Acceptability to Healthcare Professionals of Home-Based HPV Self-Sampling for Cervical Screening: A French Qualitative Study Conducted in an Area with Low Access to Health Services
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Semi-Structured Interviews
2.2. Population
2.3. Process and Analyses
3. Results
3.1. Participants
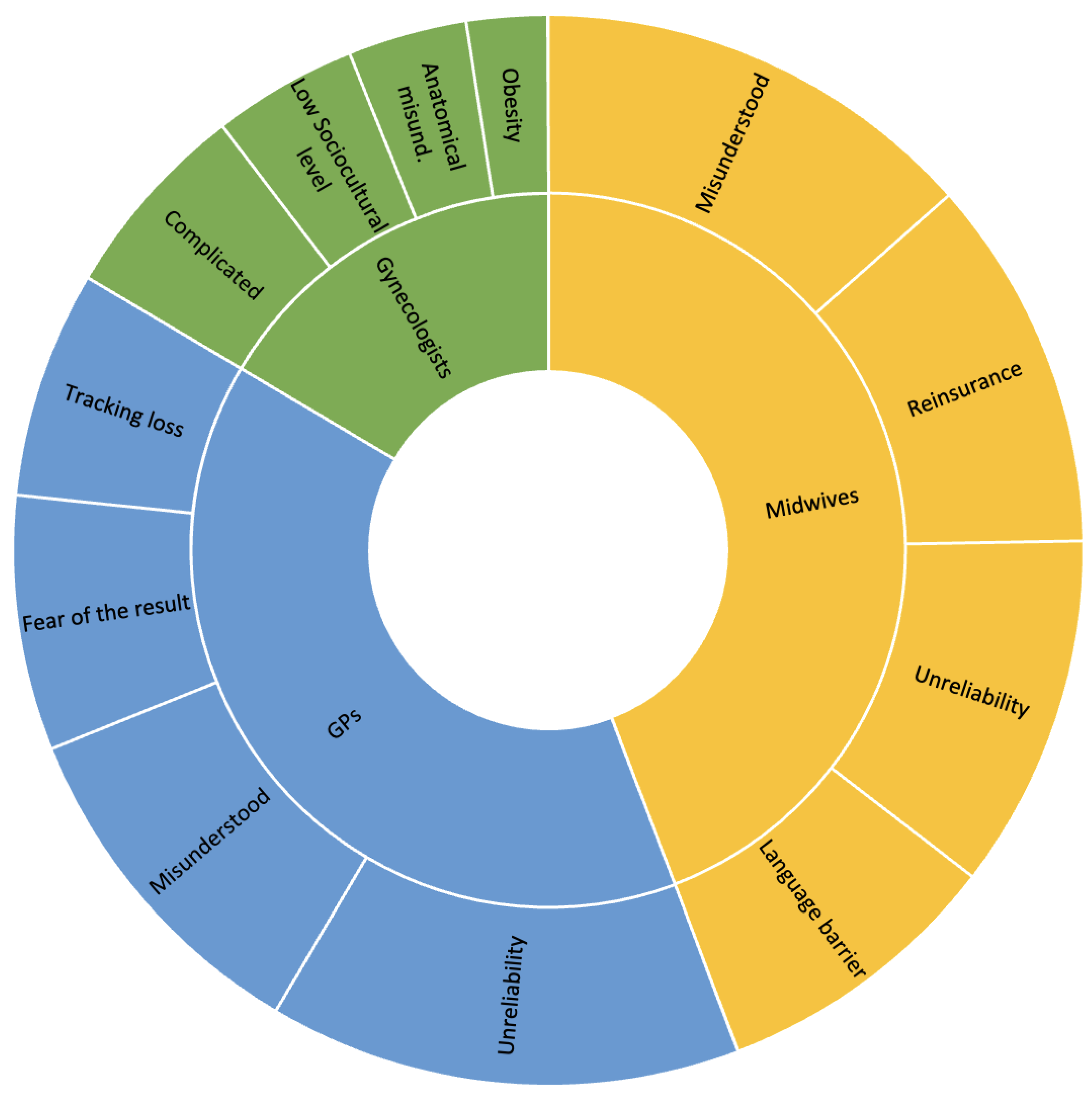
3.2. Barriers to Cervical Screening
3.2.1. Gynecologists
3.2.2. General Practitioners
3.2.3. Midwives
3.3. Barriers to Self-Sampling According to Healthcare Professionals
3.3.1. Gynecologists
3.3.2. General Practitioners
3.3.3. Midwives
3.4. Benefits of Self-Sampling
Specificities Regarding Urine and Vaginal Self-Samplings
4. Discussion
- –
- Continue sending out invitation letters as part of the routine screening program;
- –
- Provide information for the public: television campaigns, articles in women’s magazines;
- –
- Pursue face-to-face meetings with women (nursing homes, depist-bus mobile screening unit);
- –
- Make the kits free of charge;
- –
- Continue to inform and train healthcare professionals in the use of these kits;
- –
- Raise GP awareness of cervical screening;
- –
- Include easily understandable user instructions in the sent self-sampling kits;
- –
- Offer USS to specific populations;
- –
- Reassure women about their ability to carry out self-sampling;
- –
- Involve healthcare professionals when conceiving strategies including self-sampling: raising awareness, informing women (the accompanying leaflet will sometimes have to be explained by a healthcare professional or translated);
- –
- Reassure women about the availability of a professional if necessary;
- –
- Improve links to healthcare professionals (who should be consulted when the HPV test result is positive);
- –
- Make it clear that self-sampling does not exempt women from consulting a health professional to have an annual gynecological examination (breast examination, cervix observation, contraception, etc.)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Semi-Structured Interview Guide for Gynecologists
- (1)
- General data
- -
- Gender
- -
- Age
- -
- Years of practice
- -
- Urban/Rural
- -
- Any patients diagnosed with cervical cancer over the past 2 years? If yes, how many?
- (2)
- Screening practice
- -
- Do you ask all your patients about the Pap smear test?
- -
- Do you have enough time to explain the importance of screening?
- -
- What do you think about the shift from a 3-year to a 5-year interval for the screening of women over 30 years? Do you have any fears?
- -
- If a patient is reluctant to be screened, what do you suggest?
- -
- Do you do a lot of tracing or just focus on abnormal follow-ups?
- -
