Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Studies
2.2. Histology
2.2.1. Hematoxylin and Eosin Stain (H&E)
2.2.2. Alcian Blue Stain
2.2.3. Immunohistochemistry and Immunofluorescence
2.2.4. Fibrosis Assessment
2.3. TMA Analysis
2.4. Isolation of tdTomato-Positive Cells
2.5. Gene Expression Analysis by Quantitative RT-PCR and qPCR Array
2.6. Cell Lines, Reagents, and Cell Culture
2.7. Chromatin Immunoprecipitation In Vitro and In Vivo
2.8. Mouse Cytokine Array
2.9. Statistics
3. Results
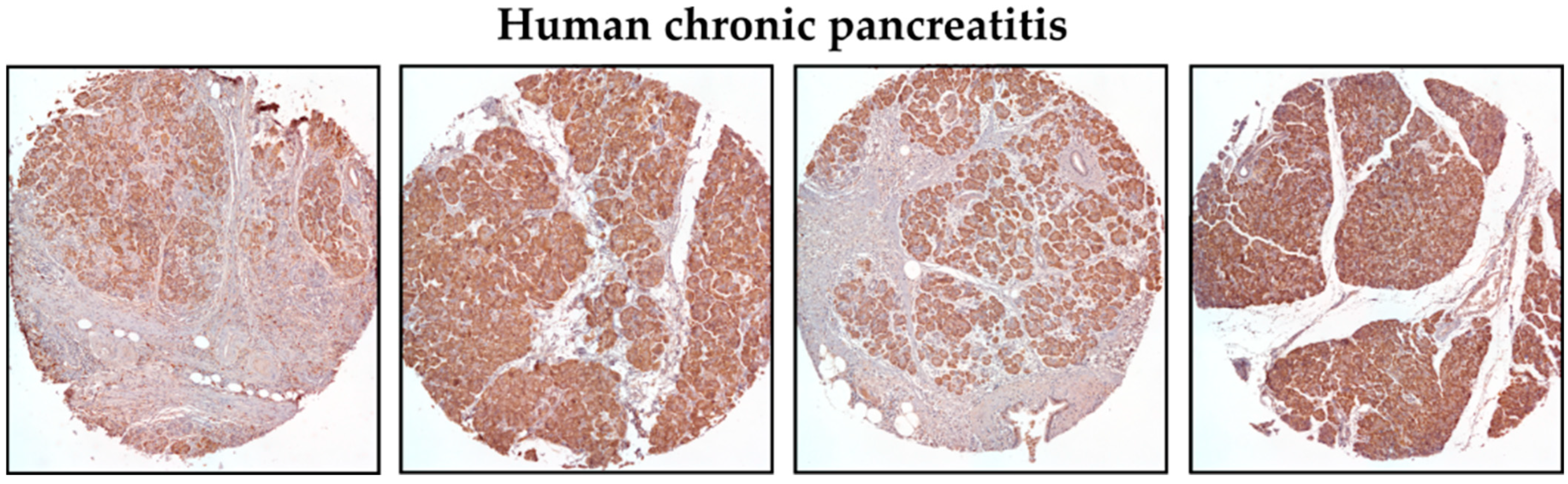
3.1. KLF5 Protein Expression Is Increased in Chronic Pancreatitis
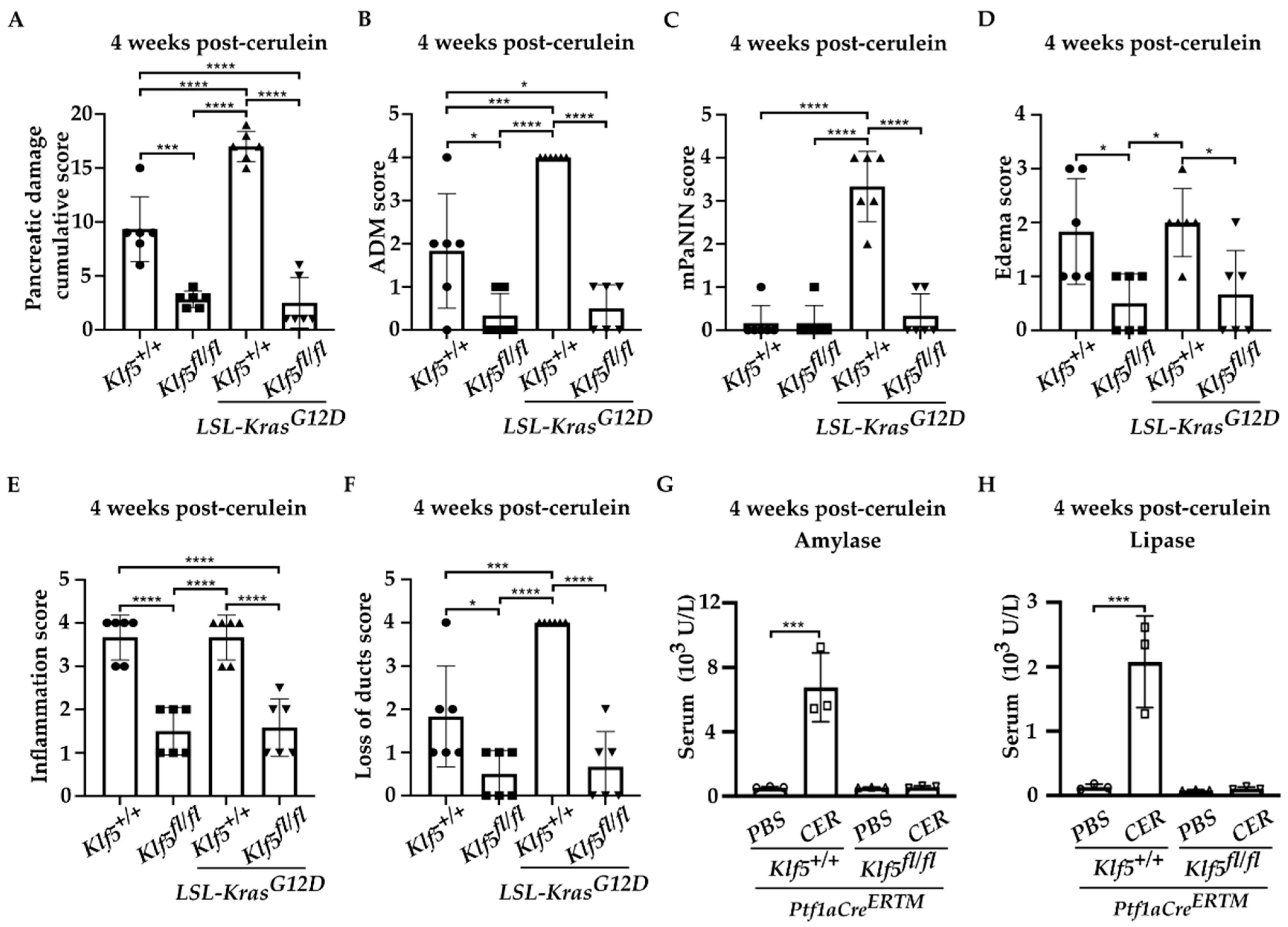
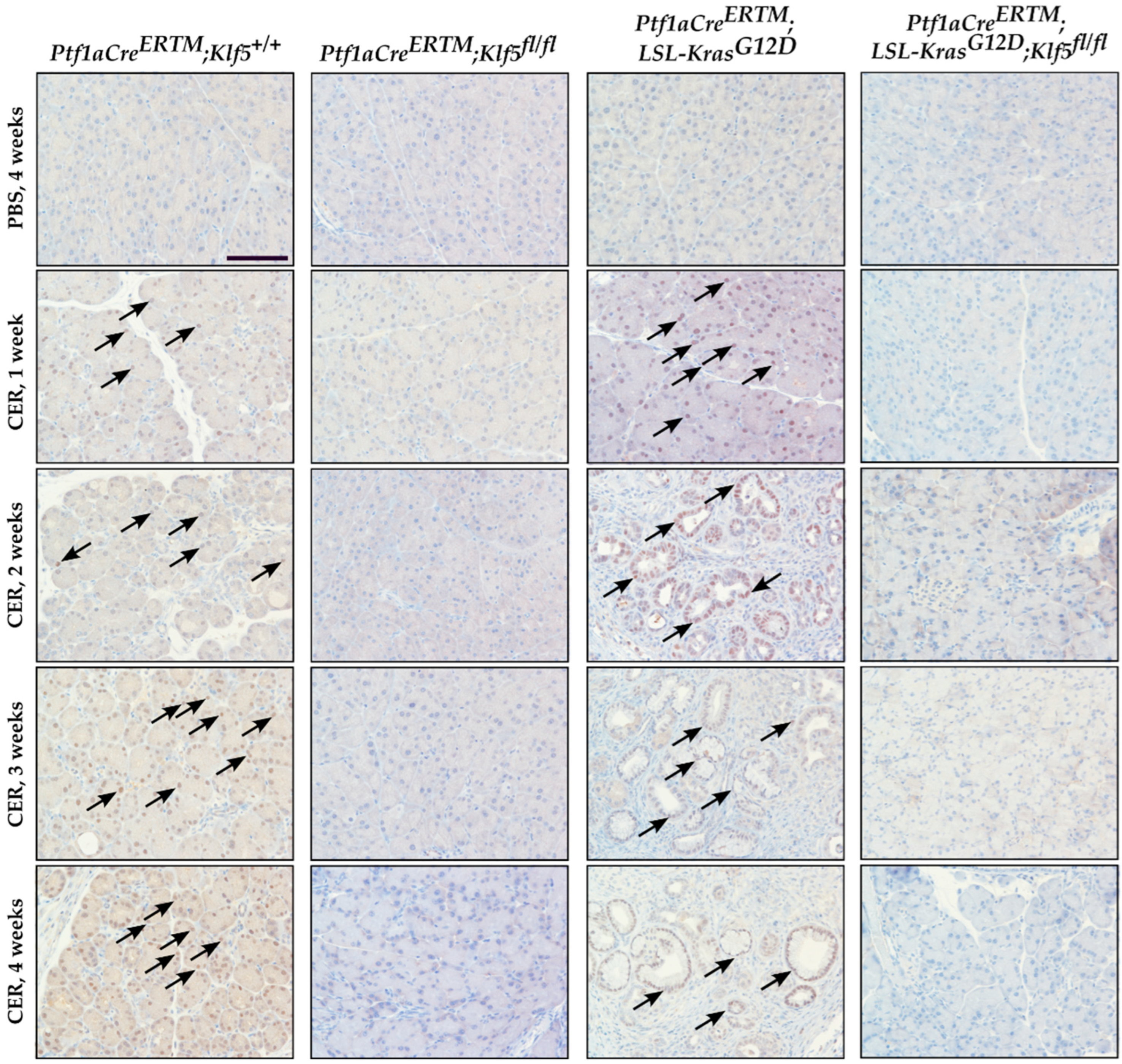
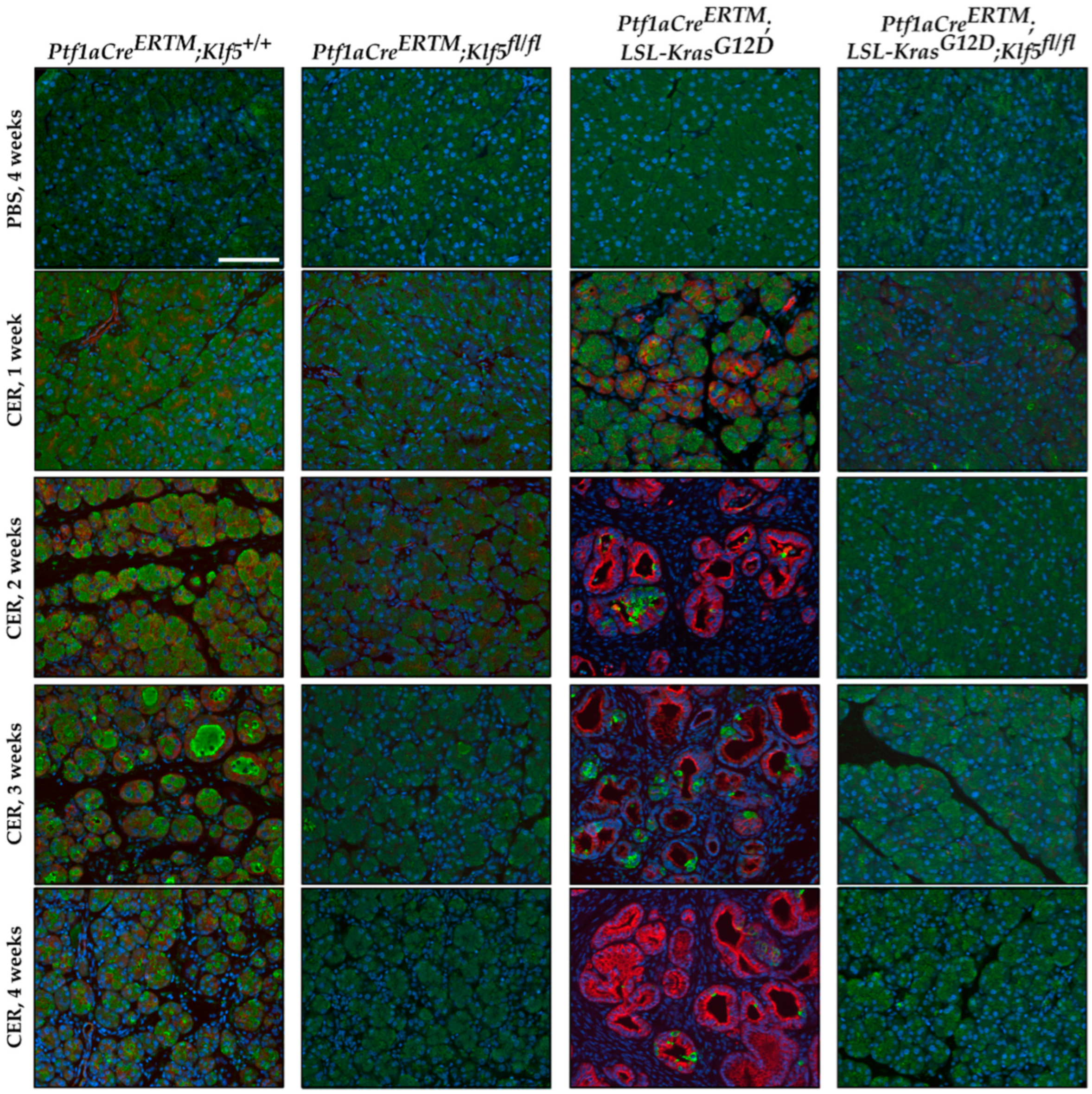
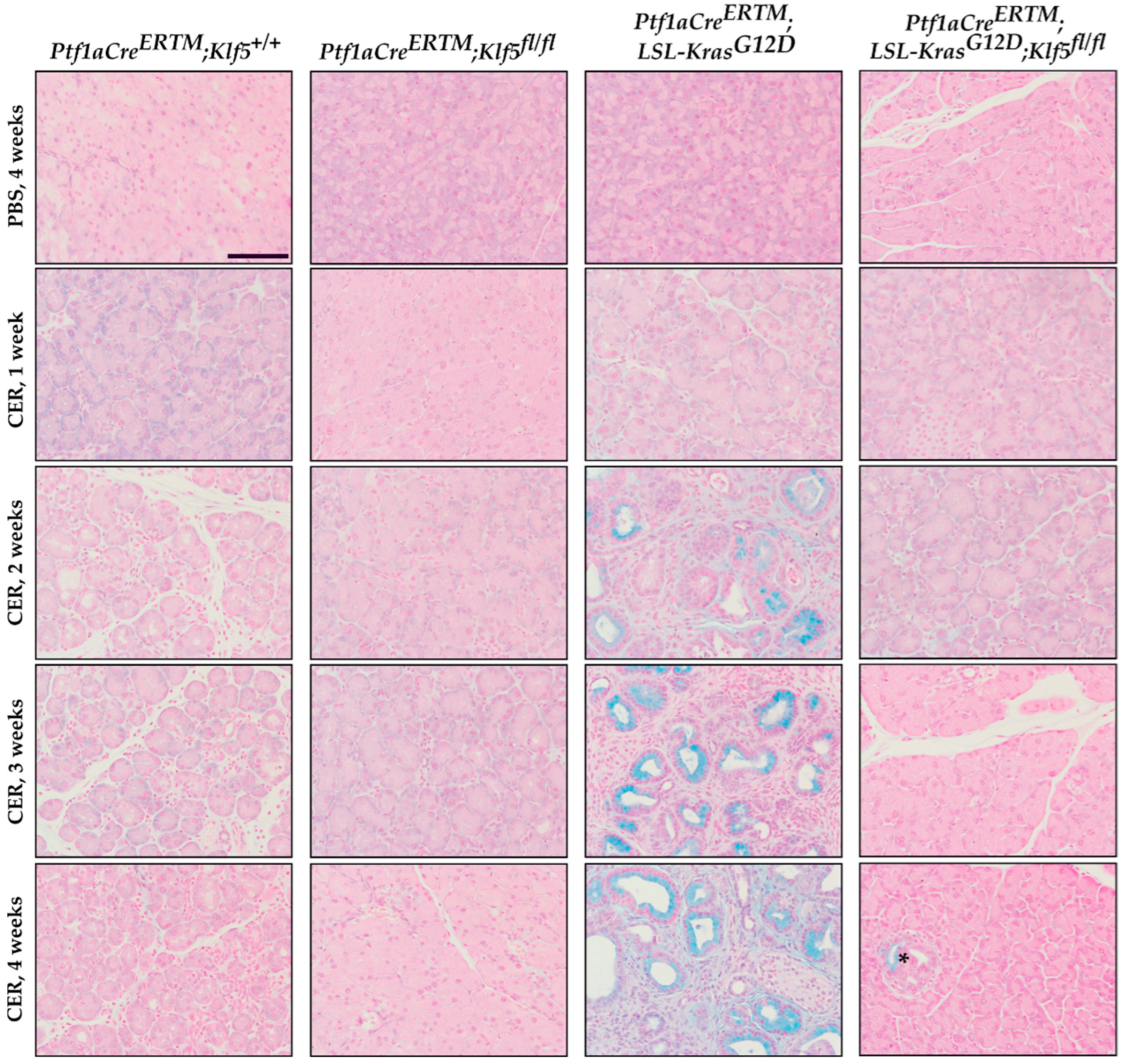
3.2. Inactivation of Klf5 in Pancreatic Acinar Cells’ Reduced Evolution to ADM and PanIN in Chronic Pancreatitis
3.3. Inactivation of Klf5 Reduces ADM and PanIN Formation in Chronic Pancreatitis
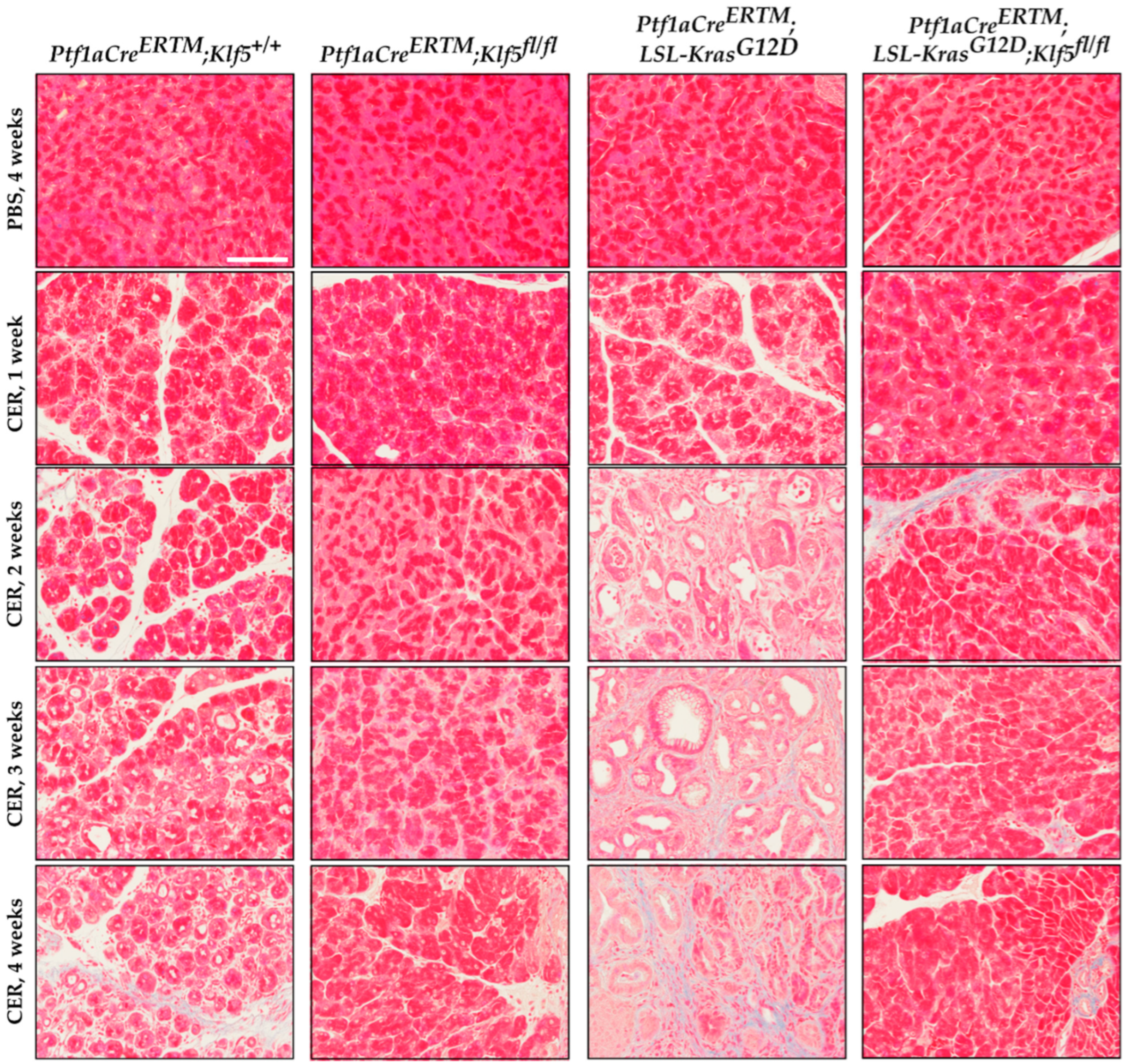
3.4. Klf5 Inactivation Reduces Fibrosis in Chronic Pancreatitis
3.5. KLF5 Regulates the Expression of Cytokines and Inflammatory Markers in Chronic Pancreatitis
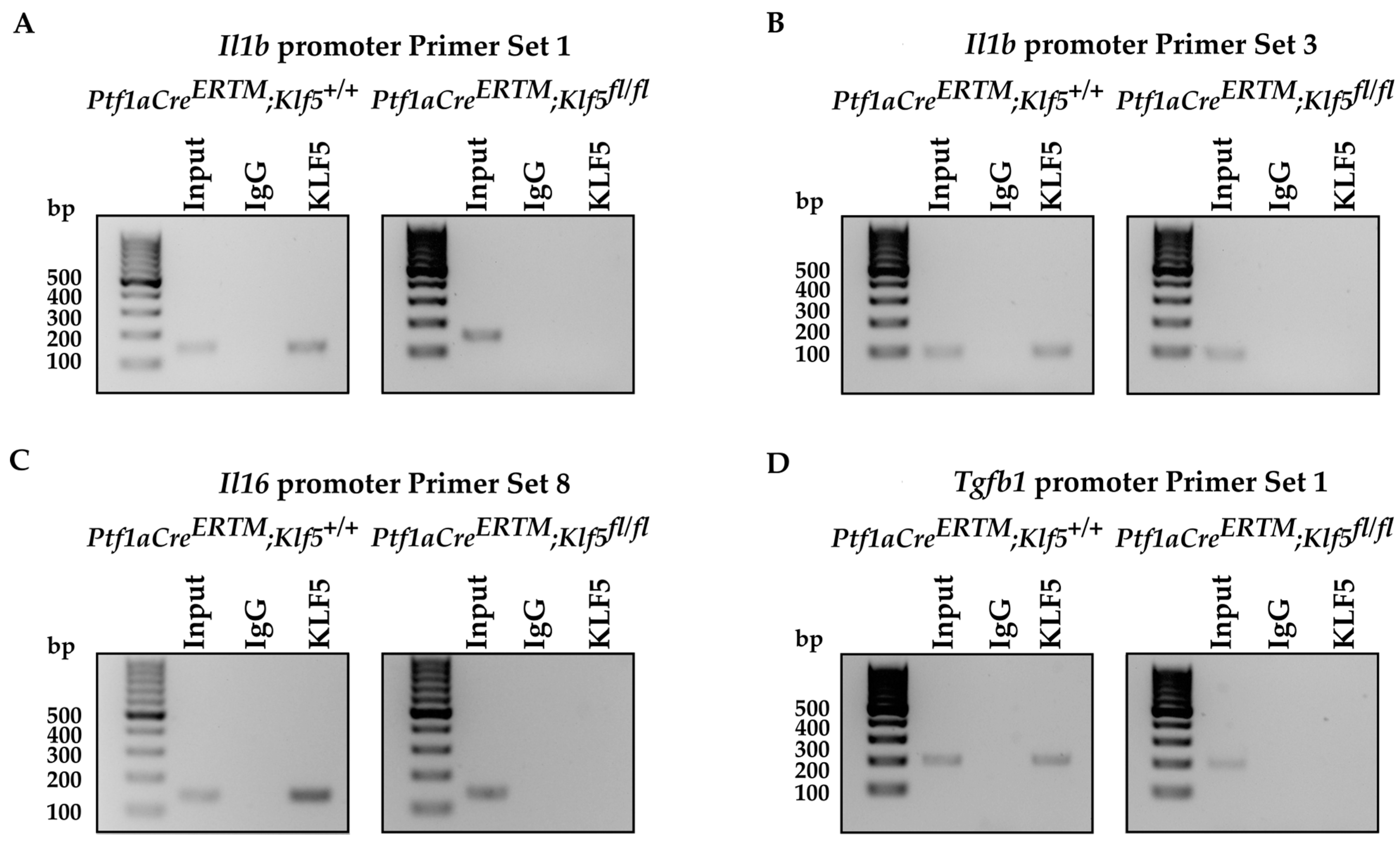
3.6. KLF5 Binds to Il1b, Il6, and Tgfb1 Promoters In Vitro and In Vivo
3.7. Deletion of the Klf5 in Pancreatic Acinar Cells Impacts Inflammatory Response in Chronic Pancreatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peery, A.F.; Crockett, S.D.; Barritt, A.S.; Dellon, E.S.; Eluri, S.; Gangarosa, L.M.; Jensen, E.T.; Lund, J.L.; Pasricha, S.; Runge, T.; et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015, 149, 1731–1741.e3. [Google Scholar] [CrossRef] [PubMed]
- Gapp, J.; Chandra, S. Acute Pancreatitis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Forsmark, C.E.; Vege, S.S.; Wilcox, C.M. Acute Pancreatitis. N. Engl. J. Med. 2016, 375, 1972–1981. [Google Scholar] [CrossRef]
- Stram, M.; Liu, S.; Singhi, A.D. Chronic Pancreatitis. Surg. Pathol. Clin. 2016, 9, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Aghdassi, A.A.; Mayerle, J.; Christochowitz, S.; Weiss, F.U.; Sendler, M.; Lerch, M.M. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair 2011, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Sharma, V.; Habtezion, A. Immune cells and immune-based therapy in pancreatitis. Immunol. Res. 2014, 58, 378–386. [Google Scholar] [CrossRef]
- Lee, B.; Zhao, Q.; Habtezion, A. Immunology of pancreatitis and environmental factors. Curr. Opin. Gastroenterol. 2017, 33, 383–389. [Google Scholar] [CrossRef]
- Chuvin, N.; Vincent, D.F.; Pommier, R.M.; Alcaraz, L.B.; Gout, J.; Caligaris, C.; Yacoub, K.; Cardot, V.; Roger, E.; Kaniewski, B.; et al. Acinar-to-Ductal Metaplasia Induced by Transforming Growth Factor Beta Facilitates KRAS(G12D)-driven Pancreatic Tumorigenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 263–282. [Google Scholar] [CrossRef]
- Wang, L.; Xie, D.; Wei, D. Pancreatic Acinar-to-Ductal Metaplasia and Pancreatic Cancer. Methods Mol. Biol. 2019, 1882, 299–308. [Google Scholar] [CrossRef]
