Prognostic Potential of Galectin-9 mRNA Expression in Chronic Lymphocytic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. PBMCs Isolation
2.3. Purification of DNA and Quantitative Assay for EBV DNA
2.4. CD19+ B-Lymphocytes Isolation
2.5. RT-qPCR for Gal-9, Ki67, and PCNA
2.6. I-FISH Analysis
2.7. CD38 and ZAP-70 Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Upregulation of mRNA Gal-9 Expression in Malignant B-Cells from CLL Patients
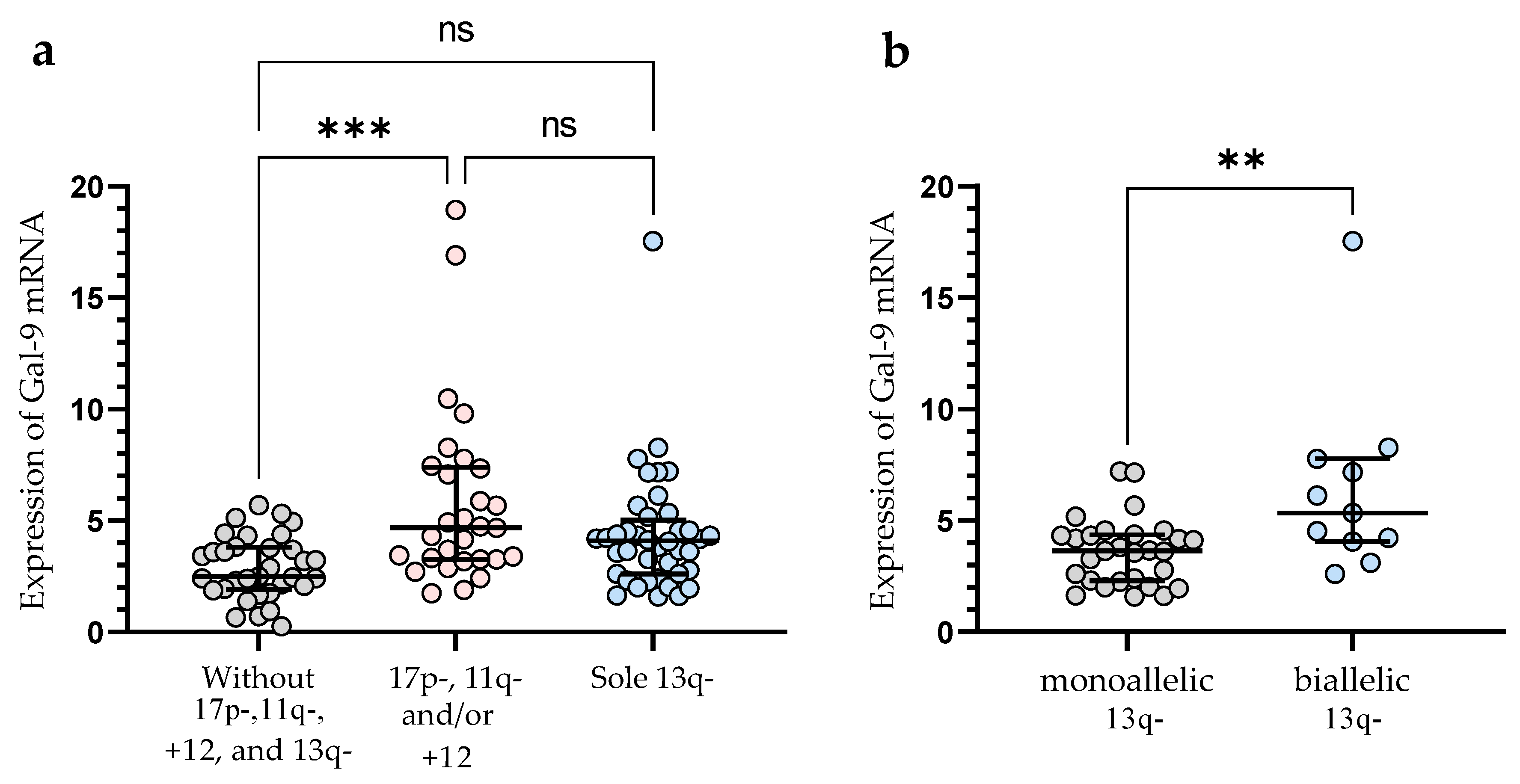
3.2. Gal-9 mRNA Relative Expression and Cytogenetic Abnormalities
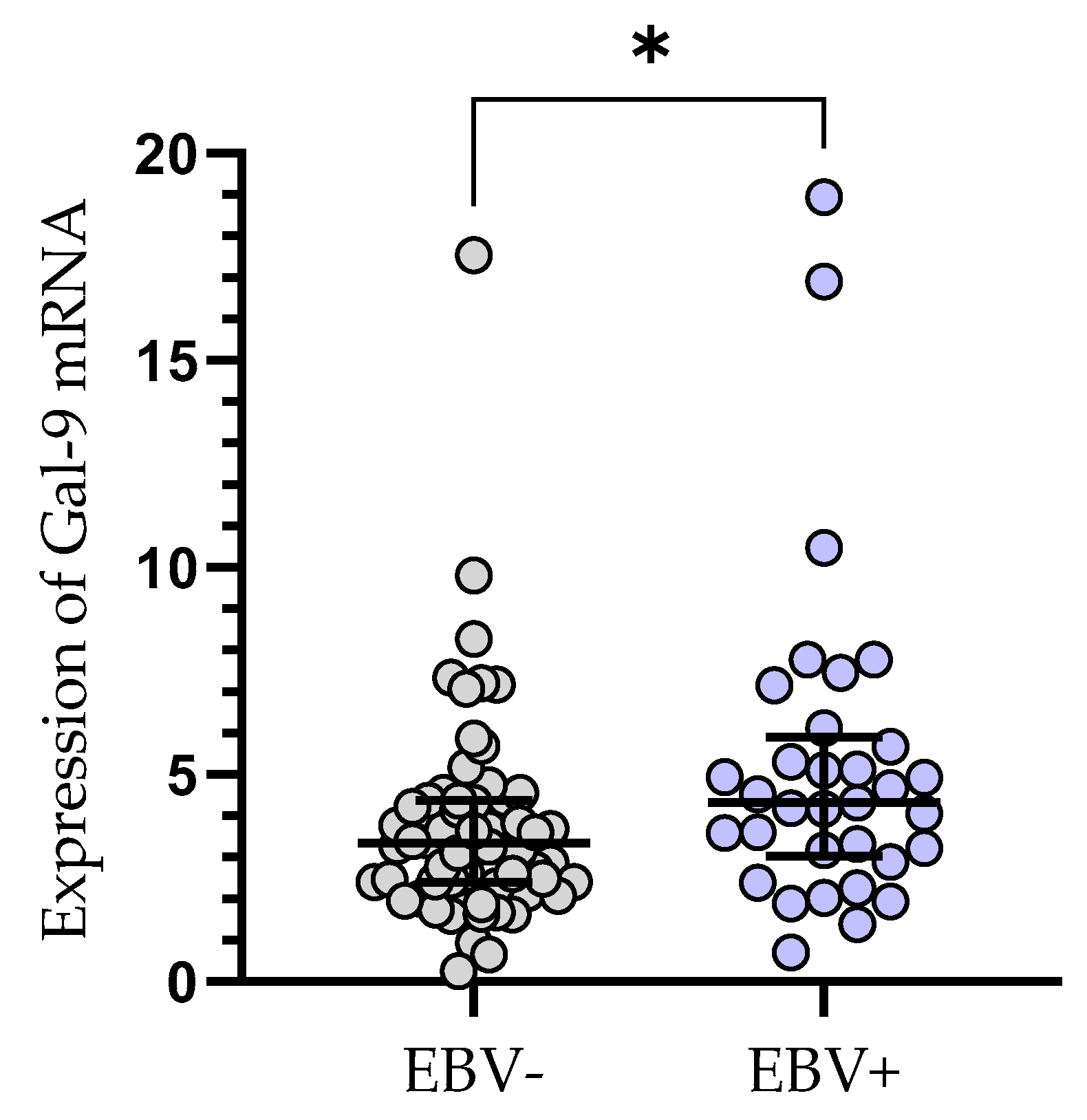
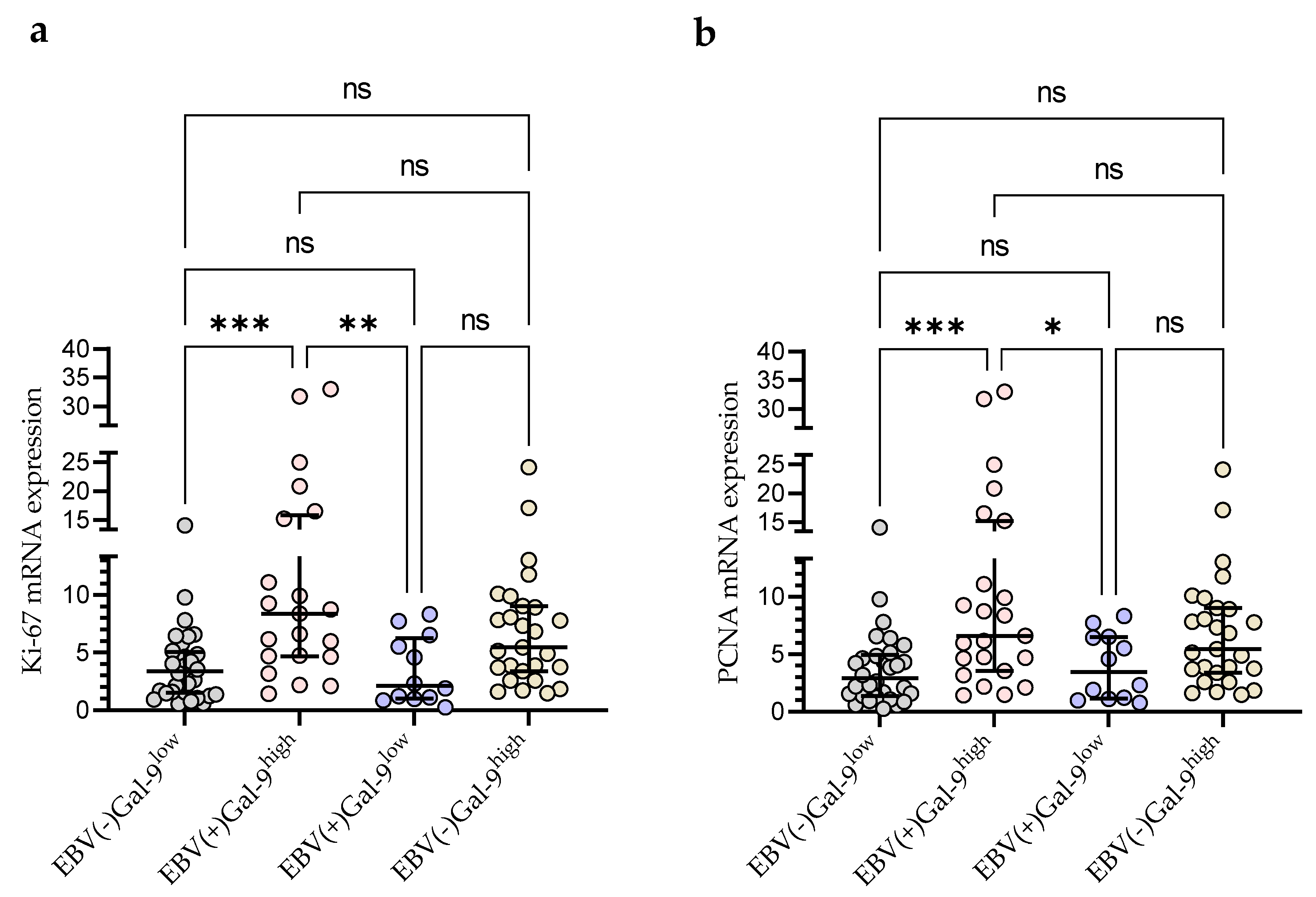
3.3. Gal-9 mRNA Expression in EBV-Positive CLL Cases
3.4. The Clinical Findings and Gal-9 mRNA Expression of CLL Patients
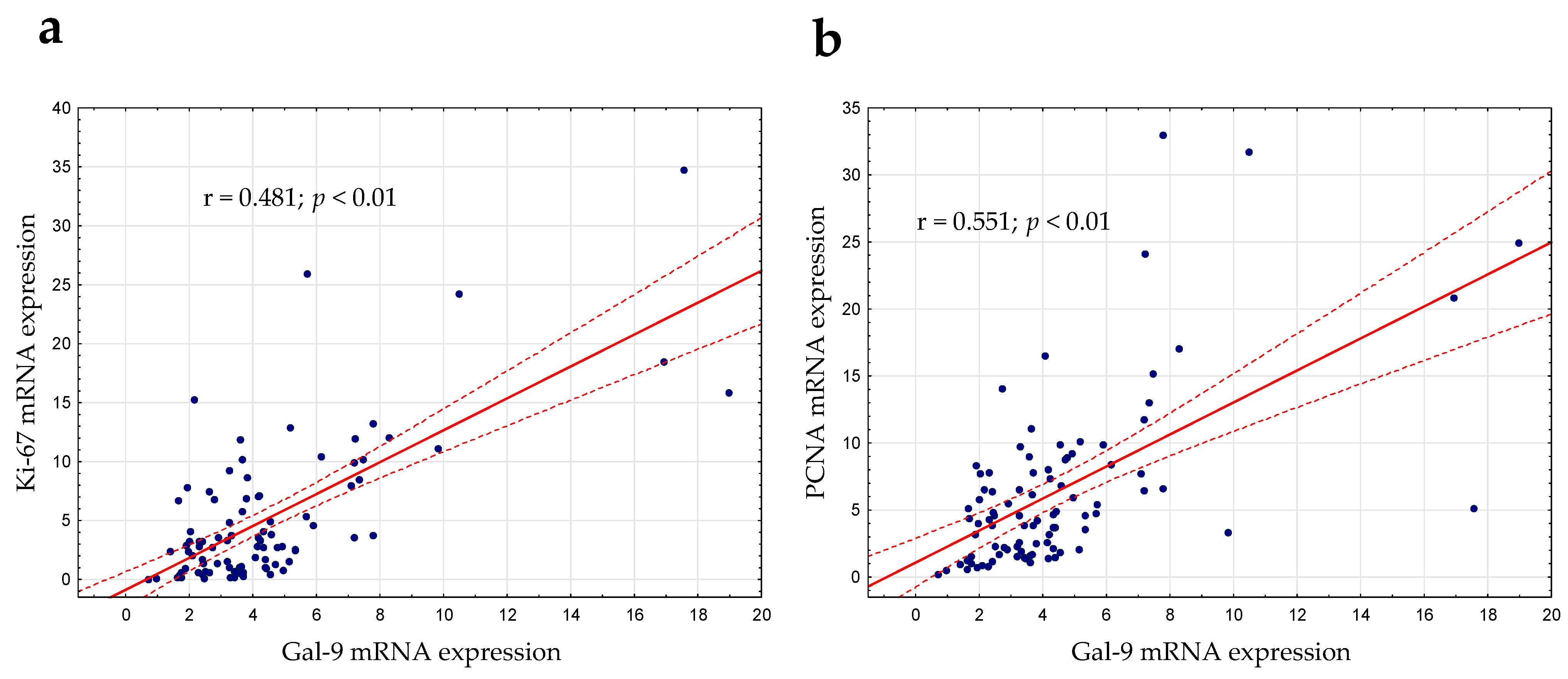
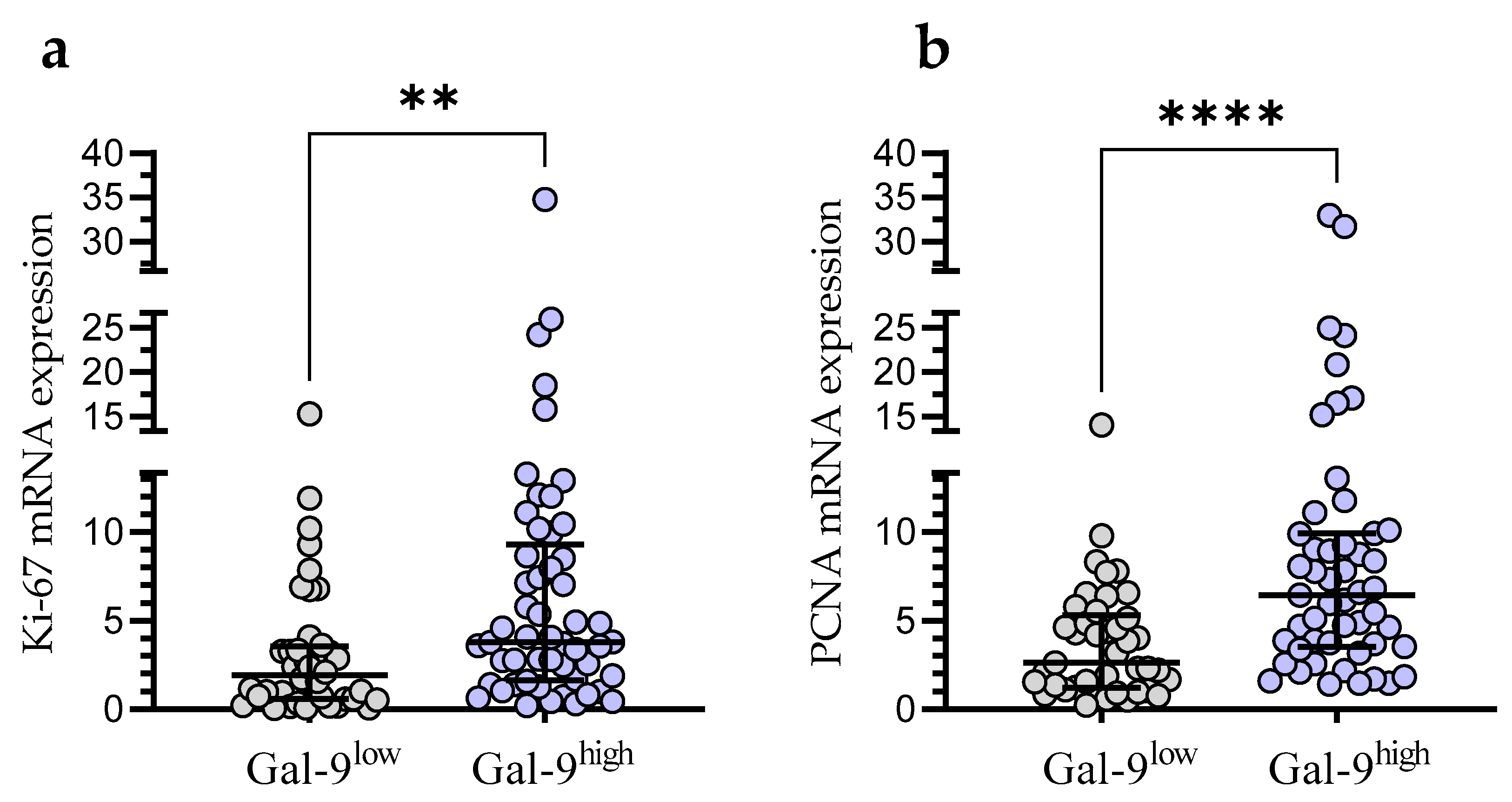
3.5. Relationship between Gal-9 and Proliferation Markers (Ki67 and PCNA) mRNA Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Dai, Q.; Li, Y.; Tang, Z.; She, T. Association between High Galectin Expression and Poor Prognosis in Hematologic Cancers: A Systematic Review and Meta-Analysis. Hematology 2023, 28, 2227494. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, H.; Li, C. Galectins and Galectin-Mediated Autophagy Regulation: New Insights into Targeted Cancer Therapy. Biomark. Res. 2023, 11, 22. [Google Scholar] [CrossRef]
- Moar, P.; Tandon, R. Galectin-9 as a Biomarker of Disease Severity. Cell. Immunol. 2021, 361, 104287. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.M.; Wiersma, V.R.; Samplonius, D.F.; Gerssen, J.; van Ginkel, R.J.; Nijman, H.W.; Hirashima, M.; Niki, T.; Eggleton, P.; Helfrich, W.; et al. Galectin-9 Activates and Expands Human T-Helper 1 Cells. PLoS ONE 2013, 8, e65616. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, X.; Ma, Y.; Du, Y.; Feng, J. A New Emerging Target in Cancer Immunotherapy: Galectin-9 (LGALS9). Genes. Dis. 2023, 10, 2366–2382. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Li, C.-F.; Wang, Y.-H.; Yao, J.; Li, H.; Yan, M.; Chang, W.-C.; Hsu, J.-M.; Cha, J.-H.; et al. Galectin-9 Interacts with PD-1 and TIM-3 to Regulate T Cell Death and Is a Target for Cancer Immunotherapy. Nat. Commun. 2021, 12, 832. [Google Scholar] [CrossRef]
- Nakajima, R.; Miyagaki, T.; Kamijo, H.; Oka, T.; Shishido-Takahashi, N.; Suga, H.; Sugaya, M.; Sato, S. Possible Therapeutic Applicability of Galectin-9 in Cutaneous T-Cell Lymphoma. J. Dermatol. Sci. 2019, 96, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 Signaling Pathway: An Emerging Target for Cancer Immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef]
- Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. [Google Scholar] [CrossRef]
