Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment
2.3. Efficacy and Outcome
2.4. Body Composition Parameters
2.5. Statistical Analysis
3. Results
3.1. Comprehensive Characterization of aGC Patient Cohort: Clinical, Epidemiological, and Body Composition Profiles with Emphasis on Visceral and Subcutaneous Fat Ratios
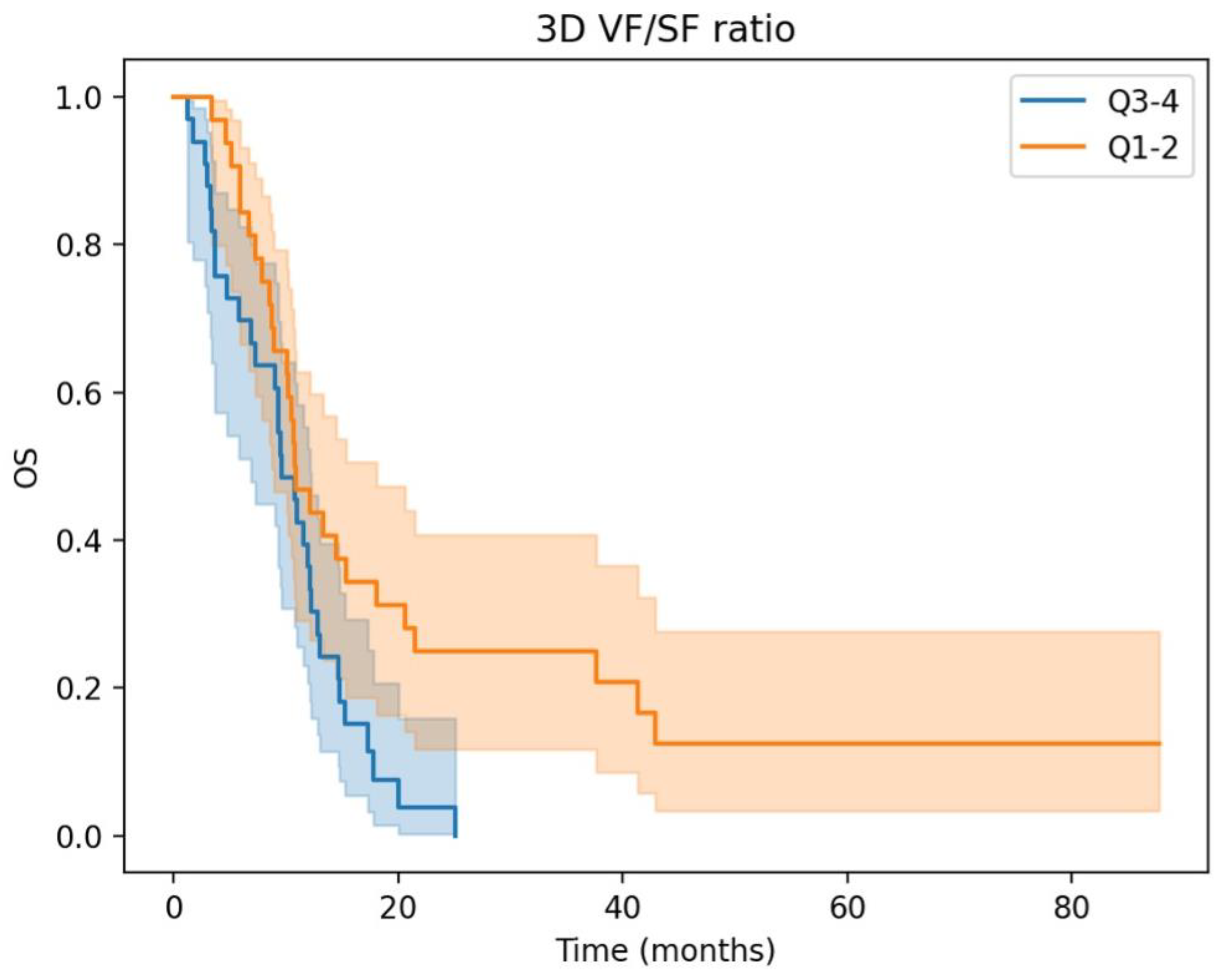
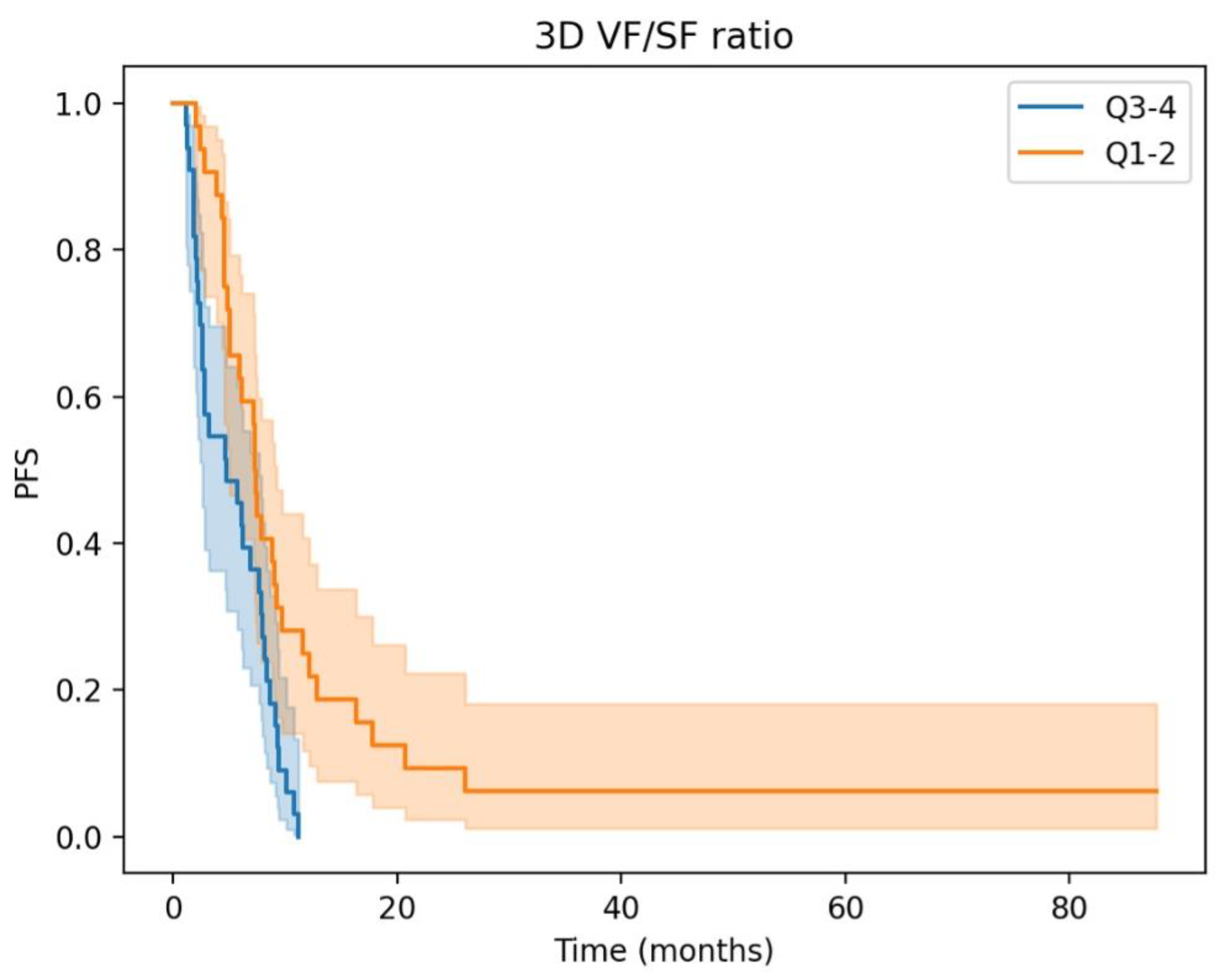
3.2. Prognostic Significance and Body Composition Parameters in aGC: Insights from Univariate and Multivariate Cox Regression Analyses
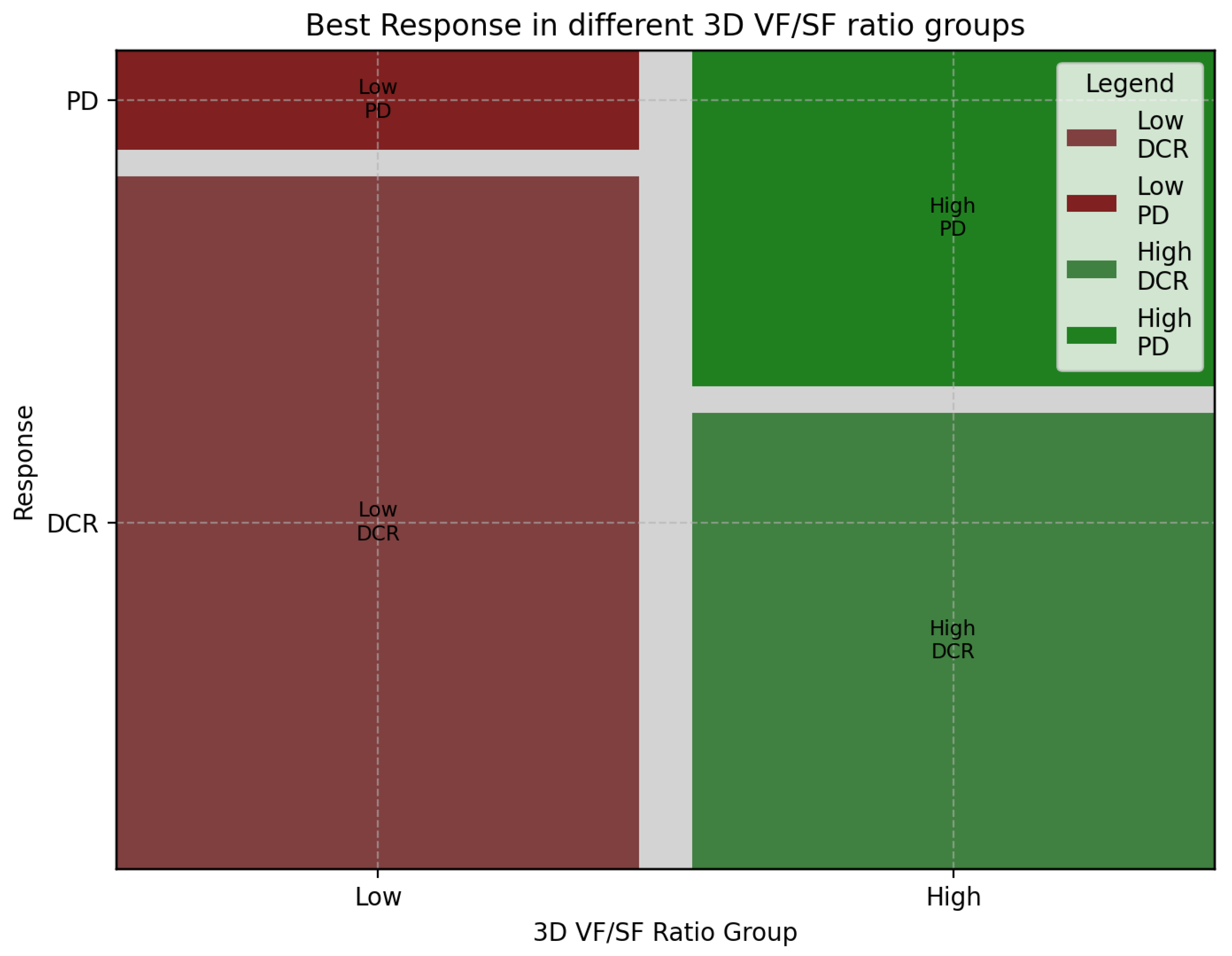
3.3. Association of 3D Fat Ratio with Treatment Response in aGC: Insights from First-Line Chemotherapy Outcomes
3.4. Chemotherapy Tolerance and Adverse Events in aGC Patients: Impact of 3D Fat Ratio on Treatment Exposure and Side Effect Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Körfer, J.; Lordick, F.; Hacker, U.T. Molecular Targets for Gastric Cancer Treatment and Future Perspectives from a Clinical and Translational Point of View. Cancers 2021, 13, 5216. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020, 18, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more ‘personalized’ approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Riihimaki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic spread in patients with gastric cancer. Oncotarget 2016, 7, 52307–52316. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, G.; Liu, X.; Chen, C.; Yu, H.; Huang, X.; Zhang, Q.; Yu, J. Blood neutrophil-lymphocyte ratio predicts survival for stages III–IV gastric cancer treated with neoadjuvant chemotherapy. World J. Surg. Oncol. 2013, 11, 112. [Google Scholar] [CrossRef]
