Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. How to Translate in Imaging the Effects of Stereotactic Radiosurgery on Tumoral Cells and Surrounding Brain Cells
- DNA injury and apoptosis: ionizing radiation produces oxygen-free radicals in tumor cells, inducing cell death mainly due to the breakage of the DNA double helix; DNA repair pathways are subsequently activated, leading to cell cycle arrest and apoptosis of cells with irreversibly damaged DNA [18,19].
- Ceramide-induced apoptosis and fibrinoid necrosis: radiation directly damages the plasma membrane of several cell types (like endothelial cells), activating the enzymatic hydrolysis of sphingomyelin, which generates ceramide. Ceramide acts as a second messenger, stimulating ‘’ceramide-induced apoptosis’’ via the mitochondrial system [20]. This process leads to the production of more reactive oxygen species, which subsequently induce an inflammatory response involving cytokines and chemokines and then the formation of fibrin-platelet thrombi and fibrinoid necrosis [21].
- Demyelination and diffuse edema: astrocytes, oligodendrocytes, and neural progenitor cells are extremely sensitive to radiation, and radiation damage in the brain results in foci of demyelination. Moreover, necrotic tumor debris, if not readily removed, causes an inflammatory response that induces a capillary permeability defect with consequent edema. The preferential sites of this phenomenon are represented by basal nuclei, cerebral peduncles and deep white matter [22,23].
- HIF-1 and VEGF activation and neoangiogenesis: it has been demonstrated that radiation injury increases the release of HIF-1a and VEGF by astrocytes. The upregulation of HIF-1a leads to angiogenesis [24], with new fragile and leaking vessels causing perilesional edema.
