Implications of Rectal Cancer Radiotherapy on the Immune Microenvironment: Allies and Foes to Therapy Resistance and Patients’ Outcome
Abstract
:Simple Summary
Abstract
1. Introduction
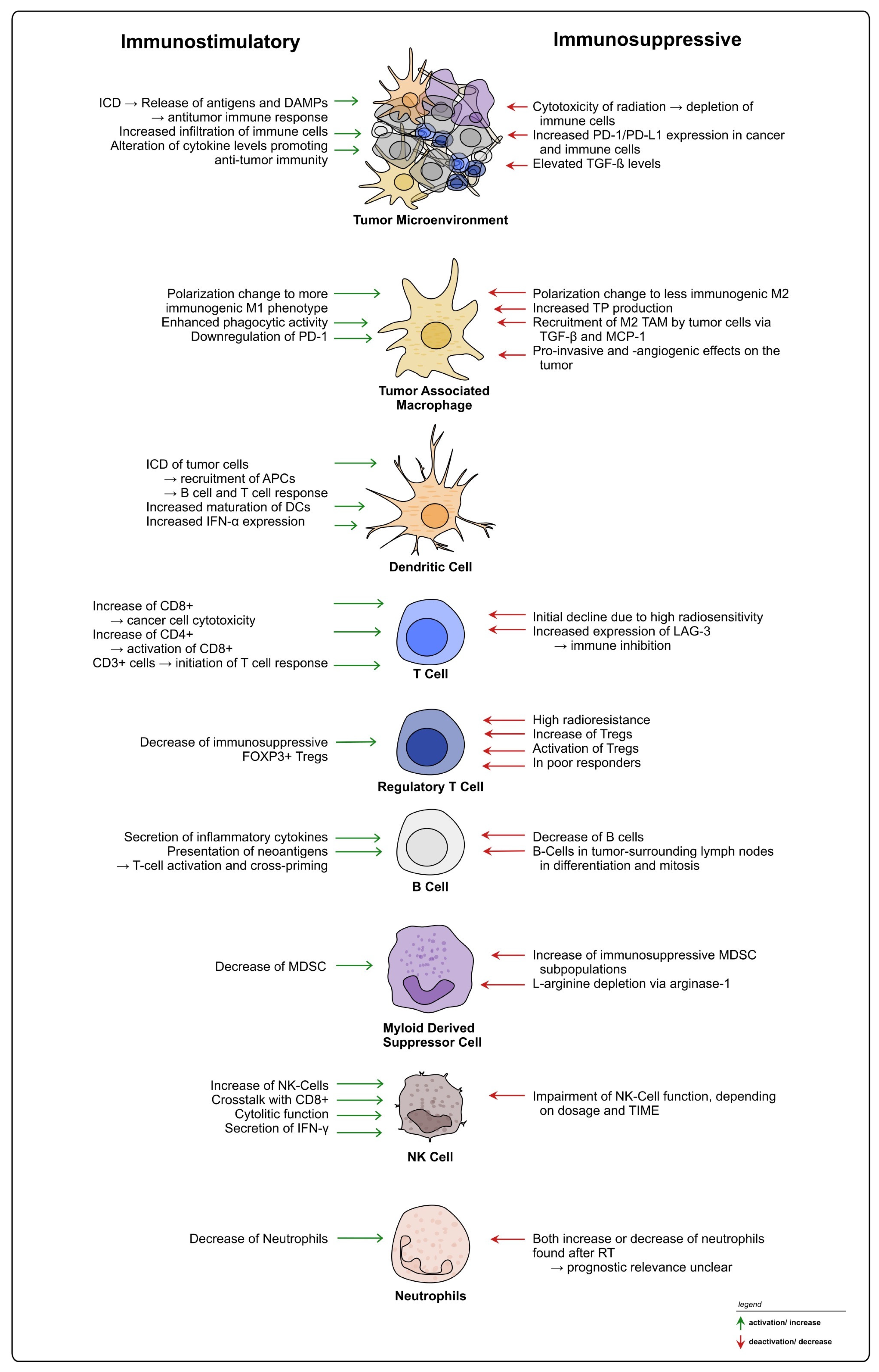
2. (Chemo-)Radiotherapy’s Dual Impact on the Rectal Cancer TIME: Immuno-Activating and -Suppressive Effects and Their Therapeutic Implications
2.1. Immune Cell Recruitment, Infiltration, and Cytokine Signaling
2.2. Treatment-Induced Effects on Innate Immunity
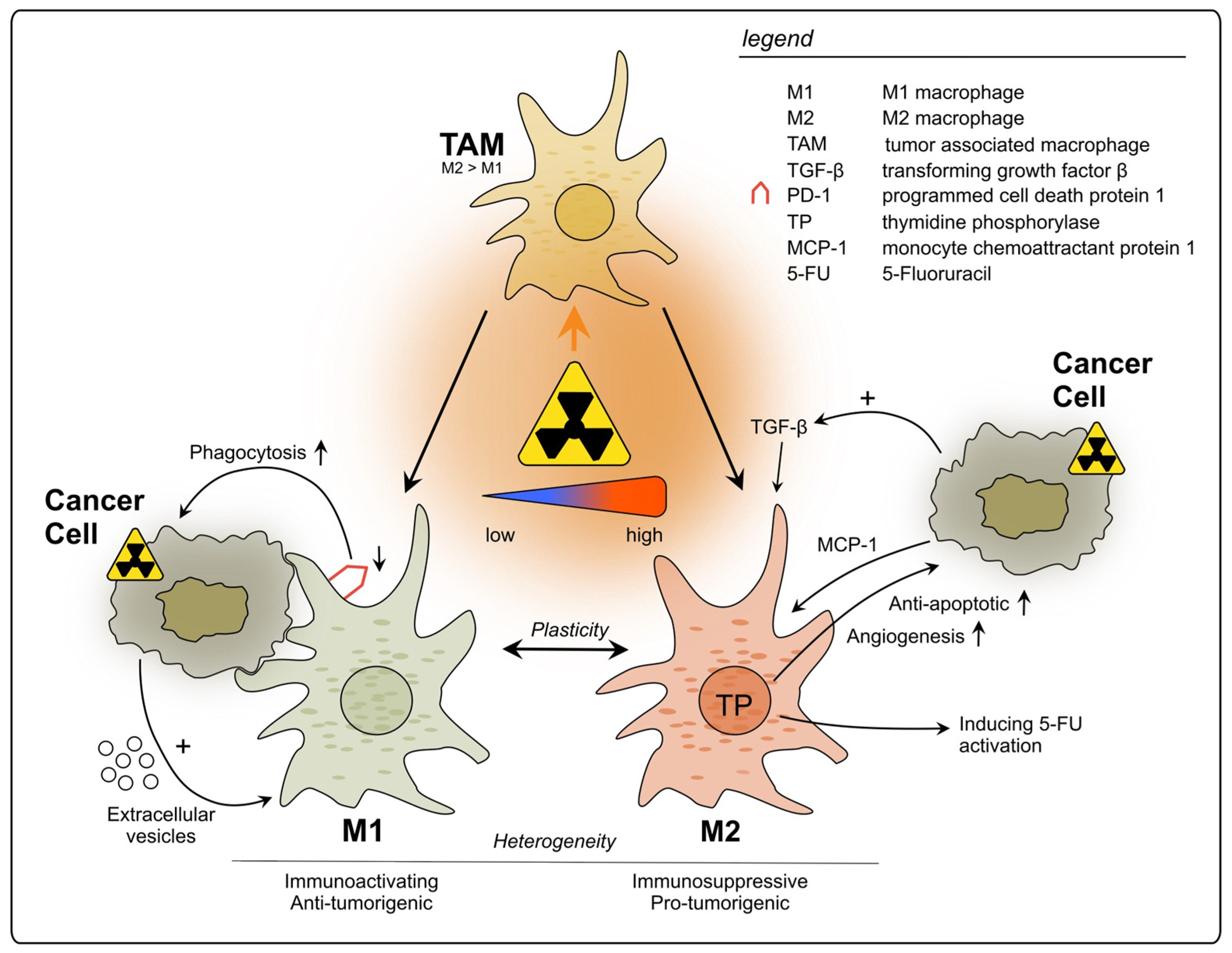
2.2.1. Tumor-Associated Macrophages
2.2.2. Neutrophils
2.2.3. Myeloid-Derived Suppressor Cells
2.2.4. Natural Killer Cells
2.2.5. Dendritic Cells
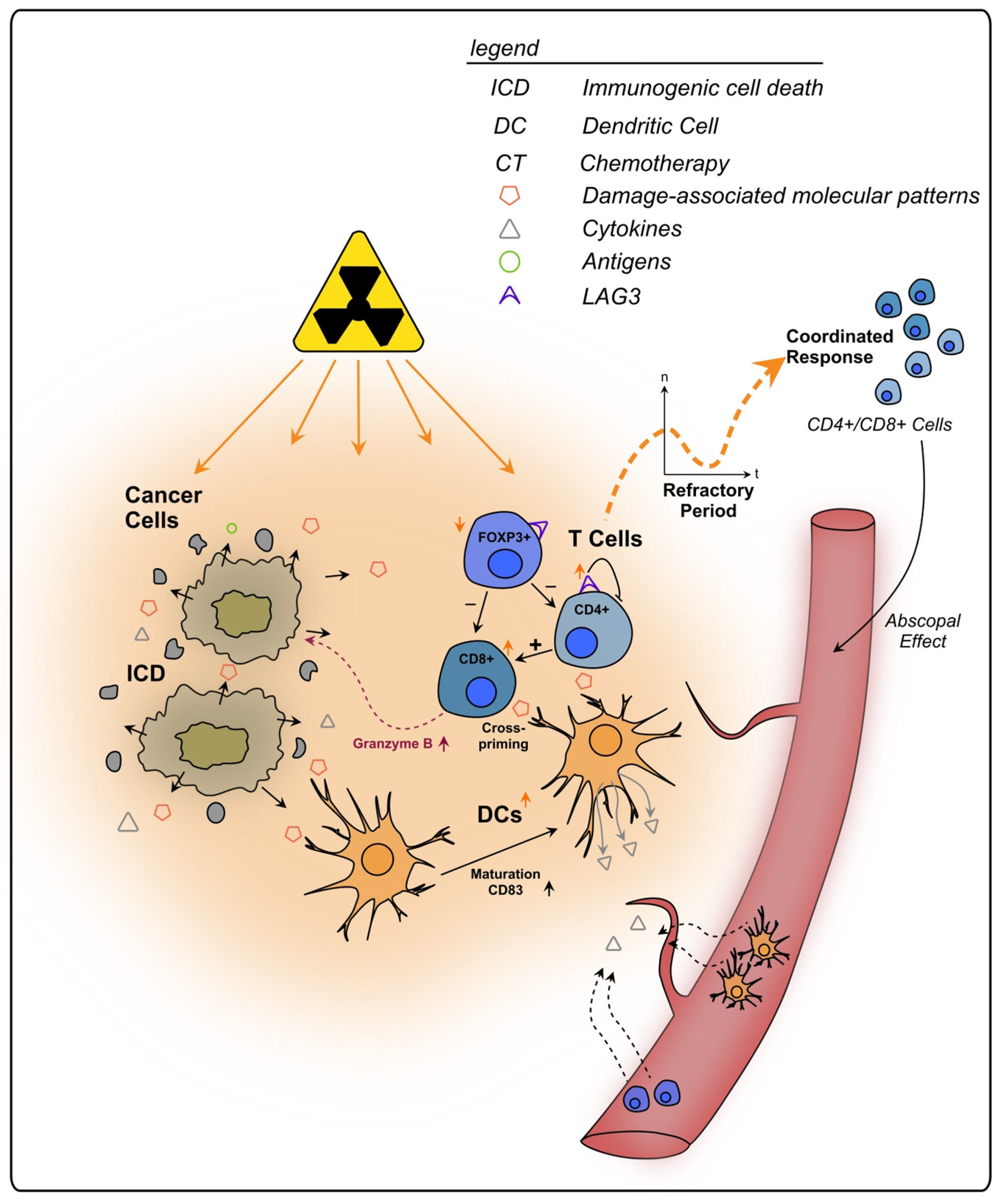
2.3. Treatment-Induced Effects on Adaptive Immunity
2.3.1. Cytotoxic T Lymphocytes and T Helper Cells
2.3.2. Regulatory T Lymphocytes
2.3.3. LAG-3+ T Cells
2.3.4. B Lymphocytes
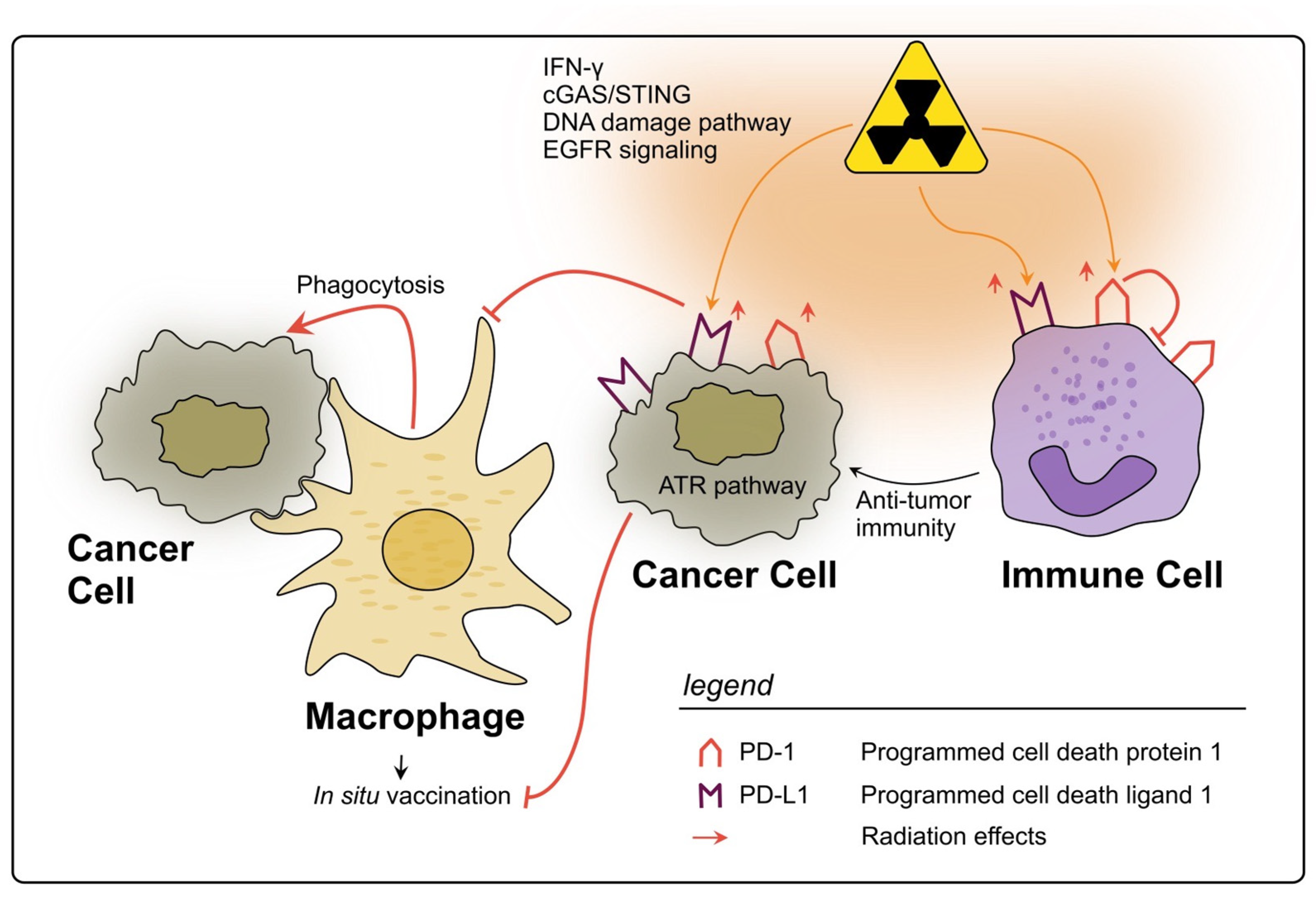
2.4. Other Therapy-Induced Immunosuppressive Effects
PD-L1 and PD-1
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| CAR-NK | Chimeric antigen receptor-transduced NK cells |
| CCL | Chemokine C-C motif chemokine ligand |
| CD | Cluster of differentiation |
| (n)CT | (Neoadjuvant) chemotherapy |
| CTL | Cytotoxic T lymphocyte |
| CRC | Colorectal cancer |
| (n)CRT | (Neoadjuvant) chemoradiotherapy |
| CXCL | C-X-C motif chemokine ligand |
| DAMP | Damage-associated molecular pattern |
| DC | Dendritic cells |
| EC | Endothelial cell |
| EGFR | Epidermal growth factor receptor |
| FOXP3 | Forkhead box P3 |
| HLA | Human leukocyte antigen |
| ICD | Immunogenic cell death |
| IFN | Interferon |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| iNOS | Nitric oxide synthase |
| LAG-3 | Lymphocyte-activating gene 3 |
| LAP | Latency-associated peptide |
| LARC | Locally advanced rectal cancer |
| LCRT | Long-course radiotherapy |
| MDSC | Myeloid-derived suppressor cell |
| MHC | Major histocompatibility complex |
| MLR | Mixed leukocyte reaction |
| M-MDSC | Monocytic myeloid-derived suppressor cell |
| NK cell | Natural killer cell |
| NLR | Neutrophil to leukocyte ratio |
| OS | Overall survival |
| pCR | Pathological complete response |
| PD-1 | Programmed cell death receptor 1 |
| pDC | Plasmacytoid dendritic cell |
| PD-L1 | Programmed cell death ligand 1 |
| PMN-MDSC | Polymorphonuclear monocytic myeloid-derived suppressor cell |
| (n)RT | (Neoadjuvant) radiotherapy |
| SCRT | Short-course radiotherapy |
| SDF-1α | Stromal cell-derived factor-1 alpha |
| slanMos | 6-sulfo LacNAc-expressing monocytes |
| SOX2 | SRY-box transcription factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAM | Tumor-associated macrophage |
| TGF-ß | Transforming growth factor-ß |
| Th cell | T helper cell |
| TIL | Tumor-infiltrating lymphocyte |
| TIME | Tumor immune microenvironment |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor α |
| TNT | Total neoadjuvant therapy |
| TP | Thymidine phosphorylase |
| Treg | Regulatory T cell |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef]
- Araghi, M.; Soerjomataram, I.; Bardot, A.; Ferlay, J.; Cabasag, C.J.; Morrison, D.S.; De, P.; Tervonen, H.; Walsh, P.M.; Bucher, O.; et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019, 4, 511–518. [Google Scholar] [CrossRef]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Wyrwicz, L.; Tiret, E.; Brown, G.; Rödel, C.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv22–iv40. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.; Goldstein, D.; Joseph, D.; Ackland, S.P.; Schache, D.; McClure, B.; McLachlan, S.A.; et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef]
- Pettersson, D.; Cedermark, B.; Holm, T.; Radu, C.; Påhlman, L.; Glimelius, B.; Martling, A. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br. J. Surg. 2010, 97, 580–587. [Google Scholar] [CrossRef]
- Ma, B.; Gao, P.; Wang, H.; Xu, Q.; Song, Y.; Huang, X.; Sun, J.; Zhao, J.; Luo, J.; Sun, Y.; et al. What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int. J. Cancer 2017, 141, 1052–1065. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Colangelo, L.H.; Beart, R.W.; Petrelli, N.J.; Allegra, C.J.; Sharif, S.; Pitot, H.C.; Shields, A.F.; Landry, J.C.; Ryan, D.P.; et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J. Clin. Oncol. 2014, 32, 1927–1934. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; Wenz, F.; Post, S.; Matzdorff, A.; Laechelt, S.; Hartmann, J.T.; Müller, L.; Link, H.; Moehler, M.; Kettner, E.; et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012, 13, 579–588. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Elbers, H.; Askoxylakis, V.; Motschall, E.; Bork, U.; Büchler, M.W.; Weitz, J.; Koch, M. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann. Surg. Oncol. 2013, 20, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Chau, I.; Brown, G.; Cunningham, D.; Tait, D.; Wotherspoon, A.; Norman, A.R.; Tebbutt, N.; Hill, M.; Ross, P.J.; Massey, A.; et al. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J. Clin. Oncol. 2006, 24, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs. Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef] [PubMed]
- Cercek, A.; Roxburgh, C.S.D.; Strombom, P.; Smith, J.J.; Temple, L.K.F.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Yaeger, R.; Stadler, Z.K.; et al. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018, 4, e180071. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Xiao, L.; Yang, S.; Liu, Q.; Gao, Y.; Chen, G.; Xiao, W. Total Neoadjuvant Therapy (TNT) versus Standard Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1555–e1566. [Google Scholar] [CrossRef]
- Fokas, E.; Glynne-Jones, R.; Appelt, A.; Beets-Tan, R.; Beets, G.; Haustermans, K.; Marijnen, C.; Minsky, B.D.; Ludmir, E.; Quirke, P.; et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020, 21, e252–e264. [Google Scholar] [CrossRef]
- Nicolas, A.M.; Pesic, M.; Engel, E.; Ziegler, P.K.; Diefenhardt, M.; Kennel, K.B.; Buettner, F.; Conche, C.; Petrocelli, V.; Elwakeel, E.; et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell 2022, 40, 168–184.e113. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Voth Park, J.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Mazeedi, M.; Almazyadi, H.A.M.; Kallmeyer, K.; Dandara, C.; Pepper, M.S.; et al. The Role of Tumor Microenvironment in Chemoresistance: To Survive, Keep Your Enemies Closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef]