- Catanese, S.; Aringhieri, G.; Vivaldi, C.; Salani, F.; Vitali, S.; Pecora, I.; Massa, V.; Lencioni, M.; Vasile, E.; Tintori, R.; et al. Role of Baseline Computed-Tomography-Evaluated Body Composition in Predicting Outcome and Toxicity from First-Line Therapy in Advanced Gastric Cancer Patients. J. Clin. Med. 2021, 10, 1079. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kuroda, S.; Kikuchi, S.; Yoshida, R.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Clinical Impact of Sarcopenia on Gastric Cancer. Anticancer. Res. 2019, 39, 2241–2249. [Google Scholar] [CrossRef]
- Dev, R.; Bruera, E.; Dalal, S. Insulin resistance and body composition in cancer patients. Ann. Oncol. 2018, 29, ii18–ii26. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yagyu, T.; Uchinaka, E.; Miyatani, K.; Hanaki, T.; Kihara, K.; Matsunaga, T.; Yamamoto, M.; Tokuyasu, N.; Honjo, S.; et al. The prognostic significance of combined geriatric nutritional risk index and psoas muscle volume in older patients with pancreatic cancer. BMC Cancer 2021, 21, 342. [Google Scholar] [CrossRef]
- Mauland, K.K.; Eng, Ø.; Ytre-Hauge, S.; Tangen, I.L.; Berg, A.; Salvesen, H.B.; Salvesen, O.; Krakstad, C.; Trovik, J.; Hoivik, E.A.; et al. High visceral fat percentage is associated with poor outcome in endometrial cancer. Oncotarget 2017, 8, 105184–105195. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE—Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliográficas 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Watanabe, M.; Mine, S.; Nishida, K.; Imamura, Y.; Kurogochi, T.; Kitagawa, Y.; Sano, T. Clinical Impact of Abdominal Fat Distribution on Prognosis After Esophagectomy for Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 23, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Kim, H.S.; Beom, S.H.; Rha, S.Y.; Chung, H.C.; Kim, J.H.; Chun, Y.J.; Lee, S.W.; Choe, E.-A.; Heo, S.J.; et al. Marked Loss of Muscle, Visceral Fat, or Subcutaneous Fat After Gastrectomy Predicts Poor Survival in Advanced Gastric Cancer: Single-Center Study from the CLASSIC Trial. Ann. Surg. Oncol. 2018, 25, 3222–3230. [Google Scholar] [CrossRef]