- Blood–brain barrier (BBB) disruption: the disruption of the BBB caused by radiation leads to cerebral vasogenic edema. Radiation furthermore induces transient vasodilatation, with variable alteration of capillary permeability generally reversible and transient [25].
3. Early Post-Treatment Assessment of Stereotactic Radiosurgery
- Key points
- Increased diffusivity could be an early sign of radiation treatment efficacy.
- The reduction of the rCBV DSC-derived parameter within the lesion has been generally considered a reference target for the effectiveness of RT.
- The reduction of the K-trans DCE-derived parameters is related to a good response to treatment due to a reduction in the pathological vascular permeability of the treated area.
3.1. Diffusion Weighted Imaging (DWI)
3.2. Dynamic Susceptibility Contrast (DSC) Perfusion MRI
3.3. Dynamic Contrast Enhanced (DCE) Perfusion MRI
4. The Role of Neuroimaging in Distinguishing True Disease Progression from Post-Treatment Radiation Effects (PTRE) Mimicking Disease Progression
- Key points
- Radionecrosis and pseudoprogression are possible post-SRS treatment changes.
- An enhancing lesion may represent both tumor recurrence and post-treatment radiation effects; T1 mapping could help in differential diagnosis with continuous but slow accumulation of contrast agent in RN in contrast to the rapid contrast agent accumulation and relatively fast clearance in tumor recurrence.
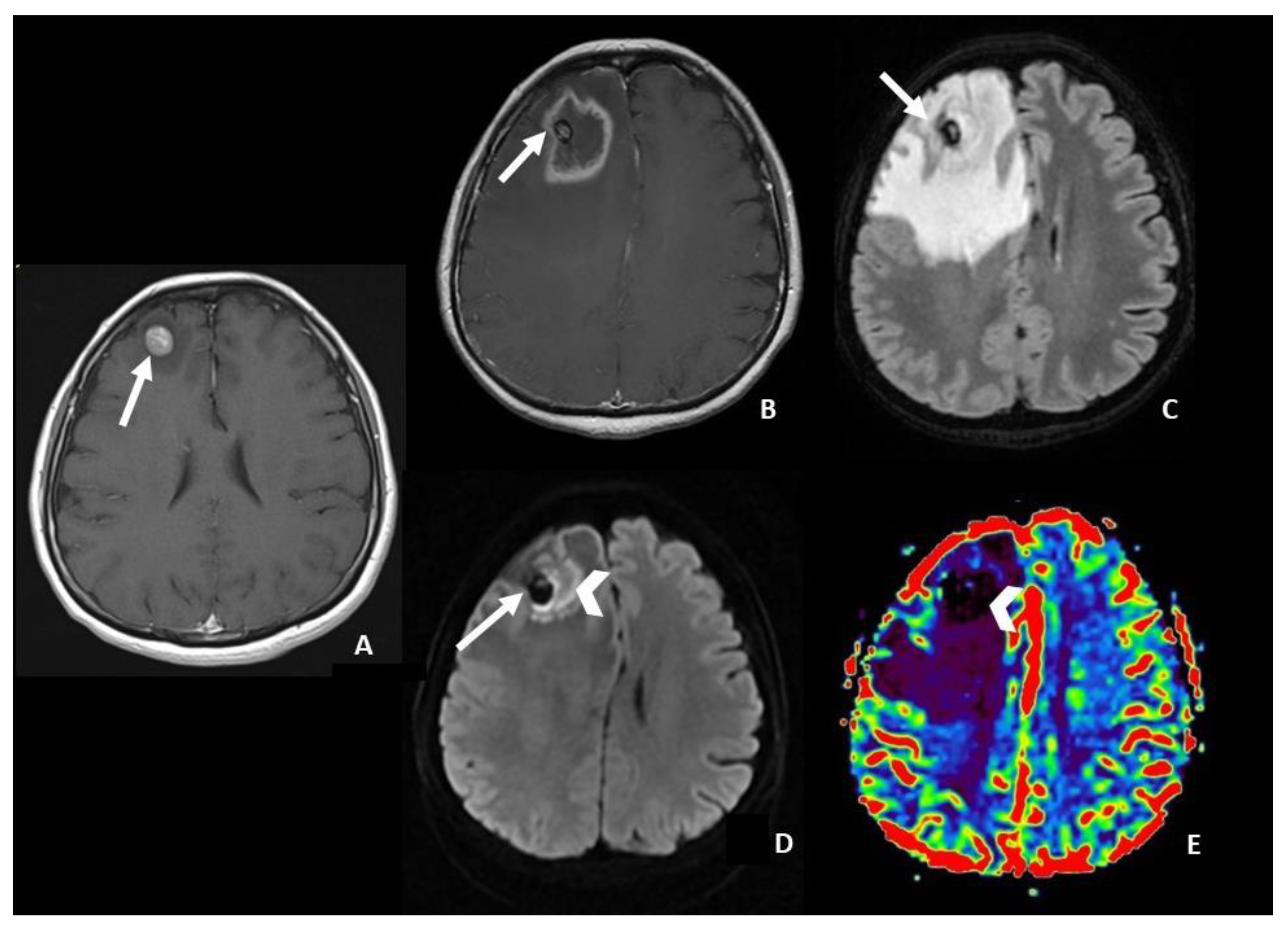
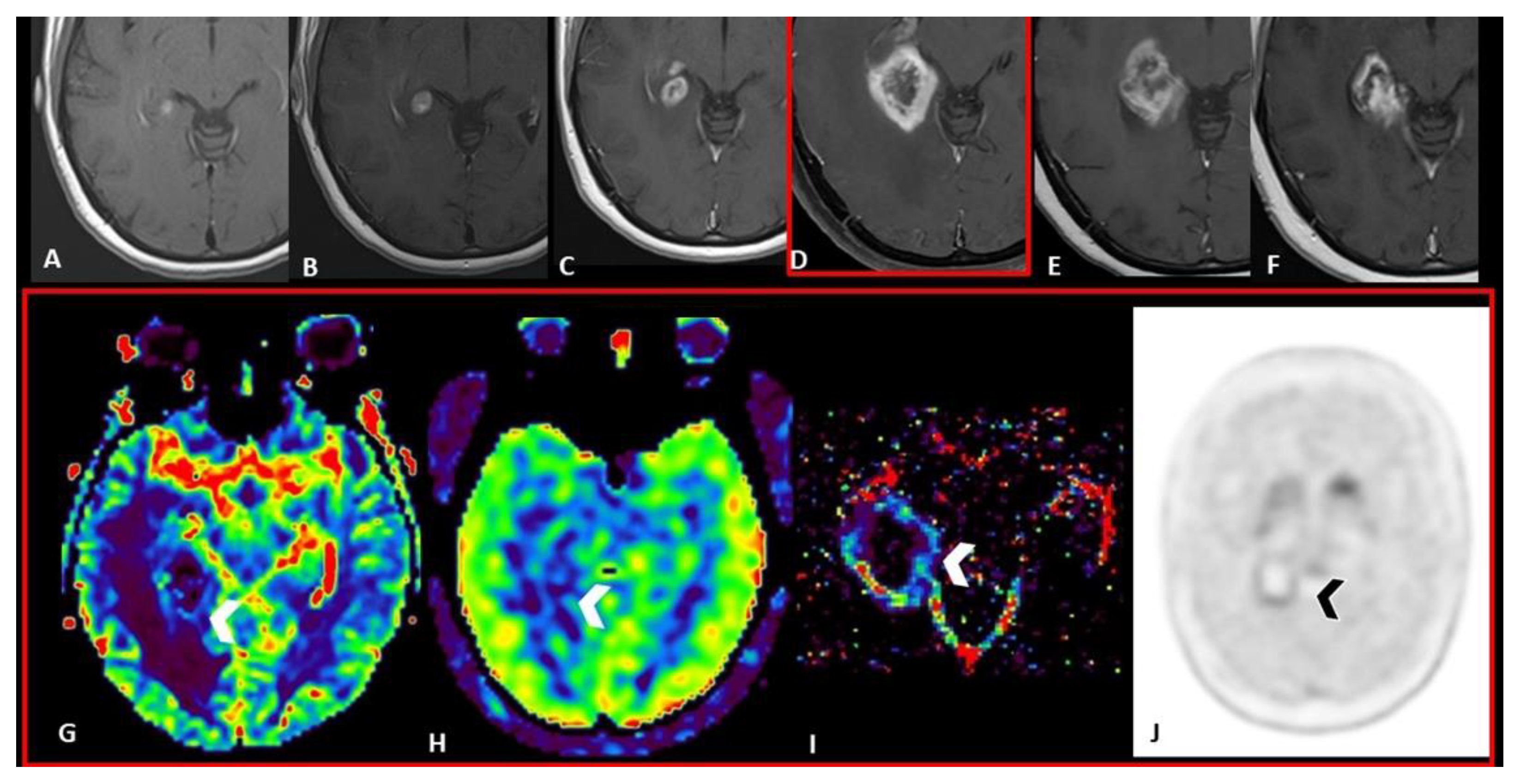
- In DWI/ADC images, “The centrally restricted diffusion sign” appeared to be due to hypercellularity in coagulative necrosis and theexpression of RN.
