Preliminary Evaluation Salivary Biomarkers in Patients with Oral Potentially Malignant Disorders (OPMD): A Case–Control Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
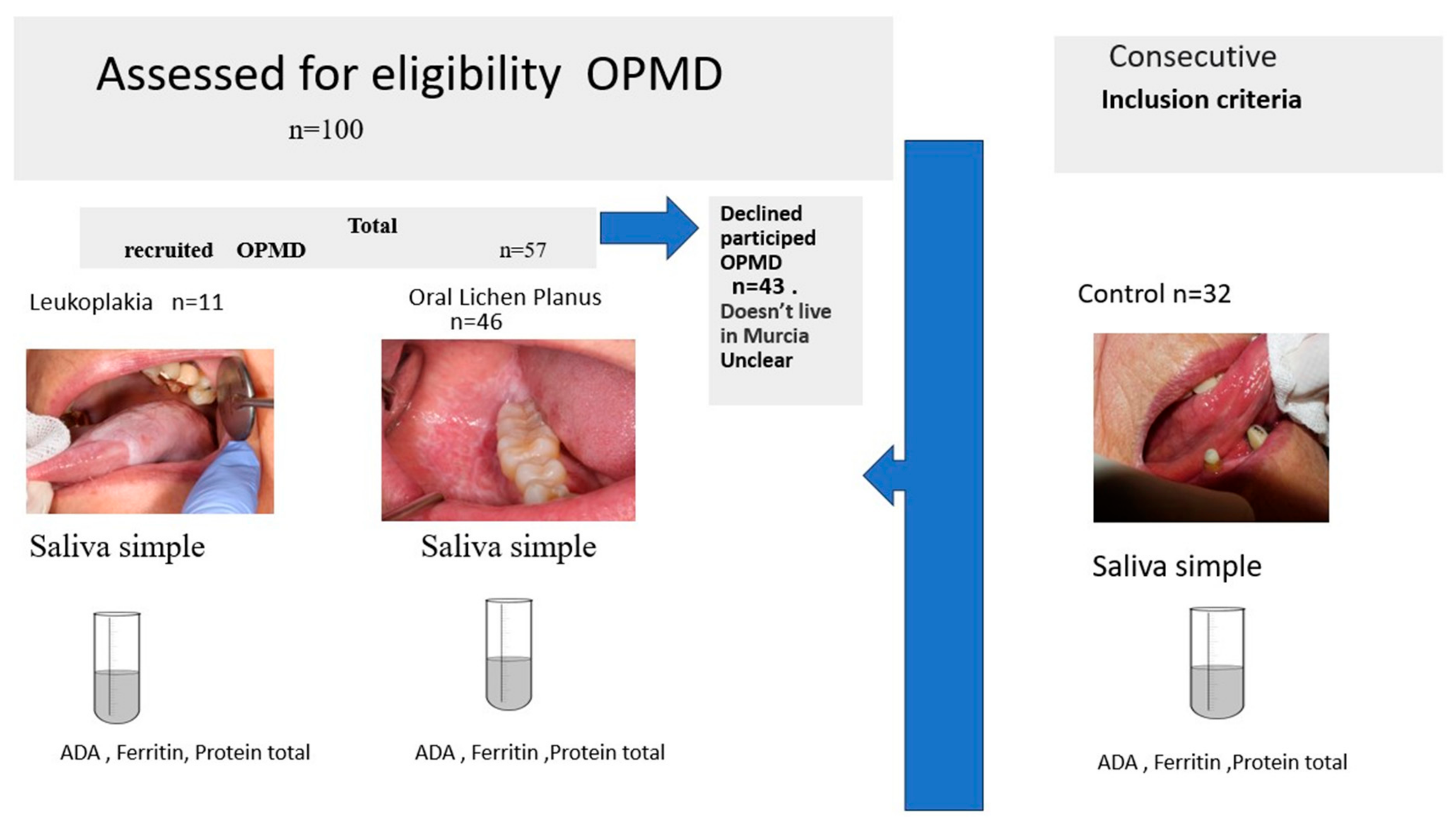
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Saliva Sampling
2.5. Biochemical Analysis
2.6. Statistical Analysis
3. Results
3.1. Salivary Biomarkers according to Study Group
3.2. Biomarkers according to Independent Factors (Table 3)
| ADA | FRR | PROT | |
|---|---|---|---|
| Gender | 0.068 (MW) | 0.256 (MW) | 0.482 (MW) |
| Age | r = 0.12 (p = 0.254) | r = 0.13 (p = 0.235) | r = 0.14 (p = 0.182) |
| Smoking | 0.231 (KW) | 0.475 (KW) | 0.399 (KW) |
| Alcohol | 0.276 (KW) | 0.176 (KW) | 0.758 (KW) |
| Time from diagnosis | r = −0.11 (p = 0.935) | r = −0.11 (p = 0.395) | r = 0.06 (p = 0.637) |
| Lichen—striated | 0.491 (MW) | 0.927 (MW) | 0.396 (MW) |
| Lichen—erosive | 0.161 (MW) | 0.317 (MW) | 0.111 (MW) |
| Location: cheek mucosal | 0.994 (MW) | 0.767 (MW) | 0.064 (MW) |
| Location: gingival mucosa | 0.944 (MW) | 0.902 (MW) | 0.934 (MW) |
| Location: lip mucosa/buccal sulcus | 0.947 (MW) | 0.664 (MW) | 0.888 (MW) |
| Location: tongue | 0.504 (MW) | 0.602 (MW) | 0.884 (MW) |
| Location: other zones | 0.947 (MW) | 0.613 (MW) | 0.431 (MW) |
| Size | 0.048 * (MW) | 0.667 (MW) | 0.045 * (MW) |
3.3. Correlations between Biomarkers
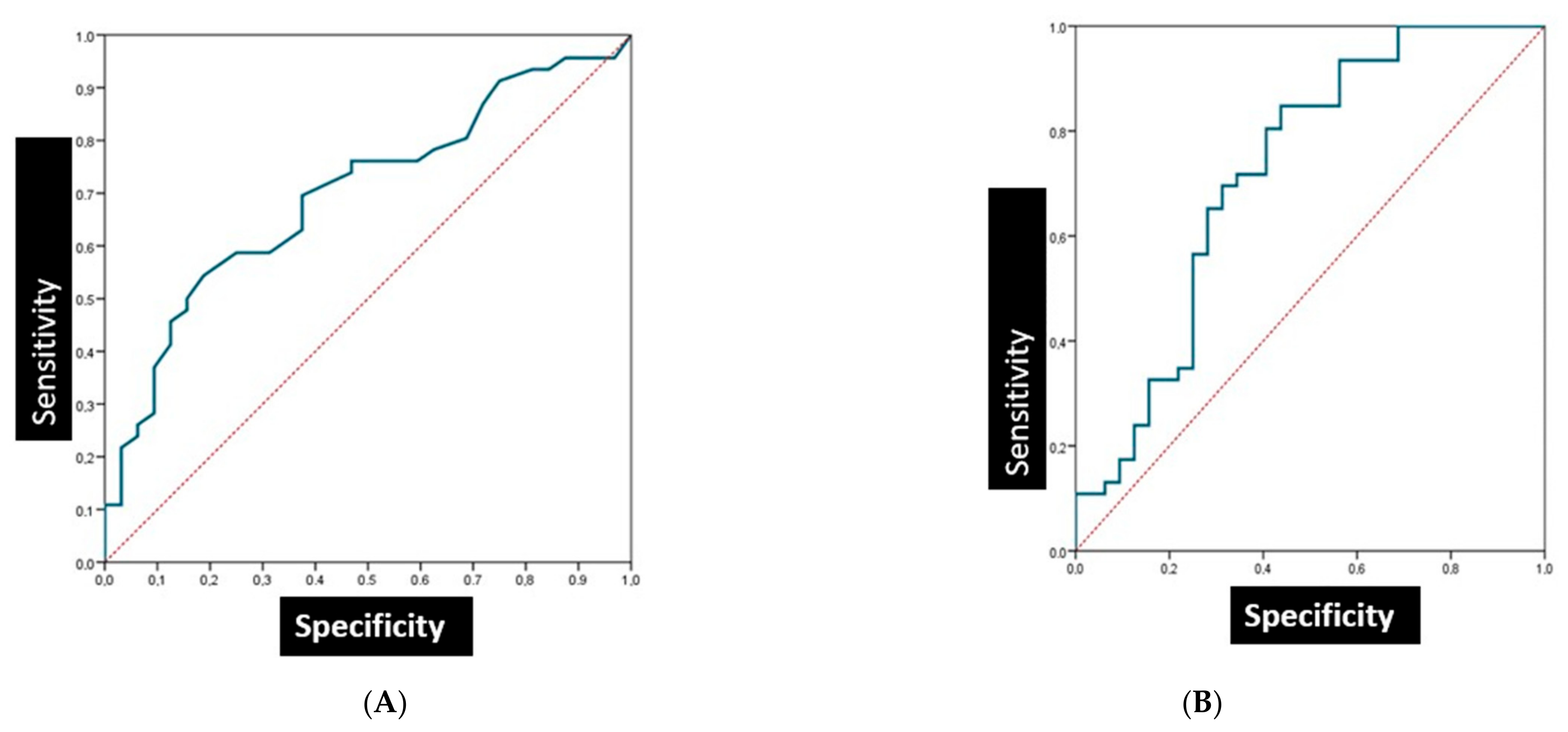
3.4. The Sensitivity and Specificity of Salivary Biomarkers in Patients with Oral Lichen Planus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre Urizar, J.M.; Bagán, J.V.; González Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Carey, B.; Diniz-Freitas, M.; Lombardi, N.; Cook, R.; Fricain, J.C.; Brailo, V.; Limeres, J.; Varoni, E.; Fenelon, M.; et al. Terminology on oral potentially malignant disorders in European languages: An appraisal. Oral Dis. 2022, 14. [Google Scholar] [CrossRef]
- Bermejo-Fenoll, A.; Sanchez-Siles, M.; Lopez-Jornet, P.; Camacho-Alonso, F.; Salazar-Sanchez, N. A retrospective clinicopathological study of 550 patients with oral lichen planus in southeastern Spain. J. Oral Pathol. Med. 2010, 39, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Troiano, G.; Cordaro, M.; Corsalini, M.; Gioco, G.; Lo Muzio, L.; Pignatelli, P.; Lajolo, C. Rate of malignant transformation of oral lichen planus: A systematic review. Oral Dis. 2019, 25, 693–709. [Google Scholar] [CrossRef]