- Hidaka, A.; Sasazuki, S.; Goto, A.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Noda, M.; Tajiri, H.; et al. Plasma insulin, C-peptide and blood glucose and the risk of gastric cancer: The Japan Public Health Center-based prospective study. Int. J. Cancer 2015, 136, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Thaete, F.L.; Simoneau, J.-A.; Kelley, D.E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997, 46, 1579–1585. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Doyle, S.L.; Reynolds, J.V. Visceral adiposity, insulin resistance and cancer risk. Diabetol. Metab. Syndr. 2011, 3, 12. [Google Scholar] [CrossRef]
- Ongaro, E.; Buoro, V.; Cinausero, M.; Caccialanza, R.; Turri, A.; Fanotto, V.; Basile, D.; Vitale, M.G.; Ermacora, P.; Cardellino, G.G.; et al. Sarcopenia in gastric cancer: When the loss costs too much. Gastric Cancer 2017, 20, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Clark, W.; Siegel, E.M.; Chen, A.Y.; Zhao, X.; Parsons, C.M.; Hernandez, J.M.; Weber, J.; Thareja, S.; Choi, J.; Shibata, D. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J. Am. Coll. Surg. 2013, 216, 1070–1081. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.S.; Ha, J.S.; Han, K.S.; Rha, K.H.; Hong, S.J.; Chung, B.H.; Koo, K.C. Subcutaneous Fat Distribution is a Prognostic Biomarker for Men with Castration Resistant Prostate Cancer. J. Urol. 2018, 200, 114–120. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chung, E.; Cho, E.-S.; Lee, J.-H.; Shin, S.-J.; Lee, H.S.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Impact of subcutaneous and visceral fat adiposity in patients with colorectal cancer. Clin. Nutr. 2021, 40, 5631–5638. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef]
