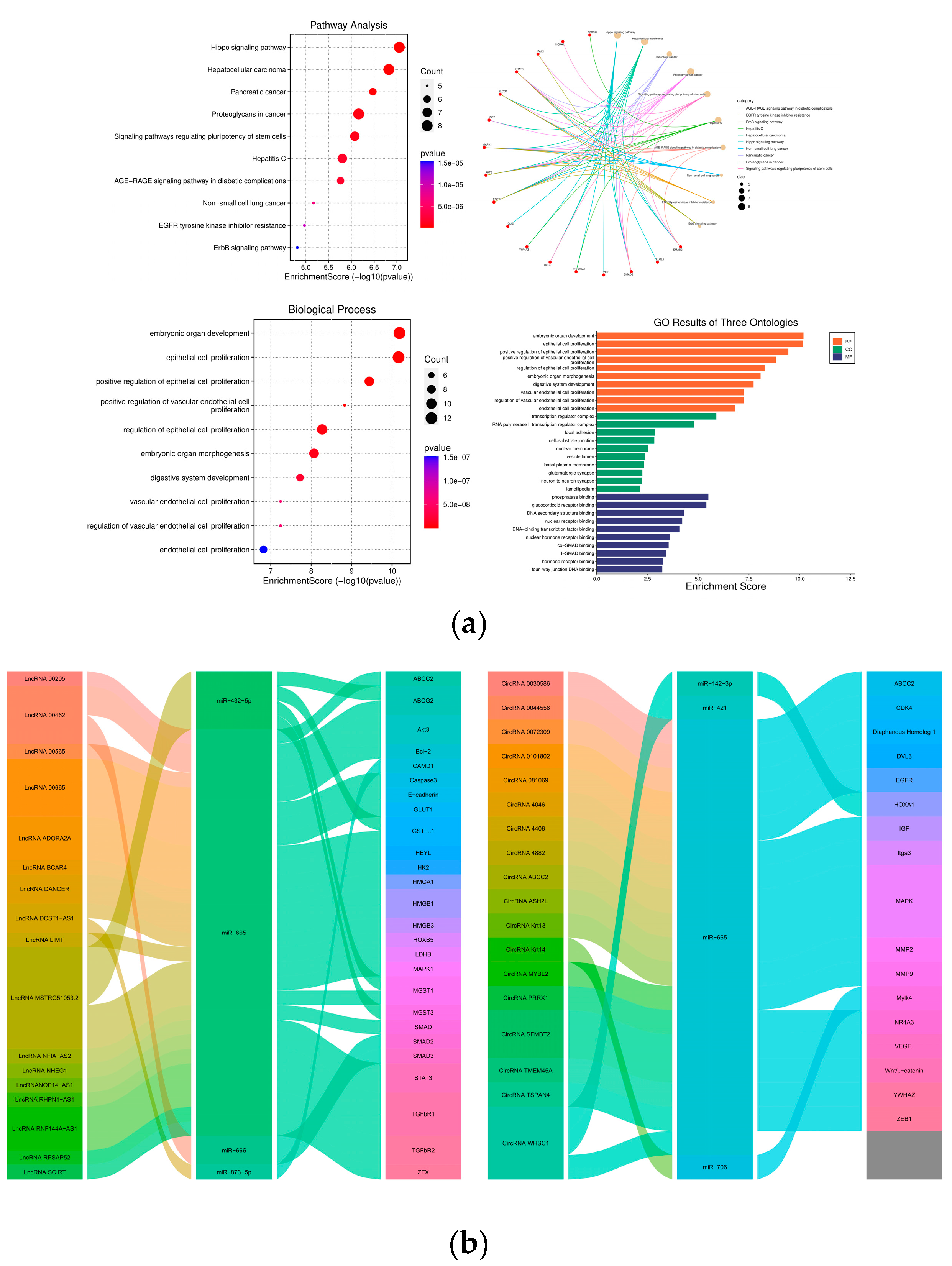
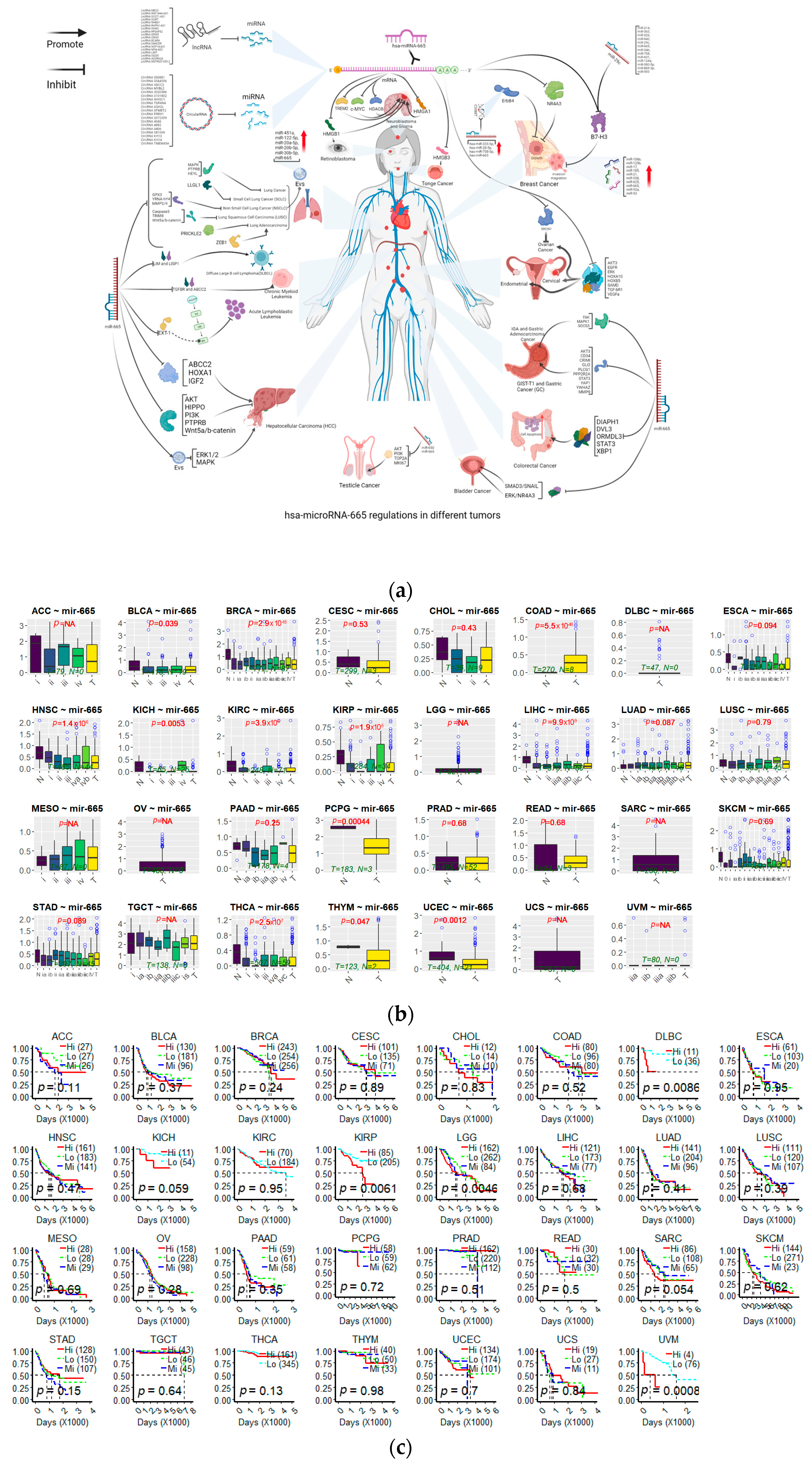
| Target Gene | Cancer Type | Regulatory Function | References |
|---|---|---|---|
| ABCC2 | Hepatocellular Cancer; Chronic Myeloid Leukemia | Inhibition of proliferation, invasion, promotion of apoptosis | [67] |
| AKT/AKT3 | Cardiomyocyte Ischemia/Reperfusion Injury; Ovarian Cancer; Gastric Cancer; HEP3B Liver Cancer Cell (HEP3B) | Inhibition of ROS, promotion of viability | [58,68,69] |
| B7-H3 | Breast Cancer | High expression | [70] |
| Caspase3 | Lung Squamous Cell Carcinoma | Inhibition of apoptosis | [20] |
| CD34 | Gastrointestinal Stromal Cancers | Inhibition of migration | [71] |
| C-MYC | Murine Neuroblastoma | Inhibition of cell cycle | [41] |
| CRIMI | Gastric Cancer | Inhibition of EMT | [28] |
| Diaphanous Homolog1 | Colorectal Cancer | Inhibition of proliferation | [72] |
| DVL3 | Colorectal Cancer | Inhibition of invasion | [73] |
| EGFR | Cervical Cancer? | Inhibition of paclitaxel resistance | [74] |
| ERK and ERK1/2 | Bladder Cancer; Acute Lymphoblastic Leukemia; Hepatocellular Carcinoma, Cervical Cancer? | Promotion of proliferation, stemness, inhibition of cancer size | [33,35,60,75] |
| EXT1/4 | Acute Lymphoblastic Leukemia | Promotion of proliferation | [33] |
| FAK | Gastric Adenocarcinoma Cancer | Promotion of EMT | [45] |
| GLI2 | gastric signet ring cell carcinoma (GSRCC)/IGA | Inhibition of GSRCC/promotion of GADC | [76] |
| GPX3 | Non-Small Cell Lung Cancer | Promotion of glycolysis | [77] |
| HDAC8 | Murine Neuroblastoma | Inhibition of cell cycle | [41] |
| HEYL | Lung Cancer | Promotion of metastasis | [34] |
| HIPPO | Hepatocellular Carcinoma | Promotion of migration, invasion | [78] |
| HMGA1 | Glioma | Inhibition of proliferation | [79] |
