Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drug Preparation and Cell Line Cultures
2.2. Cell Viability Assay
2.3. Flow Cytometric Analysis of Cell Cycle and Apoptosis
2.4. Western Blot Analysis
2.5. In Vivo Zebrafish Assays for Tumor-Induced Angiogenesis and Tumor Cell Migration
2.6. Analyses of Human VEGF, Angiopoietin 1–2, FGF-1α, PDGF (AB, AA, BB, C) Concentrations in Conditioned Media of LC Cell Lines
2.7. Statistical Analyses
3. Results
3.1. AXI Affected Viability of Human LC Cell Lines
3.2. AXI Perturbed Cell Cycle in Human LC Cell Lines
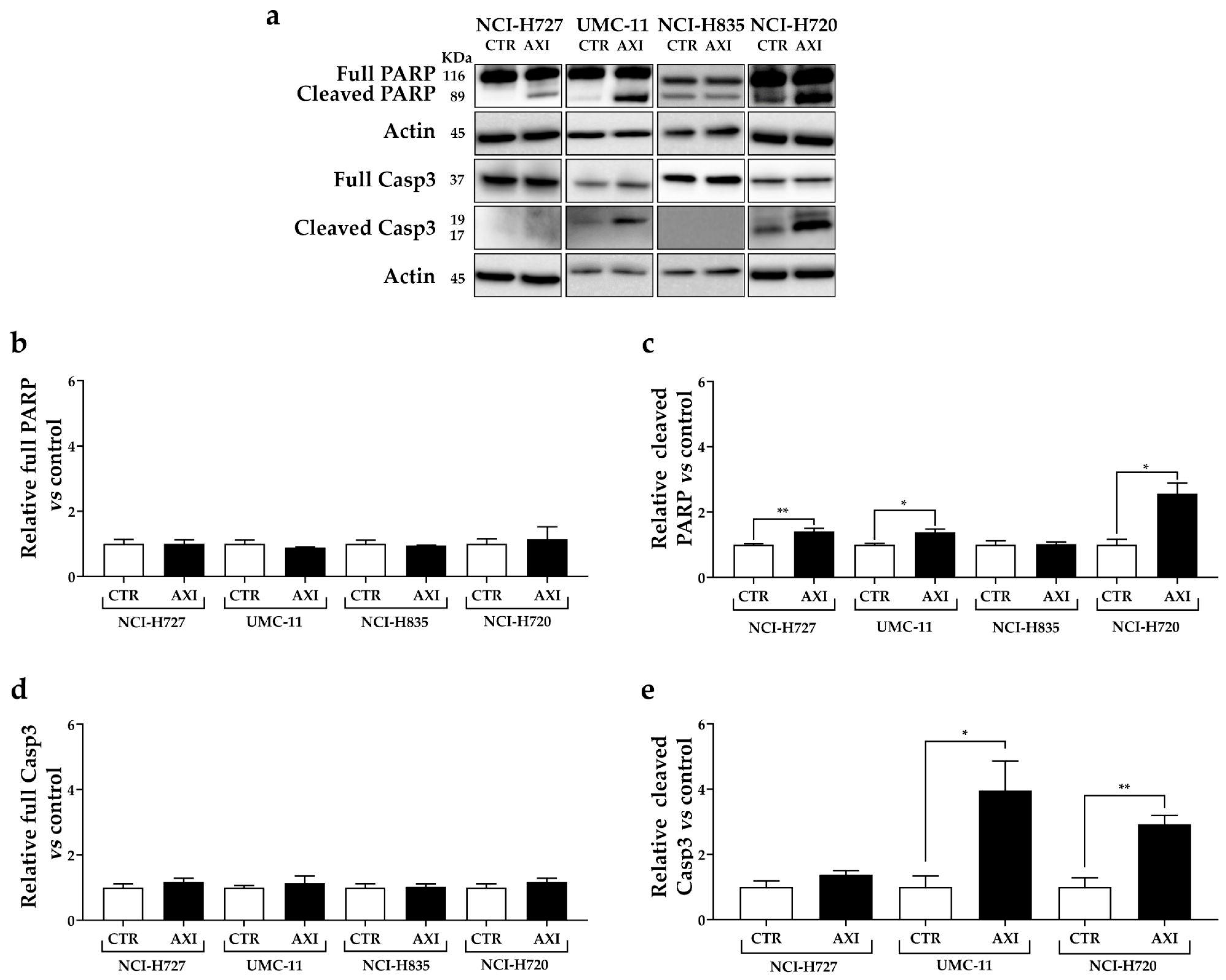
3.3. AXI Induced Cell Death in Human LC Cell Lines
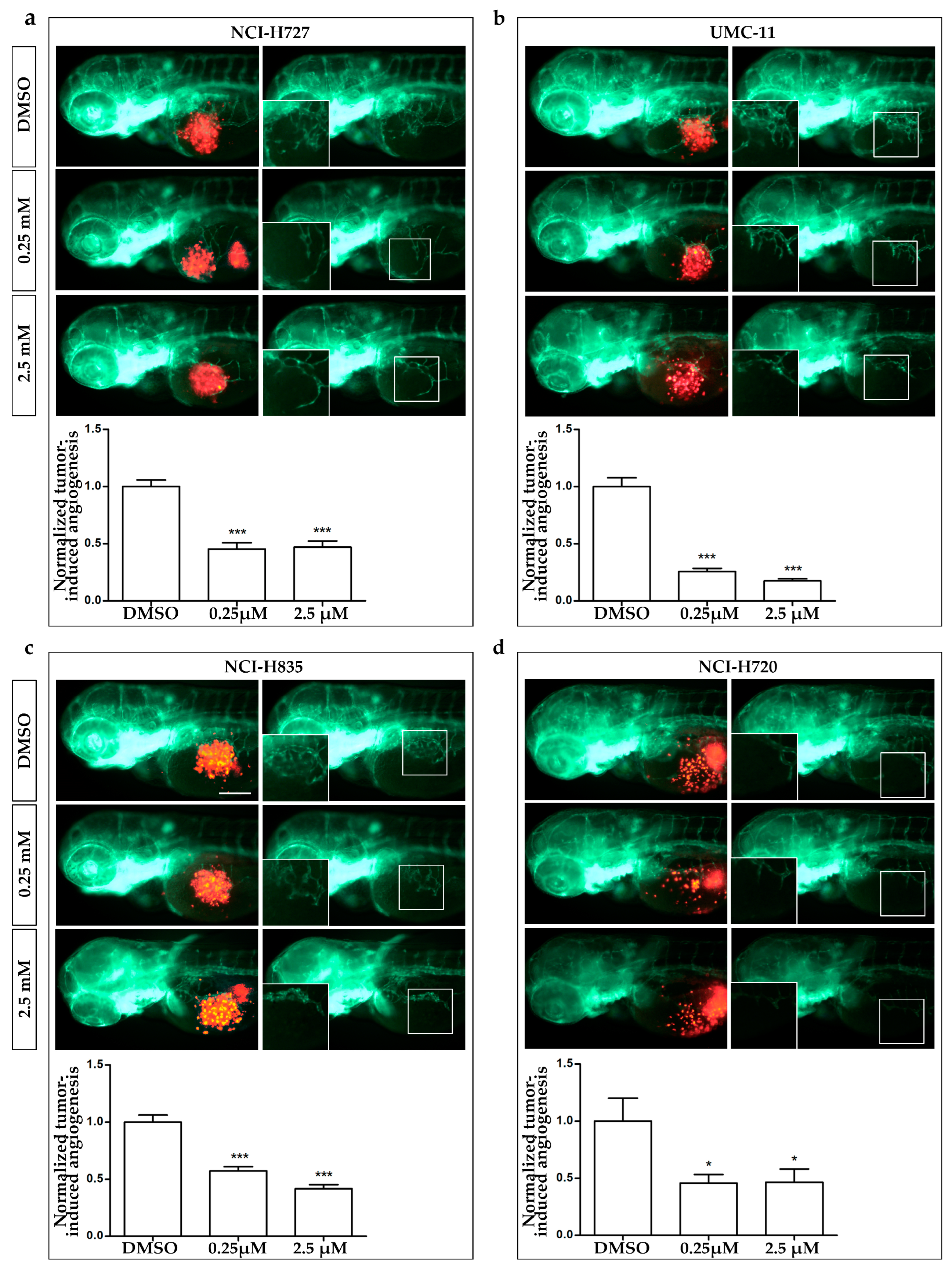
3.4. AXI Effects on Tumor-Induced Angiogenesis and Tumor Cell Migration
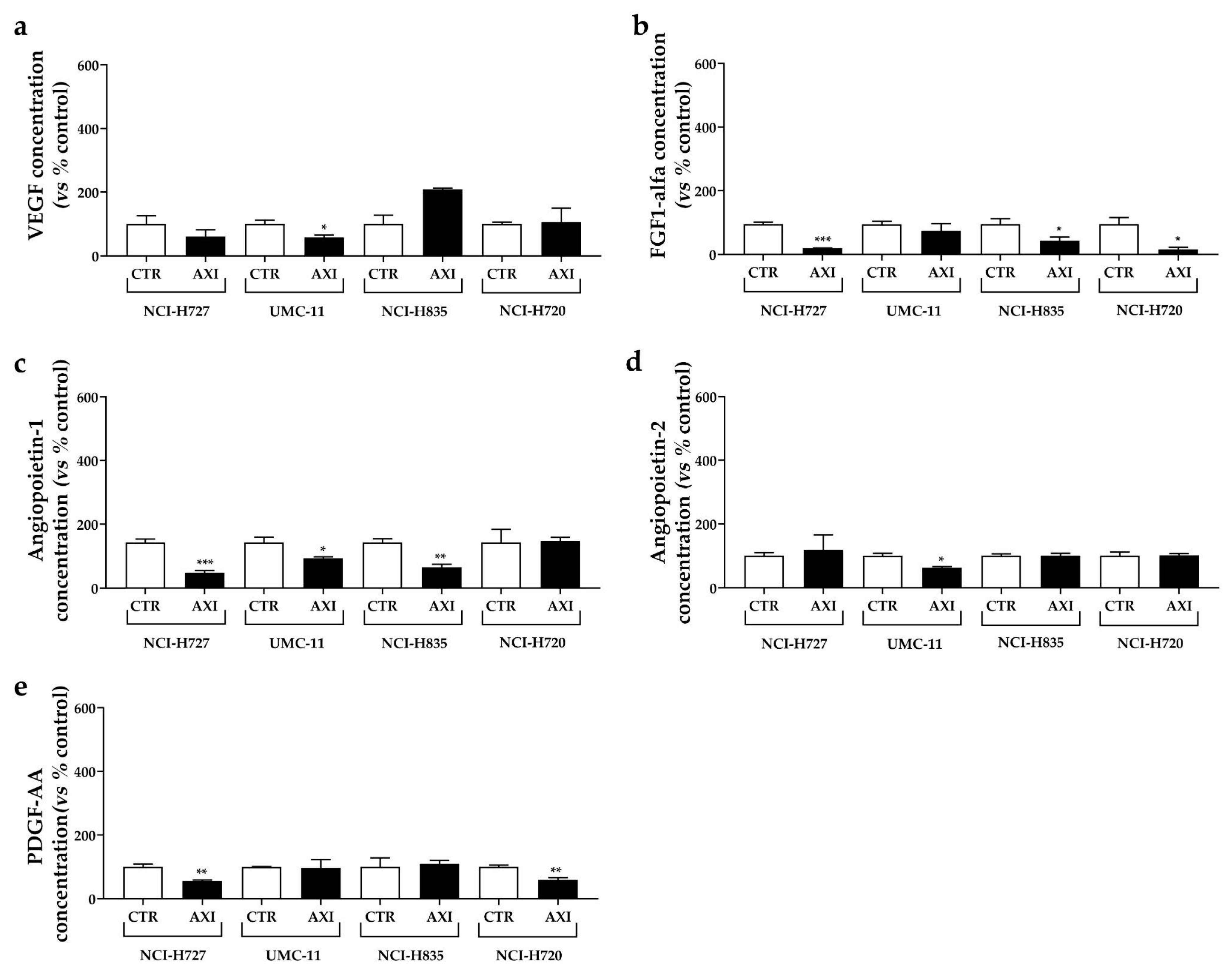
3.5. AXI Modulated Human VEGF, Angiopoietin 1–2, FGF-1α, PDGF Concentrations in Conditioned Media of LC Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melosky, B. Advanced typical and atypical carcinoid tumours of the lung: Management recommendations. Curr. Oncol. 2018, 25 (Suppl. S1), S86–S93. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Barella, M.; Bianchi, F.; Hofman, P.; Hofman, V.; Remmelink, M.; Kern, I.; Carvalho, L.; Pattini, L.; Sonzogni, A.; et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021, 478, 5–19. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Horsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, P. Medical Therapy of Pulmonary Neuroendocrine Neoplasms: Targeted, Symptomatic and Chemotherapy. Front. Horm. Res. 2015, 44, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Ferolla, P.; Brizzi, M.P.; Meyer, T.; Mansoor, W.; Mazieres, J.; Do Cao, C.; Lena, H.; Berruti, A.; Damiano, V.; Buikhuisen, W.; et al. Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 1652–1664. [Google Scholar] [CrossRef]
- Granberg, D.; Eriksson, B.; Wilander, E.; Grimfjard, P.; Fjallskog, M.L.; Oberg, K.; Skogseid, B. Experience in treatment of metastatic pulmonary carcinoid tumors. Ann. Oncol. 2001, 12, 1383–1391. [Google Scholar] [CrossRef]