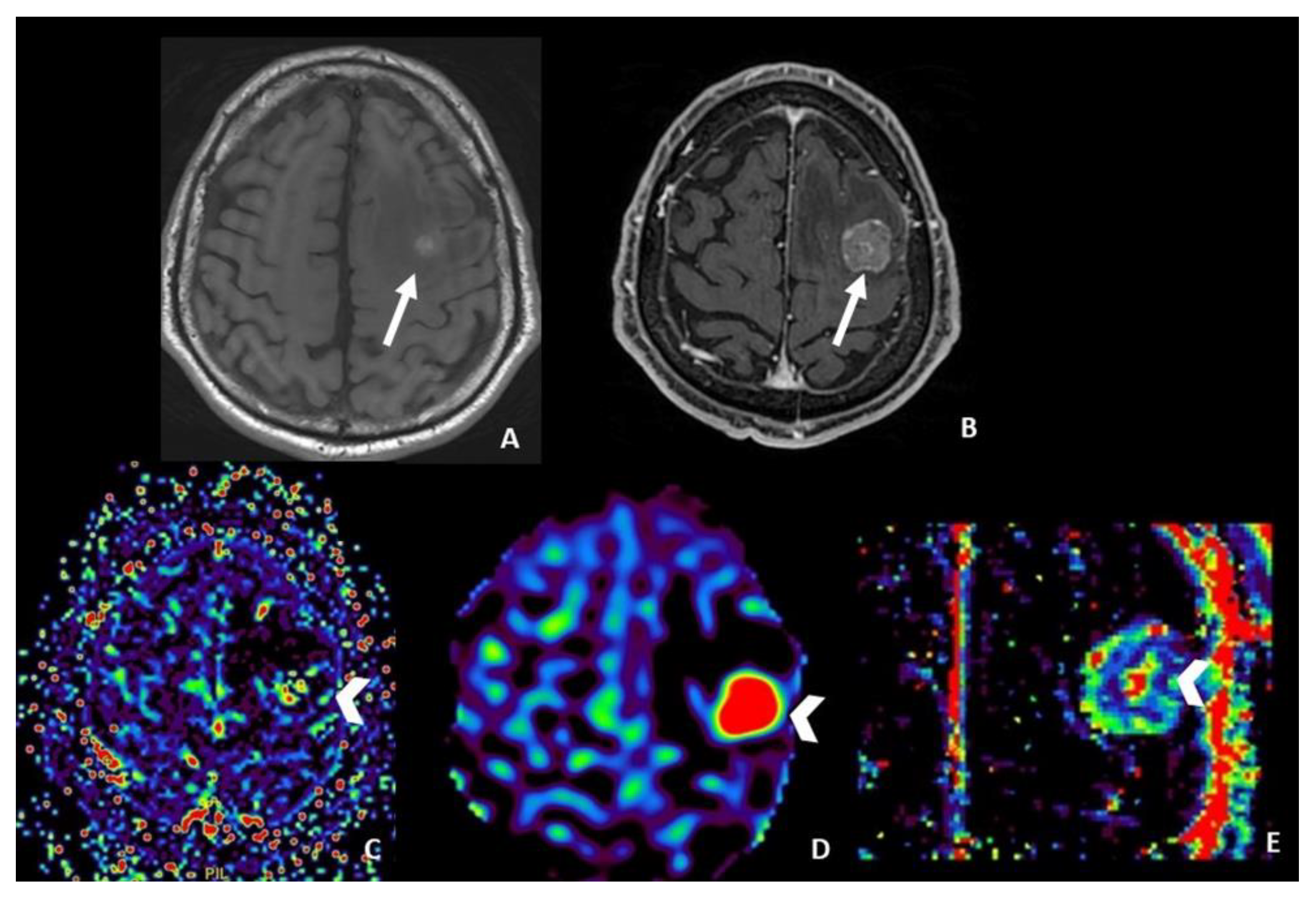
- DSC helps in differentiating pseudoprogression, or RN, from progressive disease, with the highest value of rCBV in progressive disease.