- As part of self-sampling: If the HPV test is positive, do you take an additional sample? If positive, do you perform a colposcopy?
- -
- Would you go into nursing homes to reach more of the population?
- -
- How do you see the future of screening?
- (3)
- Factors influencing screening
- -
- In your opinion, does immigrant status make a difference?
- -
- A study conducted in Nantes (France) showed that religion could be a factor for misunderstandings regarding screening: What do you think?
- -
- Are certain characteristics (e.g., obesity) barriers to screening?
- -
- Which material conditions do you need for the smear test to go well?
- -
- Which women do you have difficulty with?
- -
- Are your female patients with chronic long-term illness up to date with their screening?
- -
- Do you think you are an obstacle to the screening process?
- -
- Are there women you don’t ask about screening?
- -
- In your opinion, what can be done to promote a) screening and b) self-sampling?
- -
- Do you have any ideas about how to increase screening among women who do not come to consult?
- (4)
- Gynecologists on screening
- -
- What do you think about screening and self-sampling?
- -
- Do you think screening through self-sampling is different from other types of screening?
- -
- Do you differentiate between vaginal and urinary self-sampling?
- -
- Do you think vaginal self-sampling and urinary self-sampling are different?
- -
- In your opinion, are there specific benefits linked to self-sampling?
- (5)
- Various issues
- -
- Do any of your patients refuse screening?
- -
- Do you see any benefits in handing out self-sampling kits? If yes, which ones?
- -
- Does self-sampling seem relevant to you? If yes, why?
- -
- Regarding the delivery of self-sampling kits, would it be best to (1) deliver them yourself (2) ask for them to be sent to the regional cancer screening coordination center (3) make them available for pick-up at a pharmacy or 4) at a medical laboratory?
- -
- Do women who have received a self-sampling kit come to see you? If so, do they come because they don’t understand the kit or do they ask you to do it for them?
- -
- Regarding the kits: what do women tell you about their use? Do they have any reservations? What is their experience?
- -
- Do you trust self-sampling? What do you think? Are you aware that you will have to perform a Pap test if the HPV test comes back positive?
- -
- Would you be confident if the self-sampling recommendations only concerned vaginal self-sampling and not urinary self-sampling?
- -
- Do you think that self-sampling is easier? In your opinion, is a medical examination of the cervix mandatory?
- -
- In your opinion, does self-sampling have a future? If yes, which one?