- Gonçalves Silva, I.; Yasinska, I.M.; Sakhnevych, S.S.; Fiedler, W.; Wellbrock, J.; Bardelli, M.; Varani, L.; Hussain, R.; Siligardi, G.; Ceccone, G.; et al. The Tim-3-Galectin-9 Secretory Pathway Is Involved in the Immune Escape of Human Acute Myeloid Leukemia Cells. EBioMedicine 2017, 22, 44–57. [Google Scholar] [CrossRef]
- Wdowiak, K.; Gallego-Colon, E.; Francuz, T.; Czajka-Francuz, P.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wybraniec, M.; Chudek, J.; Wojnar, J. Increased Serum Levels of Galectin-9 in Patients with Chronic Lymphocytic Leukemia. Oncol. Lett. 2018, 17, 1019–1029. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, L.; Jing, D.; Xu, G.; Zhang, J.; Lin, L.; Zhao, J.; Yao, Z.; Lin, H. Galectin-9 Expression Predicts Favorable Clinical Outcome in Solid Tumors: A Systematic Review and Meta-Analysis. Front. Physiol. 2018, 9, 452. [Google Scholar] [CrossRef]
- Taghiloo, S.; Allahmoradi, E.; Ebadi, R.; Tehrani, M.; Hosseini-Khah, Z.; Janbabai, G.; Shekarriz, R.; Asgarian-Omran, H. Upregulation of Galectin-9 and PD-L1 Immune Checkpoints Molecules in Patients with Chronic Lymphocytic Leukemia. Asian Pac. J. Cancer Prev. 2017, 18, 2269. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Park, Y.; Kim, J.-H.; Kang, K.-W.; Lee, S.-J.; Kim, S.J.; Kim, B.S. Prognostic Value of Galectin-9 Relates to Programmed Death-Ligand 1 in Patients With Multiple Myeloma. Front. Oncol. 2021, 11, 669817. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Wu, R.; Yin, J.; Fan, M.; Xu, X. Prognostic Role of High Gal-9 Expression in Solid Tumours: A Meta-Analysis. Cell Physiol. Biochem. 2018, 45, 993–1002. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Ma, C.; Gao, W.; Song, B.; Xue, H.; Chen, W.; Chen, X.; Zhang, Y.; Shao, Q.; et al. Reduced Expression of Galectin-9 Contributes to a Poor Outcome in Colon Cancer by Inhibiting NK Cell Chemotaxis Partially through the Rho/ROCK1 Signaling Pathway. PLoS ONE 2016, 11, e0152599. [Google Scholar] [CrossRef]
- Yamauchi, A.; Kontani, K.; Kihara, M.; Nishi, N.; Yokomise, H.; Hirashima, M. Galectin-9, a Novel Prognostic Factor with Antimetastatic Potential in Breast Cancer. Breast J. 2006, 12, S196–S200. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, Y.; Xu, L.; Liu, W.; Fu, Q.; Liu, H.; Zhang, W.; Xu, J. Galectin-9 Predicts Postoperative Recurrence and Survival of Patients with Clear-Cell Renal Cell Carcinoma. Tumor Biol. 2015, 36, 5791–5799. [Google Scholar] [CrossRef]
