The Association between Proton Pump Inhibitors and the Effectiveness of CDK Inhibitors in HR+/HER- Advanced Breast Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
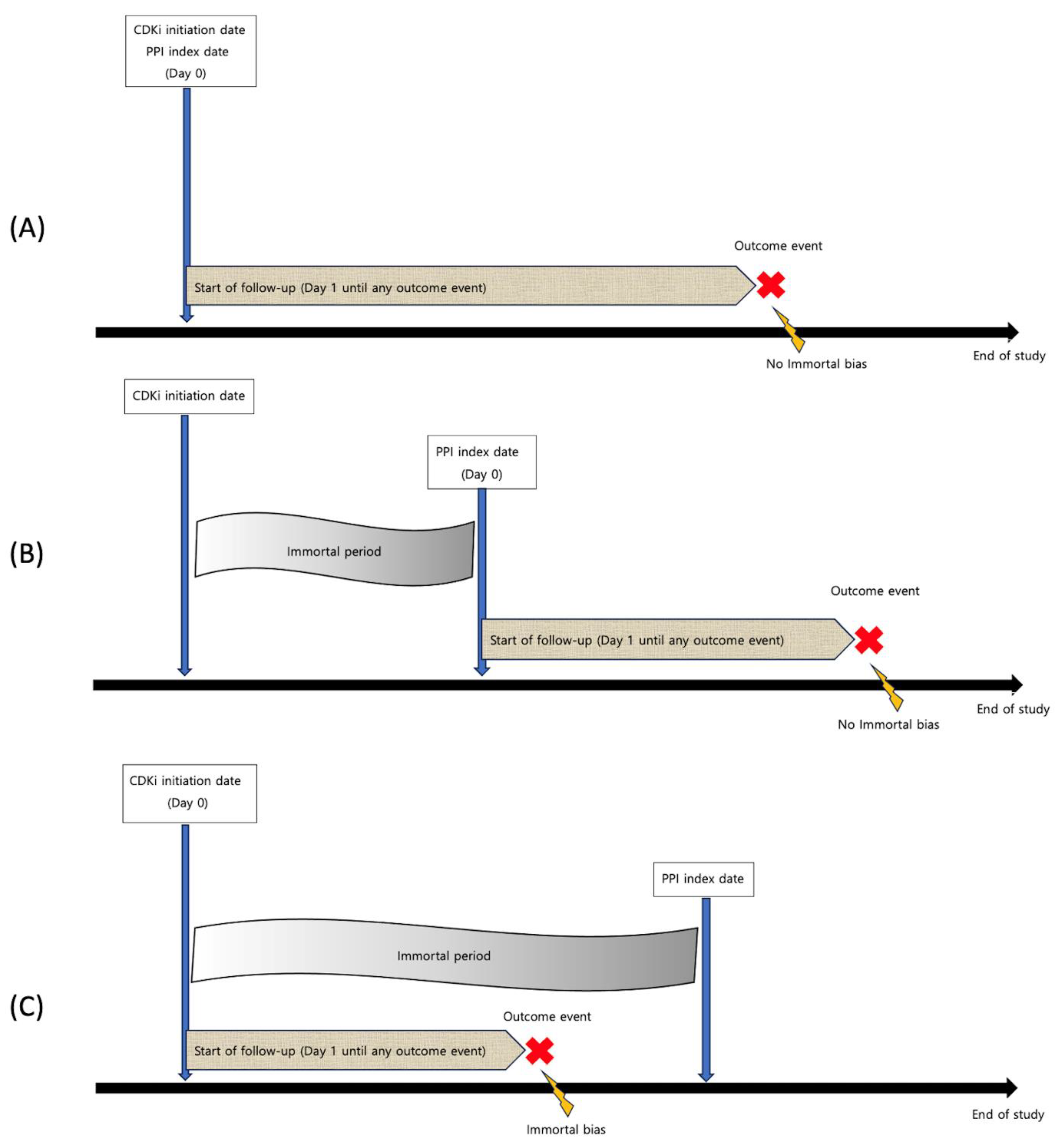
2. Materials and Methods
2.1. Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Main Outcomes and Statistical Analyses
3. Results
3.1. Study Characteristics (Table 1)
| Included Studies | Nation | Study Type | Chemotherapeutic Regimen | Sample Size, n | First Line, n (%) | PPI, n (%) | PPI Use Window | Outcome Follow-Up | Age, Mean or Median (SD or IQR) | ECOG |
|---|---|---|---|---|---|---|---|---|---|---|
| Palbociclib | ||||||||||
| Cosimo 2023 [30] | Italy | Post hoc of RCT | Palbociclib + Fulvestrant/letrozole | 416 | 416 (100) | 91 (21.9) | PPI use started together with CDKI initiation; >2/3 of the treatment | From CDKI index date | 63 (25–90) | 0–2 |
| Çağlayan 2023 [17] | Turkey | Retrospective | Palbociclib + Fulvestrant/Ai | 36 | 0 (0) | 16 (44.4) | N/A | From CDKI index date | PPI+: 55 (11.8) PPI−: 56 (13.9) | N/A |
| Lee 2023 [29] | Korea | Retrospective | Palbociclib + Fulvestrant/letrozole | 1310 | 1310 (100) | 344 (26.2) | >1/3 of the treatment | From PPI index date | N/A | N/A |
| Schieber 2023 [19] | USA | Retrospective | Palbociclib + Fulvestrant/letrozole/Tamoxifen | 82 | 82 (100) | 32 (39) | PPI use started together with CDKI initiation; >1/2 of the treatment | From CDKI index date | PPI+: 62.5 (53–68) PPI−: 68.5 (54–74) | 0–2 |
| Odabas 2023 [18] | Turkey | Retrospective | Palbociclib + Fulvestrant/letrozole | 120 | 70 (58.3) | 57 (47.5) | >2/3 of the treatment | From CDKI index date | PPI+: 60 (33–92) PPI−: 54 (25–86) | 0–3 |
| Eser 2022 [20] | Turkey | Retrospective | Palbociclib + Fulvestrant/letrozole | 105 | N/A | 65 (61.9) | >1/2 of the treatment | From CDKI index date | PPI+: 61 (32–83) PPI−: 58 (36–76) | 0–2 |
| Del Re 2021 [16] | Italy | Retrospective | Palbociclib + Fulvestrant/letrozole | 112 | 112 (100) | 56 (50) | PPI use started before CDKI initiation; >2/3 of the treatment | From CDKI index date | PPI+: 63 PPI−: 61.5 | 0–2 |
| Ribociclib | ||||||||||
| Çağlayan 2023 [17] | Turkey | Retrospective | Ribociclib + Fulvestrant/Ai | 50 | 0 (0) | 29 (58.0) | N/A | From CDKI index date | PPI+: 55 (11.8) PPI−: 56 (13.9) | N/A |
| Odabas 2023 [18] | Turkey | Retrospective | Ribociclib + Fulvestrant/letrozole | 113 | 52 (52.0) | 29 (25.7) | >2/3 of the treatment | From CDKI index date | PPI+: 60 (34–84) PPI−: 53 (31–80) | 0–3 |
| Del Re 2022 [28] | Italy | Retrospective | Ribociclib + Fulvestrant/letrozole | 128 | 128 (100) | 50 (39) | PPI use started before CDKI initiation; >2/3 of the treatment | From CDKI index date | PPI+: 64 PPI−: 58 | 0–2 |