Appendix B. Semi-Structured Interview Guide for GPs
- (1)
- General data
- -
- Gender
- -
- Age
- -
- Years of practice
- -
- Urban/Rural
- -
- Any patients diagnosed with cervical cancer over the past 2 years? If yes, how many?
- (2)
- Screening practice
- -
- Do you ask all your patients about the Pap smear test?
- -
- Do you have enough time to explain the importance of screening?
- -
- Do you think that GPs are sufficiently aware of the importance of screening?
- -
- Are you interested in gynecology? If not, who do you refer your patients to?
- (3)
- Factors influencing the performance of screening
- -
- Shyness and therapeutic alliance: Do you sometimes feel embarrassed, leading you to refer your patients to another health professional, for fear of breaking the therapeutic alliance?
- -
- In your opinion, does immigrant status make a difference?
- -
- A study conducted in Nantes (France) showed that religion could be a factor for misunderstandings regarding screening: What do you think?
- -
- Are certain characteristics (e.g., obesity) barriers to screening?
- -
- Which material conditions do you need for the smear test to go well?
- -
- Which women do you have difficulty with?
- -
- Are your female patients with chronic long-term illness up to date with their screening?
- -
- Do you think you are an obstacle to the screening process?
- -
- Are there women you don’t ask about screening?
- -
- In your opinion, what can be done to promote (a) screening and (b) self-sampling?
- -
- Do you have any ideas about how to increase screening among women who do not come to consult?
- (4)
- GPs on screening
- -
- What do you think of screening and self-sampling?
- -
- Do you think self-sampling is different from other types of screening?
- -
- Do you think vaginal self-sampling and urinary self-sampling are different?
- -
- In your opinion, are there specific benefits linked to self-sampling?
- -
- Self-sampling has been recommended since 2019. Since 2020, vaginal self-sampling is reimbursed by the social security system, together with the reminder letter. Do you see any value in these recommendations?
- (5)
- Various issues
- -
- Do any of your patients refuse any sampling?
- -
- Do you see any benefits in handing out self-sampling kits? If so, which ones?
- -
- Does handing out self-sampling kits seem relevant to you? If so, why?
- -
- Regarding the delivery of self-sampling kits, would it be best to (1) deliver them yourself (2) ask for them to be sent to the regional cancer screening coordination center (3) make them available for pick-up at a pharmacy or (4) at a medical laboratory?
- -
- Do women who have received a self-sampling kit come to see you? If so, do they come because they don’t understand the kit or because they want you to do it for them?
- -
- Regarding the kits, what do women tell you about their use? Do they have any reservations? What is their experience?
- -
- Do you trust self-sampling? What do you think? Are you aware that you will have to perform a Pap test if the HPV test comes back positive?
- -
- Would you be confident if the self-sampling recommendations only concerned vaginal self-sampling and not urinary self-sampling?
- -
- Do you think self-sampling is easier? In your opinion, is a medical examination of the cervix mandatory?
- -
- In your opinion, does self-sampling have a future? If so, which one?
Appendix C. Semi-Structured Interview Guide for Midwives
- (1)
- General data
- -
- Gender
- -
- Age
- -
- Years of practice
- -
- Urban/Rural
- -
- Any patients diagnosed with cervical cancer over the past 2 years? If so, how many?
- (2)
- Screening practice
- -
- Do you ask all your patients about the Pap smear test?
- -
- Do you have enough time to explain the importance of screening?
- -
- Do you think that midwives are sufficiently aware of screening?
- -
- Do you feel comfortable with screening?
- -
- Have you been trained in screening?
- -
- Midwives can perform colposcopies. What do you think?
- -
- How do you see the future of screening?
- (3)
- Factors influencing the performance of screening
- -
- Shyness and the therapeutic alliance: Do you sometimes feel embarrassed, leading you to refer your patients to another healthcare professional, for fear of breaking the therapeutic alliance?
- -
- In your opinion, does immigrant status make a difference?
- -
- A study conducted in Nantes (France) showed that religion could be a factor for misunderstandings regarding screening: What do you think?
- -
- Are certain characteristics (e.g., obesity) barriers to screening?
- -
- Which material conditions do you need for the smear test to go well?
- -
- Which women do you have difficulty with?
- -
- Are your female patients with chronic long-term illness up to date with their screening?
- -
- Do you think you are an obstacle to the screening process?