- Dama, P.; Tang, M.; Fulton, N.; Kline, J.; Liu, H. Gal9/Tim-3 Expression Level Is Higher in AML Patients Who Fail Chemotherapy. J. Immunother. Cancer 2019, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Alimu, X.; Zhang, J.; Pang, N.; Zhang, R.; Chen, R.; Zeng, X.; Tudahong, S.; Chen, G.; Muhashi, M.; Zhao, F.; et al. Galectin-9 and Myeloid-derived Suppressor Cell as Prognostic Indicators for Chronic Lymphocytic Leukemia. Immun. Inflam. Dis. 2023, 11, e853. [Google Scholar] [CrossRef]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wojnar, J. Galectin Targeted Therapy in Oncology: Current Knowledge and Perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, R.; Huang, D.; Tang, Y.; Ping, L.; Huang, B.; Huang, H.; Busson, P.; Li, J. Galectin-9 Facilitates Epstein-Barr Virus Latent Infection and Lymphomagenesis in Human B Cells. Microbiol. Spectr. 2023, 11, e04932-22. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.-M.; Keating, M.J.; Bueso-Ramos, C.E.; Kurzrock, R. Epstein-Barr Virus in Patients with Chronic Lymphocytic Leukemia: A Pilot Study. Leuk. Lymphoma 2006, 47, 827–836. [Google Scholar] [CrossRef]
- Tarrand, J.J.; Keating, M.J.; Tsimberidou, A.M.; O’Brien, S.; LaSala, R.P.; Han, X.-Y.; Bueso-Ramos, C.E. Epstein-Barr Virus Latent Membrane Protein 1 mRNA Is Expressed in a Significant Proportion of Patients with Chronic Lymphocytic Leukemia. Cancer 2010, 116, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Grywalska, E.; Roliński, J.; Pasiarski, M.; Korona-Glowniak, I.; Maj, M.; Surdacka, A.; Grafka, A.; Stelmach-Gołdyś, A.; Zgurski, M.; Góźdź, S.; et al. High Viral Loads of Epstein-Barr Virus DNA in Peripheral Blood of Patients with Chronic Lymphocytic Leukemia Associated with Unfavorable Prognosis. PLoS ONE 2015, 10, e0140178. [Google Scholar] [CrossRef]
- Grywalska, E.; Pasiarski, M.; Sosnowska-Pasiarska, B.; Macek, P.; Rolińska, A.; Samardakiewicz, M.; Ludian, J.; Góźdź, S.; Roliński, J. Programmed Cell Death 1 Expression and Epstein-Barr Virus Infection in Chronic Lymphocytic Leukaemia: A Prospective Cohort Study. Cancer Manag. Res. 2019, 11, 7605–7618. [Google Scholar] [CrossRef]
- Visco, C.; Falisi, E.; Young, K.H.; Pascarella, M.; Perbellini, O.; Carli, G.; Novella, E.; Rossi, D.; Giaretta, I.; Cavallini, C.; et al. Epstein-Barr Virus DNA Load in Chronic Lymphocytic Leukemia Is an Independent Predictor of Clinical Course and Survival. Oncotarget 2015, 6, 18653–18663. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.J.; Montserrat, E.; Rai, K.R.; et al. Guidelines for the Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute–Working Group 1996 Guidelines. Blood 2008, 111, 5446–5456. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical Staging of Chronic Lymphocytic Leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef]