- Abdul, N.S. Role of Advanced Diagnostic Aids in the Detection of Potentially Malignant Disorders and Oral Cancer at an Early Stage. Cureus 2023, 15, e34113. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; López, S.P.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 2020, 42, 539–555. [Google Scholar] [CrossRef]
- Wetzel, S.L.; Wollenberg, J. Oral Potentially Malignant Disorders. Dent. Clin. N. Am. 2020, 64, 25–37. [Google Scholar] [CrossRef]
- Ojeda, D.; Huber, M.A.; Kerr, A.R. Oral Potentially Malignant Disorders and Oral Cavity Cancer. Dermatol. Clin. 2020, 38, 507–521. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of Salivary Biomarkers in Oral Cancer Detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar] [CrossRef]
- Deng, S.; Wang, S.; Shi, X.; Zhou, H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8940. [Google Scholar] [CrossRef] [PubMed]
- Dikova, V.R.; Principe, S.; Bagan, J.V. Salivary inflammatory proteins in patients with oral potentially malignant disorders. J. Clin. Exp. Dent. 2019, 11, e659–e664. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2019, 23, 1407–1414. [Google Scholar] [CrossRef]
- Rai, B.; Kaur, J.; Jacobs, R.; Anand, S.C. Adenosine deaminase in saliva as a diagnostic marker of squamous cell carcinoma of tongue. Clin. Oral Investig. 2011, 15, 347–349. [Google Scholar] [CrossRef]
- Kelgandre, D.C.; Pathak, J.; Patel, S.; Ingale, P.; Swain, N. Adenosine Deaminase—A Novel Diagnostic and Prognostic Biomarker for Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2016, 17, 1865–1868. [Google Scholar] [CrossRef]
- Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation, and tumorigenesis. Biochim. Biophys. Acta 2013, 1836, 245–254. [Google Scholar] [CrossRef]
- Min Pang, B.S.; Connor, J.R. Role of ferritin in cancer biology. J. Cancer Sci. Ther. 2015, 7, 155–160. [Google Scholar]
- Khanna, V.; Karjodkar, F.; Robbins, S.; Behl, M.; Arya, S.; Tripathi, A. Estimation of serum ferritin level in potentially malignant disorders, oral squamous cell carcinoma, and treated cases of oral squamous cell carcinoma. J. Cancer Res. Ther. 2017, 13, 550–555. [Google Scholar] [CrossRef]
- van der Waal, I. Oral leukoplakia: A diagnostic challenge for clinicians and pathologists. Oral Dis. 2019, 25, 348–349. [Google Scholar] [CrossRef]