- -
- Are there women you don’t ask about screening?
- -
- In your opinion, what can be done to promote (a) screening and (b) self-sampling?
- -
- Do you have any ideas about how to increase screening among women who do not come to consult?
- (4)
- Midwives on screening
- -
- What do you think about screening and self-sampling?
- -
- Do you think that self-sampling is different from other types of screening?
- -
- Do you think vaginal self-sampling and urinary self-sampling are different?
- -
- In your opinion, are there specific benefits linked to self-sampling?
- -
- Self-sampling has been recommended since 2019. Since 2020, vaginal self-sampling is reimbursed by the social security system, together with the reminder letter. Do you see any value in these recommendations?
- (5)
- Various issues
- -
- Do any of your patients refuse sampling?
- -
- Do you see any benefits in providing self-sampling kits? If so, which ones?
- -
- Does handing out self-sampling kits seem relevant to you? If so, why?
- -
- Regarding the delivery of self-sampling kits, would it be best to (1) deliver them yourself (2) ask for them to be sent to the regional cancer screening coordination center (3) make them available for pick-up at a pharmacy (4) or at a medical laboratory?
- -
- Do women who have received a self-sampling kit come and see you? If so, do they come because they don’t understand the kit or because they want you to do it for them?
- -
- Regarding the kits, what do women tell you about their use? Do they have any reservations? What is their experience?
- -
- Do you trust self-sampling? What do you think? Are you aware that you will have to perform a Pap test if the HPV test comes back positive?
- -
- Would you be confident if the self-sampling recommendations only concerned vaginal self-sampling and not urinary self-sampling?
- -
- Do you find self-sampling easier? In your opinion, is a medical examination of the cervix mandatory?
- -
- In your opinion, does self-sampling have a future? If so, which one?
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30, F88–F99. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef]
- Caleia, A.I.; Pires, C.; Pereira, J.F.; Pinto-Ribeiro, F.; Longatto-Filho, A. Self-Sampling as a Plausible Alternative to Screen Cervical Cancer Precursor Lesions in a Population with Low Adherence to Screening: A Systematic Review. Acta Cytol. 2020, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef]
- World Health Organization. Guideline on Self-Care Interventions for Health and Well-Being. 2022. Available online: https://www.who.int/publications/i/item/9789240052192 (accessed on 13 June 2023).
- Costa, S.; Verberckmoes, B.; Castle, P.E.; Arbyn, M. Offering HPV self-sampling kits: An updated meta-analysis of the effectiveness of strategies to increase participation in cervical cancer screening. Br. J. Cancer 2023, 128, 805–813. [Google Scholar] [CrossRef]
- Hamers, F.F.; Poullié, A.I.; Arbyn, M. Updated evidence-based recommendations for cervical cancer screening in France. Eur. J. Cancer Prev. 2022, 31, 279–286. [Google Scholar] [CrossRef]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K. Accuracy of Urinary Human Papillomavirus Testing for Presence of Cervical HPV: Systematic Review and Meta-Analysis. BMJ 2014, 349, g5264. [Google Scholar] [CrossRef]
- John, J.H.; Halder, A.; Purwar, S.; Pushpalatha, K.; Gupta, P.; Dubey, P. Study to determine efficacy of urinary HPV 16 & HPV 18 detection in predicting premalignant and malignant lesions of uterine cervix. Int. J. Gynecol. Obstet. 2023, 161, 79–85. [Google Scholar] [CrossRef]
- Arbyn, M.; Peeters, E.; Benoy, I.; Broeck, D.V.; Bogers, J.; De Sutter, P.; Donders, G.; Tjalma, W.; Weyers, S.; Cuschieri, K.; et al. VALHUDES: A protocol for Validation of Human papillomavirus assays and collection Devices for HPV testing on Self-samples and urine samples. J. Clin. Virol. 2018, 117, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, S.; Latsuzbaia, A.; Broeck, D.V.; De Sutter, P.; Donders, G.; Doyen, J.; Tjalma, W.A.A.; Weyers, S.; Arbyn, M.; Vorsters, A. Analytical and clinical performance of extended HPV genotyping with BD Onclarity HPV Assay in home-collected first-void urine: A diagnostic test accuracy study. J. Clin. Virol. 2022, 155, 105271. [Google Scholar] [CrossRef] [PubMed]
