Artificial Intelligence and Lung Cancer: Impact on Improving Patient Outcomes
Abstract
:Simple Summary
Abstract
1. Introduction
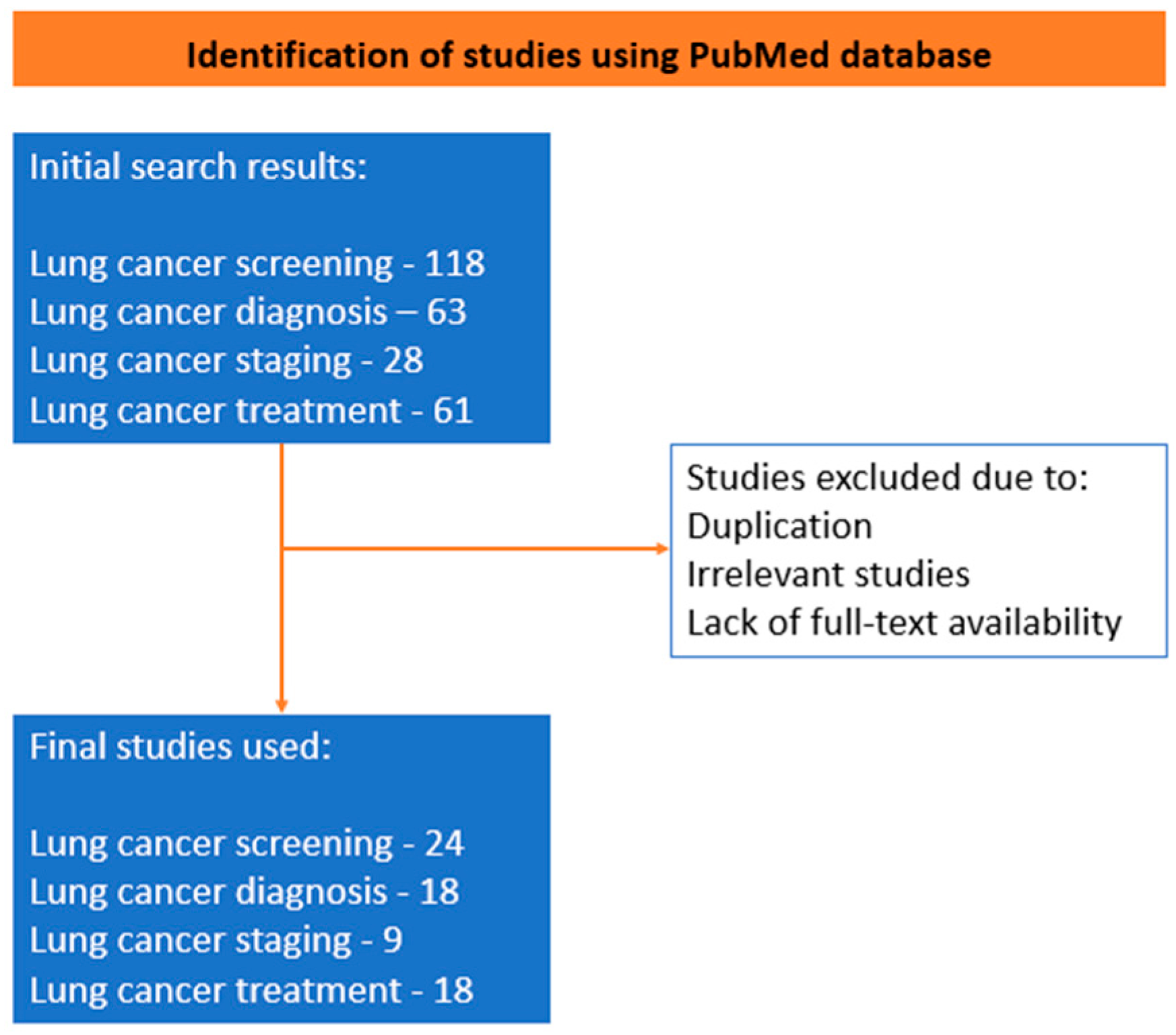
2. Methods
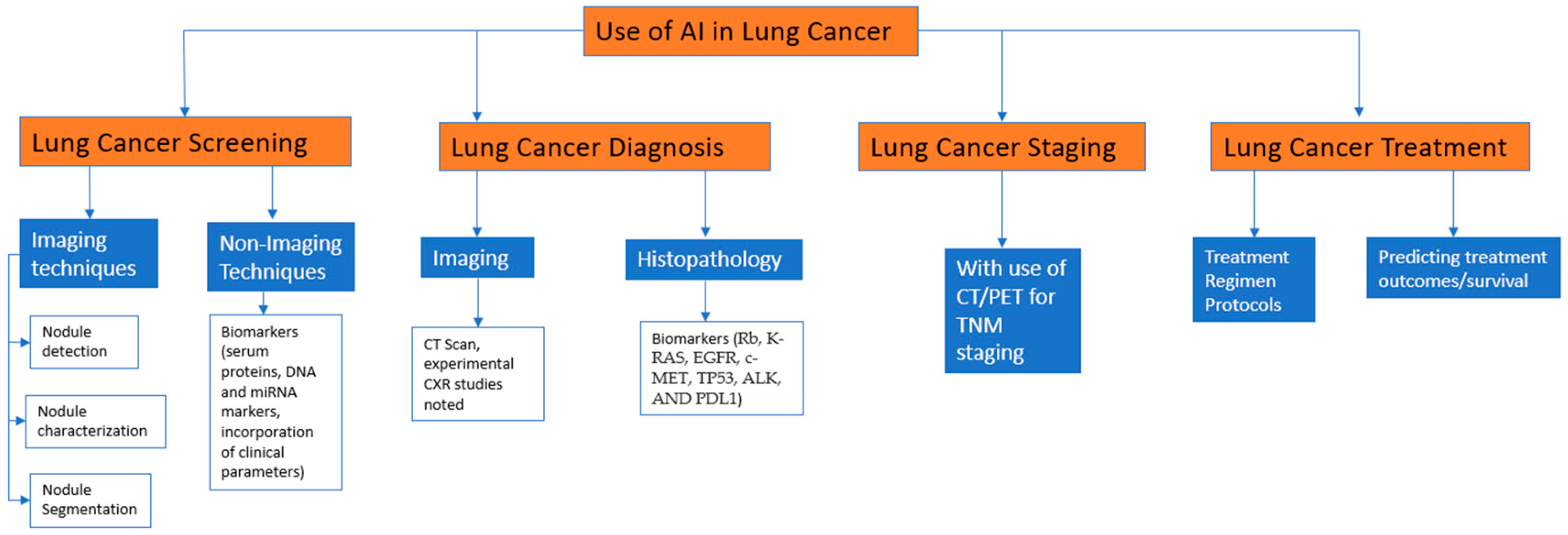
3. AI in Lung Cancer Screening
4. Imaging Techniques
4.1. Nodule Detection
4.2. Nodule Segmentation and Characterization
Non-Imaging Techniques
5. AI in Lung Cancer Diagnosis
5.1. Diagnostic Imaging
5.2. Histopathological Diagnosis
5.3. Biomarkers
6. AI in Lung Cancer Staging
7. AI in Lung Cancer Treatment
AI in Treatment Recommendations
8. AI in Predicting Treatment Outcomes
9. Limitations and Future Perspectives
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jacobs, C.; van Ginneken, B. Google’s lung cancer AI: A promising tool that needs further validation. Nat. Rev. Clin. Oncol. 2019, 16, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Bidzińska, J.; Szurowska, E. See lung cancer with an AI. Cancers 2023, 15, 1321. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Cè, M.; Irmici, G.; Ascenti, V.; Khenkina, N.; Toto-Brocchi, M.; Martinenghi, C.; Papa, S.; Carrafiello, G. Artificial intelligence in lung cancer imaging: Unfolding the future. Diagnostics 2022, 12, 2644. [Google Scholar] [CrossRef]
- Yin, X.; Liao, H.; Yun, H.; Lin, N.; Li, S.; Xiang, Y.; Ma, X. Artificial intelligence-based prediction of clinical outcome in immunotherapy and targeted therapy of lung cancer. Semin. Cancer Biol. 2022, 86 Pt 2, 146–159. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, C.; Du, T.; Gabriel, A.N.A.; Wang, X.; Li, X.; Sun, L.; Wang, N.; Jiang, X.; Zhang, Y. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed. Pharmacother. 2021, 134, 111111. [Google Scholar] [CrossRef]