- Ktrans and Vp DCE-derived parameters seem to help in differentiating progressive disease from radiation injuries; anyway, the role of DCE is still debated in the literature.
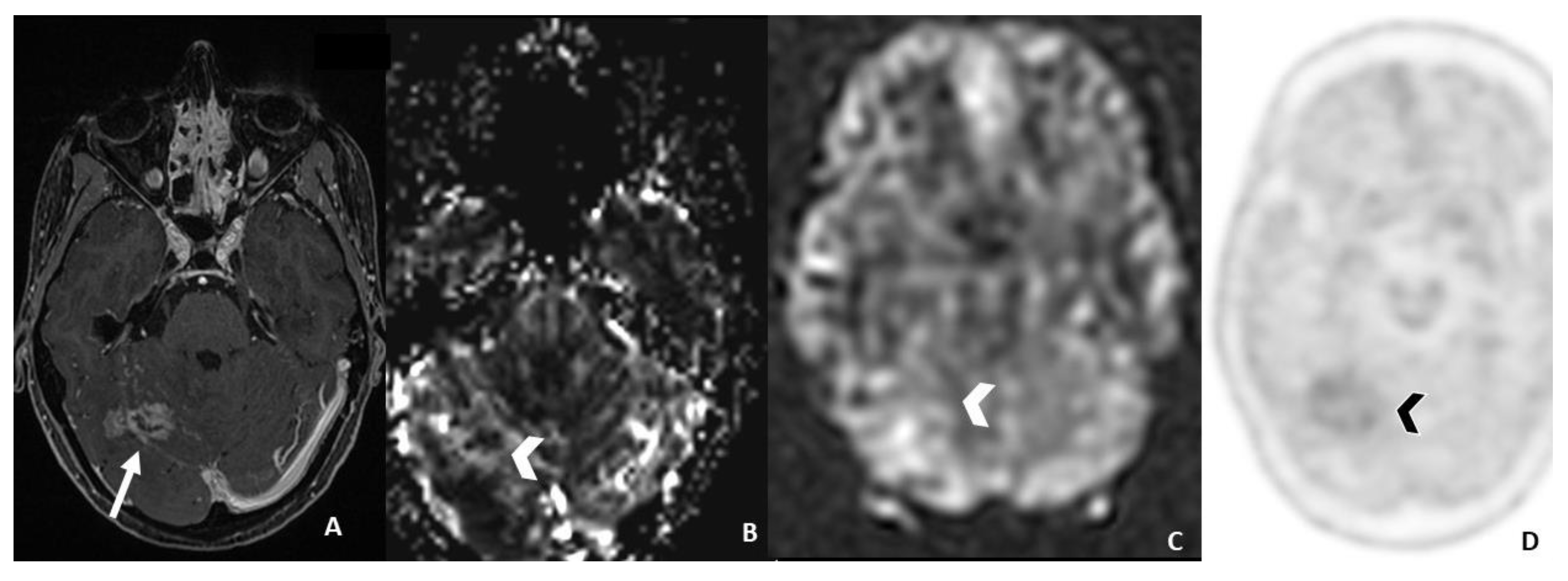
- ASL seems to be useful only in monitoring metastatic lesions characterized by high vascularity and increased CBF values, including renal cell carcinoma, melanoma and thyroid carcinoma.
- PET imaging, with 18F-fluorodeoxyglucose or amino acid tracers, represents an additional tool. Typically, high uptake of tracers is observed in tumor recurrence, while low uptake is considered a hallmark of radiation effects.
- Radiomics and AI are showing promising results in differentiating true progression from treatment effects, but they still must be validated.
4.1. Post-Contrast T1-Weighted Sequences
4.2. Diffusion Weighted Imaging (DWI)
- central restricted diffusivity (“the centrally restricted diffusion sign”) (Figure 1)
- peripheral restricted diffusivity
- both central and peripheral
- no diffusion restriction.