- Johnson, B.L.; d’Alincourt Salazar, M.; Mackenzie-Dyck, S.; D’Apuzzo, M.; Shih, H.P.; Manuel, E.R.; Diamond, D.J. Desmoplasia and oncogene driven acinar-to-ductal metaplasia are concurrent events during acinar cell-derived pancreatic cancer initiation in young adult mice. PLoS ONE 2019, 14, e0221810. [Google Scholar] [CrossRef]
- Apte, M.V.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells: A starring role in normal and diseased pancreas. Front. Physiol. 2012, 3, 344. [Google Scholar] [CrossRef]
- Masamune, A.; Watanabe, T.; Kikuta, K.; Shimosegawa, T. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin. Gastroenterol. Hepatol. 2009, 7, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Shek, F.W.; Benyon, R.C.; Walker, F.M.; McCrudden, P.R.; Pender, S.L.; Williams, E.J.; Johnson, P.A.; Johnson, C.D.; Bateman, A.C.; Fine, D.R.; et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 2002, 160, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.A.; Wu, M.J.; Kumar, R.K.; Doherty, E.; McCarroll, J.A.; Park, S.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Cell migration: A novel aspect of pancreatic stellate cell biology. Gut 2003, 52, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Apte, M.V.; Haber, P.S.; Darby, S.J.; Rodgers, S.C.; McCaughan, G.W.; Korsten, M.A.; Pirola, R.C.; Wilson, J.S. Pancreatic stellate cells are activated by proinflammatory cytokines: Implications for pancreatic fibrogenesis. Gut 1999, 44, 534–541. [Google Scholar] [CrossRef]
- Patel, M.; Fine, D.R. Fibrogenesis in the pancreas after acinar cell injury. Scand. J. Surg. 2005, 94, 108–111. [Google Scholar] [CrossRef]
- Apte, M.V.; Wilson, J.S. Stellate cell activation in alcoholic pancreatitis. Pancreas 2003, 27, 316–320. [Google Scholar] [CrossRef]
- Bynigeri, R.R.; Jakkampudi, A.; Jangala, R.; Subramanyam, C.; Sasikala, M.; Rao, G.V.; Reddy, D.N.; Talukdar, R. Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J. Gastroenterol. 2017, 23, 382–405. [Google Scholar] [CrossRef]
- Longnecker, D.S. Pathology and pathogenesis of diseases of the pancreas. Am. J. Pathol. 1982, 107, 99–121. [Google Scholar]
- Cicenas, J.; Kvederaviciute, K.; Meskinyte, I.; Meskinyte-Kausiliene, E.; Skeberdyte, A.; Cicenas, J. KRAS, TP53, CDKN2A, SMAD4, BRCA1, and BRCA2 Mutations in Pancreatic Cancer. Cancers 2017, 9, 42. [Google Scholar] [CrossRef]
- De La, O.J.; Emerson, L.L.; Goodman, J.L.; Froebe, S.C.; Illum, B.E.; Curtis, A.B.; Murtaugh, L.C. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA 2008, 105, 18907–18912. [Google Scholar] [CrossRef]
- Habbe, N.; Shi, G.; Meguid, R.A.; Fendrich, V.; Esni, F.; Chen, H.; Feldmann, G.; Stoffers, D.A.; Konieczny, S.F.; Leach, S.D.; et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18913–18918. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, C.D.; Ji, B. Ras activity in acinar cells links chronic pancreatitis and pancreatic cancer. Clin. Gastroenterol. Hepatol. 2009, 7, S40–S43. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Singh, N.; Gupta, S.; Rashid, S.; Nalika, N.; Sachdev, V.; Bal, C.S.; Datta Gupta, S.; Chauhan, S.S.; Saraya, A. Progression of Chronic Pancreatitis to Pancreatic Cancer: Is There a Role of Gene Mutations as a Screening Tool? Pancreas 2018, 47, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Daniluk, J.; Liu, Y.; Deng, D.; Chu, J.; Huang, H.; Gaiser, S.; Cruz-Monserrate, Z.; Wang, H.; Ji, B.; Logsdon, C.D. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J. Clin. Investig. 2012, 122, 1519–1528. [Google Scholar] [CrossRef]