- Luciani, A.; Blasi, M.; Provenzano, L.; Zonato, S.; Ferrari, D. Recent advances in small cell lung cancer: The future is now? Minerva Endocrinol. 2022, 47, 460–474. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Carrasco, P.; Zuazo-Gaztelu, I.; Casanovas, O. Sprouting strategies and dead ends in anti-angiogenic targeting of NETs. J. Mol. Endocrinol. 2017, 59, R77–R91. [Google Scholar] [CrossRef]
- Cives, M.; Pelle, E.; Quaresmini, D.; Rizzo, F.M.; Tucci, M.; Silvestris, F. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology 2019, 109, 83–99. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.N.; Xie, K.; Yao, J.C. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Adhikari, L.J.; Lloyd, R.V.; Rubin, J.; Haluska, P.; Carboni, J.M.; Gottardis, M.M.; Ames, M.M. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr. Relat. Cancer 2010, 17, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Mairinger, F.D.; Walter, R.F.; Werner, R.; Christoph, D.C.; Ting, S.; Vollbrecht, C.; Zarogoulidis, K.; Huang, H.; Li, Q.; Schmid, K.W.; et al. Activation of angiogenesis differs strongly between pulmonary carcinoids and neuroendocrine carinomas and is crucial for carcinoid tumourgenesis. J. Cancer 2014, 5, 465–471. [Google Scholar] [CrossRef]
- Granberg, D.; Wilander, E.; Oberg, K. Expression of tyrosine kinase receptors in lung carcinoids. Tumour Biol. 2006, 27, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Kulke, M.H.; Lenz, H.J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Lantuejoul, S.; Constantin, B.; Drabkin, H.; Brambilla, C.; Roche, J.; Brambilla, E. Expression of VEGF, semaphorin SEMA3F, and their common receptors neuropilins NP1 and NP2 in preinvasive bronchial lesions, lung tumours, and cell lines. J. Pathol. 2003, 200, 336–347. [Google Scholar] [CrossRef]
- Moody, T.W.; Lee, M.; Kris, R.M.; Bellot, F.; Bepler, G.; Oie, H.; Gazdar, A.F. Lung carcinoid cell lines have bombesin-like peptides and EGF receptors. J. Cell. Biochem. 1990, 43, 139–147. [Google Scholar] [CrossRef]