- Zarobkiewicz, M.; Kowalska, W.; Chocholska, S.; Tomczak, W.; Szymańska, A.; Morawska, I.; Wojciechowska, A.; Bojarska-Junak, A. High M-MDSC Percentage as a Negative Prognostic Factor in Chronic Lymphocytic Leukaemia. Cancers 2020, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chocholska, S.; Zarobkiewicz, M.; Szymańska, A.; Lehman, N.; Woś, J.; Bojarska-Junak, A. Prognostic Value of the miR-17~92 Cluster in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2023, 24, 1705. [Google Scholar] [CrossRef]
- Hus, I.; Podhorecka, M.; Bojarska-Junak, A.; Roliński, J.; Schmitt, M.; Sieklucka, M.; Wąsik-Szczepanek, E.; Dmoszyńska, A. The Clinical Significance of ZAP-70 and CD38 Expression in B-Cell Chronic Lymphocytic Leukaemia. Ann. Oncol. 2006, 17, 683–690. [Google Scholar] [CrossRef]
- Woś, J.; Chocholska, S.; Kowalska, W.; Tomczak, W.; Szymańska, A.; Karczmarczyk, A.; Szuster-Ciesielska, A.; Wojciechowska, A.; Bojarska-Junak, A. Prognostic Value of Tie2-Expressing Monocytes in Chronic Lymphocytic Leukemia Patients. Cancers 2021, 13, 2817. [Google Scholar] [CrossRef] [PubMed]
- Durak Aras, B.; Isik, S.; Uskudar Teke, H.; Aslan, A.; Yavasoglu, F.; Gulbas, Z.; Demirkan, F.; Ozen, H.; Cilingir, O.; Inci, N.S.; et al. Which Prognostic Marker Is Responsible for the Clinical Heterogeneity in CLL with 13q Deletion? Mol. Cytogenet. 2021, 14, 2. [Google Scholar] [CrossRef]
- Chen, J.; Sathiaseelan, V.; Moore, A.; Tan, S.; Chilamakuri, C.S.R.; Roamio Franklin, V.N.; Shahsavari, A.; Jakwerth, C.A.; Hake, S.B.; Warren, A.J.; et al. ZAP-70 Constitutively Regulates Gene Expression and Protein Synthesis in Chronic Lymphocytic Leukemia. Blood 2021, 137, 3629–3640. [Google Scholar] [CrossRef]
- Burger, J.A.; Gribben, J.G. The Microenvironment in Chronic Lymphocytic Leukemia (CLL) and Other B Cell Malignancies: Insight into Disease Biology and New Targeted Therapies. Semin. Cancer Biol. 2014, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Caligaris-Cappio, F.; Bertilaccio, M.T.S.; Scielzo, C. How the Microenvironment Wires the Natural History of Chronic Lymphocytic Leukemia. Semin. Cancer Biol. 2014, 24, 43–48. [Google Scholar] [CrossRef]
- Gemenetzi, K.; Agathangelidis, A.; Zaragoza-Infante, L.; Sofou, E.; Papaioannou, M.; Chatzidimitriou, A.; Stamatopoulos, K. B Cell Receptor Immunogenetics in B Cell Lymphomas: Immunoglobulin Genes as Key to Ontogeny and Clinical Decision Making. Front. Oncol. 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, J.U.; Yin, Y.; Urban, A.; Myklebust, C.V.; Karlsen, L.; Melvold, K.; Tveita, A.A.; Taskén, K.; Munthe, L.A.; Tjønnfjord, G.E.; et al. A Tumor Microenvironment Model of Chronic Lymphocytic Leukemia Enables Drug Sensitivity Testing to Guide Precision Medicine. Cell Death Discov. 2023, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, A.; Pepper, C.; Mitchell, S.; Pepper, A. NF-kB and the CLL Microenvironment. Front. Oncol. 2023, 13, 1169397. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Asgarian-Omran, H. Current Approaches of Immune Checkpoint Therapy in Chronic Lymphocytic Leukemia. Curr. Treat. Options Oncol. 2023, 24, 1408–1438. [Google Scholar] [CrossRef]
