Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Search Methodology
3. BI-RADS Overview
3.1. Updates of BI-RADS
3.2. BI-RADS Assessment Categories and Recommendations
3.3. BI-RADS Lexicon
4. BI-RADS Supported Modalities: Findings and Updated Techniques
4.1. BI-RADS Mammography Findings
4.2. Advancements in Mammographic Imaging Techniques
4.3. BI-RADS Ultrasound Findings
4.4. Advancements in US Techniques
| Score | USE Score | CEUS Score |
|---|---|---|
| 1 | The entire lesion is uniformly colored in green | Ring-like enhancement, no internal enhancement. |
| 2 | The lesion is shadowed in green with focal blue spots | Iso- and synchronous enhancement of the lesion with the surrounding tissue. No clear outline. |
| 3 | The half of the lesion is green and half blue | Earlier enhancement of the lesion than neighboring tissue either heterogeneous or homogeneous. Clear margin. The lesion size is nearly equal to that demonstrated in a 2D image. Regular shape. |
| 4 | The whole lesion is blue or predominantly blue with a minimum green | Earlier enhancement of the lesion than neighboring tissue, typically heterogeneous. The lesion size is larger than that in the 2D image, the lesion still reveals a clear margin with or without a perfusion defect inside the lesions, no crab claw-like enhancement. Irregular shape. |
| 5 | The whole lesion and its neighboring area ware blue or blue with focal green spots | Heterogeneous enhancement of the lesion with a larger size than that in the 2D image. With or without perfusion defect. Crab claw-like enhancement with an unclear margin. |
4.5. BI-RADS MRI Findings
4.6. Advancements in MRI Techniques
5. Microwave Breast Imaging
6. The Role of AI in the Detection and Diagnosis of Breast Cancer
- True negative (TN): both the classifier’s prediction and the test case were negative.
- True positive (TP): both the classifier’s prediction and the test case were negative.
- False negative (FN): the test cases yielded positive results, but the classifier’s prediction was negative
- False positive (FP): the test cases turned out to be negative, but the prediction was positive.
6.1. Svm-Based Detection/Classification Methods
6.2. DT/Rf-Based Detection/Classification Methods
6.3. ANN/AE-Based Detection/Classification Methods
6.4. CNN-Based Detection/Classification Methods
7. Molecular Breast Cancer Subtypes and Imaging Techniques
7.1. Molecular Breast Cancer Subtypes
- Luminal A: positive ER and PR, negative Her2, and low proliferation index.
- Luminal B: positive ER, and either positive Her2 or high proliferation index.
- Her 2 enriched: negative ER and PR, and positive Her2.
- Triple-negative: negative ER, PR, and Her2.
7.2. Molecular Breast Imaging (MBI)
7.2.1. PET-CT
- i.
- Axillary nodal metastasis: When staging axillary lymph nodes (ALNs), sentinel node biopsy (SNB) remains the gold standard [158]. It is defined as the initial site to receive breast lymphatic drainage and represents the primary location for ALN infiltration [159,160]. This sentinel node can be identified using various methods, including blue dye, radioisotopes, ICG (indocyanine green), or their combination, and subsequently retrieved intraoperatively for histopathological examination to determine the necessity for ALN dissection [160]. In contrast to SNB, FDG PET-CT exhibits reduced sensitivity in detecting axillary lymph node (ALN) metastases [161,162]. Nevertheless, FDG PET-CT has shown comparable performance to other non-invasive imaging modalities such as ultrasound (US) and MRI for ALN detection [149]. In a previous study, PET-CT demonstrated notably higher accuracy than ultrasound (US) [163]. It is worth noting that PET-CT has better specificity than sensitivity for detecting ALN metastasis, particularly in early-stage cases [164]. SUVmax may serve as a potential prognostic factor for axillary lymph node (ALN) metastases, especially in specific breast cancer subtypes like HER2-positive and ER-positive/HER2-negative tumors [130].
- ii.
- Extra-axillary nodal metastasis: Regional extra-axillary lymph nodes, which encompass the internal mammary, infraclavicular, and supraclavicular lymph nodes, are less frequently identified through sentinel node assessment [141]. FDG PET-CT offers superior accuracy in staging by detecting extraaxillary nodal metastases, particularly excelling over ultrasound in the detection of internal mammary nodal involvement [165,166]. The discovery of unexpected metastatic lymph nodes beyond the axillary region during the initial staging using FDG PET-CT has a profound impact on patient prognosis and can potentially influence decisions regarding the extent of surgical or radiotherapeutic interventions [167].
7.2.2. Positron Emission Mammography (PEM)
8. Breast Cancer Imaging Biomarkers
9. Management of Breast Cancer
10. Assessment of Treatment Response
10.1. Assessment of Neoadjuvant Therapy Response
10.2. Assessment of Response in Metastatic Breast Cancer
10.3. The Role of AI in the Assessment of Treatment Response
11. Conclusions
- Structured BI-RADS reports provide assessment categories that encompass breast density, a description of detected findings, and recommendations for managing the identified abnormalities [8].
- Digital Mammography (DM) is the ideal method for screening and early detection of BC, but it has low sensitivity in dense breasts [28].
- Breast US lexicon has been updated to reflect advanced techniques such as elastography. Also, the “special cases” category has been extended in the BI-RADS 5th edition [7].
- Currently, MRI is the key technique for imaging breast cancer with the highest sensitivity (88–100%) among breast imaging modalities [49].
- Molecular classifications opened the door to understanding that BC is not a uniform disease. The molecular subtype affects the clinical outcomes and the response to treatment [130].
- MBI offers quantitative biomarkers, which indicate tumor receptor status, tumor aggressiveness, and treatment response [137].
- PET-CT has a critical role in systemic staging and the detection of tumor response and recurrence of BC, but PET-CT has low sensitivity to diagnose primary BC compared to other dedicated breast imaging [144].
- PEM has a great advantage over PET-CT owing to its higher spatial resolution, particularly for small and low-grade lesions, with overall 91% sensitivity and 93% specificity [178].
- Radiologists must be familiar with variable BC imaging biomarkers [185].
- Breast cancer management is multimodal depending mainly on the disease stage and the molecular profile [191].
- Response to NAC is frequently assessed by breast MRI and, to a minor extent, US to discriminate NAC response from nonresponse. MRI is superior to US in preoperative tumor size assessment after NAC [213].
- The evaluation of treatment response in metastatic breast cancer commonly relies on measuring tumor size, typically using CT scans [228].
- Utilizing ML classifiers using different extracted features (e.g., statistical [241,242,243], appearance [241,242,243,244,245,246,247,248,249], morphological [241,242,243,246,247,248,249,250], texture [241,242,243,244,245,246,250,251,252,253,254,255], etc.), various investigated methods were applied to various modalities/databases (e.g., ultrasound, elastography, cell tissue characteristics, patient records, cytology images, etc.). The outcomes of these ML-based techniques highlight the potential of utilizing ML classifiers for BC detection and diagnosis [76,81,92].
- On various modalities/databases (e.g., US, mammography, elastography, histopathology, DEC-MRI, T2w-MRI, multi-parametric data, etc.), various DL technologies (e.g., augmentation, spatial drop-out, transfer learning, fusion, ensemble learning, etc.) were utilized. The results of these DL methods demonstrate the possibility of using CNN and DL models to assist radiologists in BC identification and/or diagnosis [100,118].
- The fusion of the extracted AI features from multiparametric modalities can improve the performance of BC classification [118].
- ML/AI components are able to provide quantifiable, objective measures for BC detection and diagnosis and can help with pre-treatment tumor response prediction to NAC. Therefore, their findings have the potential to enhance the effectiveness of the healthcare systems for BC [233,235,236,238,239,240].
- There is a need for an updated BI-RADS lexicon for the proper application of evolving imaging modalities, such as contrast-enhanced mammography and molecular breast imaging (MBI).
- Further investigations into the role of DTI in BC diagnosis are required.
- Monitoring treatment response with PET-CT in metastatic BC may improve metastatic patient management, however further investigation is needed.
- Further investigation for utilizing AI/ML CAD systems based on alternative nonionized modalities (other than the ionized mammograms) should be explored to reach acceptable clinical performance [86].
- Constructing large standard online databases for the purpose of evaluating developed AI-based systems for BC detection, diagnosis, classification, and/or treatment prediction can help to evolve the evolution of AI in this field.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| ACR | American College of Radiology |
| AdaBoost | Adaptive Boosting |
| ADC | Apparent diffusion coefficient |
| AE | Autoencoder |
| ALN | Axillary lymph node |
| ANN | Artificial neural networks |
| AR | Association rules |
| AUC | Area under the ROC curve |
| BI-RADS | Breast Imaging Reporting and Data System |
| BC | Breast cancer |
| BCDR | Breast cancer digital repository |
| BLR | Bayesian logistic regression |
| BreaKHis | Breast cancer histopathological database |
| CAE | Contractive autoencoder |
| CAD | Computer aided diagnosis |
| CART | Classification and Regression Trees |
| CEM | Contrast-enhanced mammography |
| CEUS | Contrast-enhanced ultrasound |
| CNN | Convolutional neural network |
| DAE | Denoising autoencoder |
| DCE-MRI | Dynamic contrast-enhanced magnetic resonance imaging |
| DDSM | Digital database for screening mammography |
| DL | Deep learning |
| DT | Decision Tree |
| DTI-MRI | Diffusion tensor magnetic resonance imaging |
| DSS | Disease-specific survival |
| DW-MRI | Diffusion weighted magnetic resonance imaging |
| EANN | Evolutionary artificial neural network |
| EP | Evolutionary programming |
| ER | Estrogen receptor |
| FA | Fractional anisotropy |
| FN | False negative |
| FP | False positive |
| GA | Genetic algorithm |
| GAC | Geometric active contour |
| GBoost | Gradient Boosting |
| GLCM | Gray level co-occurrence matrix |
| GLMNet | Generalized linear regression with elastic net |
| GNB | Gaussian Naïve Bayes |
| GWO | Grey wolf optimization |
| HER | Electronic health record |
| HER2 | Human epidermal growth factor receptor 2 |
| HOMA | homeostatic model assessment |
| ID3 | Iterative Dichotomiser 3 |
| JNUH | Jeonbuk national university hospital |
| KNN | K-Nearest Neighbor |
| MA-CNN | Multiscale all CNN |
| MBI | Molecular breast imaging |
| LDA | Linear discriminant analysis |
| LR | Logistic Regression |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIAS | Mammographic image analysis society database |
| ML | Machine learning |
| MLP | Multi-layer perceptron |
| MRI | Magnetic Resonance Imaging |
| MRMR | Maximum relevance minimum redundancy |
| MSER | Maximally stable extremal regions |
| NAC | Neoadjuvant chemotherapy |
| NCI | National Cancer Institute |
| NB | Naïve Bayes |
| PDE | Pareto-differential evolution algorithm |
| PEM | Positron emission mammography. |
| RF | Random forest |
| RFE-RF | Recursive feature elimination random forest |
| RFS | Recurrence-free survival |
| pCR | Pathological complete response |
| PGBM | Point-wise gated Boltzmann machine |
| PR | Progesterone receptor |
| QDA | Quadratic Discriminant Analysis |
| ACR | American College of Radiology |
| RBM | Restricted Boltzmann machine |
| RCB | Residual cancer burden |
| ROC | Receiver operating characteristic |
| ROI | Region of interest |
| SAE | Sparse autoencoder |
| SGD | Stochastic gradient descent |
| SOM | Self organization map |
| SSAE | Stacked sparse autoencoder |
| SWE | Shear-wave elastography |
| TN | True negative |
| TP | True positive |
| UCI | University of California Irvine |
| US | Ultrasonography |