- Barker, H.E.; Paget, J.T.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- Tamas, K.; Walenkamp, A.M.; de Vries, E.G.; van Vugt, M.A.; Beets-Tan, R.G.; van Etten, B.; de Groot, D.J.; Hospers, G.A. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Paschke, S.; Jafarov, S.; Staib, L.; Kreuser, E.D.; Maulbecker-Armstrong, C.; Roitman, M.; Holm, T.; Harris, C.C.; Link, K.H.; Kornmann, M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 2577. [Google Scholar] [CrossRef] [PubMed]
- Mezheyeuski, A.; Micke, P.; Martín-Bernabé, A.; Backman, M.; Hrynchyk, I.; Hammarström, K.; Ström, S.; Ekström, J.; Edqvist, P.H.; Sundström, M.; et al. The Immune Landscape of Colorectal Cancer. Cancers 2021, 13, 5545. [Google Scholar] [CrossRef]
- Lin, A.; Zhang, J.; Luo, P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front. Immunol. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Salem, M.E.; Weinberg, B.A.; Xiu, J.; El-Deiry, W.S.; Hwang, J.J.; Gatalica, Z.; Philip, P.A.; Shields, A.F.; Lenz, H.J.; Marshall, J.L. Comparative molecular analyses of left-sided colon, right-sided colon, and rectal cancers. Oncotarget 2017, 8, 86356–86368. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Park, C.; Wright, E.G. Radiation and the microenvironment—Tumorigenesis and therapy. Nat. Rev. Cancer 2005, 5, 867–875. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.E.; Hong, Y.S.; Kim, S.Y.; Kim, J.; Ryu, Y.M.; Kim, S.Y.; Kim, T.W. Comprehensive evaluation of the tumor immune microenvironment and its dynamic changes in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy: From the phase II ADORE study. Oncoimmunology 2022, 11, 2148374. [Google Scholar] [CrossRef] [PubMed]
- Matsutani, S.; Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Hirakawa, K.; Ohira, M. Significance of tumor-infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci. 2018, 109, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Mu, D.; Meng, X.; Kong, L.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemoradiotherapy and its clinical utility for rectal cancer. Am. J. Cancer Res. 2015, 5, 2064–2074. [Google Scholar] [PubMed]
- Teng, F.; Meng, X.; Kong, L.; Mu, D.; Zhu, H.; Liu, S.; Zhang, J.; Yu, J. Tumor-infiltrating lymphocytes, forkhead box P3, programmed death ligand-1, and cytotoxic T lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl. Res. 2015, 166, 721–732.e721. [Google Scholar] [CrossRef]
- Shinto, E.; Hase, K.; Hashiguchi, Y.; Sekizawa, A.; Ueno, H.; Shikina, A.; Kajiwara, Y.; Kobayashi, H.; Ishiguro, M.; Yamamoto, J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S414–S421. [Google Scholar] [CrossRef]
- Seo, I.; Lee, H.W.; Byun, S.J.; Park, J.Y.; Min, H.; Lee, S.H.; Lee, J.S.; Kim, S.; Bae, S.U. Neoadjuvant chemoradiation alters biomarkers of anticancer immunotherapy responses in locally advanced rectal cancer. J. Immunother. Cancer 2021, 9, e001610. [Google Scholar] [CrossRef]
- Boustani, J.; Derangère, V.; Bertaut, A.; Adotevi, O.; Morgand, V.; Charon-Barra, C.; Ghiringhelli, F.; Mirjolet, C. Radiotherapy Scheme Effect on PD-L1 Expression for Locally Advanced Rectal Cancer. Cells 2020, 9, 2071. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Kim, S.; Jeon, S.-R.; Chie, E.K.; Kim, K.; Kang, G.H.; Han, S.-W.; Kim, T.-Y.; Jeong, S.-Y.; et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8+ Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 1216–1224. [Google Scholar] [CrossRef]
- Hsieh, R.C.; Krishnan, S.; Wu, R.C.; Boda, A.R.; Liu, A.; Winkler, M.; Hsu, W.H.; Lin, S.H.; Hung, M.C.; Chan, L.C.; et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci. Immunol. 2022, 7, eabl9330. [Google Scholar] [CrossRef]
- Corrò, C.; Dutoit, V.; Koessler, T. Emerging Trends for Radio-Immunotherapy in Rectal Cancer. Cancers 2021, 13, 1374. [Google Scholar] [CrossRef]
- Otegbeye, E.E.; Mitchem, J.B.; Park, H.; Chaudhuri, A.A.; Kim, H.; Mutch, M.G.; Ciorba, M.A. Immunity, immunotherapy, and rectal cancer: A clinical and translational science review. Transl. Res. 2021, 231, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Marciscano, A.E.; Formenti, S.C. Radiation Therapy and the In Situ Vaccination Approach. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Bravo-San Pedro, J.M.; Breckpot, K.; Brough, D.; Chaurio, R.; Cirone, M.; et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front. Immunol. 2015, 6, 588. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef]
- Peng, W.; Yang, J.; Xia, L.; Qian, X.; Long, G.; Zhang, H.; Xie, J.; Zhao, J.; Zhang, L.; Pan, W. Immunogenic cell death-associated biomarkers classification predicts prognosis and immunotherapy efficacy in pancreatic ductal adenocarcinoma. Front. Oncol. 2023, 13, 1178966. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Liu, F.; Ke, D.; Wang, X.; Pan, D.; Xu, W.; Zhou, L.; He, W. An Immunogenic Cell Death-Related Classification Predicts Prognosis and Response to Immunotherapy in Head and Neck Squamous Cell Carcinoma. Front. Immunol. 2021, 12, 781466. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Seager, R.J.; Hajal, C.; Spill, F.; Kamm, R.D.; Zaman, M.H. Dynamic interplay between tumour, stroma and immune system can drive or prevent tumour progression. Converg. Sci. Phys. Oncol. 2017, 3, 034002. [Google Scholar] [CrossRef]
- Walle, T.; Martinez Monge, R.; Cerwenka, A.; Ajona, D.; Melero, I.; Lecanda, F. Radiation effects on antitumor immune responses: Current perspectives and challenges. Ther. Adv. Med. Oncol. 2018, 10, 1758834017742575. [Google Scholar] [CrossRef]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef]
- Baeten, C.I.; Castermans, K.; Lammering, G.; Hillen, F.; Wouters, B.G.; Hillen, H.F.; Griffioen, A.W.; Baeten, C.G. Effects of radiotherapy and chemotherapy on angiogenesis and leukocyte infiltration in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1219–1227. [Google Scholar] [CrossRef]
- Hallahan, D.; Kuchibhotla, J.; Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996, 56, 5150–5155. [Google Scholar] [PubMed]
- Lim, J.Y.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Wang, B.; Kawashima, N.; Braunstein, S.; Badura, M.; Cameron, T.O.; Babb, J.S.; Schneider, R.J.; Formenti, S.C.; Dustin, M.L.; et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 2008, 181, 3099–3107. [Google Scholar] [CrossRef]