- Guerra, C.; Schuhmacher, A.J.; Canamero, M.; Grippo, P.J.; Verdaguer, L.; Perez-Gallego, L.; Dubus, P.; Sandgren, E.P.; Barbacid, M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007, 11, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Carriere, C.; Young, A.L.; Gunn, J.R.; Longnecker, D.S.; Korc, M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. 2009, 382, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.P.t.; Cano, D.A.; Sekine, S.; Wang, S.C.; Hebrok, M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Investig. 2010, 120, 508–520. [Google Scholar] [CrossRef]
- McConnell, B.B.; Yang, V.W. Mammalian Kruppel-like factors in health and diseases. Physiol. Rev. 2010, 90, 1337–1381. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Chen, X.; Yang, V.W. Intestinal-enriched Kruppel-like factor (Kruppel-like factor 5) is a positive regulator of cellular proliferation. J. Biol. Chem. 2001, 276, 6897–6900. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I.; Tobe, K.; Tsushima, K.; Shindo, T.; Fujiu, K.; Nishimura, G.; Maemura, K.; Yamauchi, T.; Kubota, N.; et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell. Metab. 2005, 1, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.G.; Chao, H.H.; Yang, Y.; Yermolina, Y.A.; Tobias, J.W.; Katz, J.P. Overexpression of Kruppel-like factor 5 in esophageal epithelia in vivo leads to increased proliferation in basal but not suprabasal cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1784–G1792. [Google Scholar] [CrossRef]
- McConnell, B.B.; Bialkowska, A.B.; Nandan, M.O.; Ghaleb, A.M.; Gordon, F.J.; Yang, V.W. Haploinsufficiency of Kruppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. 2009, 69, 4125–4133. [Google Scholar] [CrossRef]
- Shieh, J.; Chu, T.H.; Liu, Y.; Kim, J.; Ruiz de Sabando, A.; Kobayashi, S.; Zee, S.Y.; Sheridan, B.S.; Bialkowska, A.B.; Yang, V.W. KLF5 protects the intestinal epithelium against Th17 immune response in a murine colitis model. JCI Insight 2022, 7, e153488. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Saxena, M.; Maharjan, K.; Song, J.J.; Shroyer, K.R.; Bialkowska, A.B.; Shivdasani, R.A.; Yang, V.W. Kruppel-like Factor 5 Regulates Stemness, Lineage Specification, and Regeneration of Intestinal Epithelial Stem Cells. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 587–609. [Google Scholar] [CrossRef]
- Liu, Y.; Chidgey, M.; Yang, V.W.; Bialkowska, A.B. Kruppel-like factor 5 is essential for maintenance of barrier function in mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G478–G491. [Google Scholar] [CrossRef]
- Kim, C.K.; He, P.; Bialkowska, A.B.; Yang, V.W. SP and KLF Transcription Factors in Digestive Physiology and Diseases. Gastroenterology 2017, 152, 1845–1875. [Google Scholar] [CrossRef]