| VAE | Variational autoencoder |
| WBCD | Wisconsin breast cancer database |
| WBCO | Wisconsin breast cancer original database |
| WSAW | Weighted simple additive weighting |
| WSE | Wrapper subset evaluator |
| XGBoost | Extreme gradient boosting |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Mao, F.; Yao, R.; Sun, Q. Ki-67 index, progesterone receptor expression, histologic grade and tumor size in predicting breast cancer recurrence risk: A consecutive cohort study. Cancer Commun. 2020, 40, 181–193. [Google Scholar] [CrossRef]
- Hekal, A.A.; Elnakib, A.; Moustafa, H.E.D. Breast Cancer Detection, Diagnosis, and Prediction. Int. J. Inf. 2020, 9, 38–42. [Google Scholar]
- Bartolotta, T.V.; Orlando, A.A.M.; Spatafora, L.; Dimarco, M.; Gagliardo, C.; Taibbi, A. S-Detect characterization of focal breast lesions according to the US BI RADS lexicon: A pictorial essay. J. Ultrasound 2020, 23, 207–215. [Google Scholar] [CrossRef]
- An, J.Y.; Unsdorfer, K.M.L.; Weinreb, J.C. BI-RADS, C-RADS, CAD-RADS, LI-RADS, Lung-RADS, NI-RADS, O-RADS, PI-RADS, TI-RADS: Reporting and Data Systems. RadioGraphics 2019, 39, 1435–1436. [Google Scholar] [CrossRef] [PubMed]
- Sickles, E.A.; D’Orsi, C.J.; Bassett, L.W.; Appleton, C.M.; Berg, W.A.; Burnside, E.S. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013; pp. 39–48. [Google Scholar]
- Rao, A.A.; Feneis, J.; Lalonde, C.; Ojeda-Fournier, H. A Pictorial Review of Changes in the BI-RADS Fifth Edition. RadioGraphics 2016, 36, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Eghtedari, M.; Chong, A.; Rakow-Penner, R.; Ojeda-Fournier, H. Current Status and Future of BI-RADS in Multimodality Imaging, From the AJR Special Series on Radiology Reporting and Data Systems. Am. J. Roentgenol. 2021, 216, 860–873. [Google Scholar] [CrossRef]
- D’Orsi, C.; Bassett, L.; Feig, S. Breast imaging reporting and data system (BI-RADS). In Breast Imaging Atlas, 4th ed.; American College of Radiology: Reston, VA, USA, 2018. [Google Scholar]
- Varas, X.; Leborgne, J.H.; Leborgne, F.; Mezzera, J.; Jaumandreu, S.; Leborgne, F. Revisiting the Mammographic Follow-Up of BI-RADS Category 3 Lesions. Am. J. Roentgenol. 2002, 179, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Talati, N.; Oudsema, R.; Steinberger, S.; Margolies, L.R. BI-RADS 3: Current and Future Use of Probably Benign. Curr. Radiol. Rep. 2018, 6, 2. [Google Scholar] [CrossRef]
- Berg, W.A.; Berg, J.M.; Sickles, E.A.; Burnside, E.S.; Zuley, M.L.; Rosenberg, R.D.; Lee, C.S. Cancer Yield and Patterns of Follow-up for BI-RADS Category 3 after Screening Mammography Recall in the National Mammography Database. Radiology 2020, 296, 32–41. [Google Scholar] [CrossRef]
- Spick, C.; Bickel, H.; Polanec, S.H.; Baltzer, P.A. Breast lesions classified as probably benign (BI-RADS 3) on magnetic resonance imaging: A systematic review and meta-analysis. Eur. Radiol. 2017, 28, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, J.H.; Han, K.; Kim, E.K.; Kim, M.J.; Moon, H.J.; Yoon, J.H.; Park, V.Y. BI-RADS category 3, 4, and 5 lesions identified at preoperative breast MRI in patients with breast cancer: Implications for management. Eur. Radiol. 2020, 30, 2773–2781. [Google Scholar] [CrossRef]
- Elezaby, M.; Li, G.; Bhargavan-Chatfield, M.; Burnside, E.S.; DeMartini, W.B. ACR BI-RADS Assessment Category 4 Subdivisions in Diagnostic Mammography: Utilization and Outcomes in the National Mammography Database. Radiology 2018, 287, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Knogler, T.; Homolka, P.; Hoernig, M.; Leithner, R.; Langs, G.; Waitzbauer, M.; Pinker, K.; Leitner, S.; Helbich, T.H. Application of BI-RADS Descriptors in Contrast-Enhanced Dual-Energy Mammography: Comparison with MRI. Breast Care 2017, 12, 212–216. [Google Scholar] [CrossRef]
- Travieso-Aja, M.; Maldonado-Saluzzi, D.; Naranjo-Santana, P.; Fernández-Ruiz, C.; Severino-Rondón, W.; Rodríguez, M.R.; Luzardo, O. Evaluation of the applicability of BI-RADS® MRI for the interpretation of contrast-enhanced digital mammography. Radiologia 2019, 61, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Pinker, K.; Chin, J.; Melsaether, A.N.; Morris, E.A.; Moy, L. Precision Medicine and Radiogenomics in Breast Cancer: New Approaches toward Diagnosis and Treatment. Radiology 2018, 287, 732–747. [Google Scholar] [CrossRef]
- Narayanan, D.; Madsen, K.S.; Kalinyak, J.E.; Berg, W.A. Interpretation of Positron Emission Mammography and MRI by Experienced Breast Imaging Radiologists: Performance and Observer Reproducibility. Am. J. Roentgenol. 2011, 196, 971–981. [Google Scholar] [CrossRef]
- Conners, A.L.; Maxwell, R.W.; Tortorelli, C.L.; Hruska, C.B.; Rhodes, D.J.; Boughey, J.C.; Berg, W.A. Gamma Camera Breast Imaging Lexicon. Am. J. Roentgenol. 2012, 199, W767–W774. [Google Scholar] [CrossRef]
- Perry, H.; Phillips, J.; Dialani, V.; Slanetz, P.J.; Fein-Zachary, V.J.; Karimova, E.J.; Mehta, T.S. Contrast-Enhanced Mammography: A Systematic Guide to Interpretation and Reporting. Am. J. Roentgenol. 2019, 212, 222–231. [Google Scholar] [CrossRef]
- Destounis, S.V.; Santacroce, A.; Arieno, A. Update on Breast Density, Risk Estimation, and Supplemental Screening. Am. J. Roentgenol. 2020, 214, 296–305. [Google Scholar] [CrossRef]
- Houssami, N.; Lee, C.I. The impact of legislation mandating breast density notification—Review of the evidence. Breast 2018, 42, 102–112. [Google Scholar] [CrossRef]
- Coşar, Z.S.; Çetin, M.; Tepe, T.K.; Çetin, R.; Zarali, A.C. Concordance of mammographic classifications of microcalcifications in breast cancer diagnosis. Clin. Imaging 2005, 29, 389–395. [Google Scholar] [CrossRef]
- van den Biggelaar, F.J.; Kessels, A.G.; van Engelshoven, J.M.; Flobbe, K. Strategies for digital mammography interpretation in a clinical patient population. Int. J. Cancer 2009, 125, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Fallenberg, E.M.; Dromain, C.; Diekmann, F.; Engelken, F.; Krohn, M.; Singh, J.M.; Ingold-Heppner, B.; Winzer, K.J.; Bick, U.; Renz, D.M. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur. Radiol. 2013, 24, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Franken, E.A.; Garg, M.; Fajardo, L.L.; Niklason, L.T. Breast Tomosynthesis: Present Considerations and Future Applications. RadioGraphics 2007, 27, S231–S240. [Google Scholar] [CrossRef]
- Helvie, M.A. Digital Mammography Imaging: Breast Tomosynthesis and Advanced Applications. Radiol. Clin. N. Am. 2010, 48, 917–929. [Google Scholar] [CrossRef]
- Lee, W.K.; Chung, J.; Cha, E.S.; Lee, J.E.; Kim, J.H. Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur. J. Radiol. 2016, 85, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.M.; Turner, E.; Sickles, E.A.; Joe, B.N. Suspicious Findings at Digital Breast Tomosynthesis Occult to Conventional Digital Mammography: Imaging Features and Pathology Findings. Breast J. 2015, 21, 538–542. [Google Scholar] [CrossRef]
- Basha, M.A.A.; Safwat, H.K.; Eldin, A.M.A.; Dawoud, H.A.; Hassanin, A.M. The added value of digital breast tomosynthesis in improving diagnostic performance of BI-RADS categorization of mammographically indeterminate breast lesions. Insights Imaging 2020, 11, 26. [Google Scholar] [CrossRef]
- Lewin, J.M.; Patel, B.K.; Tanna, A. Contrast-Enhanced Mammography: A Scientific Review. J. Breast Imaging 2019, 2, 7–15. [Google Scholar] [CrossRef]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Helbich, T.H.; Baltzer, P.A.T. Contrast-enhanced Mammography versus Contrast-enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, M.; Heuts, E.; Moossdorff, M.; van Nijnatten, T. Contrast enhanced mammography (CEM) versus magnetic resonance imaging (MRI) for staging of breast cancer: The pro CEM perspective. Eur. J. Radiol. 2021, 142, 109883. [Google Scholar] [CrossRef] [PubMed]
- Sogani, J.; Mango, V.L.; Keating, D.; Sung, J.S.; Jochelson, M.S. Contrast-enhanced mammography: Past, present, and future. Clin. Imaging 2021, 69, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lewin, J. Comparison of Contrast-Enhanced Mammography and Contrast-Enhanced Breast MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Helal, M.H.; Mansour, S.M.; Haggag, M.A.; Nada, O.M.; Farahat, I.G.; Alieldin, N.H. Can we apply the MRI BI-RADS lexicon morphology descriptors on contrast-enhanced spectral mammography? Br. J. Radiol. 2016, 89, 20160157. [Google Scholar] [CrossRef]
- Carlino, G.; Rinaldi, P.; Giuliani, M.; Rella, R.; Bufi, E.; Padovano, F.; Ciardi, C.; Romani, M.; Belli, P.; Manfredi, R. Ultrasound-guided preoperative localization of breast lesions: A good choice. J. Ultrasound 2018, 22, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hille, H.; Vetter, M.; Hackelöer, B. The Accuracy of BI-RADS Classification of Breast Ultrasound as a First-Line Imaging Method. Ultraschall Der Med. Eur. J. Ultrasound 2011, 33, 160–163. [Google Scholar] [CrossRef]
- Chang, J.M.; Moon, W.K.; Cho, N.; Yi, A.; Koo, H.R.; Han, W.; Noh, D.Y.; Moon, H.G.; Kim, S.J. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res. Treat. 2011, 129, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; David, E.; Barr, R.G.; Radzina, M.; de Soccio, V.; Elia, D.; Felice, C.D.; Pediconi, F.; Gigli, S.; Occhiato, R.; et al. US-Elastography for Breast Lesion Characterization: Prospective Comparison of US BIRADS, Strain Elastography and Shear wave Elastography. Ultraschall Der Med. Eur. J. Ultrasound 2020, 42, 533–540. [Google Scholar] [CrossRef]
- Park, C.S.; Kim, S.H.; Jung, N.Y.; Choi, J.J.; Kang, B.J.; Jung, H.S. Interobserver variability of ultrasound elastography and the ultrasound BI-RADS lexicon of breast lesions. Breast Cancer 2013, 22, 153–160. [Google Scholar] [CrossRef]
- Zhi, H.; Ou, B.; Xiao, X.Y.; Peng, Y.L.; Wang, Y.; Liu, L.S.; Xiao, Y.; Liu, S.J.; Wu, C.J.; Jiang, Y.X.; et al. Ultrasound Elastography of Breast Lesions in Chinese Women: A Multicenter Study in China. Clin. Breast Cancer 2013, 13, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Xiao, X.Y.; Ou, B.; Zhong, W.J.; Zhao, Z.Z.; Zhao, X.B.; Yang, H.Y.; Luo, B.M. Could ultrasonic elastography help the diagnosis of small (≤2cm) breast cancer with the usage of sonographic BI-RADS classification? Eur. J. Radiol. 2012, 81, 3216–3221. [Google Scholar] [CrossRef]
- Wan, C.; Du, J.; Fang, H.; Li, F.; Wang, L. Evaluation of breast lesions by contrast enhanced ultrasound: Qualitative and quantitative analysis. Eur. J. Radiol. 2012, 81, e444–e450. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, X.Y.; Zhu, S.Y.; Kang, L.K.; Xiao, Y.J.; Zheng, H.Y. Meta-analysis of contrast-enhanced ultrasound for the differentiation of benign and malignant breast lesions. Acta Radiol. 2015, 56, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Jiang, Q.; Wu, H.; Guan, X.; Qin, W.; Luo, B. Diagnosis of sub-centimetre breast lesions: Combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound—a preliminary study in China. Eur. Radiol. 2016, 27, 2443–2450. [Google Scholar] [CrossRef]
- Xiao, X.; Ou, B.; Yang, H.; Wu, H.; Luo, B. Breast Contrast-Enhanced Ultrasound: Is a Scoring System Feasible?—A Preliminary Study in China. PLoS ONE 2014, 9, e105517. [Google Scholar] [CrossRef]
- Lunkiewicz, M.; Forte, S.; Freiwald, B.; Singer, G.; Leo, C.; Kubik-Huch, R.A. Interobserver variability and likelihood of malignancy for fifth edition BI-RADS MRI descriptors in non-mass breast lesions. Eur. Radiol. 2019, 30, 77–86. [Google Scholar] [CrossRef]
- Edwards, S.D.; Lipson, J.A.; Ikeda, D.M.; Lee, J.M. Updates and Revisions to the BI-RADS Magnetic Resonance Imaging Lexicon. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Bieling, H.B.; Gieseke, J.; Kreft, B.P.; Sommer, T.; Lutterbey, G.; Schild, H.H. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997, 203, 137–144. [Google Scholar] [CrossRef]
- Müller-Schimpfle, M.; Ohmenhaüser, K.; Stoll, P.; Dietz, K.; Claussen, C.D. Menstrual cycle and age: Influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997, 203, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bryce, Y.; Zheng, J.; Sung, J.S.; Comstock, C.E.; Moskowitz, C.; Morris, E.A. Outcome of Screening MRI in Premenopausal Women as a Function of the Week of the Menstrual Cycle. Am. J. Roentgenol. 2020, 214, 1175–1181. [Google Scholar] [CrossRef]
- Rahbar, H.; Partridge, S.C. Multiparametric MR Imaging of Breast Cancer. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 223–238. [Google Scholar] [CrossRef]