4.3. Dynamic Susceptibility Contrast (DSC) Perfusion MRI
4.4. Dynamic Contrast Enhanced (DCE) Perfusion MRI
4.5. Arterial Spin Labeling (ASL) Perfusion MRI
4.6. Positron Emission Tomography (PET) Imaging
4.7. Radiomics and Artificial Intelligence
5. The Impact of Systemic Treatment in Neuroimaging Changes
- Key points
- Immunotherapy leads to new challenges in the imaging interpretation of post-SRS BM, with a high rate of post-treatment changes, especially pseudoprogression.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, E.L.; Wefel, J.S.; Maor, M.H.; Hassenbusch, S.J., 3rd; Mahajan, A.; Lang, F.F.; Woo, S.Y.; Mathews, L.A.; Allen, P.K.; Shiu, A.S.; et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 2007, 60, 277–284. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Peterson, A.M.; Meltzer, C.C.; Evanson, E.J.; Flickinger, J.C.; Kondziolka, D. MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology 1999, 211, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Knitter, J.R.; Erly, W.K.; Stea, B.D.; Lemole, G.M.; Germano, I.M.; Doshi, A.H.; Nael, K. Interval Change in Diffusion and Perfusion MRI Parameters for the Assessment of Pseudoprogression in Cerebral Metastases Treated With Stereotactic Radiation. AJR Am. J. Roentgenol. 2018, 211, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, K.; Nakasu, Y.; Horiguchi, S.; Harada, H.; Nishimura, T.; Bando, E.; Okawa, H.; Furukawa, Y.; Hirai, T.; Endo, M. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J. Neuro-Oncol. 2010, 99, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2012, 53, 1367–1374. [Google Scholar]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Stockham, A.L.; Suh, J.H.; Chao, S.T.; Barnett, G.H. Management of recurrent brain metastasis after radiosurgery. Prog. Neurol. Surg. 2012, 25, 273–286. [Google Scholar]
- Patel, T.R.; McHugh, B.J.; Bi, W.L.; Minja, F.J.; Knisely, J.P.; Chiang, V.L. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am. J. Neuroradiol. 2011, 32, 1885–1892. [Google Scholar] [CrossRef]
- Kerkhof, M.; Ganeff, I.; Wiggenraad, R.G.J.; Lycklama À Nijeholt, G.J.; Hammer, S.; Taphoorn, M.J.B.; Dirven, L.; Vos, M.J. Clinical applicability of and changes in perfusion MR imaging in brain metastases after stereotactic radiotherapy. J. Neuro-Oncol. 2018, 138, 133–139. [Google Scholar] [CrossRef]
- Sawlani, V.; Davies, N.; Patel, M.; Flintham, R.; Fong, C.; Heyes, G.; Cruickshank, G.; Steven, N.; Peet, A.; Hartley, A.; et al. Evaluation of Response to Stereotactic Radiosurgery in Brain Metastases Using Multiparametric Magnetic Resonance Imaging and a Review of the Literature. Clin. Oncol. 2019, 31, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Chou, H.H.; Tu, H.T.; Yang, M.S.; Lee, J.K.; Lin, L.Y. Diffusion magnetic resonance imaging as an evaluation of the response of brain metastases treated by stereotactic radiosurgery. Surg. Neurol. 2008, 69, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.; O’Connor, J.P.; Parker, G.J.; Jayson, G.C. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3449–3459. [Google Scholar] [CrossRef]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef]
- Badiyan, S.N.; Regine, W.F.; Mehta, M. Stereotactic Radiosurgery for Treatment of Brain Metastases. J. Oncol. Pract. 2016, 12, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Miller, J.A.; Kotecha, R.; Xiao, R.; Juloori, A.; Ward, M.C.; Ahluwalia, M.S.; Mohammadi, A.M.; Peereboom, D.M.; Murphy, E.S.; et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J. Neuro-Oncol. 2017, 133, 357–368. [Google Scholar] [CrossRef]
- Kondziolka, D.; Shin, S.M.; Brunswick, A.; Kim, I.; Silverman, J.S. The biology of radiosurgery and its clinical applications for brain tumors. Neuro-Oncol. 2015, 17, 29–44. [Google Scholar] [CrossRef]
- Kirkpatrick, J.P.; Meyer, J.J.; Marks, L.B. The Linear-Quadratic Model Is Inappropriate to Model High Dose per Fraction Effects in Radiosurgery. Semin. Radiat. Oncol. 2008, 18, 240–243. [Google Scholar] [CrossRef]
- Ali, F.S.; Arevalo, O.; Zorofchian, S.; Patrizz, A.; Riascos, R.; Tandon, N.; Blanco, A.; Ballester, L.Y.; Esquenazi, Y. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr. Oncol. Rep. 2019, 21, 66. [Google Scholar] [CrossRef]
- Kolesnick, R.; Fuks, Z. Radiation and Ceramide-induced Apoptosis. Oncogene 2003, 22, 5897–5906. [Google Scholar] [CrossRef]