- Peng, P.; Hyder, O.; Firoozmand, A.; Kneuertz, P.; Schulick, R.D.; Huang, D.; Makary, M.; Hirose, K.; Edil, B.; Choti, M.A.; et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 1478–1486. [Google Scholar] [CrossRef]
- Voron, T.; Tselikas, L.; Pietrasz, D.; Pigneur, F.; Laurent, A.; Compagnon, P.; Salloum, C.; Luciani, A.; Azoulay, D. Sarcopenia Impacts on Short- and Long-term Results of Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2015, 261, 1173–1183. [Google Scholar] [CrossRef]
- Buettner, S.; Wagner, D.; Kim, Y.; Margonis, G.A.; Makary, M.A.; Wilson, A.; Sasaki, K.; Amini, N.; Gani, F.; Pawlik, T.M. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J. Am. Coll. Surg. 2016, 222, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.-D. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef]
- Tegels, J.J.; van Vugt, J.L.; Reisinger, K.W.; Hulsewé, K.W.; Hoofwijk, A.G.; Derikx, J.P.; Stoot, J.H. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J. Surg. Oncol. 2015, 112, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kuwada, K.; Kuroda, S.; Kikuchi, S.; Yoshida, R.; Nishizaki, M.; Kagawa, S.; Fujiwara, T. Sarcopenia and Comorbidity in Gastric Cancer Surgery as a Useful Combined Factor to Predict Eventual Death from Other Causes. Ann. Surg. Oncol. 2018, 25, 1160–1166. [Google Scholar] [CrossRef]
- Huang, D.-D.; Chen, X.-Y.; Wang, S.-L.; Shen, X.; Chen, X.-L.; Yu, Z.; Zhuang, C.-L. Sarcopenia predicts 1-year mortality in elderly patients undergoing curative gastrectomy for gastric cancer: A prospective study. J. Cancer Res. Clin. Oncol. 2016, 142, 2347–2356. [Google Scholar] [CrossRef]