- Latsuzbaia, A.; Van Keer, S.; Vanden Broeck, D.; Weyers, S.; Donders, G.; De Sutter, P.; Tjalma, W.; Doyen, J.; Vorsters, A.; Arbyn, M. Comparison of the Accuracy of Alinity m HR HPV Assay on Self-Versus Clinician-Taken Samples Using the VALHUDES Protocol. J. Mol. Diagn. 2023; in press. [Google Scholar]
- Lefeuvre, C.; De Pauw, H.; Le Duc Banaszuk, A.S.; Pivert, A.; Ducancelle, A.; Rexand-Galais, F.; Arbyn, M. Study Protocol: Randomised Controlled Trial Assessing the Efficacy of Strategies Involving Self-Sampling in Cervical Cancer Screening. Int. J. Public Health 2022, 67, 1604284. [Google Scholar] [CrossRef] [PubMed]
- Lefeuvre, C.; Pivert, A.; Le Guillou-Guillemette, H.; Lunel-Fabiani, F.; Veillon, P.; Le Duc-Banaszuk, A.-S.; Ducancelle, A. Urinary HPV DNA Testing as a Tool for Cervical Cancer Screening in Women Who Are Reluctant to Have a Pap Smear in France. J. Infect. 2020, 81, 248–254. [Google Scholar] [CrossRef]
- Santé Publique France. Couverture du Dépistage du Cancer du col de L’utérus en France. 2019. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-col-de-l-uterus/documents/couverture-du-depistage-du-cancer-du-col-de-l-uterus-en-france (accessed on 25 September 2023).
- Observatoire Géode. Santé Publique France. 2018–2020. Available online: https://geodes.santepubliquefrance.fr/#bbox=534584,6249210,886419,473249&c=indicator&i=depistage_ccu.couverture_stand&s=2018-2020&t=a01&view=map2 (accessed on 25 September 2023).
- Institut National du Cancer. Le Programme de Dépistage Organisé du Cancer du col de L’utérus—Dépistage du Cancer du col de L’utérus. 2022. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Depistage-et-detection-precoce/Depistage-du-cancer-du-col-de-l-uterus/Le-programme-de-depistage-organise (accessed on 25 September 2023).
- Agence Régionale de Santé Pays de La Loire. Zonage Médecin Pour Les Pays de la Loire. 2018. Available online: https://www.pays-de-la-loire.ars.sante.fr/system/files/2018-01/carte-zonage-pays-de-la-loire-2018-medecin-departement.pdf (accessed on 25 September 2023).
- Mao, C.; Kulasingam, S.L.; Whitham, H.K.; Hawes, S.E.; Lin, J.; Kiviat, N.B. Clinician and Patient Acceptability of Self-Collected Human Papillomavirus Testing for Cervical Cancer Screening. J. Women’s Health 2017, 26, 609–615. [Google Scholar] [CrossRef]
- Katz, M.L.; Zimmermann, B.J.; Moore, D.; Paskett, E.D.; Reiter, P.L. Perspectives from health-care providers and women about completing human papillomavirus (HPV) self-testing at home. Women Health 2017, 57, 1161–1177. [Google Scholar] [CrossRef]
- Zelli, J.; Hum, S.; Lofters, A.; Dunn, S. Clinician acceptability of self-collected human papillomavirus swabs as a primary cervical cancer screening method. Can. Fam. Physician 2022, 68, e31–e38. [Google Scholar] [CrossRef]
- Pourette, D.; Cripps, A.; Guerrien, M.; Desprès, C.; Opigez, E.; Bardou, M.; Dumont, A. Assessing the Acceptability of Home-Based HPV Self-Sampling: A Qualitative Study on Cervical Cancer Screening Conducted in Reunion Island Prior to the RESISTE Trial. Cancers 2022, 14, 1380. [Google Scholar] [CrossRef]
- Vasileiou, K.; Barnett, J.; Thorpe, S.; Young, T. Characterising and justifying sample size sufficiency in interview-based studies: Systematic analysis of qualitative health research over a 15-year period. BMC Med. Res. Methodol. 2018, 18, 148. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.C.; Hébert, M.; Gallagher, F.; Tribble, D.S.C. La théorisation ancrée. Méthodes qualitatives, quantitatives et mixtes. In La Recherche en Sciences Humaines, Sociales et de la Santé, 2nd ed.; Corbière, M., Larivière, N., Eds.; Presses de l’Université du Québec: Québec City, QC, Canada, 2020; pp. 97–122. [Google Scholar]
- Paillé, P.; Mucchielli, A. L’analyse thématique. In L’Analyse Qualitative en Sciences Humaines et Sociales; Paillé, P., Mucchielli, A., Eds.; Armand Colin: Paris, France, 2016; pp. 235–312. [Google Scholar]
- Strauss, A.; Corbin, J. Basics of Qualitative Research Techniques; Sage Publications: Thousand Oaks, CA, USA, 1998. [Google Scholar]
- Institut National du Cancer. Les Freins au Dépistage: Sensibiliser et Convaincre. 2022. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Depistage-et-detection-precoce/Depistage-du-cancer-du-col-de-l-uterus/Les-freins-au-depistage-sensibiliser-et-convaincre (accessed on 25 September 2023).