- Perry, A.; Schmidt, R.E. Cancer therapy-associated CNS neuropathology: An update and review of the literature. Acta Neuropathol. 2006, 111, 197–212. [Google Scholar] [CrossRef]
- Fike, J.R.; Rosi, S.; Limoli, C.L. Neural Precursor Cells and Central Nervous System Radiation Sensitivity. Semin. Radiat. Oncol. 2009, 19, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Suzuki, Y.; Noda, S.E.; Mizui, T.; Shirai, K.; Okamoto, M.; Kaminuma, T.; Yoshida, Y.; Shirao, T.; Nakano, T. Comparison of the Radiosensitivities of Neurons and Glial Cells Derived from the Same Rat Brain. Exp. Ther. Med. 2014, 8, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Nagy, A.; Pintilie, M.; Wong, C.S. Hypoxia and Hypoxia-inducible Factor-1 Target Genes in Central Nervous System Radiation Injury: A Role for Vascular Endothelial Growth Factor. Clin. Cancer Res. 2004, 10, 3342–3353. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.; Odé, Z.; Derieppe, M.P.P.; Groenink, L.; Heymans, M.W.; Otten, R.; Lequin, M.H.; Janssens, G.O.R.; Hoving, E.W.; van Vuurden, D.G. Blood-brain barrier permeability following conventional photon radiotherapy—A systematic review and meta-analysis of clinical and preclinical studies. Clin. Transl. Radiat. Oncol. 2022, 35, 44–55. [Google Scholar] [CrossRef]
- Huang, J.; Milchenko, M.; Rao, Y.J.; LaMontagne, P.; Abraham, C.; Robinson, C.G.; Huang, Y.; Shimony, J.S.; Rich, K.M.; Benzinger, T. A Feasibility Study to Evaluate Early Treatment Response of Brain Metastases One Week After Stereotactic Radiosurgery Using Perfusion Weighted Imaging. PLoS ONE 2020, 15, e0241835. [Google Scholar] [CrossRef]
- Chen, Z.; Zu, J.; Li, L.; Lu, X.; Ni, J.; Xu, J.L. Assessment of Stereotactic Radiosurgery Treatment Response for Brain Metastases Using MRI Based Diffusion Index. Eur. J. Radiol. Open 2017, 4, 84–88. [Google Scholar] [CrossRef]
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of Brain Tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef]
- Di, N.; Cheng, W.; Jiang, X.; Liu, X.; Zhou, J.; Xie, Q.; Chu, Z.; Chen, H.; Wang, B. Can Dynamic Contrast-enhanced MRI Evaluate VEGF Expression in Brain Glioma? An MRI-guided Stereotactic Biopsy Study. J. Neuroradiol. 2019, 46, 186–192. [Google Scholar] [CrossRef]
- Taunk, N.K.; Oh, J.H.; Shukla-Dave, A.; Beal, K.; Vachha, B.; Holodny, A.; Hatzoglou, V. Early posttreatment assessment of MRI perfusion biomarkers can predict long-term response of lung cancer brain metastases to stereotactic radiosurgery. Neuro-Oncol. 2018, 20, 567–575. [Google Scholar] [CrossRef]
- Ruzevick, J.; Kleinberg, L.; Rigamonti, D. Imaging Changes Following Stereotactic Radiosurgery for Metastatic Intracranial Tumors: Differentiating Pseudoprogression from Tumor Progression and its Effect on Clinical Practice. Neurosurg. Rev. 2014, 37, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, V.; Tisnado, J.; Mehta, A.; Peck, K.K.; Daras, M.; Omuro, A.M.; Beal, K.; Holodny, A.I. Dynamic Contrast-enhanced MRI Perfusion for Differentiating Between Melanoma and Lung Cancer Brain Metastases. Cancer Med. 2017, 6, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Parvez, A.; Zadeh, G. The Diagnosis and Treatment of Pseudoprogression, Radiation Necrosis and Brain Tumor Recurrence. Int. J. Mol. Sci. 2014, 15, 11832–11846. [Google Scholar] [CrossRef]
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Zhao, B.; Zhao, P.; Ge, M.; Gao, M.; Ding, F.; Xu, S.; Liu, Y. Postcontrast T1 Mapping for Differential Diagnosis of Recurrence and Radionecrosis after Gamma Knife Radiosurgery for Brain Metastasis. Am. J. Neuroradiol. 2018, 39, 1025–1031. [Google Scholar] [CrossRef]
- Zach, L.; Guez, D.; Last, D.; Daniels, D.; Grober, Y.; Nissim, O.; Hoffmann, C.; Nass, D.; Talianski, A.; Spiegelmann, R.; et al. Delayed contrast extravasation MRI: A new paradigm in neuro-oncology. Neuro-Oncol. 2015, 17, 457–465. [Google Scholar] [CrossRef]
- Hainc, N.; Alsafwani, N.; Gao, A.; O’Halloran, P.J.; Kongkham, P.; Zadeh, G.; Gutierrez, E.; Shultz, D.; Krings, T.; Alcaide-Leon, P. The Centrally Restricted Diffusion Sign on MRI for Assessment of Radiation Necrosis in Metastases Treated with Stereotactic Radiosurgery. J. Neurooncol. 2021, 155, 325–333. [Google Scholar] [CrossRef]