- Luo, X.; Zang, X.; Yang, L.; Huang, J.; Liang, F.; Rodriguez-Canales, J.; Wistuba, I.I.; Gazdar, A.; Xie, Y.; Xiao, G. Comprehensive Computational Pathological Image Analysis Predicts Lung Cancer Prognosis. J. Thorac. Oncol. 2017, 12, 501–509. [Google Scholar] [CrossRef]
- Yu, K.H.; Zhang, C.; Berry, G.J.; Altman, R.B.; Re, C.; Rubin, D.L.; Snyder, M. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat. Commun. 2016, 7, 12474. [Google Scholar] [CrossRef]
- Amisha; Malik, P.; Pathania, M.; Rathaur, V.K. Overview of artificial intelligence in medicine. J. Fam. Med. Prim. Care 2019, 8, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef]
- Goyal, H.; Mann, R.; Gandhi, Z.; Perisetti, A.; Zhang, Z.; Sharma, N.; Saligram, S.; Inamdar, S.; Tharian, B. Application of artificial intelligence in pancreaticobiliary diseases. Clin. Med. Insights Gastroenterol. 2021, 14, 263177452199305. [Google Scholar] [CrossRef]
- Goyal, H.; Mann, R.; Gandhi, Z.; Perisetti, A.; Ali, A.; Ali, K.A.; Sharma, N.; Saligram, S.; Tharian, B.; Inamdar, S. Scope of artificial intelligence in screening and diagnosis of colorectal cancer. JCM 2020, 9, 3313. [Google Scholar] [CrossRef]
- Yang, Y.J.; Bang, C.S. Application of artificial intelligence in gastroenterology. World J. Gastroenterol. 2019, 25, 1666–1683. [Google Scholar] [CrossRef] [PubMed]
- Ruffle, J.K.; Farmer, A.D.; Aziz, Q. Artificial Intelligence-Assisted Gastroenterology—Promises and Pitfalls. Am. J. Gastroenterol. 2019, 114, 422–428. [Google Scholar] [CrossRef]