- Rickman, O.B.; Vohra, P.K.; Sanyal, B.; Vrana, J.A.; Aubry, M.C.; Wigle, D.A.; Thomas, C.F., Jr. Analysis of ErbB receptors in pulmonary carcinoid tumors. Clin. Cancer Res. 2009, 15, 3315–3324. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Li, Y.F.; Zhang, C.Y.; Zhang, S.L.; Lv, Z.Y.; Dong, S.; Chen, H.J.; Zhang, X.C.; Wu, Y.L.; Yang, J.J. Response to Icotinib Plus Chemotherapy in Pulmonary Atypical Carcinoid Harboring the EGFR L858R Mutation: A Brief Report. JTO Clin. Res. Rep. 2021, 2, 100258. [Google Scholar] [CrossRef]
- Dicitore, A.; Cantone, M.C. Targeting receptor tyrosine kinases in neuroendocrine neoplasm: What’s going on with lung carcinoids? Minerva Endocrinol. 2022, 47, 261–263. [Google Scholar] [CrossRef]
- Grande, E.; Capdevila, J.; Castellano, D.; Teule, A.; Duran, I.; Fuster, J.; Sevilla, I.; Escudero, P.; Sastre, J.; Garcia-Donas, J.; et al. Pazopanib in pretreated advanced neuroendocrine tumors: A phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE). Ann. Oncol. 2015, 26, 1987–1993. [Google Scholar] [CrossRef]
- Hu-Lowe, D.D.; Zou, H.Y.; Grazzini, M.L.; Hallin, M.E.; Wickman, G.R.; Amundson, K.; Chen, J.H.; Rewolinski, D.A.; Yamazaki, S.; Wu, E.Y.; et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin. Cancer Res. 2008, 14, 7272–7283. [Google Scholar] [CrossRef] [PubMed]
- Tzogani, K.; Skibeli, V.; Westgaard, I.; Dalhus, M.; Thoresen, H.; Slot, K.B.; Damkier, P.; Hofland, K.; Borregaard, J.; Ersboll, J.; et al. The European Medicines Agency approval of axitinib (Inlyta) for the treatment of advanced renal cell carcinoma after failure of prior treatment with sunitinib or a cytokine: Summary of the scientific assessment of the committee for medicinal products for human use. Oncologist 2015, 20, 196–201. [Google Scholar] [CrossRef] [PubMed]
- McNamara, M.G.; Le, L.W.; Horgan, A.M.; Aspinall, A.; Burak, K.W.; Dhani, N.; Chen, E.; Sinaei, M.; Lo, G.; Kim, T.K.; et al. A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma. Cancer 2015, 121, 1620–1627. [Google Scholar] [CrossRef]
- Schiller, J.H.; Larson, T.; Ou, S.H.; Limentani, S.; Sandler, A.; Vokes, E.; Kim, S.; Liau, K.; Bycott, P.; Olszanski, A.J.; et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: Results from a phase II study. J. Clin. Oncol. 2009, 27, 3836–3841. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Cives, M.; Hwang, J.; Weber, T.; Nickerson, M.; Atreya, C.E.; Venook, A.; Kelley, R.K.; Valone, T.; Morse, B.; et al. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 411–418. [Google Scholar] [CrossRef]