- Llaó Cid, L.; Wong, J.K.; Wierz, M.; Paul, Y.; Roider, T.; Fernandez Botana, I.; Gonder, S.; Floerchinger, A.; Colomer, D.; Campo, E.; et al. Single-Cell Omics Analyses Identify the TIM-3 Ligand Galectin-9 As Novel Immunotherapy Target for Chronic Lymphocytic Leukemia. Blood 2022, 140, 1805–1806. [Google Scholar] [CrossRef]
- Pang, N.; Alimu, X.; Chen, R.; Muhashi, M.; Ma, J.; Chen, G.; Zhao, F.; Wang, L.; Qu, J.; Ding, J. Activated Galectin-9/Tim3 Promotes Treg and Suppresses Th1 Effector Function in Chronic Lymphocytic Leukemia. FASEB J. 2021, 35, e21556. [Google Scholar] [CrossRef]
- Iwasaki-Hozumi, H.; Chagan-Yasutan, H.; Ashino, Y.; Hattori, T. Blood Levels of Galectin-9, an Immuno-Regulating Molecule, Reflect the Severity for the Acute and Chronic Infectious Diseases. Biomolecules 2021, 11, 430. [Google Scholar] [CrossRef]
- Meggyes, M.; Nagy, D.U.; Balassa, T.; Godony, K.; Peterfalvi, A.; Szereday, L.; Polgar, B. Influence of Galectin-9 Treatment on the Phenotype and Function of NK-92MI Cells in the Presence of Different Serum Supplements. Biomolecules 2021, 11, 1066. [Google Scholar] [CrossRef]
- Jafarkhani, S.; Hossein-Nataj, H.; Eslami-Jouybari, M.; Ghoreishi, M.; Asgarian-Omran, H. PD-1 and TIM-3 blocking cannot enhance apoptosis of chronic lymphocytic leukemia cells induced by peripheral blood CD8+ T cells. Exp. Oncol. 2023, 44, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, H.; Astaneh, M.; Tehrani, M.; Hossein-Nataj, H.; Zaboli, E.; Shekarriz, R.; Asgarian-Omran, H. Blockade of PD-1 and TIM-3 Immune Checkpoints Fails to Restore the Function of Exhausted CD8+ T Cells in Early Clinical Stages of Chronic Lymphocytic Leukemia. Immunol. Res. 2020, 68, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Griggio, V.; Perutelli, F.; Salvetti, C.; Boccellato, E.; Boccadoro, M.; Vitale, C.; Coscia, M. Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia. Front. Immunol. 2020, 11, 594556. [Google Scholar] [CrossRef]
- Bozorgmehr, N.; Hnatiuk, M.; Peters, A.C.; Elahi, S. Depletion of Polyfunctional CD26highCD8+ T Cells Repertoire in Chronic Lymphocytic Leukemia. Exp. Hematol. Oncol. 2023, 12, 13. [Google Scholar] [CrossRef]