- Lin, T.F.; Chen, J. Effect of physician gender on demand for Pap tests. Econ. Res. Int. 2014, 2014, 647169. [Google Scholar] [CrossRef]
- Chandrakumar, A.; Hoon, E.; Benson, J.; Stocks, N. Barriers and facilitators to cervical cancer screening for women from culturally and linguistically diverse backgrounds; a qualitative study of GPs. BMJ Open 2022, 12, e062823. [Google Scholar] [CrossRef] [PubMed]
- Urbute, A.; Kjaer, S.K.; Kesmodel, U.S.; Frederiksen, K.; Thomsen, L.T. Women with obesity participate less in cervical cancer screening and are more likely to have unsatisfactory smears: Results from a nationwide Danish cohort study. Prev. Med. 2022, 159, 107072. [Google Scholar] [CrossRef]
- Gressier, M.; Leroux, S.; Vincent, Y. Déterminants de la Participation aux Dépistages Organisés des Cancers sur l’Ile de Noirmoutier: Une Etude par Focus Groups Auprès des Habitants et par Entretiens Semi-Directifs Auprès des Professionnels de Santé (Étude NO.DOC). Master’s Thesis, Nantes, France, 2022. [Google Scholar]
- Institut National du Cancer (INCa). Synthèse—Généralisation du Dépistage du Cancer du col de L’Utérus/Étude Médico-Économique/Phase 1—Ref: APDEPCCUSYN16. 2016. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Synthese-Generalisation-du-depistage-du-cancer-du-col-de-l-uterus-etude-medico-economique-Phase-1 (accessed on 25 September 2023).
- Dépistages des Cancers. Centre de Coordination Centre—Val de Loire. Étude APACHE. Available online: https://www.depistage-cancer.fr/centre/15-37/do-col-de-luterus15/403-etude-apache-3 (accessed on 25 September 2023).
- Haguenoer, K.; Boyard, J.; Sengchanh, S.; Gaudy-Graffin, C.; Fontenay, R.; Marret, H. L’Auto-Prélèvement Vaginal Est Une Méthode Efficace Pour Augmenter la Participation au Dépistage du Cancer du col de L’utérus: Un Essai Randomisé en Indre-et-Loire. 2017. Available online: https://www.santepubliquefrance.fr/regions/centre-val-de-loire/documents/article/2017/l-auto-prelevement-vaginal-est-une-methode-efficace-pour-augmenter-la-participation-au-depistage-du-cancer-du-col-de-l-uterus-un-essai-randomise (accessed on 25 September 2023).
- Teigné, D.; Banaszuk, A.S.; Grimault, C.; Abes, L.; Gaultier, A.; Rat, C. Cervical cancer screening uptake: A randomized controlled trial assessing the effect of sending invitation letters to non-adherent women combined with sending their general practitioners a list of their non-adherent patients (study protocol). Front. Public Health 2022, 10, 1035288. [Google Scholar] [CrossRef]
- Strelow, B.; O’Laughlin, D. Barriers to cervical cancer screening among immigrants. JAAPA 2022, 35, 23–27. [Google Scholar] [CrossRef]
- Agence Nationale de Lutte Contre L’Illettrisme. Les Chiffes—Niveau National. 2012. Available online: http://www.anlci.gouv.fr/Illettrisme/Les-chiffres/Niveau-national (accessed on 25 September 2023).