- Baeten, C.I.; Castermans, K.; Hillen, H.F.; Griffioen, A.W. Proliferating endothelial cells and leukocyte infiltration as prognostic markers in colorectal cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 1351–1357. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, S.B.; Beak, S.K.; Han, Y.D.; Cho, M.S.; Hur, H.; Lee, K.Y.; Kim, N.K.; Min, B.S. Temporal changes in immune cell composition and cytokines in response to chemoradiation in rectal cancer. Sci. Rep. 2018, 8, 7565. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.; Negus, R.; Morris, A.; Ziprin, P.; Goldin, R.; Allavena, P.; Peck, D.; Darzi, A. Chemokine expression is associated with the accumulation of tumour associated macrophages (TAMs) and progression in human colorectal cancer. Clin. Exp. Metastasis 2007, 24, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Su, J.; Zhao, S.; He, Y.; Li, S.; Yang, X.; Zhai, S.; Rong, S.; Zhang, X.; Xu, G.; et al. CCL3 Promotes Proliferation of Colorectal Cancer Related with TRAF6/NF-κB Molecular Pathway. Contrast Media Mol. Imaging 2022, 2022, 2387192. [Google Scholar] [CrossRef]
- Chun, E.; Lavoie, S.; Michaud, M.; Gallini, C.A.; Kim, J.; Soucy, G.; Odze, R.; Glickman, J.N.; Garrett, W.S. CCL2 Promotes Colorectal Carcinogenesis by Enhancing Polymorphonuclear Myeloid-Derived Suppressor Cell Population and Function. Cell Rep. 2015, 12, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ren, X.; Yu, H.; Zhan, Y. Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds? Front. Immunol. 2021, 12, 771210. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Heishi, T.; Incio, J.; Huang, Y.; Beech, E.Y.; Pinter, M.; Ho, W.W.; Kawaguchi, K.; Rahbari, N.N.; Chung, E.; et al. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 10455–10460. [Google Scholar] [CrossRef] [PubMed]
- Jackstadt, R.; van Hooff, S.R.; Leach, J.D.; Cortes-Lavaud, X.; Lohuis, J.O.; Ridgway, R.A.; Wouters, V.M.; Roper, J.; Kendall, T.J.; Roxburgh, C.S.; et al. Epithelial NOTCH Signaling Rewires the Tumor Microenvironment of Colorectal Cancer to Drive Poor-Prognosis Subtypes and Metastasis. Cancer Cell 2019, 36, 319–336.e317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Tian, T. TGF-β Signaling in Metastatic Colorectal Cancer (mCRC): From Underlying Mechanism to Potential Applications in Clinical Development. Int. J. Mol. Sci. 2022, 23, 14436. [Google Scholar] [CrossRef]
- Angenete, E.; Langenskiöld, M.; Palmgren, I.; Falk, P.; Öresland, T.; Ivarsson, M.-L. Transforming growth factor beta-1 in rectal tumour, mucosa and plasma in relation to radiotherapy and clinical outcome in rectal cancer patients. Int. J. Color. Dis. 2007, 22, 1331–1338. [Google Scholar] [CrossRef]
- Yasui, K.; Kondou, R.; Iizuka, A.; Miyata, H.; Tanaka, E.; Ashizawa, T.; Nagashima, T.; Ohshima, K.; Urakami, K.; Kusuhara, M.; et al. Effect of preoperative chemoradiotherapy on the immunological status of rectal cancer patients. J. Radiat. Res. 2020, 61, 766–775. [Google Scholar] [CrossRef]
- Engel, M.A.; Neurath, M.F. Anticancer properties of the IL-12 family--focus on colorectal cancer. Curr. Med. Chem. 2010, 17, 3303–3308. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Tugues, S.; Burkhard, S.H.; Ohs, I.; Vrohlings, M.; Nussbaum, K.; Vom Berg, J.; Kulig, P.; Becher, B. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015, 22, 237–246. [Google Scholar] [CrossRef]
- Henry, C.J.; Ornelles, D.A.; Mitchell, L.M.; Brzoza-Lewis, K.L.; Hiltbold, E.M. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. 2008, 181, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 6, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Frankel, T.L.; Green, M.; Zou, W. IFNγ signaling integrity in colorectal cancer immunity and immunotherapy. Cell Mol. Immunol. 2022, 19, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.R.; Merlino, G. The two faces of interferon-γ in cancer. Clin. Cancer Res. 2011, 17, 6118–6124. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Matsushita, K.; Takenouchi, T.; Shimada, H.; Tomonaga, T.; Hayashi, H.; Shioya, A.; Komatsu, A.; Matsubara, H.; Ochiai, T. Strong HLA-DR antigen expression on cancer cells relates to better prognosis of colorectal cancer patients: Possible involvement of c-myc suppression by interferon-gamma in situ. Cancer Sci. 2006, 97, 57–63. [Google Scholar] [CrossRef]
- Ni, C.; Wu, P.; Zhu, X.; Ye, J.; Zhang, Z.; Chen, Z.; Zhang, T.; Zhang, T.; Wang, K.; Wu, D.; et al. IFN-γ selectively exerts pro-apoptotic effects on tumor-initiating label-retaining colon cancer cells. Cancer Lett. 2013, 336, 174–184. [Google Scholar] [CrossRef]
- Wilkins, A.; Fontana, E.; Nyamundanda, G.; Ragulan, C.; Patil, Y.; Mansfield, D.; Kingston, J.; Errington-Mais, F.; Bottomley, D.; von Loga, K.; et al. Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer. J. Immunother. Cancer 2021, 9, e001717. [Google Scholar] [CrossRef]
- Chiang, S.F.; Huang, C.Y.; Ke, T.W.; Chen, T.W.; Lan, Y.C.; You, Y.S.; Chen, W.T.; Chao, K.S.C. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol. Immunother. 2019, 68, 283–296. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef]
- Liu, Y.J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar] [CrossRef]
- Wagner, F.; Hölig, U.; Wilczkowski, F.; Plesca, I.; Sommer, U.; Wehner, R.; Kießler, M.; Jarosch, A.; Flecke, K.; Arsova, M.; et al. Neoadjuvant Radiochemotherapy Significantly Alters the Phenotype of Plasmacytoid Dendritic Cells and 6-Sulfo LacNAc(+) Monocytes in Rectal Cancer. Front. Immunol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Hiroishi, K.; Tüting, T.; Lotze, M.T. IFN-alpha-expressing tumor cells enhance generation and promote survival of tumor-specific CTLs. J. Immunol. 2000, 164, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, J.; Hiroishi, K.; Ishii, S.; Mitamura, K. Interferon-alpha and interleukin-12 gene therapy of cancer: Interferon-alpha induces tumor-specific immune responses while interleukin-12 stimulates non-specific killing. Cancer Immunol. Immunother. 2003, 52, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, M.W.; Meyer, G.; Adam, M.; Höchtlen-Vollmar, W.; Angele, M.K.; Schalhorn, A.; Wilkowski, R.; Müller, C.; Schildberg, F.W. Detrimental immunologic effects of preoperative chemoradiotherapy in advanced rectal cancer. Dis. Colon. Rectum 2003, 46, 875–887. [Google Scholar] [CrossRef]