- Cicone, F.; Minniti, G.; Romano, A.; Papa, A.; Scaringi, C.; Tavanti, F.; Bozzao, A.; Maurizi Enrici, R.; Scopinaro, F. Accuracy of F-DOPA PET and Perfusion-MRI for Differentiating Radionecrotic from Progressive Brain Metastases After Radiosurgery. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 103–111. [Google Scholar] [CrossRef]
- Tong, E.; McCullagh, K.L.; Iv, M. Advanced Imaging of Brain Metastases: From Augmenting Visualization and Improving Diagnosis to Evaluating Treatment Response. Front. Neurol. 2020, 11, 270. [Google Scholar] [CrossRef]
- Barajas, R.F.; Chang, J.S.; Sneed, P.K.; Segal, M.R.; McDermott, M.W.; Cha, S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am. J. Neuroradiol. 2009, 30, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hatzoglou, V.; Yang, T.J.; Omuro, A.; Gavrilovic, I.; Ulaner, G.; Rubel, J.; Schneider, T.; Woo, K.M.; Zhang, Z.; Peck, K.K.; et al. A prospective trial of dynamic contrast-enhanced MRI perfusion and fluorine-18 FDG PET-CT in differentiating brain tumor progression from radiation injury after cranial irradiation. Neuro Oncol. 2016, 18, 873–880. [Google Scholar] [CrossRef]
- Morabito, R.; Alafaci, C.; Pergolizzi, S.; Pontoriero, A.; Iati’, G.; Bonanno, L.; Gaeta, M.; Salpietro, F.M.; Mormina, E.; Longo, M.; et al. DCE and DSC perfusion MRI diagnostic accuracy in the follow-up of primary and metastatic intra-axial brain tumors treated by radiosurgery with cyberknife. Radiat. Oncol. 2019, 14, 65. [Google Scholar] [CrossRef]
- Essig, M.; Nguyen, T.B.; Shiroishi, M.S.; Saake, M.; Provenzale, J.M.; Enterline, D.S.; Anzalone, N.; Dörfler, A.; Rovira, À.; Wintermark, M.; et al. Perfusion MRI: The five most frequently asked clinical questions. AJR Am. J. Roentgenol. 2013, 201, W495–W510. [Google Scholar] [CrossRef]
- Lai, G.; Mahadevan, A.; Hackney, D.; Warnke, P.C.; Nigim, F.; Kasper, E.; Wong, E.T.; Carter, B.S.; Chen, C.C. Diagnostic Accuracy of PET, SPECT, and Arterial Spin-Labeling in Differentiating Tumor Recurrence from Necrosis in Cerebral Metastasis after Stereotactic Radiosurgery. AJNR Am. J. Neuroradiol. 2015, 36, 2250–2255. [Google Scholar] [CrossRef]
- Le Rhun, E.; Dhermain, F.; Vogin, G.; Reyns, N.; Metellus, P. Radionecrosis after stereotactic radiotherapy for brain metastases. Expert. Rev. Neurother. 2016, 16, 903–914. [Google Scholar] [CrossRef]
- Lizarraga, K.J.; Allen-Auerbach, M.; Czernin, J.; DeSalles, A.A.F.; Yong, W.H.; Phelps, M.E.; Chen, W. 18F-FDOPA PET for Differentiating Recurrent or Progressive Brain Metastatic Tumors from Late or Delayed Radiation Injury After Radiation Treatment. J. Nucl. Med. 2013, 55, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Terakawa, Y.; Tsuyuguchi, N.; Iwai, Y.; Yamanaka, K.; Higashiyama, S.; Takami, T.; Ohata, K. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 2008, 49, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Parekh, V.; Huang, P.; Lin, D.D.; Sheikh, K.; Baker, B.; Kirschbaum, T.; Silvestri, F.; Son, J.; Robinson, A.; et al. Distinguishing True Progression from Radionecrosis After Stereotactic Radiation Therapy for Brain Metastases With Machine Learning and Radiomics. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 1236–1243. [Google Scholar] [CrossRef]
- Larroza, A.; Moratal, D.; Paredes-Sánchez, A.; Soria-Olivas, E.; Chust, M.L.; Arribas, L.A.; Arana, E. Support vector machine classification of brain metastasis and radiation necrosis based on texture analysis in MRI. J. Magn. Reson. Imaging 2015, 42, 1362–1368. [Google Scholar] [CrossRef]
- Mouraviev, A.; Detsky, J.; Sahgal, A.; Ruschin, M.; Lee, Y.K.; Karam, I.; Heyn, C.; Stanisz, G.J.; Martel, A.L. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro Oncol. 2020, 22, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with Ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Chiang, V.; Mahajan, A.; Zito, C.R.; Sznol, M.; Tran, T.; Weiss, S.A.; Cohen, J.V.; Yu, J.; Hegde, U.; et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J. Clinic. Oncol. 2019, 37, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10, 1758834017742575. [Google Scholar] [CrossRef]
- Galldiks, N.; Kocher, M.; Ceccon, G.; Werner, J.M.; Brunn, A.; Deckert, M.; Pope, W.B.; Soffietti, R.; Le Rhun, E.; Langen, K.J.; et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: Response, progression, and pseudoprogression. Neuro-Oncol. 2020, 22, 17–30. [Google Scholar] [CrossRef]