- Marino, M.A.; Helbich, T.; Baltzer, P.; Pinker-Domenig, K. Multiparametric MRI of the breast: A review. J. Magn. Reson. Imaging 2017, 47, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Knopp, M.; Weiss, E.; Sinn, H.; Mattern, J.; Junkermann, H.; Radeleff, J.; Magener, A.; Brix, G.; Delorme, S.; Zuna, I.; et al. Pathophysiologic basis of contrast enhancement in breast tumors. J. Magn. Reson. Imaging 1999, 10, 260–266. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K.; Mielcareck, P.; Klaschik, S.; Leutner, C.; Wardelmann, E.; Gieseke, J.; Schild, H.H. Dynamic Breast MR Imaging: Are Signal Intensity Time Course Data Useful for Differential Diagnosis of Enhancing Lesions? Radiology 1999, 211, 101–110. [Google Scholar] [CrossRef]
- Daniel, B.L.; Yen, Y.F.; Glover, G.H.; Ikeda, D.M.; Birdwell, R.L.; Sawyer-Glover, A.M.; Black, J.W.; Plevritis, S.K.; Jeffrey, S.S.; Herfkens, R.J. Breast disease: Dynamic spiral MR imaging. Radiology 1998, 209, 499–509. [Google Scholar] [CrossRef]
- Partridge, S.C.; Stone, K.M.; Strigel, R.M.; DeMartini, W.B.; Peacock, S.; Lehman, C.D. Breast DCE-MRI. Acad. Radiol. 2014, 21, 1195–1203. [Google Scholar] [CrossRef]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef]
- Bihan, D.L.; Iima, M. Diffusion Magnetic Resonance Imaging: What Water Tells Us about Biological Tissues. PLoS Biol. 2015, 13, e1002203. [Google Scholar] [CrossRef]
- Partridge, S.C.; McDonald, E.S. Diffusion Weighted Magnetic Resonance Imaging of the Breast. Magn. Reson. Imaging Clin. N. Am. 2013, 21, 601–624. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.Y.; Yao, Q.Y.; Wu, L.M.; Xu, J.R. Breast Lesions: Diagnosis Using Diffusion Weighted Imaging at 1.5T and 3.0T—Systematic Review and Meta-analysis. Clin. Breast Cancer 2018, 18, e305–e320. [Google Scholar] [CrossRef] [PubMed]
- Bogner, W.; Gruber, S.; Pinker, K.; Grabner, G.; Stadlbauer, A.; Weber, M.; Moser, E.; Helbich, T.H.; Trattnig, S. Diffusion-weighted MR for Differentiation of Breast Lesions at 3.0 T: How Does Selection of Diffusion Protocols Affect Diagnosis? Radiology 2009, 253, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.P.A.; Martins, G.; Figueiredo, E.; Domingues, M.N.A.; Domingues, R.C.; da Fonseca, L.M.B.; Gasparetto, E.L. Assessment of Breast Lesions with Diffusion-Weighted MRI: Comparing the Use of Different b Values. Am. J. Roentgenol. 2009, 193, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging 2016, 45, 337–355. [Google Scholar] [CrossRef]
- Saleh, G.A.; Elmokadem, A.H.; Razek, A.A.; El-Morsy, A.; Hamdy, O.; Eleraky, E.S.; Saleh, M. Utility of diffusion tensor imaging in differentiating benign from malignant hepatic focal lesions. Eur. Radiol. 2022, 33, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; O’Loughlin, D.; Elwahab, S.A.; Kerin, M.J. Breast Cancer Detection—A Synopsis of Conventional Modalities and the Potential Role of Microwave Imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef]
- Preece, A.W.; Craddock, I.; Shere, M.; Jones, L.; Winton, H.L. MARIA M4: Clinical evaluation of a prototype ultrawideband radar scanner for breast cancer detection. J. Med. Imaging 2016, 3, 033502. [Google Scholar] [CrossRef]
- O’Loughlin, D.; O’Halloran, M.; Moloney, B.M.; Glavin, M.; Jones, E.; Elahi, M.A. Microwave Breast Imaging: Clinical Advances and Remaining Challenges. IEEE Trans. Biomed. Eng. 2018, 65, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Massey, H.; Ridley, N.; Lyburn, I.; Taylor, S.; Schoenleber-Lewis, M.; Bannister, P.; Shere, M. Radiowave detection of breast cancer in the symptomatic clinic—A multi-centre study. In Proceedings of the International Cambridge Conference on Breast Imaging, Cambridge, UK, 3–4 July 2017; pp. 201–206. [Google Scholar]
- Fasoula, A.; Duchesne, L.; Cano, J.G.; Lawrence, P.; Robin, G.; Bernard, J.G. On-Site Validation of a Microwave Breast Imaging System, before First Patient Study. Diagnostics 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.M.; McAnena, P.F.; Elwahab, S.M.A.; Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer—Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2022, 29, S211–S222. [Google Scholar] [CrossRef] [PubMed]
- Janjic, A.; Cayoren, M.; Akduman, I.; Yilmaz, T.; Onemli, E.; Bugdayci, O.; Aribal, M.E. SAFE: A Novel Microwave Imaging System Design for Breast Cancer Screening and Early Detection—Clinical Evaluation. Diagnostics 2021, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Kotb, A.; Farag, O.; Darweesh, M.S.; Mostafa, H. Breast Cancer Diagnosis Using Image Processing and Machine Learning for Elastography Images. In Proceedings of the 2019 8th International Conference on Modern Circuits and Systems Technologies (MOCAST), Thessaloniki, Greece, 13–15 May 2019; IEEE: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Ara, S.; Das, A.; Dey, A. Malignant and Benign Breast Cancer Classification using Machine Learning Algorithms. In Proceedings of the 2021 International Conference on Artificial Intelligence (ICAI), Islamabad, Pakistan, 5–7 April 2021; IEEE: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- William Wolberg, O.M. Breast Cancer Wisconsin (Diagnostic); UCI Machine Learning Repository. 1993. Available online: https://archive.ics.uci.edu/dataset/17/breast+cancer+wisconsin+diagnostic (accessed on 20 October 2023).
- Badr, E.; Almotairi, S.; Salam, M.A.; Ahmed, H. New Sequential and Parallel Support Vector Machine with Grey Wolf Optimizer for Breast Cancer Diagnosis. Alex. Eng. J. 2022, 61, 2520–2534. [Google Scholar] [CrossRef]
- Feder, S.L. Data Quality in Electronic Health Records Research: Quality Domains and Assessment Methods. West. J. Nurs. Res. 2017, 40, 753–766. [Google Scholar] [CrossRef]
- Khan, S.U.; Islam, N.; Jan, Z.; Haseeb, K.; Shah, S.I.A.; Hanif, M. A machine learning-based approach for the segmentation and classification of malignant cells in breast cytology images using gray level co-occurrence matrix (GLCM) and support vector machine (SVM). Neural Comput. Appl. 2021, 34, 8365–8372. [Google Scholar] [CrossRef]
- Ed-daoudy, A.; Maalmi, K. Breast cancer classification with reduced feature set using association rules and support vector machine. Netw. Model. Anal. Health Inform. Bioinform. 2020, 9, 34. [Google Scholar] [CrossRef]
- El-Azizy, A.R.M.; Salaheldien, M.; Rushdi, M.A.; Gewefel, H.; Mahmoud, A.M. Morphological characterization of breast tumors using conventional B-mode ultrasound images. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Wei, M.; Du, Y.; Wu, X.; Zhu, J. Automatic Classification of Benign and Malignant Breast Tumors in Ultrasound Image with Texture and Morphological Features. In Proceedings of the 2019 IEEE 13th International Conference on Anti-counterfeiting, Security, and Identification (ASID), Xiamen, China, 25–27 October 2019; IEEE: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Tiberi, G.; Sani, L.; Vispa, A.; Raspa, G.; Duranti, M.; Ghavami, M.; Dudley, S. Machine Learning Approaches for Automated Lesion Detection in Microwave Breast Imaging Clinical Data. Sci. Rep. 2019, 9, 10510. [Google Scholar] [CrossRef]
- Sami, H.; Sagheer, M.; Riaz, K.; Mehmood, M.Q.; Zubair, M. Machine Learning-Based Approaches For Breast Cancer Detection in Microwave Imaging. In Proceedings of the 2021 IEEE USNC-URSI Radio Science Meeting (Joint with AP-S Symposium), Singapore, 4–10 May 2021; IEEE: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Utgoff, P.E. Incremental induction of decision trees. Mach. Learn. 1989, 4, 161–186. [Google Scholar] [CrossRef]
- Salzberg, S.L. C4.5: Programs for Machine Learning by J. Ross Quinlan. Morgan kaufmann publishers, inc., 1993. Mach. Learn. 1994, 16, 235–240. [Google Scholar] [CrossRef]
- Panigrahi, R.; Borah, S. Rank Allocation to J48 Group of Decision Tree Classifiers using Binary and Multiclass Intrusion Detection Datasets. Procedia Comput. Sci. 2018, 132, 323–332. [Google Scholar] [CrossRef]
- Patil, N.P.; Lathi, R.; Chitre, V. Comparison of C5.0 & CART Classification algorithms using pruning technique. Int. J. Eng. Res. Technol. 2012, 1, 4. [Google Scholar]
- Singh, S.N.; Thakral, S. Using Data Mining Tools for Breast Cancer Prediction and Analysis. In Proceedings of the 2018 4th International Conference on Computing Communication and Automation (ICCCA), Greater Noida, India, 14–15 December 2018; IEEE: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Allada, A.; Rao, G.R.K.; Chitturi, P.; Chindu, H.; Prasad, M.S.N.; Tatineni, P. Breast Cancer Prediction using Deep Learning Techniques. In Proceedings of the 2021 International Conference on Artificial Intelligence and Smart Systems (ICAIS), Coimbatore, India, 25–27 March 2021; IEEE: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Sengar, P.P.; Gaikwad, M.J.; Nagdive, A.S. Comparative Study of Machine Learning Algorithms for Breast Cancer Prediction. In Proceedings of the 2020 Third International Conference on Smart Systems and Inventive Technology (ICSSIT), Tirunelveli, India, 20–22 August 2020; IEEE: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
- Abbass, H.A. An evolutionary artificial neural networks approach for breast cancer diagnosis. Artif. Intell. Med. 2002, 25, 265–281. [Google Scholar] [CrossRef]
- Karabatak, M.; Ince, M.C. An expert system for detection of breast cancer based on association rules and neural network. Expert Syst. Appl. 2009, 36, 3465–3469. [Google Scholar] [CrossRef]
- Jafari-Marandi, R.; Davarzani, S.; Gharibdousti, M.S.; Smith, B.K. An optimum ANN-based breast cancer diagnosis: Bridging gaps between ANN learning and decision-making goals. Appl. Soft Comput. 2018, 72, 108–120. [Google Scholar] [CrossRef]
- Rouhi, R.; Jafari, M.; Kasaei, S.; Keshavarzian, P. Benign and malignant breast tumors classification based on region growing and CNN segmentation. Expert Syst. Appl. 2015, 42, 990–1002. [Google Scholar] [CrossRef]
- Suckling, J. The mammographic images analysis society digital mammogram database. In Proceedings of the Exerpta Medica. Int. Congr. Ser. 1994, 1069, 375–378. [Google Scholar]
- Heath, M.; Bowyer, K.; Kopans, D.; Moore, R.H. The Digital Database for Screening Mammography. 2007. Available online: https://api.semanticscholar.org/CorpusID:68362967 (accessed on 20 October 2023).
- Kadam, V.J.; Jadhav, S.M.; Vijayakumar, K. Breast Cancer Diagnosis Using Feature Ensemble Learning Based on Stacked Sparse Autoencoders and Softmax Regression. J. Med. Syst. 2019, 43, 263. [Google Scholar] [CrossRef]
- Arevalo, J.; Gonzalez, F.A.; Ramos-Pollan, R.; Oliveira, J.L.; Lopez, M.A.G. Convolutional neural networks for mammography mass lesion classification. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Lopez, M.A.G.; Posada, N.; Moura, D.C.; Pollán, R.R.; Jose, M.G.V.; Valiente, F.S.; Ortega, C.S.; del Solar, M.R.; Herrero, G.D.; Isabel, M.A.; et al. BCDR: A Breast Cancer Digital Repository. In Proceedings of the 15th International Conference on Experimental Mechanics, Porto, Portugal, 22–27 July 2012. [Google Scholar]
- Zhang, Q.; Xiao, Y.; Dai, W.; Suo, J.; Wang, C.; Shi, J.; Zheng, H. Deep learning based classification of breast tumors with shear-wave elastography. Ultrasonics 2016, 72, 150–157. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Li, Y.; Li, P.; Li, L.; Jiang, M. Automatic classification of breast cancer histopathological images based on deep feature fusion and enhanced routing. Biomed. Signal Process. Control 2021, 65, 102341. [Google Scholar] [CrossRef]
- Spanhol, F.A.; Oliveira, L.S.; Petitjean, C.; Heutte, L. A Dataset for Breast Cancer Histopathological Image Classification. IEEE Trans. Biomed. Eng. 2016, 63, 1455–1462. [Google Scholar] [CrossRef]
- Ting, F.F.; Tan, Y.J.; Sim, K.S. Convolutional neural network improvement for breast cancer classification. Expert Syst. Appl. 2019, 120, 103–115. [Google Scholar] [CrossRef]
- Araújo, T.; Aresta, G.; Castro, E.; Rouco, J.; Aguiar, P.; Eloy, C.; Polónia, A.; Campilho, A. Classification of breast cancer histology images using Convolutional Neural Networks. PLoS ONE 2017, 12, e0177544. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.; Aresta, G.; Castro, E.; Rouco, J.; Aguiar, P.; Eloy, C.; Polónia, A.; Campilho, A. Bioimaging Challenge 2015 Breast Histology Dataset. 2017. Available online: https://rdm.inesctec.pt/dataset/nis-2017-003 (accessed on 20 October 2023).