- Zachariah, B.; Jacob, S.S.; Gwede, C.; Cantor, A.; Patil, J.; Casey, L.; Zachariah, A.B. Effect of fractionated regional external beam radiotherapy on peripheral blood cell count. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef]
- Kachikwu, E.L.; Iwamoto, K.S.; Liao, Y.P.; DeMarco, J.J.; Agazaryan, N.; Economou, J.S.; McBride, W.H.; Schaue, D. Radiation enhances regulatory T cell representation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1128–1135. [Google Scholar] [CrossRef]
- Qu, Y.; Jin, S.; Zhang, A.; Zhang, B.; Shi, X.; Wang, J.; Zhao, Y. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat. Res. 2010, 173, 148–157. [Google Scholar] [CrossRef]
- Gennatas, C.; Dardoufas, C.; Mouratidou, D.; Tsavaris, N.; Pouli, A.; Androulakis, G.; Philippakis, M.; Voros, D.; Batalis, T.; Besbeas, S.; et al. Surgical adjuvant therapy of rectal carcinoma: A controlled evaluation of leucovorin, 5-fluorouracil and radiation therapy with or without interferon-alpha2b. Ann. Oncol. 2003, 14, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Uccello, T.P.; Lesch, M.L.; Kintzel, S.A.; Gradzewicz, L.B.; Lamrous, L.; Murphy, S.P.; Fleming, F.J.; Mills, B.N.; Murphy, J.D.; Hughson, A.; et al. New insights into the responder/nonresponder divide in rectal cancer: Damage-induced Type I IFNs dictate treatment efficacy and can be targeted to enhance radiotherapy. Cell Death Dis. 2023, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Perera, F.; Fisher, B.; Kocha, W.; Plewes, E.; Taylor, M.; Vincent, M. A Phase I pilot study of pelvic radiation and alpha-2a interferon in patients with locally advanced or recurrent rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 297–303. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Christofides, A.; Strauss, L.; Yeo, A.; Cao, C.; Charest, A.; Boussiotis, V.A. The complex role of tumor-infiltrating macrophages. Nat. Immunol. 2022, 23, 1148–1156. [Google Scholar] [CrossRef]
- Ong, S.-M.; Tan, Y.-C.; Beretta, O.; Jiang, D.; Yeap, W.-H.; Tai, J.J.Y.; Wong, W.-C.; Yang, H.; Schwarz, H.; Lim, K.-H.; et al. Macrophages in human colorectal cancer are pro-inflammatory and prime T cells towards an anti-tumour type-1 inflammatory response. Eur. J. Immunol. 2012, 42, 89–100. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, B.; Yang, Z. The Role of Tumor-Associated Macrophages in Colorectal Carcinoma Progression. Cell. Physiol. Biochem. 2018, 45, 356–365. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Norton, S.E.; Dunn, E.T.; McCall, J.L.; Munro, F.; Kemp, R.A. Gut macrophage phenotype is dependent on the tumor microenvironment in colorectal cancer. Clin. Transl. Immunology 2016, 5, e76. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.Y.; Black, A.; Qian, B.Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef]
- Stary, V.; Wolf, B.; Unterleuthner, D.; List, J.; Talic, M.; Laengle, J.; Beer, A.; Strobl, J.; Stary, G.; Dolznig, H.; et al. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J. Immunother. Cancer 2020, 8, e000667. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef] [PubMed]
- Edin, S.; Wikberg, M.L.; Dahlin, A.M.; Rutegård, J.; Öberg, Å.; Oldenborg, P.A.; Palmqvist, R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE 2012, 7, e47045. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.L.; Rios, E.; Durães, C.; Ribeiro, R.; Machado, J.C.; Mantovani, A.; Barbosa, M.A.; Carneiro, F.; Oliveira, M.J. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front. Immunol. 2019, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Teresa Pinto, A.; Laranjeiro Pinto, M.; Patrícia Cardoso, A.; Monteiro, C.; Teixeira Pinto, M.; Filipe Maia, A.; Castro, P.; Figueira, R.; Monteiro, A.; Marques, M.; et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci. Rep. 2016, 6, 18765. [Google Scholar] [CrossRef]
- Matsuura, T.; Kuratate, I.; Teramachi, K.; Osaki, M.; Fukuda, Y.; Ito, H. Thymidine phosphorylase expression is associated with both increase of intratumoral microvessels and decrease of apoptosis in human colorectal carcinomas. Cancer Res. 1999, 59, 5037–5040. [Google Scholar]
- Elamin, Y.Y.; Rafee, S.; Osman, N.; KJ, O.B.; Gately, K. Thymidine Phosphorylase in Cancer; Enemy or Friend? Cancer Microenviron. 2016, 9, 33–43. [Google Scholar] [CrossRef]
- Jakob, C.; Aust, D.E.; Meyer, W.; Baretton, G.B.; Schwabe, W.; Häusler, P.; Becker, H.; Liersch, T. Thymidylate synthase, thymidine phosphorylase, dihydropyrimidine dehydrogenase expression, and histological tumour regression after 5-FU-based neo-adjuvant chemoradiotherapy in rectal cancer. J. Pathol. 2004, 204, 562–568. [Google Scholar] [CrossRef]
- Kim, T.-D.; Li, G.; Song, K.-S.; Kim, J.-M.; Kim, J.-S.; Kim, J.-S.; Yun, E.-J.; Park, J.-I.; Park, H.-D.; Hwang, B.-D.; et al. Radiation-Induced Thymidine Phosphorylase Upregulation in Rectal Cancer Is Mediated by Tumor-Associated Macrophages by Monocyte Chemoattractant Protein–1 From Cancer Cells. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 853–860. [Google Scholar] [CrossRef]
- Derwinger, K.; Lindskog, E.B.; Palmqvist, E.; Wettergren, Y. Changes in Thymidine Phosphorylase Gene Expression Related to Treatment of Rectal Cancer. Anticancer Res. 2013, 33, 2447–2451. [Google Scholar]
- Hedrick, C.C.; Malanchi, I. Neutrophils in cancer: Heterogeneous and multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Hofheinz, R.-D.; Martin, D.; Beißbarth, T.; Arnold, D.; Hartmann, A.; von der Grün, J.; Grützmann, R.; Liersch, T.; Ströbel, P.; et al. Leukocytosis and neutrophilia as independent prognostic immunological biomarkers for clinical outcome in the CAO/ARO/AIO-04 randomized phase 3 rectal cancer trial. Int. J. Cancer 2019, 145, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, K.; Park, H.J. Meta-Analysis on the Neutrophil-Lymphocyte Ratio in Rectal Cancer Treated With Preoperative Chemoradiotherapy: Prognostic Value of Pre- and Post-Chemoradiotherapy Neutrophil-Lymphocyte Ratio. Front. Oncol. 2022, 12, 778607. [Google Scholar] [CrossRef]
- Sung, S.; Son, S.H.; Park, E.Y.; Kay, C.S. Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS ONE 2017, 12, e0173955. [Google Scholar] [CrossRef]
- Dong, Y.W.; Shi, Y.Q.; He, L.W.; Su, P.Z. Prognostic significance of neutrophil-to-lymphocyte ratio in rectal cancer: A meta-analysis. Onco Targets Ther. 2016, 9, 3127–3134. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef]