- Lawson, C.E.; Marti, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J.; et al. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Jones, G.S.; Baldwin, D.R. Recent advances in the management of lung cancer. Clin. Med. 2018, 18 (Suppl. 2), s41–s46. [Google Scholar] [CrossRef] [PubMed]
- Roosan, M.R.; Mambetsariev, I.; Pharaon, R.; Fricke, J.; Baroz, A.R.; Chao, J.; Chen, C.; Nasser, M.W.; Chirravuri-Venkata, R.; Jain, M.; et al. Evaluation of somatic mutations in solid metastatic pan-cancer patients. Cancers 2021, 13, 2776. [Google Scholar] [CrossRef] [PubMed]
- Joy Mathew, C.; David, A.M.; Joy Mathew, C.M. Artificial Intelligence and its future potential in lung cancer screening. EXCLI J. 2020, 19, 1552–1562. [Google Scholar]
- Ladbury, C.; Amini, A.; Govindarajan, A.; Mambetsariev, I.; Raz, D.J.; Massarelli, E.; Williams, T.; Rodin, A.; Salgia, R. Integration of artificial intelligence in lung cancer: Rise of the machine. Cell Rep. Med. 2023, 4, 100933. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Chao, H.S.; Chen, Y.M. Application of artificial intelligence in lung cancer. Cancers 2022, 14, 1370. [Google Scholar] [CrossRef]
- Ballard, D.H.; Burton, K.R.; Lakomkin, N.; Kim, S.; Rajiah, P.; Patel, M.J.; Mazaheri, P.; Whitman, G.J. The role of imaging in health screening: Screening for specific conditions. Acad. Radiol. 2021, 28, 548–563. [Google Scholar] [CrossRef] [PubMed]
- USPSTF Lung Cancer Screening Guidelines. Available online: https://uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening#fullrecommendationstart (accessed on 25 June 2023).