- Nandan, M.O.; Yoon, H.S.; Zhao, W.; Ouko, L.A.; Chanchevalap, S.; Yang, V.W. Kruppel-like factor 5 mediates the transforming activity of oncogenic H-Ras. Oncogene 2004, 23, 3404–3413. [Google Scholar] [CrossRef]
- Usui, S.; Sugimoto, N.; Takuwa, N.; Sakagami, S.; Takata, S.; Kaneko, S.; Takuwa, Y. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J. Biol. Chem. 2004, 279, 12300–12311. [Google Scholar] [CrossRef]
- Chanchevalap, S.; Nandan, M.O.; McConnell, B.B.; Charrier, L.; Merlin, D.; Katz, J.P.; Yang, V.W. Kruppel-like factor 5 is an important mediator for lipopolysaccharide-induced proinflammatory response in intestinal epithelial cells. Nucleic Acids Res. 2006, 34, 1216–1223. [Google Scholar] [CrossRef]
- Yang, Y.; Goldstein, B.G.; Nakagawa, H.; Katz, J.P. Kruppel-like factor 5 activates MEK/ERK signaling via EGFR in primary squamous epithelial cells. FASEB J. 2007, 21, 543–550. [Google Scholar] [CrossRef]
- Nandan, M.O.; McConnell, B.B.; Ghaleb, A.M.; Bialkowska, A.B.; Sheng, H.; Shao, J.; Babbin, B.A.; Robine, S.; Yang, V.W. Kruppel-like factor 5 mediates cellular transformation during oncogenic KRAS-induced intestinal tumorigenesis. Gastroenterology 2008, 134, 120–130. [Google Scholar] [CrossRef]
- Bialkowska, A.B.; Liu, Y.; Nandan, M.O.; Yang, V.W. A colon cancer-derived mutant of Kruppel-like factor 5 (KLF5) is resistant to degradation by glycogen synthase kinase 3beta (GSK3beta) and the E3 ubiquitin ligase F-box and WD repeat domain-containing 7alpha (FBW7alpha). J. Biol. Chem. 2014, 289, 5997–6005. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, M.P.; Yang, Y.; Katz, J.P. Kruppel-like factors in cancer. Nat. Rev. Cancer 2013, 13, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Grutzmann, R.; Boriss, H.; Ammerpohl, O.; Luttges, J.; Kalthoff, H.; Schackert, H.K.; Kloppel, G.; Saeger, H.D.; Pilarsky, C. Meta-analysis of microarray data on pancreatic cancer defines a set of commonly dysregulated genes. Oncogene 2005, 24, 5079–5088. [Google Scholar] [CrossRef]
- Shain, A.H.; Salari, K.; Giacomini, C.P.; Pollack, J.R. Integrative genomic and functional profiling of the pancreatic cancer genome. BMC Genom. 2013, 14, 624. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yang, J.W.; Yang, V.W.; Bialkowska, A.B. Kruppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice. Gastroenterology 2018, 154, 1494–1508.e13. [Google Scholar] [CrossRef]
- Bai, H.; Chen, X.; Zhang, L.; Dou, X. The effect of sulindac, a non-steroidal anti-inflammatory drug, attenuates inflammation and fibrosis in a mouse model of chronic pancreatitis. BMC Gastroenterol. 2012, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Van Laethem, J.L.; Quertinmont, E.; Degraef, C.; Delhaye, M.; Geerts, A.; Deviere, J. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1105–G1112. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mondragon, J.A.; Riudavets-Puig, R.; Rauluseviciute, I.; Lemma, R.B.; Turchi, L.; Blanc-Mathieu, R.; Lucas, J.; Boddie, P.; Khan, A.; Manosalva Perez, N.; et al. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022, 50, D165–D173. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Alberi, L.A. TF-ChIP Method for Tissue-Specific Gene Targets. Front. Cell. Neurosci. 2019, 13, 95. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Diaferia, G.R.; Balestrieri, C.; Prosperini, E.; Nicoli, P.; Spaggiari, P.; Zerbi, A.; Natoli, G. Dissection of transcriptional and cis-regulatory control of differentiation in human pancreatic cancer. EMBO J. 2016, 35, 595–617. [Google Scholar] [CrossRef]