- Rao, H.L.; Chen, J.W.; Li, M.; Xiao, Y.B.; Fu, J.; Zeng, Y.X.; Cai, M.Y.; Xie, D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar] [CrossRef]
- Berry, R.S.; Xiong, M.J.; Greenbaum, A.; Mortaji, P.; Nofchissey, R.A.; Schultz, F.; Martinez, C.; Luo, L.; Morris, K.T.; Hanson, J.A. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS ONE 2017, 12, e0188799. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Fridlender, Z.G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Marijnen, C.A.; Kranenbarg, E.K.; Mulder-Stapel, A.; Hermans, J.; van de Velde, C.J.; van Krieken, J.H. Short-term preoperative radiotherapy interferes with the determination of pathological parameters in rectal cancer. J. Pathol. 2002, 197, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hao, Y.; Du, R.; Hu, D.; Xie, J.; Zhang, J.; Deng, G.; Liang, N.; Tian, T.; Käsmann, L.; et al. Radiotherapy programs neutrophils to an antitumor phenotype by inducing mesenchymal-epithelial transition. Transl. Lung Cancer Res. 2021, 10, 1424–1443. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Pop, L.M.; Laine, A.; Iyengar, P.; Vitetta, E.S.; Hannan, R. Key role for neutrophils in radiation-induced antitumor immune responses: Potentiation with G-CSF. Proc. Natl. Acad. Sci. USA 2016, 113, 11300–11305. [Google Scholar] [CrossRef]
- Kitayama, J.; Yasuda, K.; Kawai, K.; Sunami, E.; Nagawa, H. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat. Oncol. 2010, 5, 47. [Google Scholar] [CrossRef]
- Yang, G.; Chang, J.S.; Choi, J.E.; Baek, E.S.; Kim, S.-S.; Byun, H.K.; Cho, Y.; Koom, W.S.; Yang, S.Y.; Min, B.S.; et al. Association of neutrophil-to-lymphocyte ratio, radiotherapy fractionation/technique, and risk of development of distant metastasis among patients with locally advanced rectal cancer. Radiat. Oncol. 2022, 17, 100. [Google Scholar] [CrossRef]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, G.M. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Ernstoff, M.S.; Hernandez, C.; Atkins, M.; Zabaleta, J.; Sierra, R.; Ochoa, A.C. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009, 69, 1553–1560. [Google Scholar] [CrossRef]
- Munder, M.; Schneider, H.; Luckner, C.; Giese, T.; Langhans, C.-D.; Fuentes, J.M.; Kropf, P.; Mueller, I.; Kolb, A.; Modolell, M.; et al. Suppression of T-cell functions by human granulocyte arginase. Blood 2006, 108, 1627–1634. [Google Scholar] [CrossRef]
- Bennett, J.A.; Rao, V.S.; Mitchell, M.S. Systemic bacillus Calmette-Guérin (BCG) activates natural suppressor cells. Proc. Natl. Acad. Sci. USA 1978, 75, 5142–5144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Movahedi, K.; Guilliams, M.; Van den Bossche, J.; Van den Bergh, R.; Gysemans, C.; Beschin, A.; De Baetselier, P.; Van Ginderachter, J.A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008, 111, 4233–4244. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 2008, 181, 5791–5802. [Google Scholar] [CrossRef]
- Hegde, S.; Leader, A.M.; Merad, M. MDSC: Markers, development, states, and unaddressed complexity. Immunity 2021, 54, 875–884. [Google Scholar] [CrossRef]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-Talk between Myeloid-Derived Suppressor Cells and Macrophages Subverts Tumor Immunity toward a Type 2 Response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Hasnis, E.; Dahan, A.; Khoury, W.; Duek, D.; Fisher, Y.; Beny, A.; Shaked, Y.; Chowers, Y.; Half, E.E. Intratumoral HLA-DR−/CD33+/CD11b+ Myeloid-Derived Suppressor Cells Predict Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Front. Oncol. 2020, 10, 1375. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koh, J.; Choi, M.; Kim, S.; Chie, E.K. Prognostic stratification based on the levels of tumor-infiltrating myeloid-derived suppressor cells and PD-1/PD-L1 axis in locally advanced rectal cancer. Front. Oncol. 2022, 12, 1018700. [Google Scholar] [CrossRef]
- Karakasheva, T.A.; Dominguez, G.A.; Hashimoto, A.; Lin, E.W.; Chiu, C.; Sasser, K.; Lee, J.W.; Beatty, G.L.; Gabrilovich, D.I.; Rustgi, A.K. CD38+ M-MDSC expansion characterizes a subset of advanced colorectal cancer patients. JCI Insight 2018, 3, e97022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; De Ridder, M.; Verovski, V.N.; Sonveaux, P.; Jordan, B.F.; Law, K.; Monsaert, C.; Van den Berge, D.L.; Verellen, D.; Feron, O.; et al. Activated Macrophages as a Novel Determinant of Tumor Cell Radioresponse: The Role of Nitric Oxide–Mediated Inhibition of Cellular Respiration and Oxygen Sparing. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Wu, L.; Zhang, M.; Li, W.; Ding, J.; Zhu, J.; Wei, H.; Zhao, K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 2013, 8, e57114. [Google Scholar] [CrossRef]
- Toor, S.M.; Syed Khaja, A.S.; El Salhat, H.; Bekdache, O.; Kanbar, J.; Jaloudi, M.; Elkord, E. Increased Levels of Circulating and Tumor-Infiltrating Granulocytic Myeloid Cells in Colorectal Cancer Patients. Front. Immunol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Dufait, I.; Schwarze, J.K.; Law, K.; Engels, B.; Jiang, H.; Van den Berge, D.; Gevaert, T.; Storme, G.; Verovski, V.; et al. Myeloid-derived suppressor cells reveal radioprotective properties through arginase-induced l-arginine depletion. Radiother. Oncol. 2016, 119, 291–299. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef]
- Filatenkov, A.; Baker, J.; Mueller, A.M.; Kenkel, J.; Ahn, G.O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S.; et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef]
- Cui, C.; Lan, P.; Fu, L. The role of myeloid-derived suppressor cells in gastrointestinal cancer. Cancer Commun. 2021, 41, 442–471. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Lim, S.H.; Chua, W.; Cheng, C.; Descallar, J.; Ng, W.; Solomon, M.; Bokey, L.; Wong, K.; Lee, M.T.; de Souza, P.; et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014, 34, 6505–6513. [Google Scholar]
- Sconocchia, G.; Eppenberger, S.; Spagnoli, G.C.; Tornillo, L.; Droeser, R.; Caratelli, S.; Ferrelli, F.; Coppola, A.; Arriga, R.; Lauro, D.; et al. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology 2014, 3, e952197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zeng, Z.; Li, J.; Luo, Y.; Sun, W.; Gong, Y.; Zhang, J.; Wu, Q.; Xie, C. Immunomodulation of NK Cells by Ionizing Radiation. Front. Oncol. 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Kraske, J.A.; Liao, B.; Lenoir, B.; Timke, C.; von Bohlen Und Halbach, E.; Tran, F.; Griebel, P.; Albrecht, D.; Ahmed, A.; et al. Radiotherapy orchestrates natural killer cell dependent antitumor immune responses through CXCL8. Sci. Adv. 2022, 8, eabh4050. [Google Scholar] [CrossRef]