- Adams, S.J.; Stone, E.; Baldwin, D.R.; Vliegenthart, R.; Lee, P.; Fintelmann, F.J. Lung cancer screening. Lancet 2023, 401, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.C.; Wang, Y.H.; Yang, H.C.; Bai, K.J.; Wang, H.H.; Li, Y.J. Artificial Intelligence-Based Prediction of Lung Cancer Risk Using Nonimaging Electronic Medical Records: Deep Learning Approach. J. Med. Internet Res. 2021, 23, e26256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, K. Artificial intelligence: Opportunities in lung cancer. Curr. Opin. Oncol. 2022, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, P.G.; Wohlwend, J.; Yala, A.; Karstens, L.; Xiang, J.; Takigami, A.K.; Bourgouin, P.P.; Chan, P.; Mrah, S.; Amayri, W.; et al. Sybil: A Validated Deep Learning Model to Predict Future Lung Cancer Risk From a Single Low-Dose Chest Computed Tomography. J. Clin. Oncol. 2023, 41, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Thong, L.T.; Chou, H.S.; Chew, H.S.J.; Lau, Y. Diagnostic test accuracy of artificial intelligence-based imaging for lung cancer screening: A systematic review and meta-analysis. Lung Cancer 2023, 176, 4–13. [Google Scholar] [CrossRef]
- Liu, J.A.; Yang, I.Y.; Tsai, E.B. Artificial Intelligence (AI) for Lung Nodules, From the AJR Special Series on AI Applications. AJR Am. J. Roentgenol. 2022, 219, 703–712. [Google Scholar] [CrossRef]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef]
- Setio, A.A.; Ciompi, F.; Litjens, G.; Gerke, P.; Jacobs, C.; van Riel, S.J.; Wille, M.M.; Naqibullah, M.; Sanchez, C.I.; van Ginneken, B. Pulmonary Nodule Detection in CT Images: False Positive Reduction Using Multi-View Convolutional Networks. IEEE Trans. Med. Imaging 2016, 35, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.G.; Hwang, E.J.; Kim, J.; Park, N.; Lee, E.H.; Kim, H.J.; Nam, M.; Lee, J.H.; Park, C.M.; Goo, J.M. AI improves nodule detection on chest radiographs in a health screening population: A randomized controlled trial. Radiology 2023, 307, e221894. [Google Scholar] [CrossRef]
- De Margerie-Mellon, C.; Chassagnon, G. Artificial intelligence: A critical review of applications for lung nodule and lung cancer. Diagn. Interv. Imaging 2023, 104, 11–17. [Google Scholar] [CrossRef]
- Chauvie, S.; De Maggi, A.; Baralis, I.; Dalmasso, F.; Berchialla, P.; Priotto, R.; Violino, P.; Mazza, F.; Melloni, G.; Grosso, M.; et al. Artificial intelligence and radiomics enhance the positive predictive value of digital chest tomosynthesis for lung cancer detection within SOS clinical trial. Eur. Radiol. 2020, 30, 4134–4140. [Google Scholar] [CrossRef]
- Chamberlin, J.; Kocher, M.R.; Waltz, J.; Snoddy, M.; Stringer, N.F.C.; Stephenson, J.; Sahbaee, P.; Sharma, P.; Rapaka, S.; Schoepf, U.J.; et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for lung cancer screening: Accuracy and prognostic value. BMC Med. 2021, 19, 55. [Google Scholar] [CrossRef]
- Schwyzer, M.; Messerli, M.; Eberhard, M.; Skawran, S.; Martini, K.; Frauenfelder, T. Impact of dose reduction and iterative reconstruction algorithm on the detectability of pulmonary nodules by artificial intelligence. Diagn. Interv. Imaging 2022, 103, 273–280. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, S.; Hwang, E.J.; Lee, J.H.; Jin, K.N.; Lim, K.Y.; Vu, T.H.; Sohn, J.H.; Hwang, S.; Goo, J.M.; et al. Development and Validation of Deep Learning-based Automatic Detection Algorithm for Malignant Pulmonary Nodules on Chest Radiographs. Radiology 2019, 290, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhou, M.; Yang, F.; Yang, C.; Tian, J. Multi-scale Convolutional Neural Networks for Lung Nodule Classification. Inf. Process Med. Imaging 2015, 24, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Oh, J.H.; Riyahi, S.; Liu, C.J.; Jiang, F.; Chen, W.; White, C.; Rimner, A.; Mechalakos, J.G.; Deasy, J.O.; et al. Radiomics analysis of pulmonary nodules in low-dose CT for early detection of lung cancer. Med. Phys. 2018, 45, 1537–1549. [Google Scholar] [CrossRef]