- Winter, K.; Dzieniecka, M.; Strzelczyk, J.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Malecka-Wojciesko, E. Alpha Smooth Muscle Actin (alphaSMA) Immunohistochemistry Use in the Differentiation of Pancreatic Cancer from Chronic Pancreatitis. J. Clin. Med. 2021, 10, 5804. [Google Scholar] [CrossRef]
- Van De Vlekkert, D.; Machado, E.; d’Azzo, A. Analysis of Generalized Fibrosis in Mouse Tissue Sections with Masson’s Trichrome Staining. Bio-Protocol 2020, 10, e3629. [Google Scholar] [CrossRef]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef]
- Klauss, S.; Schorn, S.; Teller, S.; Steenfadt, H.; Friess, H.; Ceyhan, G.O.; Demir, I.E. Genetically induced vs. classical animal models of chronic pancreatitis: A critical comparison. FASEB J. 2018, 32, 5778–5792. [Google Scholar] [CrossRef]
- Olesen, S.S.; Krarup, H.; Poulsen, J.L.; Christensen, J.H.; Sheel, A.R.G.; Sutton, R.; Greenhalf, W.; Halloran, C.; Drewes, A.M. Pancreas-specific plasma amylase for assessment and diagnosis of chronic pancreatitis: New insights on an old topic. United Eur. Gastroenterol. J. 2019, 7, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.C.; Kwon, C.I.; El, H., II; Easler, J.J.; Watkins, J.; Fogel, E.L.; McHenry, L.; Sherman, S.; Zimmerman, M.K.; Lehman, G.A. Low Serum Pancreatic Amylase and Lipase Values Are Simple and Useful Predictors to Diagnose Chronic Pancreatitis. Gut Liver 2017, 11, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, B.; Xu, X.F.; Jiang, T.T.; Zhang, X.Q.; Shi, Y.L.; Chen, Y.; Liu, F.; Gu, J.; Zhu, L.J.; et al. Pathophysiology of chronic pancreatitis induced by dibutyltin dichloride joint ethanol in mice. World J. Gastroenterol. 2016, 22, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, B.C.; Demcsak, A.; Geisz, A.; Sahin-Toth, M. Misfolding-induced chronic pancreatitis in CPA1 N256K mutant mice is unaffected by global deletion of Ddit3/Chop. Sci. Rep. 2022, 12, 6357. [Google Scholar] [CrossRef]
- Chen, L.; Ma, C.; Bian, Y.; Li, J.; Wang, T.; Su, L.; Lu, J. Hydrogen Treatment Protects Mice Against Chronic Pancreatitis by Restoring Regulatory T Cells Loss. Cell. Physiol. Biochem. 2017, 44, 2005–2016. [Google Scholar] [CrossRef]
- Geisz, A.; Sahin-Toth, M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat. Commun. 2018, 9, 5033. [Google Scholar] [CrossRef]