- Alderdice, M.; Dunne, P.D.; Cole, A.J.; O’Reilly, P.G.; McArt, D.G.; Bingham, V.; Fuchs, M.A.; McQuaid, S.; Loughrey, M.B.; Murray, G.I.; et al. Natural killer-like signature observed post therapy in locally advanced rectal cancer is a determinant of pathological response and improved survival. Mod. Pathol. 2017, 30, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Koda, K.; Saito, N.; Oda, K.; Seike, K.; Kondo, E.; Ishizuka, M.; Takiguchi, N.; Miyazaki, M. Natural killer cell activity and distant metastasis in rectal cancers treated surgically with and without neoadjuvant chemoradiotherapy. J. Am. Coll. Surg. 2003, 197, 254–260. [Google Scholar] [CrossRef]
- Trzonkowski, P.; Szmit, E.; Myśliwska, J.; Dobyszuk, A.; Myśliwski, A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin. Immunol. 2004, 112, 258–267. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, X.; Li, H.; Niu, C.; Yu, D.; Yang, G.; Liang, X.; Wen, X.; Li, M.; Cui, J. Validating the pivotal role of the immune system in low-dose radiation-induced tumor inhibition in Lewis lung cancer-bearing mice. Cancer Med. 2018, 7, 1338–1348. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Feng, X.; Han, Z. CAR-NK cells for cancer immunotherapy: From bench to bedside. Biomark. Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Steinman, R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296. [Google Scholar] [CrossRef]
- Hart, D.N. Dendritic cells: Unique leukocyte populations which control the primary immune response. Blood 1997, 90, 3245–3287. [Google Scholar] [CrossRef]
- Graham Martínez, C.; Barella, Y.; Kus Öztürk, S.; Ansems, M.; Gorris, M.A.J.; van Vliet, S.; Marijnen, C.A.M.; Nagtegaal, I.D. The immune microenvironment landscape shows treatment-specific differences in rectal cancer patients. Front. Immunol. 2022, 13, 1011498. [Google Scholar] [CrossRef]
- Schäkel, K.; Kannagi, R.; Kniep, B.; Goto, Y.; Mitsuoka, C.; Zwirner, J.; Soruri, A.; von Kietzell, M.; Rieber, E. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity 2002, 17, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Döbel, T.; Schmitz, M.; Schäkel, K. Current Concepts on 6-sulfo LacNAc Expressing Monocytes (slanMo). Front. Immunol. 2019, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic cells in the cancer microenvironment. J. Cancer 2013, 4, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.W.; Lee, J.H.; Kim, H.R. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch. Surg. 2012, 147, 366–372. [Google Scholar] [CrossRef]
- Yang, T.J.; Oh, J.H.; Apte, A.; Son, C.H.; Deasy, J.O.; Goodman, K.A. Clinical and dosimetric predictors of acute hematologic toxicity in rectal cancer patients undergoing chemoradiotherapy. Radiother. Oncol. 2014, 113, 29–34. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.S.; Cho, M.J.; Song, K.S.; Yoon, W.H. Preoperative chemoradiation using oral capecitabine in locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 403–408. [Google Scholar] [CrossRef]
- Jarosch, A.; Sommer, U.; Bogner, A.; Reißfelder, C.; Weitz, J.; Krause, M.; Folprecht, G.; Baretton, G.B.; Aust, D.E. Neoadjuvant radiochemotherapy decreases the total amount of tumor infiltrating lymphocytes, but increases the number of CD8+/Granzyme B+ (GrzB) cytotoxic T-cells in rectal cancer. Oncoimmunology 2018, 7, e1393133. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Cullen, S.P.; Martin, S.J. Mechanisms of granule-dependent killing. Cell Death Differ. 2008, 15, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Masson, D.; Nabholz, M.; Estrade, C.; Tschopp, J. Granules of cytolytic T-lymphocytes contain two serine esterases. EMBO J. 1986, 5, 1595–1600. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Borst, J.; Ahrends, T.; Bąbała, N.; Melief, C.J.M.; Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 2018, 18, 635–647. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Soh, H.; Seyedin, S.; Cortez, M.A.; Krishnan, S.; Massarelli, E.; Hong, D.; Naing, A.; Diab, A.; et al. Combining radiation and immunotherapy: A new systemic therapy for solid tumors? Cancer Immunol. Res. 2014, 2, 831–838. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef]
- Bains, S.J.; Abrahamsson, H.; Flatmark, K.; Dueland, S.; Hole, K.H.; Seierstad, T.; Redalen, K.R.; Meltzer, S.; Ree, A.H. Immunogenic cell death by neoadjuvant oxaliplatin and radiation protects against metastatic failure in high-risk rectal cancer. Cancer Immunol. Immunother. 2020, 69, 355–364. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Roncador, G.; Brown, P.J.; Maestre, L.; Hue, S.; Martínez-Torrecuadrada, J.L.; Ling, K.L.; Pratap, S.; Toms, C.; Fox, B.C.; Cerundolo, V.; et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 2005, 35, 1681–1691. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Thornton, A.M.; Shevach, E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998, 188, 287–296. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Mirjolet, C.; Charon-Barra, C.; Ladoire, S.; Arbez-Gindre, F.; Bertaut, A.; Ghiringhelli, F.; Leroux, A.; Peiffert, D.; Borg, C.; Bosset, J.F.; et al. Tumor lymphocyte immune response to preoperative radiotherapy in locally advanced rectal cancer: The LYMPHOREC study. Oncoimmunology 2018, 7, e1396402. [Google Scholar] [CrossRef]
- McCoy, M.J.; Hemmings, C.; Miller, T.J.; Austin, S.J.; Bulsara, M.K.; Zeps, N.; Nowak, A.K.; Lake, R.A.; Platell, C.F. Low stromal Foxp3+ regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br. J. Cancer 2015, 113, 1677–1686. [Google Scholar] [CrossRef]
- Napolitano, M.; D’Alterio, C.; Cardone, E.; Trotta, A.M.; Pecori, B.; Rega, D.; Pace, U.; Scala, D.; Scognamiglio, G.; Tatangelo, F.; et al. Peripheral myeloid-derived suppressor and T regulatory PD-1 positive cells predict response to neoadjuvant short-course radiotherapy in rectal cancer patients. Oncotarget 2015, 6, 8261–8270. [Google Scholar] [CrossRef]
- Ji, D.; Song, C.; Li, Y.; Xia, J.; Wu, Y.; Jia, J.; Cui, X.; Yu, S.; Gu, J. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J. ImmunoTherapy Cancer 2020, 8, e000826. [Google Scholar] [CrossRef]