- Dudurych, I.; Garcia-Uceda, A.; Saghir, Z.; Tiddens, H.A.W.M.; Vliegenthart, R.; de Bruijne, M. Creating a training set for artificial intelligence from initial segmentations of airways. Eur. Radiol. Exp. 2021, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Khalifa, F.; Elnakib, A.; Abou El-Ghar, M.; Dunlap, N.; Wang, B.; Gimel’farb, G.; Keynton, R.; El-Baz, A. Accurate Lungs Segmentation on CT Chest Images by Adaptive Appearance-Guided Shape Modeling. IEEE Trans. Med. Imaging 2017, 36, 263–276. [Google Scholar] [CrossRef]
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357. [Google Scholar] [CrossRef]
- Hirales Casillas, C.E.; Flores Fernández, J.M.; Padilla Camberos, E.; Herrera López, E.J.; Leal Pacheco, G.; Martínez Velázquez, M. Current status of circulating protein biomarkers to aid the early detection of lung cancer. Future Oncol. 2014, 10, 1501–1513. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H.; Zhang, G.; Li, H.; Ni, R.; Yu, Y.; Zhang, Y.; Wu, Y.; Liu, H. Diagnostic value of circulating genetically abnormal cells to support computed tomography for benign and malignant pulmonary nodules. BMC Cancer 2022, 22, 382. [Google Scholar] [CrossRef]
- Liu, M.; Wu, J.; Wang, N.; Zhang, X.; Bai, Y.; Guo, J.; Zhang, L.; Liu, S.; Tao, K. The value of artificial intelligence in the diagnosis of lung cancer: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0273445. [Google Scholar] [CrossRef]
- Pei, Q.; Luo, Y.; Chen, Y.; Li, J.; Xie, D.; Ye, T. Artificial intelligence in clinical applications for lung cancer: Diagnosis, treatment and prognosis. Clin. Chem. Lab. Med. 2022, 60, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Shen, N.; Xu, Q.; Zhao, Q. Artificial intelligence in lung cancer diagnosis and prognosis: Current application and future perspective. Semin. Cancer Biol. 2023, 89, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Delzell, D.A.P.; Magnuson, S.; Peter, T.; Smith, M.; Smith, B.J. Machine learning and feature selection methods for disease classification with application to lung cancer screening image data. Front. Oncol. 2019, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Schwyzer, M.; Ferraro, D.A.; Muehlematter, U.J.; Curioni-Fontecedro, A.; Huellner, M.W.; von Schulthess, G.K.; Kaufmann, P.A.; Burger, I.A.; Messerli, M. Automated detection of lung cancer at ultralow dose PET/CT by deep neural networks—Initial results. Lung Cancer 2018, 126, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tong, L.; Zheng, X.; Wang, H.; Zhou, H.; Zhu, X.; Zhou, C.; Zhao, P.; Wang, Y.; Wang, Q.; et al. A classifier for improving early lung cancer diagnosis incorporating artificial intelligence and liquid biopsy. Front. Oncol. 2022, 12, 853801. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, C.; Jin, L.; Gao, P.; Zhao, W.; Ma, W.; Tan, M.; Wu, W.; Duan, S.; Shan, Y.; et al. Radiomics for lung adenocarcinoma manifesting as pure ground-glass nodules: Invasive prediction. Eur. Radiol. 2020, 30, 3650–3659. [Google Scholar] [CrossRef]
- Feng, B.; Chen, X.; Chen, Y.; Li, Z.; Hao, Y.; Zhang, C.; Li, R.; Liao, Y.; Zhang, X.; Huang, Y.; et al. Differentiating minimally invasive and invasive adenocarcinomas in patients with solitary sub-solid pulmonary nodules with a radiomics nomogram. Clin. Radiol. 2019, 74, 570.e1–570.e11. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther. Onkol. 2020, 196, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Aydın, N.; Çelik, Ö.; Aslan, A.F.; Odabaş, A.; Dündar, E.; Şahin, M.C. Detection of lung cancer on computed tomography using artificial intelligence applications developed by deep learning methods and the contribution of deep learning to the classification of lung carcinoma. Curr. Med. Imaging 2021, 17, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Chen, Z.; Ye, N.; Mambetsariev, I.; Fricke, J.; Daniel, E.; Wang, G.; Wong, C.W.; Rockne, R.C.; Colen, R.R.; et al. Differentiating Peripherally-Located Small Cell Lung Cancer From Non-small Cell Lung Cancer Using a CT Radiomic Approach. Front. Oncol. 2020, 10, 593. [Google Scholar] [CrossRef]