| Eser 2022 [20] | Turkey | Retrospective | Ribociclib + Fulvestrant/letrozole | 112 | N/A | 61 (54.4) | >1/2 of the treatment | From CDKI index date | PPI+: 57 (38–87) PPI−: 49 (32–87) | 0–2 |
3.2. Risk of Bias Assessment (Figure S1 in Supplementary Materials)
3.3. Survival Association between PPIs and Palbociclib
3.4. Survival Association between PPIs and Ribociclib
3.5. CDKI Dose Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strand, D.S.; Kim, D.; Peura, D.A. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; McKinnon, R.A.; Abuhelwa, A.Y.; Logan, J.M.; Badaoui, S.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer 2022, 126, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.J.; Ettinger, D.S.; Aston, J.; Barbour, S.; Bergsbaken, J.; Bierman, P.J.; Brandt, D.; Dolan, D.E.; Ellis, G.; Kim, E.J.; et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Haastrup, P.F.; Thompson, W.; Søndergaard, J.; Jarbøl, D.E. Side Effects of Long-Term Proton Pump Inhibitor Use: A Review. Basic Clin. Pharmacol. Toxicol. 2018, 123, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Hilton, J.F.; Tu, D.; Seymour, L.; Shepherd, F.A.; Bradbury, P.A. An evaluation of the possible interaction of gastric acid suppressing medication and the EGFR tyrosine kinase inhibitor erlotinib. Lung Cancer 2013, 82, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, K.; Dickinson, P.A.; Bui, K.; Cassier, P.A.; Greystoke, A.; Lisbon, E.; Moreno, V.; So, K.; Thomas, K.; Weilert, D.; et al. The Effect of Food or Omeprazole on the Pharmacokinetics of Osimertinib in Patients with Non-Small-Cell Lung Cancer and in Healthy Volunteers. J. Clin. Pharmacol. 2018, 58, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Mir, O.; Touati, N.; Lia, M.; Litière, S.; Le Cesne, A.; Sleijfer, S.; Blay, J.Y.; Leahy, M.; Young, R.; Mathijssen, R.H.J.; et al. Impact of Concomitant Administration of Gastric Acid-Suppressive Agents and Pazopanib on Outcomes in Soft-Tissue Sarcoma Patients Treated within the EORTC 62043/62072 Trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1479–1485. [Google Scholar] [CrossRef]
- Chang, Y.; Lin, W.Y.; Chang, Y.C.; Huang, C.H.; Tzeng, H.E.; Abdul-Lattif, E.; Wang, T.H.; Tseng, T.H.; Kang, Y.N.; Chi, K.Y. The Association between Baseline Proton Pump Inhibitors, Immune Checkpoint Inhibitors, and Chemotherapy: A Systematic Review with Network Meta-Analysis. Cancers 2022, 15, 284. [Google Scholar] [CrossRef]
- Lin, W.Y.; Wang, S.S.; Kang, Y.N.; Porpiglia, A.S.; Chang, Y.; Huang, C.H.; Bhimani, R.; Abdul-Lattif, E.; Azmat, M.; Wang, T.H.; et al. Do proton pump inhibitors affect the effectiveness of chemotherapy in colorectal cancer patients? A systematic review with meta-analysis. Front. Pharmacol. 2022, 13, 1048980. [Google Scholar] [CrossRef]