- Van der Meij, E.H.; van der Waal, I. Lack of clinicopathologic correla-tion in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J. Oral Pathol. Med. 2003, 32, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Delacour, H.; Bousquet, A.; Fontan, E.; Ceppa, F. Ammonia does not interfere with the Diazyme adenosine deaminase test. Clin. Chem. Lab. Med. 2013, 51, e225–e226. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Beigi, K.; Doroodizadeh, T.; Haghnegahdar, M.; Golfeshan, F.; Ranjbar, R.; Tebyanian, H. Therapeutic applications of herbal/synthetic/bio-drug in oral cancer: An update. Eur. J. Pharmacol. 2021, 890, 173657. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Hussain, A.; Tebyaniyan, H. Exploring animal models in oral cancer research and clinical intervention: A critical review. Vet. Med. Sci. 2023, 9, 1833–1847. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef]
- Adeoye, J.; Brennan, P.A.; Thomson, P. “Search less, verify more”-Reviewing salivary biomarkers in oral cancer detection. J. Oral Pathol. Med. 2020, 49, 711–719. [Google Scholar] [CrossRef]
- Wang, X.; Kaczor-Urbanowicz, K.; Wong, D.T. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Ashok, K.J.; Pinto, G.J.O.; Kavitha, A.K.; Kavitha, A.K.; Palathra, M.J. The diagnostic and prognostic value of serum adenosine deaminase levels in head and neck cancer. J. Clin. Diag. Res. 2008, 3, 833–837. [Google Scholar]
- Mishra, R.; Agarwal, M.K.; Chansuria, J.P.N. Serum adenosine deaminase levels as an index of tumor growth in head and neck malignancy. Indian J. Otolaryngol. Head Neck Surg. 2000, 52, 360–363. [Google Scholar] [CrossRef]
- Dhankhar, R.; Dahiya, K.; Sharma, T.K.; Ghalaut, V.S.; Atri, R.; Kaushal, V. Diagnostic significance of adenosine deaminase, uric acid and C-reactive protein levels in patients of head and neck carcinoma. Clin. Lab. 2011, 57, 795–798. [Google Scholar]
- Saracoglu, U.; Guven, O.; Durak, I. Adenosine deaminase and 5’-nucleotidase activities in saliva from patients with oral and laryngeal cancer. Oral Dis. 2005, 11, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Maxim, P.E.; Veltri, R.W. Serum ferritin as a tumor marker in patients with squamous cell carcinoma of the head and neck. Cancer 1986, 57, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, M.; Manifar, S.; Akkafan, R.; Mortazavi, H.; Sabour, S. Serum levels of ferritin, copper, and zinc in patients with oral cancer. Biomed. J. 2014, 37, 331–336. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, K.; Tang, H.; Chen, D. Diagnostic values of serum tumor markers Cyfra21-1, SCCAg, ferritin, CEA, CA19-9, and AFP in oral/oropharyngeal squamous cell carcinoma. OncoTargets Ther. 2016, 9, 3381–3386. [Google Scholar]