- Teramoto, A.; Tsukamoto, T.; Kiriyama, Y.; Fujita, H. Automated classification of lung cancer types from cytological images using deep convolutional neural networks. Biomed. Res. Int. 2017, 2017, 4067832. [Google Scholar] [CrossRef] [PubMed]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Flores-Fernández, J.M.; Herrera-López, E.J.; Sánchez-Llamas, F.; Rojas-Calvillo, A.; Cabrera-Galeana, P.A.; Leal-Pacheco, G.; González-Palomar, M.G.; Femat, R.; Martínez-Velázquez, M. Development of an optimized multi-biomarker panel for the detection of lung cancer based on principal component analysis and artificial neural network modeling. Expert Syst. Appl. 2012, 39, 10851–10856. [Google Scholar] [CrossRef]
- Saad, M.; Choi, T.S. Computer-assisted subtyping and prognosis for non-small cell lung cancer patients with unresectable tumor. Comput. Med. Imaging Graph. 2018, 67, 1–8. [Google Scholar] [CrossRef]
- Scott, A.; Salgia, R. Biomarkers in lung cancer: From early detection to novel therapeutics and decision making. Biomark. Med. 2008, 2, 577–586. [Google Scholar] [CrossRef]
- Zhong, L.; Coe, S.P.; Stromberg, A.J.; Khattar, N.H.; Jett, J.R.; Hirschowitz, E.A. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J. Thorac. Oncol. 2006, 1, 513–519. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Funaguchi, N.; Nakano, T. Chemotherapy for advanced non-small cell lung cancer with a focus on squamous cell carcinoma. J. Cancer Res. Ther. 2016, 12, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, L.T. Staging of non-small cell lung cancer. Semin. Respir. Crit. Care Med. 2008, 29, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, B.; Zhang, L.; Greuter, M.J.W.; de Bock, G.H.; Zhang, H.; Zhang, H.; Xie, X. Lung nodule detectability of artificial intelligence-assisted CT image reading in lung cancer screening. Curr. Med. Imaging 2022, 18, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Setio, A.A.A.; Traverso, A.; de Bel, T.; Berens, M.S.N.; Bogaard, C.V.D.; Cerello, P.; Chen, H.; Dou, Q.; Fantacci, M.E.; Geurts, B.; et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: The LUNA16 challenge. Med. Image Anal. 2017, 42, 1–13. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Bennani, S.; Revel, M.P. Imagerie par tomodensitométrie du cancer bronchique non à petites cellules [Computed tomography imaging of non-small cell lung cancer]. Cancer Radiother. 2016, 20, 694. [Google Scholar] [CrossRef]
- Masood, A.; Sheng, B.; Li, P.; Hou, X.; Wei, X.; Qin, J.; Feng, D. Computer-assisted decision support system in pulmonary cancer detection and stage classification on CT images. J. Biomed. Inform. 2018, 79, 117–128. [Google Scholar] [CrossRef]
- Baker, S.R.; Patel, R.H.; Yang, L.; Lelkes, V.M.; Castro, A., 3rd. Malpractice suits in chest radiology: An evaluation of the histories of 8265 radiologists. J. Thorac. Imaging 2013, 28, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, J.; Sun, Y.; Li, C.; Wu, W.; Jin, L.; Yang, Z.; Ni, B.; Gao, P.; Wang, P.; et al. 3D deep learning from CT scans predicts tumor invasiveness of subcentimeter pulmonary adenocarcinomas. Cancer Res. 2018, 78, 6881–6889. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Zhao, Z.; Zhang, Q.; Shao, J.; Wang, C.; Qiu, Z.; Li, W. Artificial intelligence-assisted decision making for prognosis and drug efficacy prediction in lung cancer patients: A narrative review. J. Thorac. Dis. 2021, 13, 7021–7033. [Google Scholar] [CrossRef]
- Rabbani, M.; Kanevsky, J.; Kafi, K.; Chandelier, F.; Giles, F.J. Role of artificial intelligence in the care of patients with nonsmall cell lung cancer. Eur. J. Clin. Investig. 2018, 48, e12901. [Google Scholar] [CrossRef]