- Akeus, P.; Langenes, V.; Kristensen, J.; von Mentzer, A.; Sparwasser, T.; Raghavan, S.; Quiding-Järbrink, M. Treg-cell depletion promotes chemokine production and accumulation of CXCR3+ conventional T cells in intestinal tumors. Eur. J. Immunol. 2015, 45, 1654–1666. [Google Scholar] [CrossRef]
- Son, C.H.; Bae, J.H.; Shin, D.Y.; Lee, H.R.; Jo, W.S.; Yang, K.; Park, Y.S. Combination effect of regulatory T-cell depletion and ionizing radiation in mouse models of lung and colon cancer. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 390–398. [Google Scholar] [CrossRef]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-31. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 Expression Defines a Subset of CD4+CD25highFoxp3+ Regulatory T Cells That Are Expanded at Tumor Sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef]
- Tayshetye, P.; Friday, A.J.; Omstead, A.N.; Verma, T.; Miller, S.; Zheng, P.; Jani, P.; Zaidi, A.; Finley, G. Tumor Microenvironment before and after Chemoradiation in Locally Advanced Rectal Cancer: Beyond PD-L1. Cancers 2022, 15, 276. [Google Scholar] [CrossRef]
- Peng, Q.Q.; Li, J.L.; Xin, P.L.; Du, K.X.; Lin, X.Y.; Wu, J.X.; Zhang, M.T.; Kong, X.Q. Assessment of the expression and response of PD-1, LAG-3, and TIM-3 after neoadjuvant radiotherapy in rectal cancer. Neoplasma 2021, 68, 742–750. [Google Scholar] [CrossRef]
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.A.; Golan, T.; Geva, R.; Wermke, M.; de Miguel, M.; et al. A first-in-human study of the anti-LAG-3 antibody favezelimab plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. ESMO Open 2022, 7, 100639. [Google Scholar] [CrossRef]
- LeBien, T.W.; Tedder, T.F. B lymphocytes: How they develop and function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef]
- Sharonov, G.V.; Serebrovskaya, E.O.; Yuzhakova, D.V.; Britanova, O.V.; Chudakov, D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 2020, 20, 294–307. [Google Scholar] [CrossRef]
- Downs-Canner, S.M.; Meier, J.; Vincent, B.G.; Serody, J.S. B Cell Function in the Tumor Microenvironment. Annu. Rev. Immunol. 2022, 40, 169–193. [Google Scholar] [CrossRef]
- Edin, S.; Kaprio, T.; Hagström, J.; Larsson, P.; Mustonen, H.; Böckelman, C.; Strigård, K.; Gunnarsson, U.; Haglund, C.; Palmqvist, R. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci. Rep. 2019, 9, 19997. [Google Scholar] [CrossRef] [PubMed]
- Franiak-Pietryga, I.; Miyauchi, S.; Kim, S.S.; Sanders, P.D.; Sumner, W.; Zhang, L.; Mundt, A.J.; Califano, J.A.; Sharabi, A.B. Activated B Cells and Plasma Cells Are Resistant to Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 514–528. [Google Scholar] [CrossRef]
- Sendoya, J.M.; Iseas, S.; Coraglio, M.; Golubicki, M.; Robbio, J.; Salanova, R.; Kujaruk, M.; Mikolaitis, V.; Rizzolo, M.; Ruiz, G.; et al. Pre-Existing Tumoral B Cell Infiltration and Impaired Genome Maintenance Correlate with Response to Chemoradiotherapy in Locally Advanced Rectal Cancer. Cancers 2020, 12, 2227. [Google Scholar] [CrossRef]
- Kolotova, N.M.; Maiborodin, I.V.; Fursov, S.A.; Lushnikova, E.L.; Zarubenkov, O.A.; Maiborodina, V.I. Morphology of Pararectal Lymph Nodes in Rectal Cancer after Neoadjuvant Therapy. Bull. Exp. Biol. Med. 2010, 149, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Barbera-Guillem, E.; Nelson, M.B.; Barr, B.; Nyhus, J.K.; May, K.F., Jr.; Feng, L.; Sampsel, J.W. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol. Immunother. 2000, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef]
- Kythreotou, A.; Siddique, A.; Mauri, F.A.; Bower, M.; Pinato, D.J. PD-L1. J. Clin. Pathol. 2018, 71, 189–194. [Google Scholar] [CrossRef]
- Baretti, M.; Zhu, Q.; Fu, W.; Meyer, J.; Wang, H.; Anders, R.A.; Azad, N.S. Chemoradiation-induced alteration of programmed death-ligand 1, CD8+ tumor-infiltrating lymphocytes and mucin expression in rectal cancer. Oncotarget 2022, 13, 907–917. [Google Scholar] [CrossRef]
- Wang, N.H.; Lei, Z.; Yang, H.N.; Tang, Z.; Yang, M.Q.; Wang, Y.; Sui, J.D.; Wu, Y.Z. Radiation-induced PD-L1 expression in tumor and its microenvironment facilitates cancer-immune escape: A narrative review. Ann. Transl. Med. 2022, 10, 1406. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef]
- Hecht, M.; Büttner-Herold, M.; Erlenbach-Wünsch, K.; Haderlein, M.; Croner, R.; Grützmann, R.; Hartmann, A.; Fietkau, R.; Distel, L.V. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur. J. Cancer 2016, 65, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Akiyoshi, T.; Yamamoto, N.; Kawachi, H.; Ishikawa, Y.; Mori, S.; Oba, K.; Nagino, M.; Fukunaga, Y.; Ueno, M. Pattern of programmed cell death-ligand 1 expression and CD8-positive T-cell infiltration before and after chemoradiotherapy in rectal cancer. Eur. J. Cancer 2018, 91, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.T.; Liao, C.K.; Chen, T.C.; Lai, C.C.; Chiang, S.F.; Chiang, J.M. A high density of PD-L1-expressing immune cells is significantly correlated with favorable disease free survival in nonmetastatic colorectal cancer. Medicine (Baltimore) 2022, 101, e28573. [Google Scholar] [CrossRef] [PubMed]
- Droeser, R.A.; Hirt, C.; Viehl, C.T.; Frey, D.M.; Nebiker, C.; Huber, X.; Zlobec, I.; Eppenberger-Castori, S.; Tzankov, A.; Rosso, R.; et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur. J. Cancer 2013, 49, 2233–2242. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Kitagawa, Y.; Akiyoshi, T.; Yamamoto, N.; Mukai, T.; Hiyoshi, Y.; Yamaguchi, T.; Nagasaki, T.; Fukunaga, Y.; Hirota, T.; Noda, T.; et al. Tumor-Infiltrating PD-1+ Immune Cell Density is Associated with Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Clin. Color. Cancer 2022, 21, e1–e11. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Dai, W.; Cai, G.; Xu, Y.; Li, X.; Li, Q.; Cai, S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 2016, 15, 55. [Google Scholar] [CrossRef]
- Lee, L.H.; Cavalcanti, M.S.; Segal, N.H.; Hechtman, J.F.; Weiser, M.R.; Smith, J.J.; Garcia-Aguilar, J.; Sadot, E.; Ntiamoah, P.; Markowitz, A.J.; et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod. Pathol. 2016, 29, 1433–1442. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiang, S.-F.; Ke, T.-W.; Chen, T.-W.; Lan, Y.-C.; You, Y.-S.; Shiau, A.-C.; Chen, W.T.-L.; Chao, K.S.C. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1+ TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol. Immunother. 2018, 67, 551–562. [Google Scholar] [CrossRef]