- Kooi, T.; Litjens, G.; van Ginneken, B.; Gubern-Mérida, A.; Sánchez, C.I.; Mann, R.; den Heeten, A.; Karssemeijer, N. Large scale deep learning for computer aided detection of mammographic lesions. Med. Image Anal. 2017, 35, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Sim, K.S.; Ting, F.F. Breast cancer detection using convolutional neural networks for mammogram imaging system. In Proceedings of the 2017 International Conference on Robotics, Automation and Sciences (ICORAS), Melaka, Malaysia, 27–29 November 2017; IEEE: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Agnes, S.A.; Anitha, J.; Pandian, S.I.A.; Peter, J.D. Classification of Mammogram Images Using Multiscale all Convolutional Neural Network (MA-CNN). J. Med. Syst. 2019, 44, 30. [Google Scholar] [CrossRef]
- Muduli, D.; Dash, R.; Majhi, B. Automated diagnosis of breast cancer using multi-modal datasets: A deep convolution neural network based approach. Biomed. Signal Process. Control. 2021, 71, 102825. [Google Scholar] [CrossRef]
- Moreira, I.C.; Amaral, I.; Domingues, I.; Cardoso, A.; Cardoso, M.J.; Cardoso, J.S. INbreast. Acad. Radiol. 2012, 19, 236–248. [Google Scholar] [CrossRef]
- Al-Dhabyani, W.; Gomaa, M.; Khaled, H.; Fahmy, A. Dataset of breast ultrasound images. Data Brief 2020, 28, 104863. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Ali, H.; Wang, H.Y.; Lei, C.; Ali, H. Feature fusion and Ensemble learning-based CNN model for mammographic image classification. J. King Saud Univ. Comput. Inf. Sci. 2022, 34, 3310–3318. [Google Scholar] [CrossRef]
- Huynh, B.Q.; Li, H.; Giger, M.L. Digital mammographic tumor classification using transfer learning from deep convolutional neural networks. J. Med. Imaging 2016, 3, 034501. [Google Scholar] [CrossRef]
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet classification with deep convolutional neural networks. Commun. ACM 2012, 60, 84–90. [Google Scholar] [CrossRef]
- Hu, Q.; Whitney, H.M.; Giger, M.L. A deep learning methodology for improved breast cancer diagnosis using multiparametric MRI. Sci. Rep. 2020, 10, 10536. [Google Scholar] [CrossRef]
- Hassan, S.A.; Sayed, M.S.; Abdalla, M.I.; Rashwan, M.A. Breast cancer masses classification using deep convolutional neural networks and transfer learning. Multimed. Tools Appl. 2020, 79, 30735–30768. [Google Scholar] [CrossRef]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; IEEE: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Hassan, S.A.; Sayed, M.S.; Abdalla, M.I.; Rashwan, M.A. Detection of breast cancer mass using MSER detector and features matching. Multimed. Tools Appl. 2019, 78, 20239–20262. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and Operating a Public Information Repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Wang, Y.; Choi, E.J.; Choi, Y.; Zhang, H.; Jin, G.Y.; Ko, S.B. Breast Cancer Classification in Automated Breast Ultrasound Using Multiview Convolutional Neural Network with Transfer Learning. Ultrasound Med. Biol. 2020, 46, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, C.; Vanhoucke, V.; Ioffe, S.; Shlens, J.; Wojna, Z. Rethinking the Inception Architecture for Computer Vision. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Las Vegas, NV, USA, 27–30 June 2016; IEEE: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Hekal, A.A.; Elnakib, A.; Moustafa, H.E.D. Automated early breast cancer detection and classification system. Signal Image Video Process. 2021, 15, 1497–1505. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Hekal, A.A.; Moustafa, H.E.D.; Elnakib, A. Ensemble deep learning system for early breast cancer detection. Evol. Intell. 2023, 16, 1045–1054. [Google Scholar] [CrossRef]
- Moreau, N.; Rousseau, C.; Fourcade, C.; Santini, G.; Brennan, A.; Ferrer, L.; Lacombe, M.; Guillerminet, C.; Colombié, M.; Jézéquel, P.; et al. Automatic Segmentation of Metastatic Breast Cancer Lesions on 18F-FDG PET/CT Longitudinal Acquisitions for Treatment Response Assessment. Cancers 2021, 14, 101. [Google Scholar] [CrossRef]
- Ramos-Pollán, R.; Guevara-López, M.A.; Suárez-Ortega, C.; Díaz-Herrero, G.; Franco-Valiente, J.M.; del Solar, M.R.; de Posada, N.G.; Vaz, M.A.P.; Loureiro, J.; Ramos, I. Discovering Mammography-based Machine Learning Classifiers for Breast Cancer Diagnosis. J. Med. Syst. 2011, 36, 2259–2269. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.H.; Kim, S.; Kang, T.; Bae, Y.T. Tumour 18 F-FDG Uptake on preoperative PET/CT may predict axillary lymph node metastasis in ER-positive/HER2-negative and HER2-positive breast cancer subtypes. Eur. Radiol. 2014, 25, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, A.; Ricardo, S.; Milanezi, F.; Carneiro, V.; Amendoeira, I.; Vieira, D.; Cameselle-Teijeiro, J.; Schmitt, F. Nottingham Prognostic Index in Triple-Negative Breast Cancer: A reliable prognostic tool? BMC Cancer 2011, 11, 299. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.; Coates, A.; Gelber, R.; Thürlimann, B.; Senn, H.J. Strategies for subtypes—dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Pernas, S.; Tolaney, S.M. HER2-positive breast cancer: New therapeutic frontiers and overcoming resistance. Ther. Adv. Med. Oncol. 2019, 11, 175883591983351. [Google Scholar] [CrossRef] [PubMed]
- Goutsouliak, K.; Veeraraghavan, J.; Sethunath, V.; Angelis, C.D.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Towards personalized treatment for early stage HER2-positive breast cancer. Nat. Rev. Clin. Oncol. 2019, 17, 233–250. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Delord, M.; de Roquancourt, A.; Merlet, P.; Hamy, A.S.; Espié, M.; Hindié, E. Prognostic impact of 18F-FDG PET/CT staging and of pathological response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 42, 377–385. [Google Scholar] [CrossRef]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Riedl, C.C.; Dickler, M.N.; Jhaveri, K.; Pandit-Taskar, N.; Weber, W. Molecular Imaging of Biomarkers in Breast Cancer. J. Nucl. Med. 2016, 57, 53S–59S. [Google Scholar] [CrossRef]
- Shermis, R.B.; Redfern, R.E.; Burns, J.; Kudrolli, H. Molecular Breast Imaging in Breast Cancer Screening and Problem Solving. RadioGraphics 2017, 37, 1309–1606. [Google Scholar] [CrossRef]
- Lebron, L.; Greenspan, D.; Pandit-Taskar, N. PET Imaging of Breast Cancer. PET Clin. 2015, 10, 159–195. [Google Scholar] [CrossRef]
- Tchou, J.; Sonnad, S.S.; Bergey, M.R.; Basu, S.; Tomaszewski, J.; Alavi, A.; Schnall, M. Degree of Tumor FDG Uptake Correlates with Proliferation Index in Triple Negative Breast Cancer. Mol. Imaging Biol. 2009, 12, 657–662. [Google Scholar] [CrossRef]
- Ulaner, G.A. PET/CT for Patients with Breast Cancer: Where Is the Clinical Impact? Am. J. Roentgenol. 2019, 213, 254–265. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET Image Acquisition and Quantitative Data Analysis. J. Nucl. Med. 2009, 50, 11S–20S. [Google Scholar] [CrossRef]
- Jacene, H.A.; Leboulleux, S.; Baba, S.; Chatzifotiadis, D.; Goudarzi, B.; Teytelbaum, O.; Horton, K.M.; Kamel, I.; Macura, K.J.; Tsai, H.L.; et al. Assessment of Interobserver Reproducibility in Quantitative 18F-FDG PET and CT Measurements of Tumor Response to Therapy. J. Nucl. Med. 2009, 50, 1760–1769. [Google Scholar] [CrossRef] [PubMed]
- Lebron-Zapata, L.; Jochelson, M.S. Overview of Breast Cancer Screening and Diagnosis. PET Clin. 2018, 13, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Krammer, J.; Schnitzer, A.; Kaiser, C.G.; Buesing, K.A.; Sperk, E.; Brade, J.; Wasgindt, S.; Suetterlin, M.; Schoenberg, S.O.; Sutton, E.J.; et al. 18F-FDG PET/CT for initial staging in breast cancer patients—Is there a relevant impact on treatment planning compared to conventional staging modalities? Eur. Radiol. 2015, 25, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Kalinyak, J.E.; Berg, W.A.; Schilling, K.; Madsen, K.S.; Narayanan, D.; Tartar, M. Breast cancer detection using high-resolution breast PET compared to whole-body PET or PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, 260–275. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Chen, S.; Ibrahim, N.K.; Yan, Y.; Wong, S.T.; Wang, H.; Wong, F.C. Risk stratification in patients with advanced-stage breast cancer by pretreatment [18F]FDG PET/CT. Cancer 2015, 121, 3965–3974. [Google Scholar] [CrossRef]
- Cochet, A.; Dygai-Cochet, I.; Riedinger, J.M.; Humbert, O.; Berriolo-Riedinger, A.; Toubeau, M.; Guiu, S.; Coutant, C.; Coudert, B.; Fumoleau, P.; et al. 18F-FDG PET/CT provides powerful prognostic stratification in the primary staging of large breast cancer when compared with conventional explorations. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, 428–437. [Google Scholar] [CrossRef]
- An, Y.S.; Kang, D.K.; Jung, Y.S.; Han, S.; Kim, T.H. Tumor metabolism and perfusion ratio assessed by 18F-FDG PET/CT and DCE-MRI in breast cancer patients: Correlation with tumor subtype and histologic prognostic factors. Eur. J. Radiol. 2015, 84, 1365–1370. [Google Scholar] [CrossRef] [PubMed]
- Tural, D.; Salim, D.K.; Mutlu, H.; Erkilic, M.; Gunduz, S.; Karakurt, M.; Musri, F.; Tuna, S.; Boz, A.; Aydin, F.; et al. Is there any relation between PET-CT SUVmax value and prognostic factors in locally advanced breast cancer. J. BUON 2015, 20, 1282–1286. [Google Scholar] [PubMed]
- Arslan, E.; Çermik, T.F.; Trabulus, F.D.; Talu, E.C.; Başaran, Ş. Role of 18F-FDG PET/CT in evaluating molecular subtypes and clinicopathological features of primary breast cancer. Nucl. Med. Commun. 2018, 39, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.E.; Kim, J.Y.; Lee, S.H.; Kim, S.; Kang, T. Preoperative 18F-FDG PET/CT predicts disease-free survival in patients with primary invasive ductal breast cancer. Acta Radiol. 2014, 56, 1463–1470. [Google Scholar] [CrossRef]