- Coroller, T.P.; Agrawal, V.; Narayan, V.; Hou, Y.; Grossmann, P.; Lee, S.W.; Mak, R.H.; Aerts, H.J. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother. Oncol. 2016, 119, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, N.; Abidi, S.S.; Blouin, C. A Predictive Model for Personalized Therapeutic Interventions in Non-small Cell Lung Cancer. IEEE J. Biomed. Health Inform. 2016, 20, 424–431. [Google Scholar] [CrossRef]
- Tian, P.; He, B.; Mu, W.; Liu, K.; Liu, L.; Zeng, H.; Liu, Y.; Jiang, L.; Zhou, P.; Huang, Z.; et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics 2021, 11, 2098–2107. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Wu, F.; Xie, M.; Feng, Y.; Hu, C. Using Artificial Intelligence (Watson for Oncology) for Treatment Recommendations Amongst Chinese Patients with Lung Cancer: Feasibility Study. J. Med. Internet Res. 2018, 20, e11087. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, H.Y.; Kho, B.G.; Park, C.K.; Oh, I.J.; Kim, Y.C.; Kim, S.; Yun, J.S.; Song, S.Y.; Na, K.J.; et al. Artificial intelligence and lung cancer treatment decision: Agreement with recommendation of multidisciplinary tumor board. Transl. Lung Cancer Res. 2020, 9, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, G.; Varela, G.; Novoa, N.; Jiménez, M.F. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Artif. Intell. Med. 2004, 30, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Dercle, L.; Fronheiser, M.; Lu, L.; Du, S.; Hayes, W.; Leung, D.K.; Roy, A.; Wilkerson, J.; Guo, P.; Fojo, A.T.; et al. Identification of Non-Small Cell Lung Cancer Sensitive to Systemic Cancer Therapies Using Radiomics. Clin. Cancer Res. 2020, 26, 2151–2162. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, Y.; Zhang, J.; Ren, J.; Zhao, Z.; Zhang, X.; Li, S.; Deng, L.; Zhou, J. Predicting EGFR mutation status in lung adenocarcinoma: Development and validation of a computed tomography-based radiomics signature. Am. J. Cancer Res. 2021, 11, 546. [Google Scholar]
- Mu, W.; Jiang, L.; Zhang, J.; Shi, Y.; Gray, J.E.; Tunali, I.; Gao, C.; Sun, Y.; Tian, J.; Zhao, X.; et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat. Commun. 2020, 11, 5228. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.; Ye, Z.; Dong, D.; Yu, D.; Zhou, M.; Liu, Y.; Gevaert, O.; Wang, K.; Zhu, Y.; et al. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur. Respir. J. 2019, 53, 1800986. [Google Scholar] [CrossRef]
- Song, J.; Wang, L.; Ng, N.N.; Zhao, M.; Shi, J.; Wu, N.; Li, W.; Liu, Z.; Yeom, K.W.; Tian, J. Development and Validation of a Machine Learning Model to Explore Tyrosine Kinase Inhibitor Response in Patients With Stage IV EGFR Variant-Positive Non-Small Cell Lung Cancer. JAMA Netw. Open 2020, 3, e2030442. [Google Scholar] [CrossRef]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The artificial intelligence and machine learning in lung cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hosny, A.; Zeleznik, R.; Parmar, C.; Coroller, T.; Franco, I.; Mak, R.H.; Aerts, H.J.W.L. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin. Cancer Res. 2019, 25, 3266–3275. [Google Scholar] [CrossRef] [PubMed]
- Real-World Evidence |FDA. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 23 October 2023).
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef]
| Author, Year | Dataset | AI Algorithm | Outcomes | Results |
|---|---|---|---|---|
| Ardila et al., 2019 [26] | Low-dose CT scan | Deep learning algorithm | Diagnosis of lung cancer | AUC = 0.94 |
| Delzell et al., 2019 [47] | CT scan of 200 lung nodules | Radiomics | Verify nodules as benign or malignant | AUC = 0.72 |
| Schwyzer et al., 2018 [48] | FDG-PET imaging | Deep machine learning | Diagnosis of lung cancer using ultra-low-dose PET scans | Sensitivity = 95.9% Specificity = 98.1% |
| Liu et al., 2023 [44] | Images and radiological features of 5251 patients from 14 studies | ANN SVM | Diagnosis of lung cancer | Sensitivity = 87% Specificity = 87% |
| Zheng et al., 2022 [49] | CT images of 9 NSCLC studies | Radiomics Deep learning | To diagnose whether patient had NSCLC | AUROC = 0.78 |
| Sun et al., 2020 [50] | Pure ground glass nodules of 385 patients | Radiomics | Invasiveness prediction | AUC = 0.77 |