- Corley, D.A. Safety and Complications of Long-Term Proton Pump Inhibitor Therapy: Getting Closer to the Truth. Gastroenterology 2019, 157, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Klamerus, K.J.; Yuhas, L.M.; Pawlak, S.; Plotka, A.; O’Gorman, M.; Kirkovsky, L.; Kosa, M.; Wang, D. Impact of Acid-Reducing Agents on the Pharmacokinetics of Palbociclib, a Weak Base With pH-Dependent Solubility, With Different Food Intake Conditions. Clin. Pharmacol. Drug Dev. 2017, 6, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Samant, T.S.; Dhuria, S.; Lu, Y.; Laisney, M.; Yang, S.; Grandeury, A.; Mueller-Zsigmondy, M.; Umehara, K.; Huth, F.; Miller, M.; et al. Ribociclib Bioavailability Is Not Affected by Gastric pH Changes or Food Intake: In Silico and Clinical Evaluations. Clin. Pharmacol. Ther. 2018, 104, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Omarini, C.; Diodati, L.; Palleschi, M.; Meattini, I.; Crucitta, S.; Lorenzini, G.; Isca, C.; Fontana, A.; Livi, L.; et al. Drug-drug interactions between palbociclib and proton pump inhibitors may significantly affect clinical outcome of metastatic breast cancer patients. ESMO Open 2021, 6, 100231. [Google Scholar] [CrossRef] [PubMed]
- Çağlayan, D.; Koçak, M.Z.; Geredeli, Ç.; Tatlı, A.M.; Göksu, S.S.; Eryılmaz, M.K.; Araz, M.; Artaç, M. The effect of concomitant use of proton pump inhibitors with CDK 4/6 inhibitors on survival in metastatic breast cancer. Eur. J. Clin. Pharmacol. 2023, 79, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Odabas, H.; Dogan, A.; Ozcelik, M.; Yildirim, S.; Ozkerim, U.; Turan, N.; Yildirim, M.E.; Gumus, M. Does Proton Pump Inhibitors Decrease the Efficacy of Palbociclib and Ribociclib in Patients with Metastatic Breast Cancer? Medicina 2023, 59, 557. [Google Scholar] [CrossRef] [PubMed]
- Schieber, T.; Steele, S.; Collins, S.; Berger, M.; Fleming, M.; McLaughlin, E.; Sudheendra, P.; Vargo, C. Effect of Concurrent Proton Pump Inhibitors with Palbociclib Tablets for Metastatic Breast Cancer. Clin. Breast Cancer 2023, 23, 658–663. [Google Scholar] [CrossRef]
- Eser, K.; Önder, A.H.; Sezer, E.; Çil, T.; İnal, A.; Öztürk, B.; Erçolak, V.; Duman, B.B.; Çelik, H.; Köşeci, T.; et al. Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study. BMC Cancer 2022, 22, 516. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Harville, D.A. Maximum Likelihood Approaches to Variance Component Estimation and to Related Problems. J. Am. Stat. Assoc. 1977, 72, 320–338. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Crucitta, S.; Omarini, C.; Bargagna, I.; Mongillo, M.; Palleschi, M.; Stucci, S.; Meattini, I.; D’Onofrio, R.; Lorenzini, G.; et al. Concomitant administration of proton pump inhibitors does not significantly affect clinical outcomes in metastatic breast cancer patients treated with ribociclib. Breast 2022, 66, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kwon, S.H.; Kwon, S.; Jung, H.I.; Nam, J.H.; Lee, E.K. Concomitant Use of Proton Pump Inhibitors and Palbociclib Among Patients with Breast Cancer. JAMA Netw. Open 2023, 6, e2324852. [Google Scholar] [CrossRef] [PubMed]