- Anvari, A.; Sasanpour, P.; Kheradmardi, M.R. Radiotherapy and Immunotherapy in Melanoma Brain Metastases. Hematol. Oncol. Stem. Cell Ther. 2023, 16, 1–20. [Google Scholar] [CrossRef]
- Kaul, D.; Berghoff, A.S.; Grosu, A.L.; Lucas, C.W.; Guckenberger, M. Focal Radiotherapy of Brain Metastases in Combination With Immunotherapy and Targeted Drug Therapy. Dtsch. Arztebl. Int. 2021, 118, 759–766. [Google Scholar] [CrossRef]
- Colaco, R.J.; Martin, P.; Kluger, H.M.; Yu, J.B.; Chiang, V.L. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J. Neurosurg. 2016, 125, 17–23. [Google Scholar] [CrossRef]
- Lancellotta, V.; Del Regno, L.; Di Stefani, A.; Fionda, B.; Marazzi, F.; Rossi, E.; Balducci, M.; Pampena, R.; Morganti, A.G.; Mangoni, M. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: Results of a systematic review. Radiol. Medica 2022, 127, 773–783. [Google Scholar] [CrossRef]
- Cohen, J.V.; Alomari, A.K.; Vortmeyer, A.O.; Jilaveanu, L.B.; Goldberg, S.B.; Mahajan, A.; Chiang, V.L.; Kluger, H.M. Melanoma Brain Metastasis Pseudoprogression after Pembrolizumab Treatment. Cancer Immunol. Res. 2016, 4, 179–182. [Google Scholar] [CrossRef]
- Trommer-Nestler, M.; Marnitz, S.; Kocher, M.; Rueß, D.; Schlaak, M.; Theurich, S.; Von Bergwelt-Baildon, M.; Morgenthaler, J.; Jablonska, K.; Celik, E.; et al. Robotic Stereotactic Radiosurgery in Melanoma Patients with Brain Metastases under Simultaneous Anti-PD-1 Treatment. Int. J. Mol. Sci. 2018, 19, 2653. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Radon, M.; Mills, S.; Mitchell, D.; Palmieri, C.; Chung, C.; Jenkinson, M.D. The Role of the Immune Response in Brain Metastases: Novel Imaging Biomarkers for Immunotherapy. Front. Oncol. 2021, 11, 711405. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, R.; Das, K.; Radon, M.; Bhojak, M.; Rudland, P.R.; Sluming, V.; Jenkinson, M.D. Diffusion-weighted MRI characteristics of the cerebral metastasis to brain boundary predicts patient outcomes. BMC Med. Imaging 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Hirai, T.; Morishita, S.; Kitajima, M.; Murakami, R.; Korogi, Y.; Makino, K.; Nakamura, H.; Ikushima, I.; Yamura, M.; et al. Diffusion-weighted imaging of metastatic brain tumors: Comparison with histologic type and tumor cellularity. AJNR Am. J. Neuroradiol. 2006, 27, 1419–1425. [Google Scholar] [PubMed]
- Larkin, J.R.; Simard, M.A.; de Bernardi, A.; Johanssen, V.A.; Perez-Balderas, F.; Sibson, N.R. Improving Delineation of True Tumor Volume With Multimodal MRI in a Rat Model of Brain Metastasis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Wang, D.; Peck, K.K.; Flynn, J.; Zhang, Z.; Fatovic, R.; Anderson, E.S.; Beal, K.; Shoushtari, A.N. DCE-MRI perfusion predicts pseudoprogression in metastatic melanoma treated with immunotherapy. J. Neuro-Oncol. 2020, 146, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Du Four, S.; Janssen, Y.; Michotte, A.; Van Binst, A.M.; Van den Begin, R.; Duerinck, J.; Neyns, B. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med. 2018, 7, 4870–4879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Moltoni, G.; Blandino, A.; Palizzi, S.; Romano, A.; de Rosa, G.; De Blasi Palma, L.; Monopoli, C.; Guarnera, A.; Minniti, G.; et al. Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment. Cancers 2023, 15, 5092. https://doi.org/10.3390/cancers15205092
Romano A, Moltoni G, Blandino A, Palizzi S, Romano A, de Rosa G, De Blasi Palma L, Monopoli C, Guarnera A, Minniti G, et al. Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment. Cancers. 2023; 15(20):5092. https://doi.org/10.3390/cancers15205092
Chicago/Turabian StyleRomano, Andrea, Giulia Moltoni, Antonella Blandino, Serena Palizzi, Allegra Romano, Giulia de Rosa, Lara De Blasi Palma, Cristiana Monopoli, Alessia Guarnera, Giuseppe Minniti, and et al. 2023. "Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment" Cancers 15, no. 20: 5092. https://doi.org/10.3390/cancers15205092
APA StyleRomano, A., Moltoni, G., Blandino, A., Palizzi, S., Romano, A., de Rosa, G., De Blasi Palma, L., Monopoli, C., Guarnera, A., Minniti, G., & Bozzao, A. (2023). Radiosurgery for Brain Metastases: Challenges in Imaging Interpretation after Treatment. Cancers, 15(20), 5092. https://doi.org/10.3390/cancers15205092