- Ahn, S.G.; Park, J.T.; Lee, H.M.; Lee, H.W.; Jeon, T.J.; Han, K.; Lee, S.A.; Dong, S.M.; Ryu, Y.H.; Son, E.J.; et al. Standardized uptake value of 18F-fluorodeoxyglucose positron emission tomography for prediction of tumor recurrence in breast cancer beyond tumor burden. Breast Cancer Res. 2014, 16, 502. [Google Scholar] [CrossRef]
- Jung, N.Y.; Kim, S.H.; Choi, B.B.; Kim, S.H.; Sung, M.S. Associations between the standardized uptake value of 18F-FDG PET/CT and the prognostic factors of invasive lobular carcinoma: In comparison with invasive ductal carcinoma. World J. Surg. Oncol. 2015, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A. Predictive factors affecting axillary lymph node involvement in patients with breast cancer in Duhok: Cross-sectional study. Ann. Med. Surg. 2019, 44, 87–90. [Google Scholar] [CrossRef]
- Chung, H.L.; Le-Petross, H.T.; Leung, J.W.T. Imaging Updates to Breast Cancer Lymph Node Management. RadioGraphics 2021, 41, 1283–1299. [Google Scholar] [CrossRef]
- Moo, T.A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef]
- Hamdy, O.; El-Badrawy, A.; Saleh, G.A.; Metwally, I.H.; Abdelwahab, K.; Farouk, O.; Denewer, A.; Setit, A. Preoperative localization of sentinel lymph node in breast cancer patients by silver wire insertion or liquid charcoal injection guided by CT lymphography. Breast J. 2019, 26, 617–624. [Google Scholar] [CrossRef]
- Hamdy, O.; Farouk, O.; El-Badrawy, A.; Denewer, A.T.; Setit, A. Sentinel lymph node biopsy in breast cancer—An updated overview. Eur. Surg. 2020, 52, 268–276. [Google Scholar] [CrossRef]
- Hindié, E.; Groheux, D.; Brenot-Rossi, I.; Rubello, D.; Moretti, J.L.; Espié, M. The Sentinel Node Procedure in Breast Cancer: Nuclear Medicine as the Starting Point. J. Nucl. Med. 2011, 52, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, K.I.; Julian, J.A.; Holloway, C.M.; McCready, D.; Gulenchyn, K.Y.; George, R.; Hodgson, N.; Lovrics, P.; Perera, F.; Elavathil, L.; et al. Prospective Study of 2-[18F]Fluorodeoxyglucose Positron Emission Tomography in the Assessment of Regional Nodal Spread of Disease in Patients with Breast Cancer: An Ontario Clinical Oncology Group Study. J. Clin. Oncol. 2012, 30, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Riegger, C.; Koeninger, A.; Hartung, V.; Otterbach, F.; Kimmig, R.; Forsting, M.; Bockisch, A.; Antoch, G.; Heusner, T.A. Comparison of the Diagnostic Value of FDG-PET/CT and Axillary Ultrasound for the Detection of Lymph Node Metastases in Breast Cancer Patients. Acta Radiol. 2012, 53, 1092–1098. [Google Scholar] [CrossRef]
- Machida, Y.; Kubota, K.; Katayama, T.; Toriihara, A.; Shibuya, H. Diagnostic performance of fluorodeoxyglucose-positron emission tomography/computed tomography combined with ultrasonography-guided fine needle aspiration cytology for identifying axillary lymph node status in patients with breast cancer. Eur. J. Surg. Oncol. (EJSO) 2013, 39, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Jochelson, M.S.; Lebron, L.; Jacobs, S.S.; Zheng, J.; Moskowitz, C.S.; Powell, S.N.; Sacchini, V.; Ulaner, G.A.; Morris, E.A.; Dershaw, D.D. Detection of Internal Mammary Adenopathy in Patients with Breast Cancer by PET/CT and MRI. Am. J. Roentgenol. 2015, 205, 899–904. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Lee, A.W. Comparisons of Positron Emission Tomography/Computed Tomography and Ultrasound Imaging for Detection of Internal Mammary Lymph Node Metastases in Patients with Breast Cancer and Pathologic Correlation by Ultrasound-Guided Biopsy Procedures. J. Ultrasound Med. 2015, 34, 1385–1394. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Espié, M.; Vercellino, L.; Hamy, A.S.; Delord, M.; Berenger, N.; Toubert, M.E.; Misset, J.L.; Hindié, E. The Yield of 18F-FDG PET/CT in Patients with Clinical Stage IIA, IIB, or IIIA Breast Cancer: A Prospective Study. J. Nucl. Med. 2011, 52, 1526–1534. [Google Scholar] [CrossRef]
- Salaün, P.Y.; Abgral, R.; Malard, O.; Querellou-Lefranc, S.; Quere, G.; Wartski, M.; Coriat, R.; Hindie, E.; Taieb, D.; Tabarin, A.; et al. Good clinical practice recommendations for the use of PET/CT in oncology. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 28–50. [Google Scholar] [CrossRef]
- Wu, S.G.; Li, H.; Tang, L.Y.; Sun, J.Y.; Zhang, W.W.; Li, F.Y.; Chen, Y.X.; He, Z.Y. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: A SEER database analysis. Tumor Biol. 2017, 39, 101042831770508. [Google Scholar] [CrossRef]
- Teke, F.; Teke, M.; Inal, A.; Kaplan, M.A.; Kucukoner, M.; Aksu, R.; Urakci, Z.; Tasdemir, B.; Isikdogan, A. Significance of Hormone Receptor Status in Comparison of 18F -FDG-PET/CT and 99mTc-MDP Bone Scintigraphy for Evaluating Bone Metastases in Patients with Breast Cancer: Single center Experience. Asian Pac. J. Cancer Prev. 2015, 16, 387–391. [Google Scholar] [CrossRef]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Vogsen, M.; Jensen, J.D.; Christensen, I.Y.; Gerke, O.; Jylling, A.M.B.; Larsen, L.B.; Braad, P.E.; Søe, K.L.; Bille, C.; Ewertz, M.; et al. FDG-PET/CT in high-risk primary breast cancer—A prospective study of stage migration and clinical impact. Breast Cancer Res. Treat. 2020, 185, 145–153. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Xu, T.; Ding, H.; Li, Y.; Liu, H.; Huang, Y.; Liu, L.; Du, T.; Zhao, Y.; et al. Comparison of Diagnostic Efficacy of [68Ga]Ga-FAPI-04 and [18F]FDG PET/CT for Staging and Restaging of Gastric Cancer. Front. Oncol. 2022, 12, 925100. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Meng, T.; Shang, Q.; Pang, Y.; Chen, H. Uncommon Metastases From Occult Breast Cancer Revealed by 18F-FDG and 68Ga-FAPI PET/CT. Clin. Nucl. Med. 2022, 47, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Eshet, Y.; Tau, N.; Apter, S.; Nissan, N.; Levanon, K.; Bernstein-Molho, R.; Globus, O.; Itay, A.; Shapira, T.; Oedegaard, C.; et al. The Role of 68Ga-FAPI PET/CT in Detection of Metastatic Lobular Breast Cancer. Clin. Nucl. Med. 2023, 48, 228–232. [Google Scholar] [CrossRef]
- Yanai, A.; Itoh, M.; Hirakawa, H.; Yanai, K.; Tashiro, M.; Harada, R.; Yoshikawa, A.; Yamamoto, S.; Ohuchi, N.; Ishida, T. Newly-Developed Positron Emission Mammography (PEM) Device for the Detection of Small Breast Cancer. Tohoku J. Exp. Med. 2018, 245, 13–19. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Kumar, V.; Morse, D.L.; Gatenby, R.A. Molecular and Functional Imaging of Breast Cancer. Cancer Control 2010, 17, 143–155. [Google Scholar] [CrossRef]
- Glass, S.B.; Shah, Z.A. Clinical Utility of Positron Emission Mammography. Bayl. Univ. Med. Cent. Proc. 2013, 26, 314–319. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Lima, E.N.P.; Macedo, B.R.C.; Conrado, J.L.F.A.; Marques, E.F.; Chojniak, R. Can positron emission mammography help to identify clinically significant breast cancer in women with suspicious calcifications on mammography? Eur. Radiol. 2016, 27, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Kalles, V.; Zografos, G.C.; Provatopoulou, X.; Koulocheri, D.; Gounaris, A. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer 2012, 20, 123–130. [Google Scholar] [CrossRef]
- Weaver, O.; Leung, J.W.T. Biomarkers and Imaging of Breast Cancer. Am. J. Roentgenol. 2018, 210, 271–278. [Google Scholar] [CrossRef]
- Harris, L.; Fritsche, H.; Mennel, R.; Norton, L.; Ravdin, P.; Taube, S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. American Society of Clinical Oncology 2007 Update of Recommendations for the Use of Tumor Markers in Breast Cancer. J. Clin. Oncol. 2007, 25, 5287–5312. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Liu, J.; Hayashi, N.; Mittendorf, E.A.; Gong, Y.; Palla, S.L.; Tokuda, Y.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Ueno, N.T. Loss of Human Epidermal Growth Factor Receptor 2 (HER2) Expression in Metastatic Sites of HER2-Overexpressing Primary Breast Tumors. J. Clin. Oncol. 2012, 30, 593–599. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Ang, J.E.; Kaye, S.; Banerji, U. Tissue-Based Approaches to Study Pharmacodynamic Endpoints in Early Phase Oncology Clinical Trials. Curr. Drug Targets 2012, 13, 1525–1534. [Google Scholar] [CrossRef]
- Drew, Y.; Ledermann, J.; Hall, G.; Rea, D.; Glasspool, R.; Highley, M.; Jayson, G.; Sludden, J.; Murray, J.; Jamieson, D.; et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br. J. Cancer 2016, 114, 723–730. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Pryma, D.A.; Clark, A.S. Molecular Imaging Biomarkers for Oncology Clinical Trials. J. Nucl. Med. 2014, 55, 525–528. [Google Scholar] [CrossRef]
- Marinelli, B.; Espinet-Col, C.; Ulaner, G.A.; McArthur, H.L.; Gonen, M.; Jochelson, M.; Weber, W.A. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am. J. Nucl. Med. Mol. Imaging 2016, 6, 120. [Google Scholar]
- Humbert, O.; Cochet, A.; Coudert, B.; Berriolo-Riedinger, A.; Kanoun, S.; Brunotte, F.; Fumoleau, P. Role of positron emission tomography for the monitoring of response to therapy in breast cancer. Oncologist 2015, 20 2, 94–104. [Google Scholar] [CrossRef]
- Dewidar, S.A.; Hamdy, O.; Eltantawy, A.; El-Mesery, M.; Gayar, A.M.E.; Soliman, M.M. Effect of concomitant use of pitavastatin with neoadjuvant chemotherapy protocols in breast cancer patients: A randomized controlled clinical trial. Saudi Pharm. J. 2022, 30, 1486–1496. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef] [PubMed]
- Lagendijk, M.; van Maaren, M.C.; Saadatmand, S.; Strobbe, L.J.; Poortmans, P.M.; Koppert, L.B.; Tilanus-Linthorst, M.M.; Siesling, S. Breast conserving therapy and mastectomy revisited: Breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int. J. Cancer 2017, 142, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Awan, U.A.; Saeed, R.F.; Qazi, A.S.; Mumtaz, S.; Rubnawaz, S. Utility of Personalized Medicine in the Treatment of Different Subtypes of Breast Cancer. In Breast Cancer: From Bench to Personalized Medicine; Springer Nature: Singapore, 2022; pp. 337–366. [Google Scholar] [CrossRef]