- Zhuang, C.-L.; Huang, D.-D.; Pang, W.-Y.; Zhou, C.-J.; Wang, S.-L.; Lou, N.; Ma, L.-L.; Yu, Z.; Shen, X. Sarcopenia is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival after Radical Gastrectomy for Gastric Cancer: Analysis from a Large-Scale Cohort. Medicine 2016, 95, e3164. [Google Scholar] [CrossRef] [PubMed]
- Kudou, K.; Saeki, H.; Nakashima, Y.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Tsutsumi, R.; Nishimura, S.; Nakaji, Y.; Akiyama, S.; et al. Prognostic Significance of Sarcopenia in Patients with Esophagogastric Junction Cancer or upper Gastric Cancer. Ann. Surg. Oncol. 2017, 24, 1804–1810. [Google Scholar] [CrossRef]
- Stojcev, Z.; Matysiak, K.; Duszewski, M.; Banasiewicz, T. The role of dietary nutrition in stomach cancer. Contemp. Oncol. 2013, 17, 343–345. [Google Scholar] [CrossRef]
- Sugiyama, K.; Narita, Y.; Mitani, S.; Honda, K.; Masuishi, T.; Taniguchi, H.; Kadowaki, S.; Ura, T.; Ando, M.; Tajika, M.; et al. Baseline Sarcopenia and Skeletal Muscle Loss During Chemotherapy Affect Survival Outcomes in Metastatic Gastric Cancer. Anticancer. Res. 2018, 38, 5859–5866. [Google Scholar] [CrossRef]
- Hayashi, N.; Ando, Y.; Gyawali, B.; Shimokata, T.; Maeda, O.; Fukaya, M.; Goto, H.; Nagino, M.; Kodera, Y. Low skeletal muscle density is associated with poor survival in patients who receive chemotherapy for metastatic gastric cancer. Oncol. Rep. 2016, 35, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
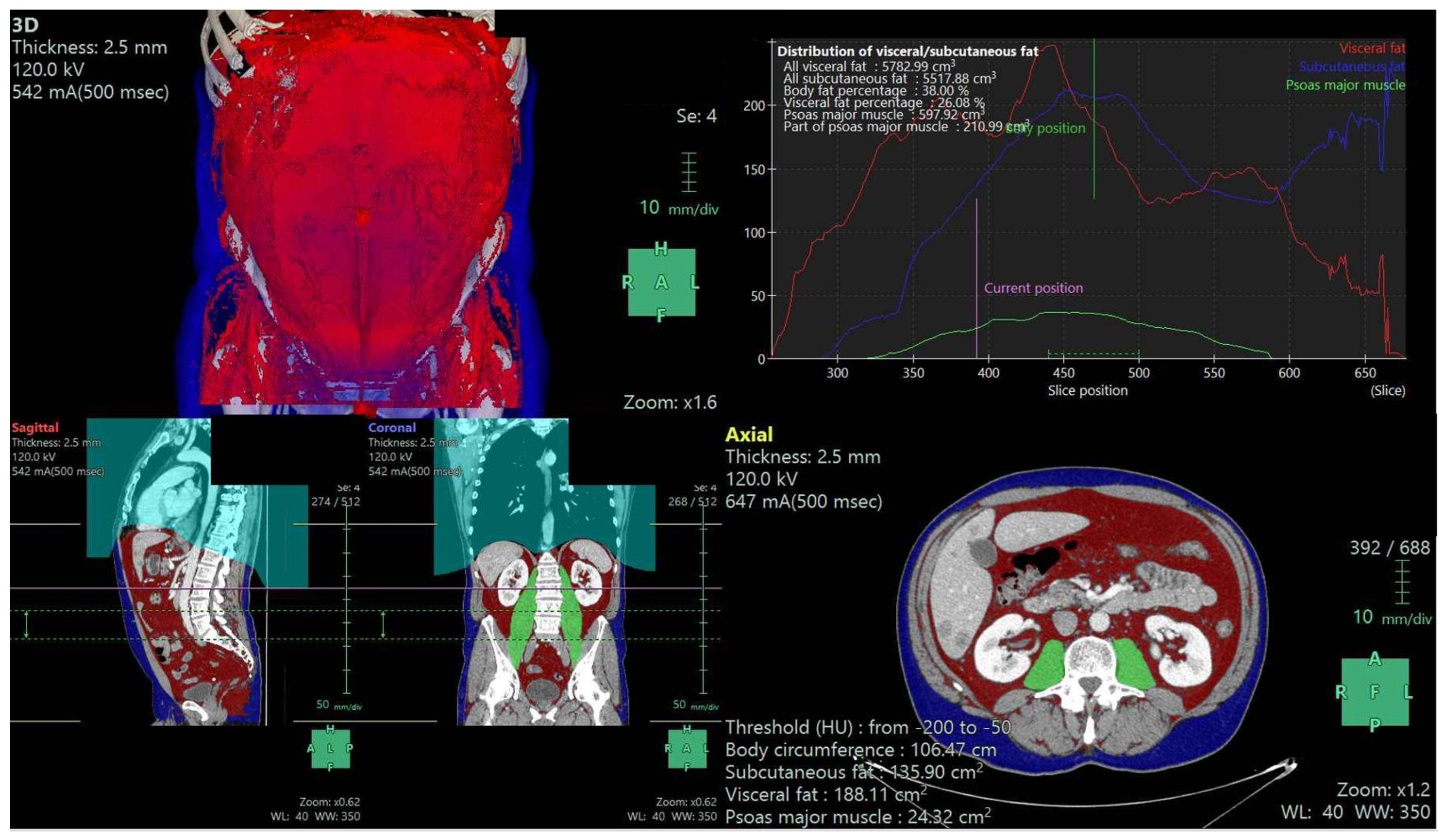

| Characteristics | All Patients (N = 65) | 3D Fat Ratio Low (N = 32) | High (N = 33) | p |
|---|---|---|---|---|
| Age, years Median (range) | 67 (59.25–74.75) | 67 (61–74) | 67 (57–75) | 0.474 |
| Sex (female/male) | 19 (29)/46 (71) | 17 (53)/15 (47) | 2 (6)/31 (94) | <0.001 |
| ECOG PS 0 vs. 1–2 | 30 (52)/28 (48) | 16 (50)/16 (50) | 14 (42)/19 (57) | 0.622 |
| Primary tumor site EGJ/GP/GD | 25 (38)/23 (35)/17 (26) | 7 (22)/13 (41)/12 (37) | 18 (54)/10 (30)/5(15) | 0.017 |