- Kang, S.; Ahn, I.E. Prognostic Markers in the Era of Targeted Therapies. Acta Haematol. 2023; 1–14, online ahead of print. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, J.; Bi, Y.; Wang, H. ZAP-70 in Chronic Lymphocytic Leukemia: A Meta-Analysis. Clin. Chim. Acta 2018, 483, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Chanaga, C.I.; Rassenti, L.Z. Biomarkers in Chronic Lymphocytic Leukemia: Clinical Applications and Prognostic Markers. Best. Pract. Res. Clin. Haematol. 2016, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Mollstedt, J.; Mansouri, L.; Rosenquist, R. Precision Diagnostics in Chronic Lymphocytic Leukemia: Past, Present and Future. Front. Oncol. 2023, 13, 1146486. [Google Scholar] [CrossRef] [PubMed]
- Rassenti, L.Z.; Jain, S.; Keating, M.J.; Wierda, W.G.; Grever, M.R.; Byrd, J.C.; Kay, N.E.; Brown, J.R.; Gribben, J.G.; Neuberg, D.S.; et al. Relative Value of ZAP-70, CD38, and Immunoglobulin Mutation Status in Predicting Aggressive Disease in Chronic Lymphocytic Leukemia. Blood 2008, 112, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Rassenti, L.Z.; Huynh, L.; Toy, T.L.; Chen, L.; Keating, M.J.; Gribben, J.G.; Neuberg, D.S.; Flinn, I.W.; Rai, K.R.; Byrd, J.C.; et al. ZAP-70 Compared with Immunoglobulin Heavy-Chain Gene Mutation Status as a Predictor of Disease Progression in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2004, 351, 893–901. [Google Scholar] [CrossRef]
- Miao, Y.; Miao, Y.; Shi, K.; Sun, Q.; Zhao, S.-S.; Xia, Y.; Qin, S.-C.; Qiu, H.-R.; Yang, H.; Xu, H.; et al. A Higher Percentage of Cells with 13q Deletion Predicts Worse Outcome in Chinese Patients with Chronic Lymphocytic Leukemia Carrying Isolated 13q Deletion. Ann. Hematol. 2018, 97, 1663–1669. [Google Scholar] [CrossRef]
- Ouillette, P.; Collins, R.; Shakhan, S.; Li, J.; Li, C.; Shedden, K.; Malek, S.N. The Prognostic Significance of Various 13q14 Deletions in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2011, 17, 6778–6790. [Google Scholar] [CrossRef]
- Garg, R.; Wierda, W.; Ferrajoli, A.; Abruzzo, L.; Pierce, S.; Lerner, S.; Keating, M.; O’Brien, S. The Prognostic Difference of Monoallelic versus Biallelic Deletion of 13q in Chronic Lymphocytic Leukemia: Monoallelic vs Biallelic Deletion of 13q. Cancer 2012, 118, 3531–3537. [Google Scholar] [CrossRef]
- Herishanu, Y.; Pérez-Galán, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The Lymph Node Microenvironment Promotes B-Cell Receptor Signaling, NF-κB Activation, and Tumor Proliferation in Chronic Lymphocytic Leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef]
- Bruey, J.-M.; Kantarjian, H.; Ma, W.; Estrov, Z.; Yeh, C.; Donahue, A.; Sanders, H.; O’Brien, S.; Keating, M.; Albitar, M. Circulating Ki-67 Index in Plasma as a Biomarker and Prognostic Indicator in Chronic Lymphocytic Leukemia. Leuk. Res. 2010, 34, 1320–1324. [Google Scholar] [CrossRef][Green Version]