- Bhatavdekar, J.M.; Vora, H.H.; Goyal, A.; Shah, N.G.; Karelia, N.H.; Trivedi, S.N. Significance of ferritin as a marker in head and neck malignancies. Tumori J. 1987, 73, 59–63. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, L.; Han, Y.; Li, F.; Zheng, A.; Xu, Y.; Wang, F.; Xiao, B.; Chen, C.; Tao, Z. Ferritin: A potential serum marker for lymph node metastasis in head and neck squamous cell carcinoma. Oncol. Lett. 2019, 17, 314–322. [Google Scholar] [CrossRef]
- Wu, Y.H.; Lin, P.Y.; Yang, J.H.; Kuo, Y.S.; Wu, Y.C. Serum levels and positive rates of tumor biomarkers in oral precancer patients. J. Formos. Med. Assoc. 2021, 120, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Lin, P.Y.; Yang, J.H.; Kuo, Y.S.; Wu, Y.C.; Chiang, C.P. Significantly higher serum tumor marker levels in patients with oral submucous fibrosis. J. Dent. Sci. 2021, 16, 846–853. [Google Scholar] [CrossRef]
- Buch, S.A.; Babu, S.G.; Castelino, R.L.; Pillai, D.S.; Bhat, S.; Devi, U.H. Ferritin levels in serum and saliva of oral cancer and oral potentially malignant disorders. Eur. J. Ther. 2022, 28, 109–114. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chiang, C.P. Significantly higher serum levels and positive rates of tumor biomarkers in patients with oral lichen planus. J. Dent. Sci. 2023, 18, 1288–1294. [Google Scholar] [CrossRef]

| Variables | CONTROL | OPMD | p Value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age Mean ± SD | 63.6 ± 8.8 | 65.0 ± 12.2 | >0.05 | |||
| Yes, <10 cig/day | 0 | 0 | 3 | 5.1 | 0.011 | |
| Ex-smoker | 7 | 21.9 | 27 | 45.8 | ||
| No | 23 | 71.9 | 25 | 42.4 | ||
| Alcohol | Yes, severe | 0 | 0 | 3 | 5.1 | |
| Yes, moderate | 4 | 12.5 | 10 | 16.9 | >0.05 | |
| Yes, mild | 9 | 20.1 | 19 | 32.2 | ||
| No | 19 | 59.4 | 27 | 45.8 | ||
| Type of lesion | Oral lichen planus | 46 | 80.7 | |||
| Leukoplakia | - | - | 11 | 19.3 | ||
| Lichen planus | Reticular | - | - | 30 | 65.2 | |
| Erosive | - | - | 16 | 34.8 | ||
| Location | Cheek mucosa | - | - | 30 | 50.8 | |