- Wei, D.; Wang, L.; Yan, Y.; Jia, Z.; Gagea, M.; Li, Z.; Zuo, X.; Kong, X.; Huang, S.; Xie, K. KLF4 Is Essential for Induction of Cellular Identity Change and Acinar-to-Ductal Reprogramming during Early Pancreatic Carcinogenesis. Cancer Cell. 2016, 29, 324–338. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Z.; Wang, J.; Zhou, L.; Zhang, J.; Yao, B.; Dou, J.; Qiu, Z.; Huang, C. Kruppel-Like Factor 4 Inhibits Pancreatic Cancer Epithelial-to-Mesenchymal Transition and Metastasis by Down-Regulating Caveolin-1 Expression. Cell. Physiol. Biochem. 2018, 46, 238–252. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Lin, Y.; Wang, X.; Cui, X.; Zhang, Z.; Xian, G.; Qin, C. Novel crosstalk between KLF4 and ZEB1 regulates gemcitabine resistance in pancreatic ductal adenocarcinoma. Int. J. Oncol. 2017, 51, 1239–1248. [Google Scholar] [CrossRef]
- Guo, K.; Cui, J.; Quan, M.; Xie, D.; Jia, Z.; Wei, D.; Wang, L.; Gao, Y.; Ma, Q.; Xie, K. The Novel KLF4/MSI2 Signaling Pathway Regulates Growth and Metastasis of Pancreatic Cancer. Clin. Cancer Res. 2017, 23, 687–696. [Google Scholar] [CrossRef]
- Jin, G.; Hong, W.; Guo, Y.; Bai, Y.; Chen, B. Molecular Mechanism of Pancreatic Stellate Cells Activation in Chronic Pancreatitis and Pancreatic Cancer. J. Cancer 2020, 11, 1505–1515. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gukovsky, I.; Zaninovic, V.; Song, M.; Sandoval, D.; Gukovsky, S.; Pandol, S.J. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J. Clin. Investig. 1997, 100, 1853–1862. [Google Scholar] [CrossRef]
- Bottinger, E.P.; Jakubczak, J.L.; Roberts, I.S.; Mumy, M.; Hemmati, P.; Bagnall, K.; Merlino, G.; Wakefield, L.M. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997, 16, 2621–2633. [Google Scholar] [CrossRef]
- Bhatia, M. Inflammatory response on the pancreatic acinar cell injury. Scand. J. Surg. 2005, 94, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wildi, S.; Kleeff, J.; Mayerle, J.; Zimmermann, A.; Bottinger, E.P.; Wakefield, L.; Buchler, M.W.; Friess, H.; Korc, M. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut 2007, 56, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, Y.; Noe, M.; Li, Q.; Pasricha, P.J. Neuronal Transforming Growth Factor beta Signaling via SMAD3 Contributes to Pain in Animal Models of Chronic Pancreatitis. Gastroenterology 2018, 154, 2252–2265.e2. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; DeCant, B.; Mascarinas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGFbeta Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Akanuma, N.; Liu, C.; Naji, A.; Halff, G.A.; Washburn, W.K.; Sun, L.; Wang, P. TGF-beta1 promotes acinar to ductal metaplasia of human pancreatic acinar cells. Sci. Rep. 2016, 6, 30904. [Google Scholar] [CrossRef]
- Muller-Pillasch, F.; Menke, A.; Yamaguchi, H.; Elsasser, H.P.; Bachem, M.; Adler, G.; Gress, T.M. TGFbeta and the extracellular matrix in pancreatitis. Hepatogastroenterology 1999, 46, 2751–2756. [Google Scholar]
- Jia, L.; Zhou, Z.; Liang, H.; Wu, J.; Shi, P.; Li, F.; Wang, Z.; Wang, C.; Chen, W.; Zhang, H.; et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene 2016, 35, 2040–2051. [Google Scholar] [CrossRef]
- Ma, D.; Chang, L.Y.; Zhao, S.; Zhao, J.J.; Xiong, Y.J.; Cao, F.Y.; Yuan, L.; Zhang, Q.; Wang, X.Y.; Geng, M.L.; et al. KLF5 promotes cervical cancer proliferation, migration and invasion in a manner partly dependent on TNFRSF11a expression. Sci. Rep. 2017, 7, 15683. [Google Scholar] [CrossRef]