- Dicitore, A.; Bacalini, M.G.; Saronni, D.; Gaudenzi, G.; Cantone, M.C.; Gelmini, G.; Grassi, E.S.; Gentilini, D.; Borghi, M.O.; Di Blasio, A.M.; et al. Role of Epigenetic Therapy in the Modulation of Tumor Growth and Migration in Human Castration-Resistant Prostate Cancer Cells with Neuroendocrine Differentiation. Neuroendocrinology 2022, 112, 580–594. [Google Scholar] [CrossRef]
- Dicitore, A.; Saronni, D.; Gaudenzi, G.; Carra, S.; Cantone, M.C.; Borghi, M.O.; Persani, L.; Vitale, G. Long-term effects of somatostatin analogues in rat GH-secreting pituitary tumor cell lines. J. Endocrinol. Investig. 2022, 45, 29–41. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef]
- Gaudenzi, G.; Albertelli, M.; Dicitore, A.; Wurth, R.; Gatto, F.; Barbieri, F.; Cotelli, F.; Florio, T.; Ferone, D.; Persani, L.; et al. Patient-derived xenograft in zebrafish embryos: A new platform for translational research in neuroendocrine tumors. Endocrine 2017, 57, 214–219. [Google Scholar] [CrossRef]
- Vitale, G.; Gaudenzi, G.; Dicitore, A.; Cotelli, F.; Ferone, D.; Persani, L. Zebrafish as an innovative model for neuroendocrine tumors. Endocr. Relat. Cancer 2014, 21, R67–R83. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Carra, S.; Gaudenzi, G.; Dicitore, A.; Cantone, M.C.; Plebani, A.; Saronni, D.; Zappavigna, S.; Caraglia, M.; Candeo, A.; Bassi, A.; et al. Modeling Lung Carcinoids with Zebrafish Tumor Xenograft. Int. J. Mol. Sci. 2022, 23, 8126. [Google Scholar] [CrossRef]
- Bodei, L.; Cwikla, J.B.; Kidd, M.; Modlin, I.M. The role of peptide receptor radionuclide therapy in advanced/metastatic thoracic neuroendocrine tumors. J. Thorac. Dis. 2017, 9 (Suppl. S15), S1511–S1523. [Google Scholar] [CrossRef] [PubMed]