| Gingival mucosa | - | - | 18 | 30.5 | ||
| Lip | - | - | 10 | 16.9 | ||
| Tongue | - | - | 13 | 22 | ||
| other zones | - | - | 16 | 27.1 | ||
| Lesion size | <2 cm | - | - | 18 | 30.5 | |
| >2 cm | - | - | 41 | 69.5 | ||
| Total (n = 89) | Control (n = 32) | OPMD (n = 57) | p-Value | ||
|---|---|---|---|---|---|
| ADA (IU/L) | Mean ± SD | 0.80 ± 2.02 | 0.71 ± 1.72 | 0.85 ± 2.18 | 0.934 |
| Median (IQR) | 0.28 (0.2–14.9) | 0.30 (0.10–0.57) | 0.28 (0.11–0.77) | ||
| FRR (µg/L) | Mean ± SD | 10.74 ± 9.20 | 7.19 ± 4.44 | 12.66 ± 10.50 | 0.001 |
| Median (IQR) | 9.40 (2.5–53.9) | 6.20 (4.50–8.15) | 9.40 (6.50–14.50) | ||
| Proteins (mg/dL) | Mean ± SD | 20.16 ± 17.44 | 14.15 ± 15.19 | 23.41 ± 17.83 | 0.001 |
| Median (IQR) | 14.8 (4–86.1) | 9.9 (3.0–21.0) | 18.9 (11.5–27.4) |
| Total Sample | Control | OPMD | |
|---|---|---|---|
| ADA vs. FRR | r = 0.37 (p < 0.001 ***) | r = 0.15 (p = 0.419) | r = 0.52 (p < 0.001 ***) |
| ADA vs. PROT | r = 0.53 (p < 0.001 ***) | r = 0.61 (p < 0.001 ***) | r = 0.53 (p < 0.001 ***) |
| FRR vs. PROT | r = 0.44 (p < 0.001 ***) | r = 0.41 (p = 0.020 *) | r = 0.35 (p = 0.007 **) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Jornet, P.; Olmo-Monedero, A.; Peres-Rubio, C.; Pons-Fuster, E.; Tvarijonaviciute, A. Preliminary Evaluation Salivary Biomarkers in Patients with Oral Potentially Malignant Disorders (OPMD): A Case–Control Study. Cancers 2023, 15, 5256. https://doi.org/10.3390/cancers15215256
López-Jornet P, Olmo-Monedero A, Peres-Rubio C, Pons-Fuster E, Tvarijonaviciute A. Preliminary Evaluation Salivary Biomarkers in Patients with Oral Potentially Malignant Disorders (OPMD): A Case–Control Study. Cancers. 2023; 15(21):5256. https://doi.org/10.3390/cancers15215256
Chicago/Turabian StyleLópez-Jornet, Pia, Aitana Olmo-Monedero, Camila Peres-Rubio, Eduardo Pons-Fuster, and Asta Tvarijonaviciute. 2023. "Preliminary Evaluation Salivary Biomarkers in Patients with Oral Potentially Malignant Disorders (OPMD): A Case–Control Study" Cancers 15, no. 21: 5256. https://doi.org/10.3390/cancers15215256
APA StyleLópez-Jornet, P., Olmo-Monedero, A., Peres-Rubio, C., Pons-Fuster, E., & Tvarijonaviciute, A. (2023). Preliminary Evaluation Salivary Biomarkers in Patients with Oral Potentially Malignant Disorders (OPMD): A Case–Control Study. Cancers, 15(21), 5256. https://doi.org/10.3390/cancers15215256