- Novovic, S.; Borch, A.; Werge, M.; Karran, D.; Gluud, L.; Schmidt, P.N.; Hansen, E.F.; Nojgaard, C.; Jensen, A.B.; Jensen, F.K.; et al. Characterisation of the fibroinflammatory process involved in progression from acute to chronic pancreatitis: Study protocol for a multicentre, prospective cohort study. BMJ Open 2019, 9, e028999. [Google Scholar] [CrossRef] [PubMed]
- Beyer, G.; Habtezion, A.; Werner, J.; Lerch, M.M.; Mayerle, J. Chronic pancreatitis. Lancet 2020, 396, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhu, Z.; Homer, R.J.; Gerard, C.; Strieter, R.; Elias, J.A. The C10/CCL6 chemokine and CCR1 play critical roles in the pathogenesis of IL-13-induced inflammation and remodeling. J. Immunol. 2004, 172, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, B.; Oshima, K.; Rodriguez, M.G.; Cox, R.D.; Neuschwander-Tetri, B.A. Differences in the degree of cerulein-induced chronic pancreatitis in C57BL/6 mouse substrains lead to new insights in identification of potential risk factors in the development of chronic pancreatitis. Am. J. Pathol. 2013, 183, 692–708. [Google Scholar] [CrossRef]
- Alexeev, V.; Donahue, A.; Uitto, J.; Igoucheva, O. Chemotaxis-driven disease-site targeting of therapeutic adult stem cells in dystrophic epidermolysis bullosa. Stem Cell Res. Ther. 2016, 7, 124. [Google Scholar] [CrossRef]
- Chao, G.Y.; Wallis, R.H.; Marandi, L.; Ning, T.; Sarmiento, J.; Paterson, A.D.; Poussier, P. Iddm30 controls pancreatic expression of Ccl11 (Eotaxin) and the Th1/Th2 balance within the insulitic lesions. J. Immunol. 2014, 192, 3645–3653. [Google Scholar] [CrossRef]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Mishra, A. Role of eosinophils in the initiation and progression of pancreatitis pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G211–G222. [Google Scholar] [CrossRef]
- Zambirinis, C.P.; Levie, E.; Nguy, S.; Avanzi, A.; Barilla, R.; Xu, Y.; Seifert, L.; Daley, D.; Greco, S.H.; Deutsch, M.; et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015, 212, 2077–2094. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Li, Y.; Liu, Y.; Xie, X.; Wu, Y.; Zhou, Y.; Ren, J.; Zhang, J.; Zhu, H.; et al. CCL21/CCR7 Axis Contributed to CD133+ Pancreatic Cancer Stem-Like Cell Metastasis via EMT and Erk/NF-kappaB Pathway. PLoS ONE 2016, 11, e0158529. [Google Scholar] [CrossRef]
- Hirth, M.; Gandla, J.; Hoper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated with Pain in Patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef] [PubMed]
- Baldan, J.; Houbracken, I.; Rooman, I.; Bouwens, L. Adult human pancreatic acinar cells dedifferentiate into an embryonic progenitor-like state in 3D suspension culture. Sci. Rep. 2019, 9, 4040. [Google Scholar] [CrossRef]
- Araki, H.; Nishihara, T.; Matsuda, M.; Fukuhara, A.; Kihara, S.; Funahashi, T.; Kataoka, T.R.; Kamada, Y.; Kiyohara, T.; Tamura, S.; et al. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut 2008, 57, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chim. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Walsh, K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003, 14, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Bozadjieva-Kramer, N.; Shao, Y.; Lyons-Abbott, S.; Rupp, A.C.; Sandoval, D.A.; Seeley, R.J. The gut peptide Reg3g links the small intestine microbiome to the regulation of energy balance, glucose levels, and gut function. Cell. Metab. 2022, 34, 1765–1778.e1766. [Google Scholar] [CrossRef]