| Primary tumor surgery (yes/no) | 17 (26) | 10 (31) | 7 (21) | 0.407 |
| N° metastatic sites 0/1/ > =2/na | 6 (9)/42 (65)/10 (15)/7 (11) | 4 (12)/18 (56)/6 (19)/4 (12) | 2 (6)/24(73)/4 (12)/3 (9) | 0.561 |
| Metastatic sites Liver Lung Peritoneum Lymph nodes Bones | 25 (38) 6 (9) 33 (51) 42 (65) 6 (9) | 13 (41) 2 (6) 17 (53) 20 (62) 3 (9) | 12 (36) 4 (12) 16 (48) 22 (67) 3 (9) | 0.801 0.673 0.805 0.798 0.999 |
| HER2 mutant Yes/no/na | 13 (20)/36 (55)/16 (25) | 5 (16)/24 (75)/3 (50) | 8 (24)/17 (51)/8 (24) | 0.339 |
| NLR > 3 Yes/no/na | 29 (45)/28 (43)/8 (12) | 15 (47)/13 (41)/4 (12) | 14 (42)/15 (45)/4 (12) | 0.793 |
| PLR > 200 Yes/no/na | 28 (43)/28 (43)/9 (14) | 16 (50)/12 (38)/4 (12) | 12 (36)/16 (48)/5 (15) | 0.423 |
| BMI <20/20–24.9/25–29.9/>30 | 14 (22)/32 (49)/15 (23)/4 (6) | 5 (16)/17 (53)/8 (25)/2 (6) | 9 (27)/15 (45)/7 (21)/2 (6) | 0.724 |
| SMI, median (range; SD) Female Male | 48 (41–54; 9) 40 (36–45; 8) 51 (46–56; 7) | 44.40 (37.97–54.42; 9.59) 40 (34–45; 8.39) 55 (46–64.5; 10.60) | 48.42 (44.42–53.96) 42 48.48 (45.01–55.40; 7.83) | 0.202 |
| Visceral fat volume, median (range; SD) | 2154 (1450 –3920; 1969) | 1989 (1328–2949; 1500) | 2843 (1556–4445; 2994) | 0.094 |
| Subcutaneous fat volume, median (range; SD) | 3296 (1929–5367; 2694) | 4623 (2754–6048; 2775) | 2185 (1001–3857; 2169) | <0.001 |
| Visceral fat index, median (range; SD) | 438 (325–665; 387) | 421 (347–583; 332) | 530 (312–756; 434) | 0.214 |
| Subcutaneous fat index, median (range; SD) | 617 (365–957; 517) | 849 (605–1212; 546) | 429 (248–660; 387) | <0.001 |
| Psoas mass index, median (range; SD) | 59 (46–75; 20) | 55 (43–72; 18) | 63 (48–82; 22) | 0.054 |
| Abdominal fat percentage, median (range, SD) | 28.39 (21.17–35.55; 11.99) | 30.81 (26.08–37.23; 9.51) | 24.99 (16.80–33.90; 13.28) | 0.011 |