- Loi, S. The ESMO clinical practise guidelines for early breast cancer: Diagnosis, treatment and follow-up: On the winding road to personalized medicine. Ann. Oncol. 2019, 30, 1183–1184. [Google Scholar] [CrossRef] [PubMed]
- Czajka, M.L.; Pfeifer, C. Breast Cancer Surgery; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Cruz, L.D.L.; Moody, A.M.; Tappy, E.E.; Blankenship, S.A.; Hecht, E.M. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple—Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann. Surg. Oncol. 2015, 22, 3241–3249. [Google Scholar] [CrossRef]
- Wei, C.H.; Scott, A.M.; Price, A.N.; Miller, H.C.; Klassen, A.F.; Jhanwar, S.M.; Mehrara, B.J.; Disa, J.J.; McCarthy, C.; Matros, E.; et al. Psychosocial and Sexual Well-Being Following Nipple-Sparing Mastectomy and Reconstruction. Breast J. 2016, 22, 10–17. [Google Scholar] [CrossRef]
- Char, S.; Bloom, J.A.; Erlichman, Z.; Jonczyk, M.M.; Chatterjee, A. A comprehensive literature review of patient-reported outcome measures (PROMs) among common breast reconstruction options: What types of breast reconstruction score well? Breast J. 2021, 27, 322–329. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Chung, A.; Giuliano, A.E. Lymphatic Mapping and Sentinel Lymphadenectomy for Breast Cancer. In The Breast; Elsevier: Amsterdam, The Netherlands, 2018; pp. 604–630.e6. [Google Scholar] [CrossRef]
- Veronesi, P.; Corso, G. Standard and controversies in sentinel node in breast cancer patients. Breast 2019, 48, S53–S56. [Google Scholar] [CrossRef]
- Cserni, G.; Maguire, A.; Bianchi, S.; Ryska, A.; Kovács, A. Sentinel lymph node assessment in breast cancer—an update on current recommendations. Virchows Arch. 2021, 480, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Beddok, A.; Cottu, P.; Fourquet, A.; Kirova, Y. Combination of Modern Radiotherapy and New Targeted Treatments for Breast Cancer Management. Cancers 2021, 13, 6358. [Google Scholar] [CrossRef]
- Haussmann, J.; Corradini, S.; Nestle-Kraemling, C.; Bölke, E.; Njanang, F.J.D.; Tamaskovics, B.; Orth, K.; Ruckhaeberle, E.; Fehm, T.; Mohrmann, S.; et al. Recent advances in radiotherapy of breast cancer. Radiat. Oncol. 2020, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.E.; Moses, L.; Stuart, K.; Nahar, N.; Tiver, K.; Wang, T.; Ward, R.; Ahern, V. Contouring consensus guidelines in breast cancer radiotherapy: Comparison and systematic review of patterns of failure. J. Med. Imaging Radiat. Oncol. 2018, 63, 102–115. [Google Scholar] [CrossRef]
- Asaoka, M.; Gandhi, S.; Ishikawa, T.; Takabe, K. Neoadjuvant Chemotherapy for Breast Cancer: Past, Present, and Future. Breast Cancer Basic Clin. Res. 2020, 14, 117822342098037. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef]
- Denduluri, N.; Chavez-MacGregor, M.; Telli, M.L.; Eisen, A.; Graff, S.L.; Hassett, M.J.; Holloway, J.N.; Hurria, A.; King, T.A.; Lyman, G.H.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2433–2443. [Google Scholar] [CrossRef]
- Willson, M.L.; Burke, L.; Ferguson, T.; Ghersi, D.; Nowak, A.K.; Wilcken, N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst. Rev. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Krauss, K.; Stickeler, E. Endocrine Therapy in Early Breast Cancer. Breast Care 2020, 15, 337–346. [Google Scholar] [CrossRef]
- Taourel, P.; Pages, E.; Millet, I.; Bourgier, C.; Rouanet, P.; Jacot, W.; Crochet, P.; Azria, D. Magnetic resonance imaging in breast cancer management in the context of neo-adjuvant chemotherapy. Crit. Rev. Oncol. 2018, 132, 51–65. [Google Scholar] [CrossRef]
- Jochelson, M.S.; Lampen-Sachar, K.; Gibbons, G.; Dang, C.; Lake, D.; Morris, E.A.; Morrow, M. Do MRI and Mammography Reliably Identify Candidates for Breast Conservation After Neoadjuvant Chemotherapy? Ann. Surg. Oncol. 2015, 22, 1490–1495. [Google Scholar] [CrossRef]
- Taydas, O.; Durhan, G.; Akpinar, M.G.; Demirkazik, F.B. Comparison of MRI and US in Tumor Size Evaluation of Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Eur. J. Breast Health 2019, 15, 119–124. [Google Scholar] [CrossRef]
- Panorgias, A.; Zawadzki, R.J.; Capps, A.G.; Hunter, A.A.; Morse, L.S.; Werner, J.S. Multimodal Assessment of Microscopic Morphology and Retinal Function in Patients with Geographic Atrophy. Investig. Opthalmology Vis. Sci. 2013, 54, 4372. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Lee, H.B.; Lee, Y.J.; Seong, M.K.; Paik, N.; Park, W.C.; Park, S.; Jung, S.P.; and, S.Y.B. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: Comparative analysis to invasive carcinoma of no special type. Breast Cancer Res. Treat. 2020, 184, 527–542. [Google Scholar] [CrossRef]
- Vicente, A.M.G.; Mora, M.Á.C.; Martín, A.A.L.; del Mar Muñoz Sánchez, M.; Calatayud, F.R.; López, O.V.G.; Aunión, R.E.; Ageitos, A.G.; Castrejón, Á.S. Glycolytic activity with 18F-FDG PET/CT predicts final neoadjuvant chemotherapy response in breast cancer. Tumor Biol. 2014, 35, 11613–11620. [Google Scholar] [CrossRef]
- Schwarz-Dose, J.; Untch, M.; Tiling, R.; Sassen, S.; Mahner, S.; Kahlert, S.; Harbeck, N.; Lebeau, A.; Brenner, W.; Schwaiger, M.; et al. Monitoring Primary Systemic Therapy of Large and Locally Advanced Breast Cancer by Using Sequential Positron Emission Tomography Imaging with [18F]Fluorodeoxyglucose. J. Clin. Oncol. 2009, 27, 535–541. [Google Scholar] [CrossRef]
- Rousseau, C.; Devillers, A.; Sagan, C.; Ferrer, L.; Bridji, B.; Campion, L.; Ricaud, M.; Bourbouloux, E.; Doutriaux, I.; Clouet, M.; et al. Monitoring of Early Response to Neoadjuvant Chemotherapy in Stage II and III Breast Cancer by [18F]Fluorodeoxyglucose Positron Emission Tomography. J. Clin. Oncol. 2006, 24, 5366–5372. [Google Scholar] [CrossRef]
- Tian, F.; Shen, G.; Deng, Y.; Diao, W.; Jia, Z. The accuracy of 18F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: A meta-analysis and systematic review. Eur. Radiol. 2017, 27, 4786–4796. [Google Scholar] [CrossRef]
- Connolly, R.M.; Leal, J.P.; Solnes, L.; Huang, C.Y.; Carpenter, A.; Gaffney, K.; Abramson, V.; Carey, L.A.; Liu, M.C.; Rimawi, M.; et al. TBCRC026: Phase II Trial Correlating Standardized Uptake Value with Pathologic Complete Response to Pertuzumab and Trastuzumab in Breast Cancer. J. Clin. Oncol. 2019, 37, 714–722. [Google Scholar] [CrossRef]
- Weber, J.J.; Jochelson, M.S.; Eaton, A.; Zabor, E.C.; Barrio, A.V.; Gemignani, M.L.; Pilewskie, M.; Zee, K.J.V.; Morrow, M.; El-Tamer, M. MRI and Prediction of Pathologic Complete Response in the Breast and Axilla after Neoadjuvant Chemotherapy for Breast Cancer. J. Am. Coll. Surg. 2017, 225, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Sener, S.F.; Sargent, R.E.; Lee, C.; Manchandia, T.; Le-Tran, V.; Olimpiadi, Y.; Zaremba, N.; Alabd, A.; Nelson, M.; Lang, J.E. MRI does not predict pathologic complete response after neoadjuvant chemotherapy for breast cancer. J. Surg. Oncol. 2019, 120, 903–910. [Google Scholar] [CrossRef]
- Kim, J.; Han, B.K.; Ko, E.Y.; Ko, E.S.; Choi, J.S.; Park, K.W. Prediction of pathologic complete response on MRI in patients with breast cancer receiving neoadjuvant chemotherapy according to molecular subtypes. Eur. Radiol. 2022, 32, 4056–4066. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Horii, R.; Gomi, N.; Miyagi, Y.; Takahashi, S.; Ito, Y.; Akiyama, F.; Ohno, S.; Iwase, T. Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: Association with breast cancer subtype. Springerplus 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Y.; Kim, S.H.; Kang, B.J.; Lee, A.W. Treatment Response Evaluation of Breast Cancer after Neoadjuvant Chemotherapy and Usefulness of the Imaging Parameters of MRI and PET/CT. J. Korean Med. Sci. 2015, 30, 808. [Google Scholar] [CrossRef]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lin, N.U.; Guo, H.; Yap, J.T.; Mayer, I.A.; Falkson, C.I.; Hobday, T.J.; Dees, E.C.; Richardson, A.L.; Nanda, R.; Rimawi, M.F.; et al. Phase II Study of Lapatinib in Combination with Trastuzumab in Patients with Human Epidermal Growth Factor Receptor 2—Positive Metastatic Breast Cancer: Clinical Outcomes and Predictive Value of Early [18F]Fluorodeoxyglucose Positron Emission Tomography Imaging (TBCRC 003). J. Clin. Oncol. 2015, 33, 2623–2631. [Google Scholar] [CrossRef]
- Tateishi, U.; Gamez, C.; Dawood, S.; Yeung, H.W.D.; Cristofanilli, M.; Macapinlac, H.A. Bone Metastases in Patients with Metastatic Breast Cancer: Morphologic and Metabolic Monitoring of Response to Systemic Therapy with Integrated PET/CT. Radiology 2008, 247, 189–196. [Google Scholar] [CrossRef]
- Iagaru, A.; Minamimoto, R. Nuclear Medicine Imaging Techniques for Detection of Skeletal Metastases in Breast Cancer. PET Clin. 2018, 13, 383–393. [Google Scholar] [CrossRef]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef]
- Mani, S.; Chen, Y.; Arlinghaus, L.R.; Li, X.; Chakravarthy, A.B.; Bhave, S.R.; Welch, E.B.; Levy, M.A.; Yankeelov, T.E. Early prediction of the response of breast tumors to neoadjuvant chemotherapy using quantitative MRI and machine learning. AMIA Annu. Symp. Proceedings Arch. 2011, 2011, 868–877. [Google Scholar]
- Huober, J.; von Minckwitz, G.; Denkert, C.; Tesch, H.; Weiss, E.; Zahm, D.M.; Belau, A.; Khandan, F.; Hauschild, M.; Thomssen, C.; et al. Effect of neoadjuvant anthracycline—taxane-based chemotherapy in different biological breast cancer phenotypes: Overall results from the GeparTrio study. Breast Cancer Res. Treat. 2010, 124, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, A.; Wengert, G.J.; Helbich, T.H.; Bago-Horvath, Z.; Alaei, S.; Bartsch, R.; Dubsky, P.; Baltzer, P.; Clauser, P.; Kapetas, P.; et al. Impact of Machine Learning with Multiparametric Magnetic Resonance Imaging of the Breast for Early Prediction of Response to Neoadjuvant Chemotherapy and Survival Outcomes in Breast Cancer Patients. Investig. Radiol. 2019, 54, 110–117. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Hooda, N. Prediction of Pathological Complete Response after Neoadjuvant Chemotherapy for breast cancer using ensemble machine learning. Inform. Med. Unlocked 2019, 16, 100219. [Google Scholar] [CrossRef]