| Feng et al., 2019 [51] | Sub-solid nodules of 100 patients | Radiomics | Differentiate minimally invasive and invasive adenocarcinoma | AUC = 0.912 |
| Avanzo et al., 2020 [52] | Nodules of low-dose CT scan | SVM | Differentiate adenocarcinoma from focal pneumonia | Accuracy = 87.6% |
| Aydin et al., 2021 [53] | 301 lung cancer CT scans | CNN | Differentiate into squamous cell, adenocarcinoma, and small cell carcinoma | Sensitivity = 90% Specificity = 44% |
| Chen et al., 2020 [54] | CT radiomics of 69 lung cancer patients | Radiomics | Differentiate NSCLC from SCLC | AUC = 0.93 |
| Yu et al., 2016 [7] | 2480 histopathological images of lung adenocarcinoma | SVM Random forest | Distinguish malignant tumors from healthy tissue | AUC = 0.81 |
| Teramoto et al., 2017 [55] | 298 histopathological images | Conventional deep neural networks | Classified adenocarcinoma, squamous cell carcinoma, and small cell carcinoma | Accuracy = 89%, 60%, 70% respectively |
| Coudray et al., 2018 [56] | Pathological images of adenocarcinoma | Conventional deep neural networks | Predicted 10 most prevalent genes in adenocarcinoma | Accuracy = 73.3%–85.6% |
| Flores-Fernandez et al., 2012 [57] | Serum biomarkers of 63 lung cancer patients | Artificial neural network modeling | Correctly classifying lung cancer patients based on biomarker panel | Correct classification rate = 93.3% |
| Study | Dataset | AI Methods | Predicted Outcomes | Performance Metrics/Results |
|---|---|---|---|---|
| Coroller et al., 2016 [72] | NSCLC | Radiomics-based model | Predicting pathologic complete response to chemoradiation | AUC: 0.61 |
| Kureshi et al., 2016 [73] | NSCLC | Radiomics-based model | Predicting response to EGFR-TKI therapy | AUC: 0.76 |
| Tian et al., 2021 [74] | NSCLC | Radiomics and deep learning | Predict response to PD-1 and PD-L1 immunotherapy | AUC: 0.71 |
| Liu et al., 2018 [75] | NSCLC and SCLC | WFO | Feasibility in treatment recommendation | Consistency 65.8% |
| Kim et al., 2020 [76] | NSCLC and SCLC | WFO | Treatment concordance between MDT and WFO | Concordance 92.4% |
| Santos-Garcia et al., 2004 [77] | NSCLC | Neural Network | Predict postoperative cardio-respiratory | AUC: 0.98 |
| Dercle et al., 2020 [78] | NSCLC | Radiomics-based model | Treatment Sensitivity of Nivolumab | AUC: 0.77 |
| Treatment Sensitivity of Docetaxel | AUC: 0.67 | |||
| Treatment Sensitivity of Gefitinib | AUC: 0.82 | |||
| Zhang et al., 2021 [79] | Adenocarcinoma | Radiomics-based model | Predicting EGFR mutation for targeted therapy | AUC: 0.84 |
| Mu et al. 2020 [80] | NSCLC | Deep learning models | Predicting EGFR mutation for targeted therapy | AUC: 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, Z.; Gurram, P.; Amgai, B.; Lekkala, S.P.; Lokhandwala, A.; Manne, S.; Mohammed, A.; Koshiya, H.; Dewaswala, N.; Desai, R.; et al. Artificial Intelligence and Lung Cancer: Impact on Improving Patient Outcomes. Cancers 2023, 15, 5236. https://doi.org/10.3390/cancers15215236
Gandhi Z, Gurram P, Amgai B, Lekkala SP, Lokhandwala A, Manne S, Mohammed A, Koshiya H, Dewaswala N, Desai R, et al. Artificial Intelligence and Lung Cancer: Impact on Improving Patient Outcomes. Cancers. 2023; 15(21):5236. https://doi.org/10.3390/cancers15215236
Chicago/Turabian StyleGandhi, Zainab, Priyatham Gurram, Birendra Amgai, Sai Prasanna Lekkala, Alifya Lokhandwala, Suvidha Manne, Adil Mohammed, Hiren Koshiya, Nakeya Dewaswala, Rupak Desai, and et al. 2023. "Artificial Intelligence and Lung Cancer: Impact on Improving Patient Outcomes" Cancers 15, no. 21: 5236. https://doi.org/10.3390/cancers15215236
APA StyleGandhi, Z., Gurram, P., Amgai, B., Lekkala, S. P., Lokhandwala, A., Manne, S., Mohammed, A., Koshiya, H., Dewaswala, N., Desai, R., Bhopalwala, H., Ganti, S., & Surani, S. (2023). Artificial Intelligence and Lung Cancer: Impact on Improving Patient Outcomes. Cancers, 15(21), 5236. https://doi.org/10.3390/cancers15215236