- Gosain, R.; Mukherjee, S.; Yendamuri, S.S.; Iyer, R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers 2018, 10, 510. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Zhou, N.; Zhang, T.; Ren, P.; Chen, G. H727 Multicellular Spheroids and Its Resistance to Antitumor Drugs Sunitinib and Axitinib. J. Nanosci. Nanotechnol. 2018, 18, 8078–8084. [Google Scholar] [CrossRef]
- Hoeferlin, L.A.; Oleinik, N.V.; Krupenko, N.I.; Krupenko, S.A. Activation of p21-Dependent G1/G2 Arrest in the Absence of DNA Damage as an Antiapoptotic Response to Metabolic Stress. Genes Cancer 2011, 2, 889–899. [Google Scholar] [CrossRef]
- Niculescu, A.B., 3rd; Chen, X.; Smeets, M.; Hengst, L.; Prives, C.; Reed, S.I. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 1998, 18, 629–643. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef]
- Couvelard, A.; O’Toole, D.; Turley, H.; Leek, R.; Sauvanet, A.; Degott, C.; Ruszniewski, P.; Belghiti, J.; Harris, A.L.; Gatter, K.; et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: Negative correlation of microvascular density and VEGF expression with tumour progression. Br. J. Cancer 2005, 92, 94–101. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Uccella, S.; Finzi, G.; Albarello, L.; Sessa, F.; Capella, C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: Correlation with microvessel density and clinicopathologic features. Hum. Pathol. 2003, 34, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Poncet, G.; Villaume, K.; Walter, T.; Pourreyron, C.; Theillaumas, A.; Lepinasse, F.; Hervieu, V.; Cordier-Bussat, M.; Scoazec, J.Y.; Roche, C. Angiogenesis and tumor progression in neuroendocrine digestive tumors. J. Surg. Res. 2009, 154, 68–77. [Google Scholar] [CrossRef]
- Rodallec, M.; Vilgrain, V.; Couvelard, A.; Rufat, P.; O’Toole, D.; Barrau, V.; Sauvanet, A.; Ruszniewski, P.; Menu, Y. Endocrine pancreatic tumours and helical CT: Contrast enhancement is correlated with microvascular density, histoprognostic factors and survival. Pancreatology 2006, 6, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, Z.K.; Arbiser, J.L.; Cohen, C.; Gal, A.A. Neuroendocrine lung tumors: Grade correlates with proliferation but not angiogenesis. Mod. Pathol. 2001, 14, 1195–1199. [Google Scholar] [CrossRef]