- Newitt, D.; Hylton, N. Multi-center breast DCE-MRI data and segmentations from patients in the I-SPY 1/ACRIN 6657 trials. Cancer Imaging Arch. 2016, 10, 7. [Google Scholar]
- Aghaei, F.; Tan, M.; Hollingsworth, A.B.; Qian, W.; Liu, H.; Zheng, B. Computer-aided breast MR image feature analysis for prediction of tumor response to chemotherapy. Med. Phys. 2015, 42, 6520–6528. [Google Scholar] [CrossRef]
- Sutton, E.J.; Onishi, N.; Fehr, D.A.; Dashevsky, B.Z.; Sadinski, M.; Pinker, K.; Martinez, D.F.; Brogi, E.; Braunstein, L.; Razavi, P.; et al. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. 2020, 22, 57. [Google Scholar] [CrossRef]
- Vicent, C.H.; Tudela, X.; Ruiz, P.M.; Pedralva, V.; Pastor, A.J.; Ahicart, D.; Novella, S.R.; Meneu, I.; Albuixech, Á.M.; Santamaria, M.Á.; et al. Machine Learning Models and Multiparametric Magnetic Resonance Imaging for the Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancers 2022, 14, 3508. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Elsharkawy, M.; Khalifa, F.; Soliman, A.; Ghazal, M.; AlHalabi, M.; Yaghi, M.; Alrahmawy, M.; Elmougy, S.; Sandhu, H.S.; et al. Precise higher-order reflectivity and morphology models for early diagnosis of diabetic retinopathy using OCT images. Sci. Rep. 2021, 11, 4730. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, D.; Kandil, H.; Khelifi, A.; Yaghi, M.; Ghazal, M.; Sharafeldeen, A.; Mahmoud, A.; El-Baz, A. How AI Can Help in the Diagnostic Dilemma of Pulmonary Nodules. Cancers 2022, 14, 1840. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldeen, A.; Elsharkawy, M.; Shaffie, A.; Khalifa, F.; Soliman, A.; Naglah, A.; Khaled, R.; Hussein, M.M.; Alrahmawy, M.; Elmougy, S.; et al. Thyroid Cancer Diagnostic System using Magnetic Resonance Imaging. In Proceedings of the 2022 26th International Conference on Pattern Recognition (ICPR), Montreal, QC, Canada, 21–25 August 2022; IEEE: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Elsharkawy, M.; Alghamdi, N.S.; Soliman, A.; El-Baz, A. Precise Segmentation of COVID-19 Infected Lung from CT Images Based on Adaptive First-Order Appearance Model with Morphological/Anatomical Constraints. Sensors 2021, 21, 5482. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Alksas, A.; Ghazal, M.; Yaghi, M.; Khelifi, A.; Mahmoud, A.; Contractor, S.; van Bogaert, E.; El-Baz, A. Accurate Segmentation for Pathological Lung Based on Integration of 3D Appearance and Surface Models. In Proceedings of the 2023 IEEE International Conference on Image Processing (ICIP), Kuala Lumpur, Malaysia, 8–11 October 2023; IEEE: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Elsharkawy, M.; Khaled, R.; Shaffie, A.; Khalifa, F.; Soliman, A.; khalek Abdel Razek, A.A.; Hussein, M.M.; Taman, S.; Naglah, A.; et al. Texture and shape analysis of diffusion-weighted imaging for thyroid nodules classification using machine learning. Med. Phys. 2021, 49, 988–999. [Google Scholar] [CrossRef]
- Elgafi, M.; Sharafeldeen, A.; Elnakib, A.; Elgarayhi, A.; Alghamdi, N.S.; Sallah, M.; El-Baz, A. Detection of Diabetic Retinopathy Using Extracted 3D Features from OCT Images. Sensors 2022, 22, 7833. [Google Scholar] [CrossRef]
- Sharafeldeen, A.; Elgafi, M.; Elnakib, A.; Mahmoud, A.; Elgarayhi, A.; Alghamdi, N.S.; Sallah, M.; El-Baz, A. Diabetic Retinopathy Detection Using 3D OCT Features. In Proceedings of the 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena, Colombia, 18–21 April 2023; IEEE: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Haggag, S.; Elnakib, A.; Sharafeldeen, A.; Elsharkawy, M.; Khalifa, F.; Farag, R.K.; Mohamed, M.A.; Sandhu, H.S.; Mansoor, W.; Sewelam, A.; et al. A Computer-Aided Diagnostic System for Diabetic Retinopathy Based on Local and Global Extracted Features. Appl. Sci. 2022, 12, 8326. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Elmogy, M.; Sharafeldeen, A.T.; Elsharkawy, M.; El-Adawy, N.; Eltanboly, A.; Shalaby, A.; Keynton, R.; El-Baz, A. Automated Diagnosis of Diabetic Retinopathy Using Clinical Biomarkers, Optical Coherence Tomography, and Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2020, 216, 201–206. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Sharafeldeen, A.; Soliman, A.; Khalifa, F.; Ghazal, M.; El-Daydamony, E.; Atwan, A.; Sandhu, H.S.; El-Baz, A. A Novel Computer-Aided Diagnostic System for Early Detection of Diabetic Retinopathy Using 3D-OCT Higher-Order Spatial Appearance Model. Diagnostics 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.; Sharafeldeen, A.; Soliman, A.; Khalifa, F.; Ghazal, M.; El-Daydamony, E.; Atwan, A.; Sandhu, H.S.; El-Baz, A. Diabetic Retinopathy Diagnostic CAD System Using 3D-Oct Higher Order Spatial Appearance Model. In Proceedings of the 2022 IEEE 19th International Symposium on Biomedical Imaging (ISBI), Kolkata, India, 28–31 March 2022; IEEE: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Sharafeldeen, A.; Taher, F.; Shalaby, A.; Soliman, A.; Mahmoud, A.; Ghazal, M.; Khalil, A.; Alghamdi, N.S.; Razek, A.A.K.A.; et al. Early assessment of lung function in coronavirus patients using invariant markers from chest X-rays images. Sci. Rep. 2021, 11, 12095. [Google Scholar] [CrossRef]
- Farahat, I.S.; Sharafeldeen, A.; Elsharkawy, M.; Soliman, A.; Mahmoud, A.; Ghazal, M.; Taher, F.; Bilal, M.; Razek, A.A.K.A.; Aladrousy, W.; et al. The Role of 3D CT Imaging in the Accurate Diagnosis of Lung Function in Coronavirus Patients. Diagnostics 2022, 12, 696. [Google Scholar] [CrossRef]
- Alghamdi, N.S.; Taher, F.; Kandil, H.; Sharafeldeen, A.; Elnakib, A.; Soliman, A.; ElNakieb, Y.; Mahmoud, A.; Ghazal, M.; El-Baz, A. Segmentation of Infant Brain Using Nonnegative Matrix Factorization. Appl. Sci. 2022, 12, 5377. [Google Scholar] [CrossRef]

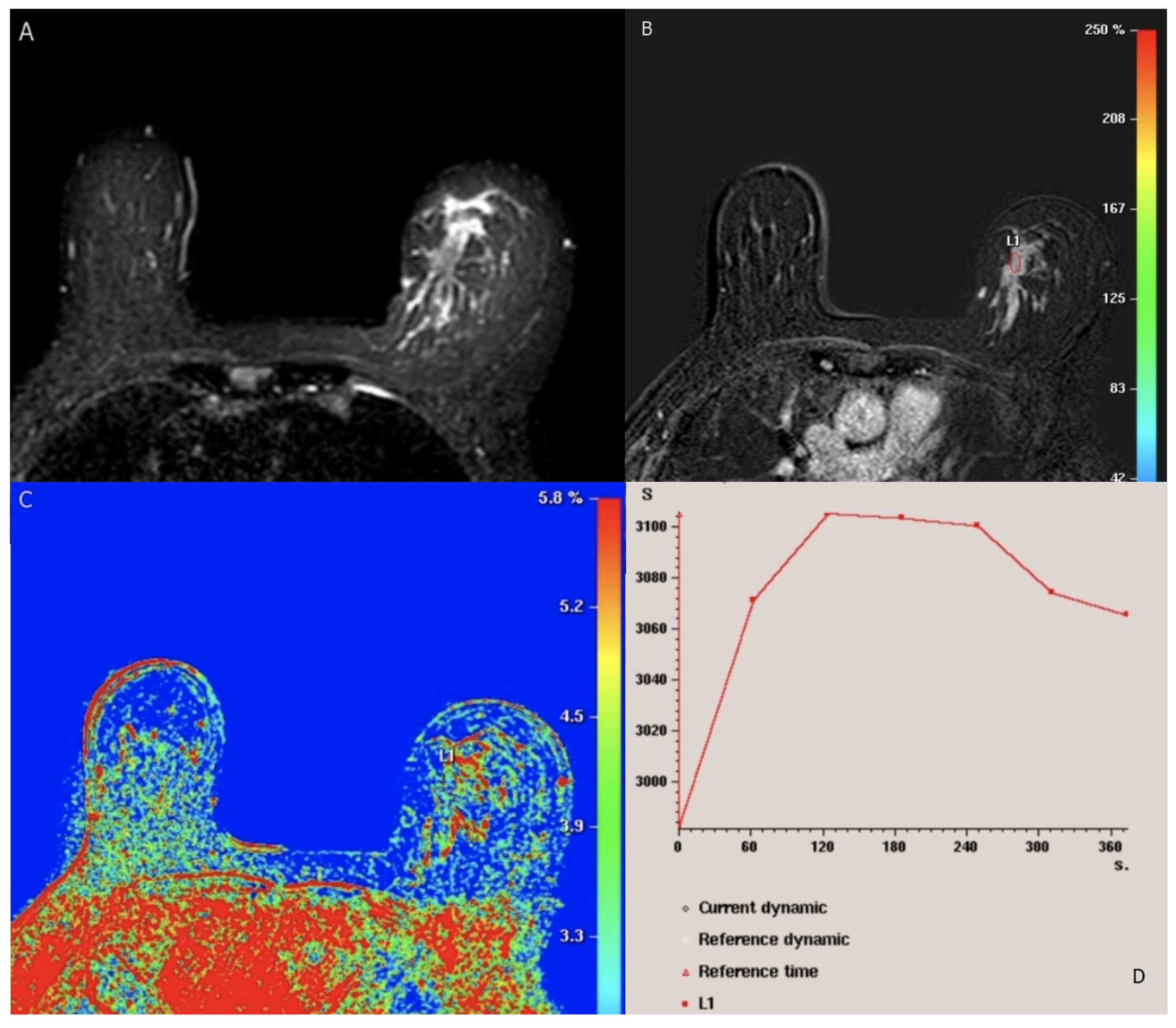
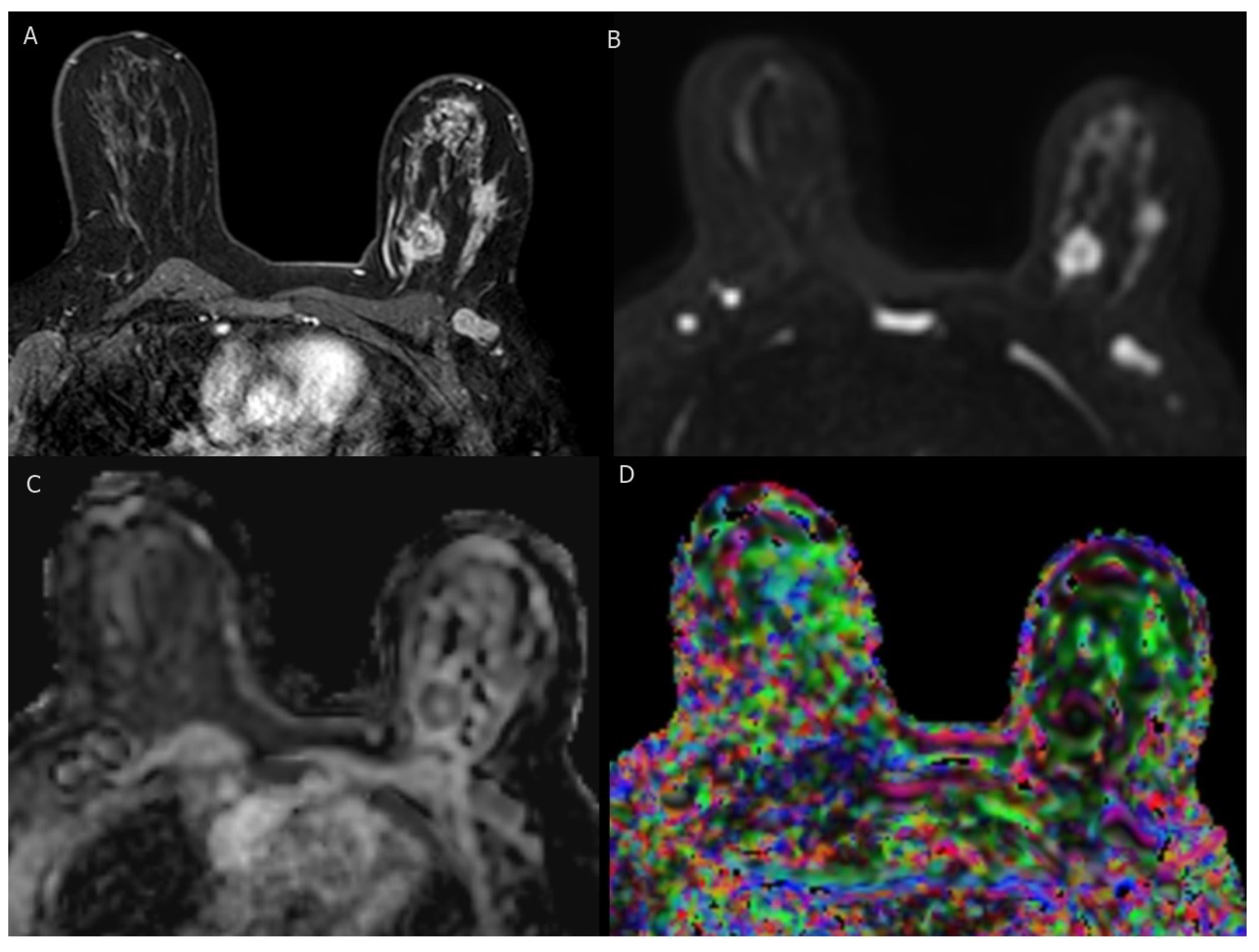
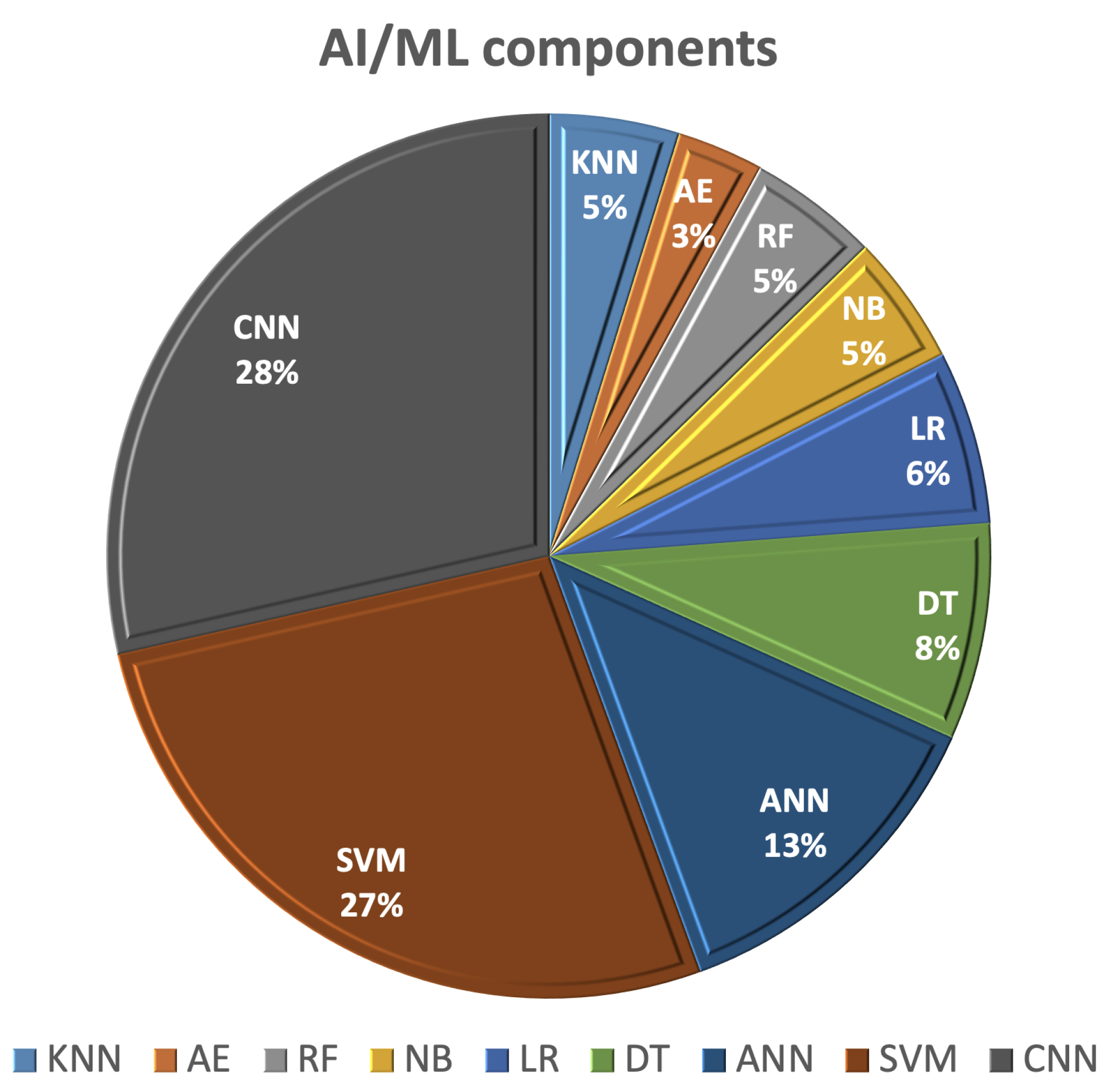
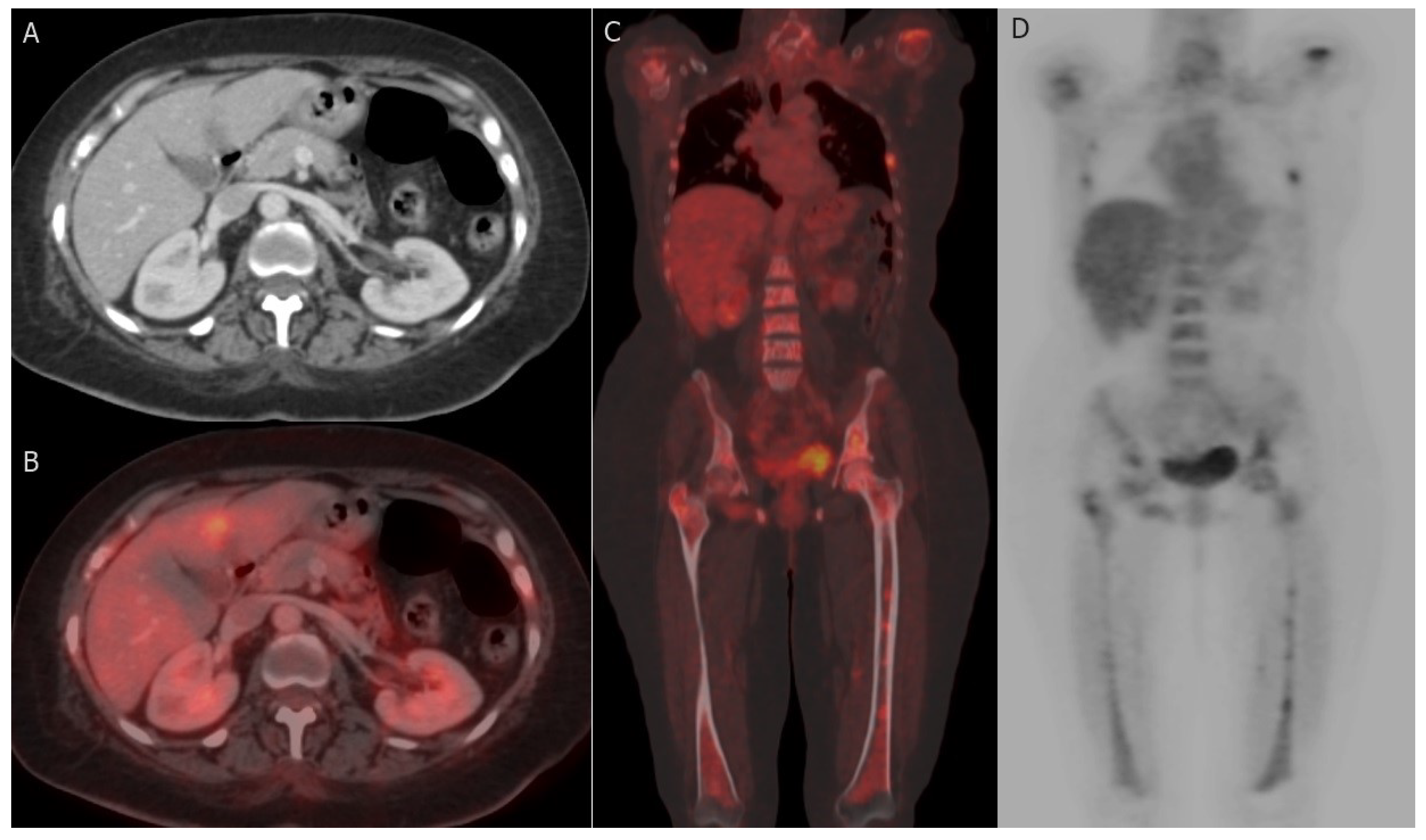
| Category | Description | Recommendation |
|---|---|---|
| 0 | Incomplete assessment:
|
|
| 1 | Negative: negative assessment | Screening within 1 year (per SBI and ACR recommendations); no expected malignancy. |
| 2 | Benign findings: to express a benign lesion that has no malignant possibility | Screening within 1 year (per SBI and ACR recommendations); similar to category 1. Category 1 is favored over category 2 whenever suitable to avoid patient and clinician anxiety and requesting unnecessary imaging examinations after the description of benign findings. |
| 3 | Probably benign finding: debatable category applied when a finding is nearly definitely benign but preferred to have a short interval follow-up; unlikely to demand biopsy. It holds a risk of malignancy up to 2%. | Short interval follow-up examinations (classically 6 months) for 24–36 months is recommended. Stability established at the end of follow-up is considered benign, thus the finding is relocated category 2. |
| 4 | Suspicious abnormality: finding not classic for malignancy, >2% to <95% chance of malignancy. On US and mammography, it split into:
| Intervention is essential, better to be image-guided tissue biopsy to create a pathologic diagnosis; follow-up of biopsy outcomes with radiology-pathology correlation is allocated to the examining radiologist. |
| 5 | Extremely evocative of malignancy: 95–100% likelihood of malignancy; typical findings of malignancy | Image-guided Core biopsy for tissue sample; benign result is deemed discordant, and further intervention is advised and may incorporate replicate image-guided vs surgical biopsy. |
| 6 | Biopsy-proven malignancy: verified cancer that has not finished definitive treatment | Properly utilized in patients receiving neoadjuvant therapy or in those who need additional staging; clinical managing of the malignancy is recommended. |
| Findings | Descriptors |
|---|---|
| Breast density |
|
| Mass: Shape Margin Density | A space-occupying 3D object. Round, oval, irregular Circumscribed, obscured, micro-lobulated, indistinct, spiculated High, equal density, low, fat-containing |
| Calcification: | |
| Typically benign | Skin, popcornlike vascular, large rodlike, milk of calcium, dystrophic, suture |
| Suspiciou morphology | Amorphous, heterogeneous, coarse, fine linear or fine-linear branching, fine pleomorphic. |
| Distribution | Diffuse, linear, segmental, regional, grouped. |
| Asymmetry | Asymmetry, global asymmetry, focal asymmetry, developing asymmetry |
| Associated features | Skin thickening, skin retraction, retracted nipple, trabecular thickening, axillary lymphadenopathy, calcifications |
| Location of lesion | Laterality, clockface and quadrant, distance from the nipple |
| Findings | Descriptors |
|---|---|
| Tissue composition/ background echotexture | Homogenous (Fat, fibroglandular). Heterogenous. |
| Mass: | |
| Shape Margin Orientation Echo pattern Posterior features | Round, oval, irregular. Circumscribed, indistinct, angular, microlobulated, spiculated. Parallel or not parallel Anechoic, hypoechoic, hyperechoic, isoechoic, heterogeneous, complex cystic and solid. No posterior acoustic features, shadowing, enhancement, combined features. |
| Calcification: | Calcifications inside a mass, intraductal calcifications outside of a mass. |