| HMGB1/3 | Glioma; Neuroblastoma; Tongue Squamous Cell Carcinoma; | Inhibition of migration, invasion, oncogenicity | [38,80,81] |
| HOXA1 | Hepatocellular Cancer | Inhibition of motility | [82] |
| HOXA10 | Ovarian Cancer | Inhibition of proliferation | [83] |
| HOXB5 | Endometrial | Inhibition of colony formation, invasion, migration | [84] |
| IGF2 | Hepatocellular Carcinoma (HCC) | Inhibition of mobility | [85] |
| LIM | Diffuse Large B Cell Lymphoma (DLBCL) | Inhibition of proliferation | [20] |
| LLGL1 | Small Cell Lung Cancer (SCLC) | Promotion of S-phase fraction | [86] |
| MAPK/ERK | Hepatocellular Carcinoma (HCC); Gastric Cancer (GC); Lung Cancer | Promotion of proliferation by Evs, inhibition of apoptosis | [27,57,75] |
| NR4A3 | Breast Cancer; Bladder Cancer | Promotion of metastasis, stemness | [19,60] |
| ORMDL3 | Inflammatory bowel disease | Promotion of inflammatory | [87] |
| PAK1 | Cardiomyocyte Ischemia/Reperfusion Injury | Inhibition of ROS, cell apoptosis | [69] |
| PLCG1 | GSRCC/IGA | Inhibition of GSRCC/promotion of GADC | [76] |
| PPP2R2A | Gastric Cancer (GC) | Inhibition of EMT | [88] |
| PRICKLE2 | Lung Adenocarcinoma | Promotion of migration | [89] |
| PTPRB | Lung Cancer; Hepatocellular Carcinoma | Promotion of proliferation | [90,91] |
| RAB23 | Osteosarcoma | Inhibition of invasion | [91] |
| SAMD1/2/3 | Cervical; Pancreatic Cancer; Bladder Cancer | Inhibition of cancer size | [32,35,92] |
| SH3LASP1 | Diffuse Large B Cell Lymphoma (DLBCL) | Inhibition of proliferation, invasion. Promotion of apoptosis | [40] |
| SNAL | Bladder Cancer | Inhibition of EMT | [32] |
| SOCS3 | Gastric Adenocarcinoma Cancer | Promotion of EMT, migration | [45] |
| SRCIN1 | Ovarian Cancer | Promotion of proliferation | [26] |