- Bakhrebah, M.A.; Nasrullah, M.; Abdulaal, W.H.; Hassan, M.A.; Siddiqui, H.; Doghaither, H.A.A.; Omar, U.M.; Helmi, N.; Fallatah, M.M.; Al-Ghafari, A.B.; et al. High Expression of Pd-1 in Circulating Cells of Patients With Advanced Colorectal Cancer Receiving Adjuvant Therapy. Technol. Cancer Res. Treat. 2020, 19, 1533033820969446. [Google Scholar] [CrossRef]
- Enkhbat, T.; Nishi, M.; Takasu, C.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Kashihara, H.; Ishikawa, D.; Shimada, M. Programmed Cell Death Ligand 1 Expression Is an Independent Prognostic Factor in Colorectal Cancer. Anticancer Res. 2018, 38, 3367–3373. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Matsutani, S.; Hirakawa, K.; Ohira, M. The Prognostic Significance of the Tumor-Infiltrating Programmed Cell Death-1+ to CD8+ Lymphocyte Ratio in Patients with Colorectal Cancer. Anticancer Res. 2017, 37, 4165–4172. [Google Scholar]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired Resistance to Fractionated Radiotherapy Can Be Overcome by Concurrent PD-L1 Blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Duggan, W.P.; Kisakol, B.; O’Connell, E.; Matveeva, A.; O’Grady, T.; McDonough, E.; Lindner, A.U.; McNamara, D.; Longley, D.; Ginty, F.; et al. Multiplexed Immunofluorescence Imaging Reveals an Immune-Rich Tumor Microenvironment in Mucinous Rectal Cancer Characterized by Increased Lymphocyte Infiltration and Enhanced Programmed Cell Death Protein 1 Expression. Dis. Colon. Rectum 2023, 66, 914–922. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Shabgah, A.G.; Aslani, S.; Alimardani, M.; Pasdar, A.; Sahebkar, A. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J. Cell. Physiol. 2019, 234, 16824–16837. [Google Scholar] [CrossRef]
- Sauce, D.; Almeida, J.R.; Larsen, M.; Haro, L.; Autran, B.; Freeman, G.J.; Appay, V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. Aids 2007, 21, 2005–2013. [Google Scholar] [CrossRef]
- Raimondi, G.; Shufesky, W.J.; Tokita, D.; Morelli, A.E.; Thomson, A.W. Regulated Compartmentalization of Programmed Cell Death-1 Discriminates CD4+CD25+ Resting Regulatory T Cells from Activated T Cells. J. Immunol. 2006, 176, 2808–2816. [Google Scholar] [CrossRef]
- Huang, X.; Venet, F.; Wang, Y.L.; Lepape, A.; Yuan, Z.; Chen, Y.; Swan, R.; Kherouf, H.; Monneret, G.; Chung, C.S.; et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 6303–6308. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Pesce, S.; Muccio, L.; Carlomagno, S.; Sivori, S.; Moretta, A.; Marcenaro, E. Features of Memory-Like and PD-1+ Human NK Cell Subsets. Front. Immunol. 2016, 7, 351. [Google Scholar] [CrossRef]
- Lin, Z.; Cai, M.; Zhang, P.; Li, G.; Liu, T.; Li, X.; Cai, K.; Nie, X.; Wang, J.; Liu, J.; et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J. Immunother. Cancer 2021, 9, e003554. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Wan, J.; Zhang, H.; Wu, R.; Wang, J.; Wang, Y.; Xu, Y.; Cai, S.; Zhang, Z.; et al. Short-course radiotherapy combined with CAPOX and Toripalimab for the total neoadjuvant therapy of locally advanced rectal cancer: A randomized, prospective, multicentre, double-arm, phase II trial (TORCH). BMC Cancer 2022, 22, 274. [Google Scholar] [CrossRef]
- Dayde, D.; Tanaka, I.; Jain, R.; Tai, M.C.; Taguchi, A. Predictive and Prognostic Molecular Biomarkers for Response to Neoadjuvant Chemoradiation in Rectal Cancer. Int. J. Mol. Sci. 2017, 18, 573. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, Q.; Venkatachalam, N.; Hofheinz, R.D.; Veldwijk, M.R.; Herskind, C.; Ebert, M.P.; Zhan, T. Predicting response to neoadjuvant chemoradiotherapy in rectal cancer: From biomarkers to tumor models. Ther. Adv. Med. Oncol. 2022, 14, 17588359221077972. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Morris, C.R.; Bates, J.H.; German, R.R. Effect of misclassified underlying cause of death on survival estimates of colon and rectal cancer. J. Natl. Cancer Inst. 2011, 103, 1130–1133. [Google Scholar] [CrossRef]
- Cattaneo, C.M.; Dijkstra, K.K.; Fanchi, L.F.; Kelderman, S.; Kaing, S.; van Rooij, N.; van den Brink, S.; Schumacher, T.N.; Voest, E.E. Tumor organoid-T-cell coculture systems. Nat. Protoc. 2020, 15, 15–39. [Google Scholar] [CrossRef]
- Sun, C.P.; Lan, H.R.; Fang, X.L.; Yang, X.Y.; Jin, K.T. Organoid Models for Precision Cancer Immunotherapy. Front. Immunol. 2022, 13, 770465. [Google Scholar] [CrossRef]
- Meraz, I.M.; Majidi, M.; Meng, F.; Shao, R.; Ha, M.J.; Neri, S.; Fang, B.; Lin, S.H.; Tinkey, P.T.; Shpall, E.J.; et al. An Improved Patient-Derived Xenograft Humanized Mouse Model for Evaluation of Lung Cancer Immune Responses. Cancer Immunol. Res. 2019, 7, 1267–1279. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, L.; Wan, J.; Zhang, H.; Wu, R.; Wang, J.; Wang, Y.; Xu, Y.; Cai, S.; Zhang, Z.; et al. Neoadjuvant chemoradiotherapy combined with immunotherapy for locally advanced rectal cancer: A new era for anal preservation. Front. Immunol. 2022, 13, 1067036. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartorius, D.; Blume, M.L.; Fleischer, J.R.; Ghadimi, M.; Conradi, L.-C.; De Oliveira, T. Implications of Rectal Cancer Radiotherapy on the Immune Microenvironment: Allies and Foes to Therapy Resistance and Patients’ Outcome. Cancers 2023, 15, 5124. https://doi.org/10.3390/cancers15215124
Sartorius D, Blume ML, Fleischer JR, Ghadimi M, Conradi L-C, De Oliveira T. Implications of Rectal Cancer Radiotherapy on the Immune Microenvironment: Allies and Foes to Therapy Resistance and Patients’ Outcome. Cancers. 2023; 15(21):5124. https://doi.org/10.3390/cancers15215124
Chicago/Turabian StyleSartorius, Dorothée, Moritz Leander Blume, Johannes Robert Fleischer, Michael Ghadimi, Lena-Christin Conradi, and Tiago De Oliveira. 2023. "Implications of Rectal Cancer Radiotherapy on the Immune Microenvironment: Allies and Foes to Therapy Resistance and Patients’ Outcome" Cancers 15, no. 21: 5124. https://doi.org/10.3390/cancers15215124
APA StyleSartorius, D., Blume, M. L., Fleischer, J. R., Ghadimi, M., Conradi, L.-C., & De Oliveira, T. (2023). Implications of Rectal Cancer Radiotherapy on the Immune Microenvironment: Allies and Foes to Therapy Resistance and Patients’ Outcome. Cancers, 15(21), 5124. https://doi.org/10.3390/cancers15215124