| Visceral fat percentage, median (range; SD) | 26.39 (18.76–36.35; 12.65) | 26.08 (20.08–31.04; 9.09) | 27.36 (17.60–41.07; 15.36) | 0.229 |
| Variable | PFS—HR (95% CI) | p | OS—HR (95% CI) | p |
|---|---|---|---|---|
| Age ≥ vs. <67 years | 1.22 (0.74–2.01) | 0.44 | 0.94 (0.56–1.59) | 0.28 |
| Sex Female vs. Male | 0.98 (0.57–1.69) | 0.94 | 1.03 (0.58–1.83) | 0.91 |
| ECOG PS >0 vs. =0 | 1.86 (1.12–3.09) | 0.02 | 1.72 (1.02–2.89) | 0.04 |
| Primary tumor surgery (curative intention) | 0.58 (0.33–1.03) | 0.07 | 0.74 (0.41–1.33) | 0.31 |
| N° metastatic sites >1 vs. = 1 | 1.22 (0.61–2.44) | 0.57 | 1.43 (0.69–2.95) | 0.34 |
| Metastatic sites Liver Peritoneum Lymph nodes Lungs Bones | 0.74 (0.44–1.24) 0.96 (0.58–1.58) 1.16 (0.69–1.95) 1.59 (0.68–3.72) 1.61 (0.69–3.77) | 0.25 0.88 0.58 0.28 0.27 | 0.54 (0.31–0.92) 1.30 (0.78–2.18) 1.17 (0.68–2.01) 1.25 (0.50–3.13) 3.76 (1.56–9.07) | 0.02 0.32 0.56 0.64 <0.005 |
| NLR > 3 Yes vs. no | 2.35 (1.31–4.24) | <0.005 | 1.97 (1.12–3.48) | 0.02 |
| PLR > 200 Yes vs. no | 1.13 (0.65–1.94) | 0.67 | 1.45 (0.83–2.54) | 0.19 |
| SMI (Martin) Yes vs. no | 0.92 (0.54–1.58) | 0.76 | 1.06 (0.61–1.85) | 0.82 |
| Psoas volume index High vs. Low | 0.88 (0.54–1.46) | 0.63 | 1.04 (0.62–1.73) | 0.89 |
| Visceral fat volume index High vs. Low | 1.11 (0.68–1.83) | 0.67 | 1.22 (0.73–2.06) | 0.45 |
| Subcutaneous fat volume index High vs. Low | 0.68 (0.41–1.11) | 0.12 | 0.98 (0.59–1.64) | 0.95 |
| Abdominal fat percentage High vs. Low | 0.95 (0.58–1.56) | 0.83 | 1.02 (0.61–1.71) | 0.94 |
| Visceral fat percentage High vs. Low | 0.84 (0.51–1.38) | 0.49 | 0.82 (0.49–1.37) | 0.44 |
| 3D Visceral/Subcutaneous fat ratio High vs. Low | 2.20 (1.28–3.78) | <0.005 | 1.96 (1.13–3.38) | 0.02 |
| Variable | PFS—HR (95% CI) | p | OS—HR (95% CI) | p |
|---|---|---|---|---|
| ECOG PS | 1.53 (1.00–2.33) | 0.05 | 1.69 (1.07–2.66) | 0.02 |