- Stevenson, F.K.; Forconi, F.; Kipps, T.J. Exploring the Pathways to Chronic Lymphocytic Leukemia. Blood 2021, 138, 827–835. [Google Scholar] [CrossRef]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, PCNA, and MCM Proteins: Markers of Proliferation in the Diagnosis of Breast Cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- del Giglio, A.; O’Brien, S.; Ford, R.; Saya, H.; Manning, J.; Keating, M.; Johnston, D.; Khetan, R.; el-Naggar, A.; Deisseroth, A. Prognostic Value of Proliferating Cell Nuclear Antigen Expression in Chronic Lymphoid Leukemia. Blood 1992, 79, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.D.; O’brien, S.; Ford, R.J.; Manning, J.; Saya, H.; Keating, M.; Johnston, D.; Chamone, D.F.; Deisseroth, A.B. Proliferating Cell Nuclear Antigen (PCNA) Expression in Chronic Lymphocytic Leukemia (CLL). Leuk. Lymphoma 1993, 10, 265–271. [Google Scholar] [CrossRef]
- Šoljić, V.; Perak, R.B.; Vukojević, K.; Saraga-Babić, M.; Bubalo, P.; Karan, D.; Todorović, J.; Batinić, D. ZAP-70 Expression and Proliferative Activity in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2013, 54, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein–Barr Virus and Cancer. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Hutcheson, R.L.; Chakravorty, A.; Sugden, B. Burkitt Lymphomas Evolve to Escape Dependencies on Epstein-Barr Virus. Front. Cell. Infect. Microbiol. 2021, 10, 606412. [Google Scholar] [CrossRef]
- Chabay, P. Advances in the Pathogenesis of EBV-Associated Diffuse Large B Cell Lymphoma. Cancers 2021, 13, 2717. [Google Scholar] [CrossRef]
- Higuchi, H.; Yamakawa, N.; Imadome, K.-I.; Yahata, T.; Kotaki, R.; Ogata, J.; Kakizaki, M.; Fujita, K.; Lu, J.; Yokoyama, K.; et al. Role of Exosomes as a Proinflammatory Mediator in the Development of EBV-Associated Lymphoma. Blood 2018, 131, 2552–2567. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-H.; Gao, R.; Xia, Y.; Gale, R.P.; Chen, R.-Z.; Yang, Y.-Q.; Wang, L.; Qu, X.-Y.; Qiu, H.-R.; Cao, L.; et al. Prognostic Impact of Epstein-Barr Virus (EBV)-DNA Copy Number at Diagnosis in Chronic Lymphocytic Leukemia. Oncotarget 2016, 7, 2135–2142. [Google Scholar] [CrossRef] [PubMed]
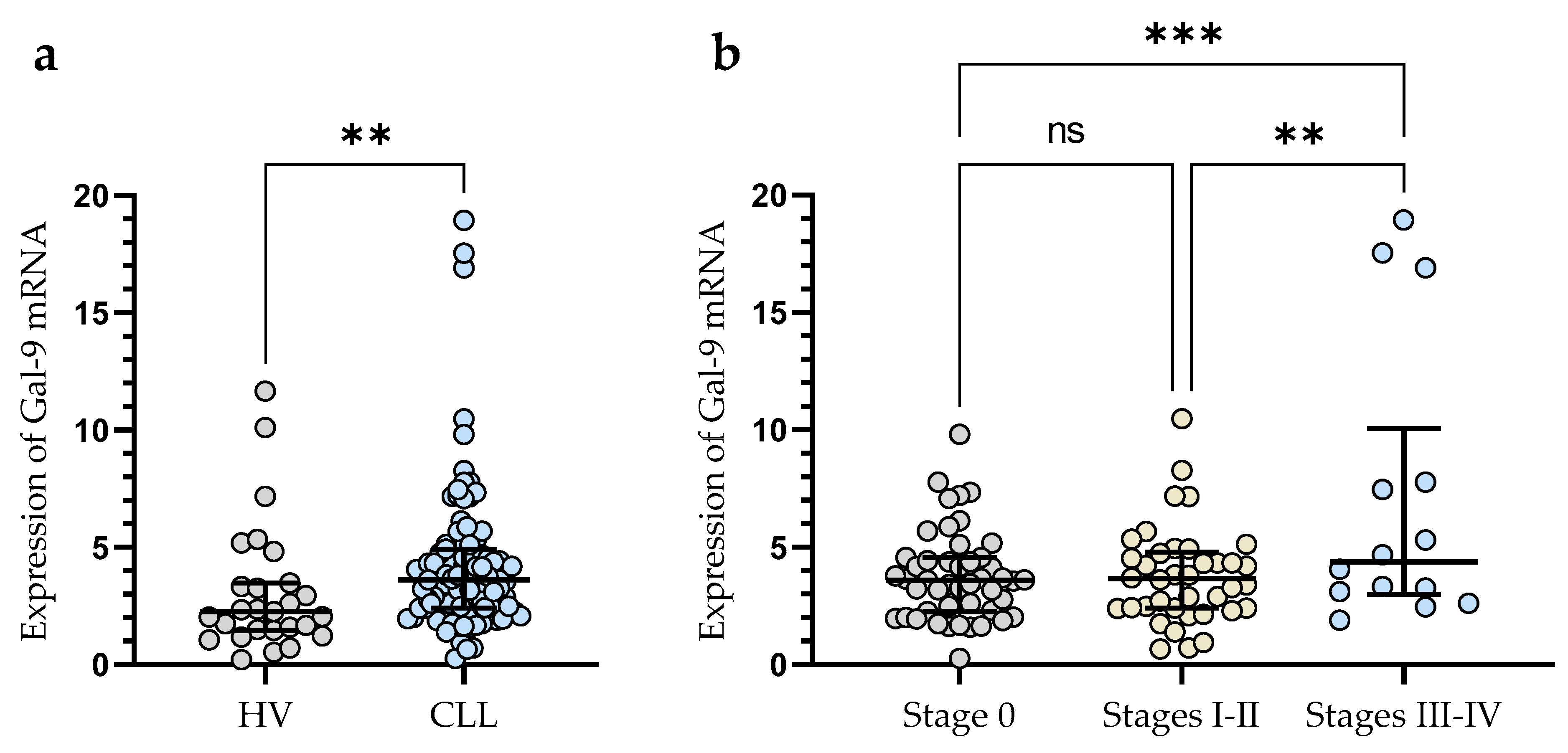



| Characteristics | n |
|---|---|
| Total number of patients | 100 |
| Rai Stage 0 | 46 |
| Rai Stages I–II | 40 |
| Rai Stages III–IV | 14 |
| ZAP-70 | |
| ≥20% | 35 |
| <20% | 65 |
| CD38 | |
| ≥30% | 39 |
| <30% | 61 |
| FISH | |
| 17p- | 5 |
| 11q- | 15 |
| +12 | 7 |
| sole 13q- | 40 |
| Heterozygous 13q- | 28/40 |
| Homozygous 13q- | 11/40 |
| Without 17p-, 11q-, +12, and 13q- | 33 |
| Patients treated during observation period | 49 |
| Complete remission (CR) | 7/49 |
| Partial remission (PR) | 27/49 |
| Stable disease (SD) | 8/49 |