- Slodkowska, J.; Sikora, J.; Androsiuk, W.; Rudzinski, P.; Radomyski, A. Lung carcinoids. Tumor angiogenesis in relation to clinicopathologic characteristics. Anal. Quant. Cytol. Histol. 1999, 21, 267–272. [Google Scholar] [PubMed]
- Telega, A.; Kos-Kudla, B.; Foltyn, W.; Blicharz-Dorniak, J.; Rosiek, V. Selected neuroendocrine tumour markers, growth factors and their receptors in typical and atypical bronchopulmonary carcinoids. Endokrynol. Pol. 2012, 63, 477–482. [Google Scholar]
- Gaudenzi, G.; Carra, S.; Dicitore, A.; Cantone, M.C.; Persani, L.; Vitale, G. Fishing for neuroendocrine tumors. Endocr. Relat. Cancer 2020, 27, R163–R176. [Google Scholar] [CrossRef]
- Hason, M.; Bartunek, P. Zebrafish Models of Cancer-New Insights on Modeling Human Cancer in a Non-Mammalian Vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]
- Chen, Y.; Tortorici, M.A.; Garrett, M.; Hee, B.; Klamerus, K.J.; Pithavala, Y.K. Clinical pharmacology of axitinib. Clin. Pharmacokinet. 2013, 52, 713–725. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicitore, A.; Gaudenzi, G.; Carra, S.; Cantone, M.C.; Oldani, M.; Saronni, D.; Borghi, M.O.; Grotteschi, J.; Persani, L.; Vitale, G. Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study. Cancers 2023, 15, 5375. https://doi.org/10.3390/cancers15225375
Dicitore A, Gaudenzi G, Carra S, Cantone MC, Oldani M, Saronni D, Borghi MO, Grotteschi J, Persani L, Vitale G. Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study. Cancers. 2023; 15(22):5375. https://doi.org/10.3390/cancers15225375
Chicago/Turabian StyleDicitore, Alessandra, Germano Gaudenzi, Silvia Carra, Maria Celeste Cantone, Monica Oldani, Davide Saronni, Maria Orietta Borghi, Jacopo Grotteschi, Luca Persani, and Giovanni Vitale. 2023. "Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study" Cancers 15, no. 22: 5375. https://doi.org/10.3390/cancers15225375
APA StyleDicitore, A., Gaudenzi, G., Carra, S., Cantone, M. C., Oldani, M., Saronni, D., Borghi, M. O., Grotteschi, J., Persani, L., & Vitale, G. (2023). Antitumor Activity of Axitinib in Lung Carcinoids: A Preclinical Study. Cancers, 15(22), 5375. https://doi.org/10.3390/cancers15225375