| Liver metastasis | 0.70 (0.39–1.24 | 0.22 | 0.50 (0.27–0.91) | 0.02 |
| Bone metastasis | 1.68 (0.67–4.17) | 0.27 | 3.96 (1.52–10.29) | <0.005 |
| Primary surgery | 0.63 (0.33–1.17) | 0.14 | 0.67 (0.35–1.28) | 0.23 |
| High 3D fat ratio | 2.09 (1.19–3.67) | 0.01 | 2.16 (1.23–3.80) | 0.01 |
| Response | High-Ratio Group | Low-Ratio Group | Sum |
|---|---|---|---|
| CR | 1 | 0 | 1 |
| PR | 8 | 12 | 20 |
| SD | 10 | 16 | 26 |
| PD | 14 | 4 | 18 |
| Sum | 33 | 32 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aringhieri, G.; Di Salle, G.; Catanese, S.; Vivaldi, C.; Salani, F.; Vitali, S.; Caccese, M.; Vasile, E.; Genovesi, V.; Fornaro, L.; et al. Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer. Cancers 2023, 15, 5391. https://doi.org/10.3390/cancers15225391
Aringhieri G, Di Salle G, Catanese S, Vivaldi C, Salani F, Vitali S, Caccese M, Vasile E, Genovesi V, Fornaro L, et al. Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer. Cancers. 2023; 15(22):5391. https://doi.org/10.3390/cancers15225391
Chicago/Turabian StyleAringhieri, Giacomo, Gianfranco Di Salle, Silvia Catanese, Caterina Vivaldi, Francesca Salani, Saverio Vitali, Miriam Caccese, Enrico Vasile, Virginia Genovesi, Lorenzo Fornaro, and et al. 2023. "Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer" Cancers 15, no. 22: 5391. https://doi.org/10.3390/cancers15225391
APA StyleAringhieri, G., Di Salle, G., Catanese, S., Vivaldi, C., Salani, F., Vitali, S., Caccese, M., Vasile, E., Genovesi, V., Fornaro, L., Tintori, R., Balducci, F., Cappelli, C., Cioni, D., Masi, G., & Neri, E. (2023). Abdominal Visceral-to-Subcutaneous Fat Volume Ratio Predicts Survival and Response to First-Line Palliative Chemotherapy in Patients with Advanced Gastric Cancer. Cancers, 15(22), 5391. https://doi.org/10.3390/cancers15225391