- Wood, B.; Lofters, A.; Vahabi, M. Strategies to reach marginalized women for cervical cancer screening: A qualitative study of stakeholder perspectives. Curr. Oncol. 2018, 25, e8–e16. [Google Scholar] [CrossRef]
- Waller, J.; Bartoszek, M.; Marlow, L.; Wardle, J. Barriers to cervical cancer screening attendance in England: A population-based survey. J. Med. Screen. 2009, 16, 199–204. [Google Scholar] [CrossRef]
- Sultana, F.; Mullins, R.; Murphy, M.; English, D.R.; Simpson, J.A.; Drennan, K.T.; Heley, S.; Wrede, C.D.; Brotherton, J.M.L.; Saville, M.; et al. Women’s views on human papillomavirus self-sampling: Focus groups to assess acceptability, invitation letters and a test kit in the Australian setting. Sex. Health 2015, 12, 279–286. [Google Scholar] [CrossRef]
- Haut Conseil à l’Egalité Entre les Hommes et les Femmes. Les Actes Sexistes Durant le Suivi Gynécologique et Obstétrical: Des Remarques aux Violences, la Nécessité de Reconnaitre, Prévenir et Condamner le Sexisme. 2018. Available online: https://www.haut-conseil-egalite.gouv.fr/sante-droits-sexuels-et-reproductifs/actualites/article/actes-sexistes-durant-le-suivi-gynecologique-et-obstetrical-reconnaitre-et (accessed on 25 September 2023).

| Sex | Working Area | |||||||
|---|---|---|---|---|---|---|---|---|
| Age | Women | Men | Rural | Urban | Rural + Urban | Years of Medical Practice | Cancer Cases | |
| Gynecologists n = 14 | 48 | 11 | 3 | / | 11 | 3 | 18 | 3 |
| Min = 32; max = 75 | ||||||||
| GPs n = 25 | 44 | 13 | 12 | 18 | 5 | 2 | 18 | 1 |
| Min = 28; max = 70 | ||||||||
| Midwives n = 20 | 44 | 19 | 1 | 11 | 9 | / | 19 | 1 |
| Min = 29; max = 60 | ||||||||
| Extract/Example | ||
|---|---|---|
| Offset low medical density | Access to care | “They already have trouble accessing care, doctors” MID14 |
| Delays for appointments | “Women who request an appointment today get it in seven months” Gyn9 | |
| Possibility of screening | “I do not offer gynecological consultation” GP7 | |
| Target | Geographic isolation | “There are places where the nearest doctor or midwife is 40 km away” MID18 |
| Demedicalization | “It simplifies the task and avoids the intrusive and invasive nature of our examinations” GP15 “There are patients who don’t want to be examined by a man” Gyn6 | |
| Comfort | “Self-sampling is private, there’s no one else” GP12, “Self-testing is better for shy women” MID15 | |
| Free of charges | “If it’s reimbursed, people will do it” MID10 | |
| Responsibility | Screening reminder | “Try to empower patients about screening (…) the person takes responsibility” GP15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Goff, J.; Le Duc-Banaszuk, A.-S.; Lefeuvre, C.; Pivert, A.; Ducancelle, A.; De Pauw, H.; Arbyn, M.; Vinay, A.; Rexand-Galais, F. Acceptability to Healthcare Professionals of Home-Based HPV Self-Sampling for Cervical Screening: A French Qualitative Study Conducted in an Area with Low Access to Health Services. Cancers 2023, 15, 5163. https://doi.org/10.3390/cancers15215163
Le Goff J, Le Duc-Banaszuk A-S, Lefeuvre C, Pivert A, Ducancelle A, De Pauw H, Arbyn M, Vinay A, Rexand-Galais F. Acceptability to Healthcare Professionals of Home-Based HPV Self-Sampling for Cervical Screening: A French Qualitative Study Conducted in an Area with Low Access to Health Services. Cancers. 2023; 15(21):5163. https://doi.org/10.3390/cancers15215163
Chicago/Turabian StyleLe Goff, Johane, Anne-Sophie Le Duc-Banaszuk, Caroline Lefeuvre, Adeline Pivert, Alexandra Ducancelle, Hélène De Pauw, Marc Arbyn, Aubeline Vinay, and Franck Rexand-Galais. 2023. "Acceptability to Healthcare Professionals of Home-Based HPV Self-Sampling for Cervical Screening: A French Qualitative Study Conducted in an Area with Low Access to Health Services" Cancers 15, no. 21: 5163. https://doi.org/10.3390/cancers15215163