- Yin, G.; Du, J.; Cao, H.; Liu, X.; Xu, Q.; Xiang, M. Reg3g Promotes Pancreatic Carcinogenesis in a Murine Model of Chronic Pancreatitis. Dig. Dis. Sci. 2015, 60, 3656–3668. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Xie, M.; Zhang, Z.; Gou, J.; Yang, J.; Tian, C.; Ma, K.; Wang, C.Y.; Lu, Y.; et al. Regenerating islet-derived protein 3 gamma (Reg3g) ameliorates tacrolimus-induced pancreatic beta-cell dysfunction in mice by restoring mitochondrial function. Br. J. Pharmacol. 2022, 179, 3078–3095. [Google Scholar] [CrossRef]
- Lindhorst, A.; Bechmann, I.; Gericke, M. Unspecific DNA recombination in AdipoqCre-ER(T2) mediated knockout approaches in transgenic mice is sex-, age- and genotype-dependent. Adipocyte 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Li, X.; Clappier, C.; Kleiter, I.; Heuchel, R. Tamoxifen affects chronic pancreatitis-related fibrogenesis in an experimental mouse model: An effect beyond Cre recombination. FEBS Open Bio 2019, 9, 1756–1768. [Google Scholar] [CrossRef]
- Hammad, S.; Othman, A.; Meyer, C.; Telfah, A.; Lambert, J.; Dewidar, B.; Werle, J.; Nwosu, Z.C.; Mahli, A.; Dormann, C.; et al. Confounding influence of tamoxifen in mouse models of Cre recombinase-induced gene activity or modulation. Arch. Toxicol. 2018, 92, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Falke, L.L.; Broekhuizen, R.; Huitema, A.; Maarseveen, E.; Nguyen, T.Q.; Goldschmeding, R. Tamoxifen for induction of Cre-recombination may confound fibrosis studies in female mice. J. Cell Commun. Signal. 2017, 11, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Reinert, R.B.; Kantz, J.; Misfeldt, A.A.; Poffenberger, G.; Gannon, M.; Brissova, M.; Powers, A.C. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS ONE 2012, 7, e33529. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alavi, M.; Mejia-Bautista, A.; Tang, M.; Bandovic, J.; Rosenberg, A.Z.; Bialkowska, A.B. Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis. Cancers 2023, 15, 5427. https://doi.org/10.3390/cancers15225427
Alavi M, Mejia-Bautista A, Tang M, Bandovic J, Rosenberg AZ, Bialkowska AB. Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis. Cancers. 2023; 15(22):5427. https://doi.org/10.3390/cancers15225427
Chicago/Turabian StyleAlavi, Maryam, Ana Mejia-Bautista, Meiyi Tang, Jela Bandovic, Avi Z. Rosenberg, and Agnieszka B. Bialkowska. 2023. "Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis" Cancers 15, no. 22: 5427. https://doi.org/10.3390/cancers15225427
APA StyleAlavi, M., Mejia-Bautista, A., Tang, M., Bandovic, J., Rosenberg, A. Z., & Bialkowska, A. B. (2023). Krüppel-like Factor 5 Plays an Important Role in the Pathogenesis of Chronic Pancreatitis. Cancers, 15(22), 5427. https://doi.org/10.3390/cancers15225427