- Cosimo, S.D.; Pérez-García, J.M.; Ezquerra, M.B.; Dalenc, F.; Gil, M.G.; Borrego, M.R.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Abstract PD13-10: PD13-10 Impact of Proton Pump Inhibitors (PPI) on Palbociclib (PAL) Outcomes in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer (HR+/HER2- ABC): Exploratory Analysis of the PARSIFAL Trial. Cancer Res. 2023, 83, PD13-10. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil-Gil, M.; Ruíz-Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Fulvestrant-Palbociclib vs Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor–Positive, ERBB2-Negative Advanced Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Smelick, G.S.; Heffron, T.P.; Chu, L.; Dean, B.; West, D.A.; Duvall, S.L.; Lum, B.L.; Budha, N.; Holden, S.N.; Benet, L.Z.; et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol. Pharm. 2013, 10, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.J.; Peters, M.; Weber, B.L.; Davis, C.B. Optimizing the Therapeutic Window of Targeted Drugs in Oncology: Potency-Guided First-in-Human Studies. Clin. Transl. Sci. 2021, 14, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Pauli-Magnus, C.; Rekersbrink, S.; Klotz, U.; Fromm, M.F. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Harish Patil, P.; Vullendula, S.K.A.; Birangal, S.; Shenoy, G.G.; Rao, M.; Jayant Dengale, S.; Bhat, K.; Puralae Channabasavaiah, J. Molecular Insights into the Mechanism of Modulatory Effects of Proton Pump Inhibitors on P-glycoprotein Mediated Drug Transport of Palbociclib and Ribociclib. Curr. Drug Metab. 2023, 24, 458–465. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-C.; Song, J.; Chang, Y.; Huang, C.-H.; Sudan, A.; Chen, P.-C.; Chi, K.-Y. The Association between Proton Pump Inhibitors and the Effectiveness of CDK Inhibitors in HR+/HER- Advanced Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 5133. https://doi.org/10.3390/cancers15215133
Chang Y-C, Song J, Chang Y, Huang C-H, Sudan A, Chen P-C, Chi K-Y. The Association between Proton Pump Inhibitors and the Effectiveness of CDK Inhibitors in HR+/HER- Advanced Breast Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(21):5133. https://doi.org/10.3390/cancers15215133
Chicago/Turabian StyleChang, Yu-Cheng, Junmin Song, Yu Chang, Chin-Hsuan Huang, Aarushi Sudan, Pei-Chin Chen, and Kuan-Yu Chi. 2023. "The Association between Proton Pump Inhibitors and the Effectiveness of CDK Inhibitors in HR+/HER- Advanced Breast Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 15, no. 21: 5133. https://doi.org/10.3390/cancers15215133