| STAT3 | Gastric Cancer (GC); Colorectal Cancer; Breast Cancer | Inhibition of proliferation, oncogenicity | [70,93,94] |
| TGF-bR1/2 | Pancreatic Cancer; Cervical Cancer; Chronic Myeloid Leukemia | Inhibition of cell cycle proliferation | [35,92,95] |
| TREM2 | Microglia Cancer | Inhibition of inflammatory processes | [96] |
| TRIM8 | Lung Squamous Cell Carcinoma (LUSC) | Promotion of proliferation | [20] |
| VEGF-α | Ovarian Cancer | Inhibition of angiogenesis | [97] |
| Wnt5a/b-catenin | Lung Squamous Cell Carcinoma (LUSC); Retinoblastoma; HEP3B | Inhibition of oncogenicity | [20] |
| XBP1 | Inflammatory bowel disease, Breast Cancer | Promotion of apoptosis | [87] |
| YAP1 | Gastric Cancer (GC) | Inhibition of migration | [98] |
| YRNA-hY4 | NSCLC | High expression | [99] |
| YWHAZ | GC | Inhibition of viability | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Pavani, K.C.; Chunduru, J.; Broeckx, B.J.G.; Van Soom, A.; Peelman, L. Hsa-miR-665 Is a Promising Biomarker in Cancer Prognosis. Cancers 2023, 15, 4915. https://doi.org/10.3390/cancers15204915
Guan X, Pavani KC, Chunduru J, Broeckx BJG, Van Soom A, Peelman L. Hsa-miR-665 Is a Promising Biomarker in Cancer Prognosis. Cancers. 2023; 15(20):4915. https://doi.org/10.3390/cancers15204915
Chicago/Turabian StyleGuan, Xuefeng, Krishna Chaitanya Pavani, Jayendra Chunduru, Bart J. G. Broeckx, Ann Van Soom, and Luc Peelman. 2023. "Hsa-miR-665 Is a Promising Biomarker in Cancer Prognosis" Cancers 15, no. 20: 4915. https://doi.org/10.3390/cancers15204915
APA StyleGuan, X., Pavani, K. C., Chunduru, J., Broeckx, B. J. G., Van Soom, A., & Peelman, L. (2023). Hsa-miR-665 Is a Promising Biomarker in Cancer Prognosis. Cancers, 15(20), 4915. https://doi.org/10.3390/cancers15204915