| Associated features | Skin thickening, edema, skin retraction, vascularity (absent, vessels in rim, internal vascularity), architectural distortion, elasticity assessment (soft, intermediate, hard). |
| Special cases | Simple cyst, complicated cyst, clustered microcysts, foreign body counting implants, mass in skin, lymph nodes (intramammary or axillary), vascular abnormalities (arteriovenous malformation, pseudoaneurysms, or Mondor disease), postoperative collection, fat necrosis. |
| Findings | Descriptors |
|---|---|
| Tissue composition | Entirely fatty breast Scattered fibroglandular tissue Heterogeneous fibroglandular tissue Marked fibroglandular tissue |
| Background parenchymal enhancement (BPE) | |
| Symmetry Level | Symmetrical/Asymmetrical Minimal/Mild/Moderate/Severe |
| Focus: | Yes/No |
| Mass: | |
| Shape Margin | Oval (+lobulated)/Round/Irregular Circumscribed |
| Irregular/Spiculated | |
| Patterns of internal enhancement | Homogenous Heterogenous Clumped Clustered ring |
| Non-mass enhancement: | |
| Distribution | Focal/Linear/Segmental/Regional/Multi-regional/Diffuse |
| Patterns of internal enhancement | Homogenous Heterogenous Clumped Clustered ring |
| Non-enhancing findings: | Cyst, non-enhancing mass, architectural distortion, ductal hyperintensity on precontrast T1 weighted images, postsurgical hematoma or seroma, posttreatment skin thickening, signal void from clips and foreign bodies. |
| Concomitant findings: | Skin retraction, skin invasion, nipple retraction, nipple invasion, pectoralis muscle invasion, chest wall invasion, inflammatory breast cancer, axillary adenopathy, architectural distortion. |
| Fat-containing lesions: | Normal or abnormal lymph nodes, hamartoma, fat necrosis, postoperative seroma encompassing fat. |
| Intra-mammary lymph nodes: | Yes/No |
| Skin lesions: | Yes/No |
| Location and depth of lesions: | |
| Implant findings: | Material of the implant, lumen type, contour, position, water droplets, intra- and extracapsular findings, peri-implant findings. |
| Kinetic signal intensity time curve assessment: | |
| Initial phase | Slow/Medium/Fast |
| Delayed phase | Persistent/Plateau/Washout |
| Name | Rule |
|---|---|
| Accuracy (Acc) | (TP + TN)/Total |
| Precision (Prec) | TP/(TP + FP) |
| Recall (Rec) or Sensitivity (Sens) or True Positive Rate (TPR) | TP/(TP + FN) |
| Specificity (Spec) | TN/(FP + TN) |
| F-Measure (F1-M) | (2 × Precision × Recall)/(Precision + Recall) |
| False Positive Rate (FPR) | |
| PR AUC | Precision-Recall Area Under Curve |
| Receiver operating characteristic curve (ROC) | An ROC curve plots TPR vs. FPR at different classification thresholds |
| Area Under the ROC Curve (AUC) | AUC measures the entire two-dimensional area underneath the entire ROC curve |
| Correlation | |
| AUC-SD | Standard deviation of the AUC |
| Study | Method | Goal | Database | Evaluation |
|---|---|---|---|---|
| Adel et al., 2019 [76] |
| Malignant/Benign BC Classification | Private data, 82 images from 34 patients (56 malignant and 26 benign) | Acc = 94.1 |
| Wei et al., 2019 [84] |
| Malignant/Benign Ultrasound BC Classification | Ultrasound dataset (472 benign, 589 malignant) |
|
| El-Azizy et al., 2019 [83] |
| Malignant/Benign Ultrasound BC Classification | Private B-mode ultrasound dataset (216 benign, 107 malignant) | Semi-automated
|
| Rana et al., 2019 [85] |
| Automated lesion detection and classification using clinical data extracted from microwave device. | Private data, 20 healthy breasts and 23 non-healthy breasts |
|
| Ed-daoudy and Maalmi, 2020 [82] |
| Malignant/Benign BC Classification | WBCD (357 benign, 212 malignant) |
|
| Khan et al., 2021 [81] |
| Malignant/Benign Cytology BC Classification | More than 4000 images from the pathology department Lady Reading Hospital Peshawar | Acc = 96.3 |
| Ara et al., 2021 [77] |
| Malignant/Benign BC Classification | WBCD (357 benign, 212 malignant) |
|
| Badr et al., 2021 [79] | GWO+SVM algorithm with equilibration scaling |
|
|
|
| Sami et al., 2022 [86] |
| Prediction of the breast lesion using microwave signals | Open-source datasets consisted of 1008 data examples obtained at the University of Manitoba. |
|
| Study | Method | Goal | Database | Evaluation |
|---|---|---|---|---|
| Singh et al., 2018 [91] | Different ML classifiers were compared | Malignant/Benign BC Classification | WBCO (456 benign, 241 malignant |
|
| Sengar et al., 2020 [93] | LR and DT classifiers were compared | Malignant/Benign BC Classification | WBCD (357 benign, 212 malignant) |
|
| Allada et al., 2021 [92] | Different ML classifiers were compared | Malignant/Benign BC Classification | WBCD (357 benign, 212 malignant) |
|
| Study | Method | Goal | Database | Evaluation |
|---|---|---|---|---|
| Abbass et al., 2002 [94] | EANN based on PDE with local search | Malignant/Benign BC Classification | WBCD | Acc = 99.1 |
| Karabatak et al., 2009 [95] |
| Malignant/Benign BC Classification | WBCD | Acc = 95.6 |
| Rouhi et al., 2015 [97] |
| Malignant/Benign Mammogram BC Classification |
| MAIS
|
| Jafari-Marandi et al., 2018 [96] | A SOM followed by an ANN | Malignant/Benign BC Classification |
|
|
| Kadam et al., 2019 [100] | Two SSAE + ensemble of softmax classifiers | Malignant/Benign BC Classification | WDBC |
|
| Study | Method | Goal | Database | Evaluation |
|---|---|---|---|---|
| Arevalo et al., 2015 [101] |
| Malignant/Benign BC Mammogram Classification | BCDR [102] (736 images from 344 patients, 426 benign, 310 malignant) | AUC = 0.86 |
| Zhang et al., 2016 [103] | Two-layer DL architecture (PGBM+RBM) | Malignant/Benign BC Classification | 227 SWE images (135 benign, 92 malignant) |
|
| Huynh et al., 2016 [116] | Soft voting for two SVM outputs; one uses transfer learning Alexnet features and the other uses handcrafted features | BC Mammogram Classification | Data from University of Chicago Medical Center (607 images, 261 benign, 346 malignant) | AUC = 0.86 |
| Araújo et al., 2017 [107] |
| Malignant/Benign BC Histopathology Classification | Online dataset [108] (249 training images, 20 testing images) |
|
| Kooi et al., 2017 [109] | Integrating CNN features with handcrafted features | Detection of solid, malignant lesions from mammogram | Local dataset of around 45,000 images | Acc = 94.1 |
| Tan et al., 2017 [110] | Preprocessing + CNN | BC Mammogram Classification | Mini-MIAS [98] (62 benign, 51 malignant, 209 normal) |
|
| Agnes et al., 2019 [111] | Preprocessing + MA-CNN | BC Mammogram Classification | Mini-MIAS [98] (62 benign, 51 malignant, 209 normal) |
|
| Ting et al., 2019 [106] |
| BC Mammogram Classification | MIAS [98] (21 benign, 27 malignant, 183 normal) |
|
| Hu et al., 2020 [118] |
| BC Classification | Multiparametric data (DCE-MRI and T2W) of 927 unique breast lesions from 616 women (199 benign, 728 malignant) |
|
| Hassan et al., 2020 [119] |
| BC Mammogram Classification | Training data
Test data
| AlexNet
|
| Wang et al., 2020 [123] | Pre-trained Inception-v3 models were applied for feature extraction from multi-view (transverse /coronal) US images | BC Ultrasound Classification | Private JNUH data (316 breast lesion, 181 benign, 135 malignant) | CNN A
|
| Wang et al., 2021 [104] |
| Malignant/Benign BC Histopathology Classification | BreaKHis [105] (135 benign, 92 malignant) | Acc = 95.6 |
| Muduli et al., 2021 [112] | Preprocessing + CNN | BC Classification | Mammogram
|
|
| Hekal et al., 2021 [125] | Otsu segmentation of suspected regions, AlexNet/ResNet for feature extraction, and SVM for classification | Classification of benign and malignant mammogram structures | CBIS-DDSM [122] detest of 3549 mammogram images (1852 benign, 1697 malignant) |
|
| Haq et al., 2022 [115] |
| BC Mammogram Classification | MIAS
| |
| Moreau et al., 2022 [128] |
| Automatic Segmentation of Metastatic Breast Cancer for Treatment Response Assessment. | Images were acquired at two sites (A-ICO, N-ICO). | SUL
|
| Hekal et al., 2023 [127] | Otsu segmentation of suspected regions followed by ensemble DL | Classification of benign and malignant mammogram structures | CBIS-DDSM [122] detest of 3549 mammogram images (1852 benign, 1697 malignant) |
|
| Modalities/Database | Features | AL/ML Components |
|---|---|---|
|
|
|
| Type of Biomarker | Examples |
|---|---|
| Diagnostic | ER, PR, HER2 and BI-RADS descriptors |
| Pharmacodynamic | Standardized uptake value (SUV) at 18-FDG PET-CT and 68Ga-FAPI SUVmax |
| Predictive | ER, PR, BRCA gene, increased mammographic breast density |
| Prognostic | Tumor stage, grade, tumor receptor status, and SUV at 18-FDG PET-CT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, G.A.; Batouty, N.M.; Gamal, A.; Elnakib, A.; Hamdy, O.; Sharafeldeen, A.; Mahmoud, A.; Ghazal, M.; Yousaf, J.; Alhalabi, M.; et al. Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review. Cancers 2023, 15, 5216. https://doi.org/10.3390/cancers15215216
Saleh GA, Batouty NM, Gamal A, Elnakib A, Hamdy O, Sharafeldeen A, Mahmoud A, Ghazal M, Yousaf J, Alhalabi M, et al. Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review. Cancers. 2023; 15(21):5216. https://doi.org/10.3390/cancers15215216
Chicago/Turabian StyleSaleh, Gehad A., Nihal M. Batouty, Abdelrahman Gamal, Ahmed Elnakib, Omar Hamdy, Ahmed Sharafeldeen, Ali Mahmoud, Mohammed Ghazal, Jawad Yousaf, Marah Alhalabi, and et al. 2023. "Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review" Cancers 15, no. 21: 5216. https://doi.org/10.3390/cancers15215216
APA StyleSaleh, G. A., Batouty, N. M., Gamal, A., Elnakib, A., Hamdy, O., Sharafeldeen, A., Mahmoud, A., Ghazal, M., Yousaf, J., Alhalabi, M., AbouEleneen, A., Tolba, A. E., Elmougy, S., Contractor, S., & El-Baz, A. (2023). Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review. Cancers, 15(21), 5216. https://doi.org/10.3390/cancers15215216