| Disease progression (PD) | 7/49 |
| Untreated patients | 51 |
| The IGHV mutational status | |
| Unmutated IGHV | 15 |
| Mutated IGHV | 22 |
| Not available | 63 |
| EBV(−) | 62 |
| EBV(+) | 33 |
| not available | 5 |
| Age in years at diagnosis, median (range) | 67 (38–85) |
| White blood cells (WBC) count (G/L), median (IQR) | 25.84 (17.28–54.80) |
| Lymphocyte count (G/L), median (IQR) | 18.30 (10.68–44.96) |
| Lactate dehydrogenase (LDH) level (IU/L), median (IQR) | 379 (324–428) |
| β2-microglobulin level (mg/dL), median (IQR) | 2.45 (2.01–3.18) |
| ZAP-70-positive CD19+/CD5+ cells (%), median (IQR) | 7.42 (3.67–20.10) |
| CD38-positive CD19+/CD5+ cells (%), median (IQR) | 6.72 (1.595–34.23) |
| Risk Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | ||||||
| ≥65 years | 1.95 | 0.971–3.936 | ns | NA | ||
| <65 years | ||||||
| ZAP-70 | ||||||
| ≥20% | 1.587 | 0.838–3.008 | <0.01 | 1.697 | 0.506–5.695 | <0.05 |
| <20% | ||||||
| CD38 | ||||||
| ≥30% | 1.785 | 0.952–3.350 | ns | NA | ||
| <30% | ||||||
| β2M | ||||||
| ≥3.5 mg/dL | 2.293 | 1.172–4.488 | <0.01 | 2.734 | 1.096–6.821 | <0.05 |
| <3.5 mg/dL | ||||||
| 17p-, 11q- or +12 | ||||||
| Positive | 2.03 | 1.051–3.923 | <0.01 | 2.327 | 0.980–5.529 | <0.05 |
| Negative | ||||||
| Sole 13q- | ||||||
| Positive | 0.482 | 0.249–0.930 | <0.05 | 0.766 | 0.300–1.953 | ns |
| Negative | ||||||
| EBV-DNA | ||||||
| Positive | 1.366 | 0.695–2.687 | ns | NA | ||
| Negative | ||||||
| Gal-9 mRNA | ||||||
| ≥3.389 | 2.162 | 1.071–4.361 | <0.05 | 0.472 | 0.232–0.956 | <0.05 |
| <3.389 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarska-Junak, A.; Kowalska, W.; Chocholska, S.; Szymańska, A.; Tomczak, W.; Zarobkiewicz, M.K.; Roliński, J. Prognostic Potential of Galectin-9 mRNA Expression in Chronic Lymphocytic Leukemia. Cancers 2023, 15, 5370. https://doi.org/10.3390/cancers15225370
Bojarska-Junak A, Kowalska W, Chocholska S, Szymańska A, Tomczak W, Zarobkiewicz MK, Roliński J. Prognostic Potential of Galectin-9 mRNA Expression in Chronic Lymphocytic Leukemia. Cancers. 2023; 15(22):5370. https://doi.org/10.3390/cancers15225370
Chicago/Turabian StyleBojarska-Junak, Agnieszka, Wioleta Kowalska, Sylwia Chocholska, Agata Szymańska, Waldemar Tomczak, Michał Konrad Zarobkiewicz, and Jacek Roliński. 2023. "Prognostic Potential of Galectin-9 mRNA Expression in Chronic Lymphocytic Leukemia" Cancers 15, no. 22: 5370. https://doi.org/10.3390/cancers15225370
APA StyleBojarska-Junak, A., Kowalska, W., Chocholska, S., Szymańska, A., Tomczak, W., Zarobkiewicz, M. K., & Roliński, J. (2023). Prognostic Potential of Galectin-9 mRNA Expression in Chronic Lymphocytic Leukemia. Cancers, 15(22), 5370. https://doi.org/10.3390/cancers15225370









