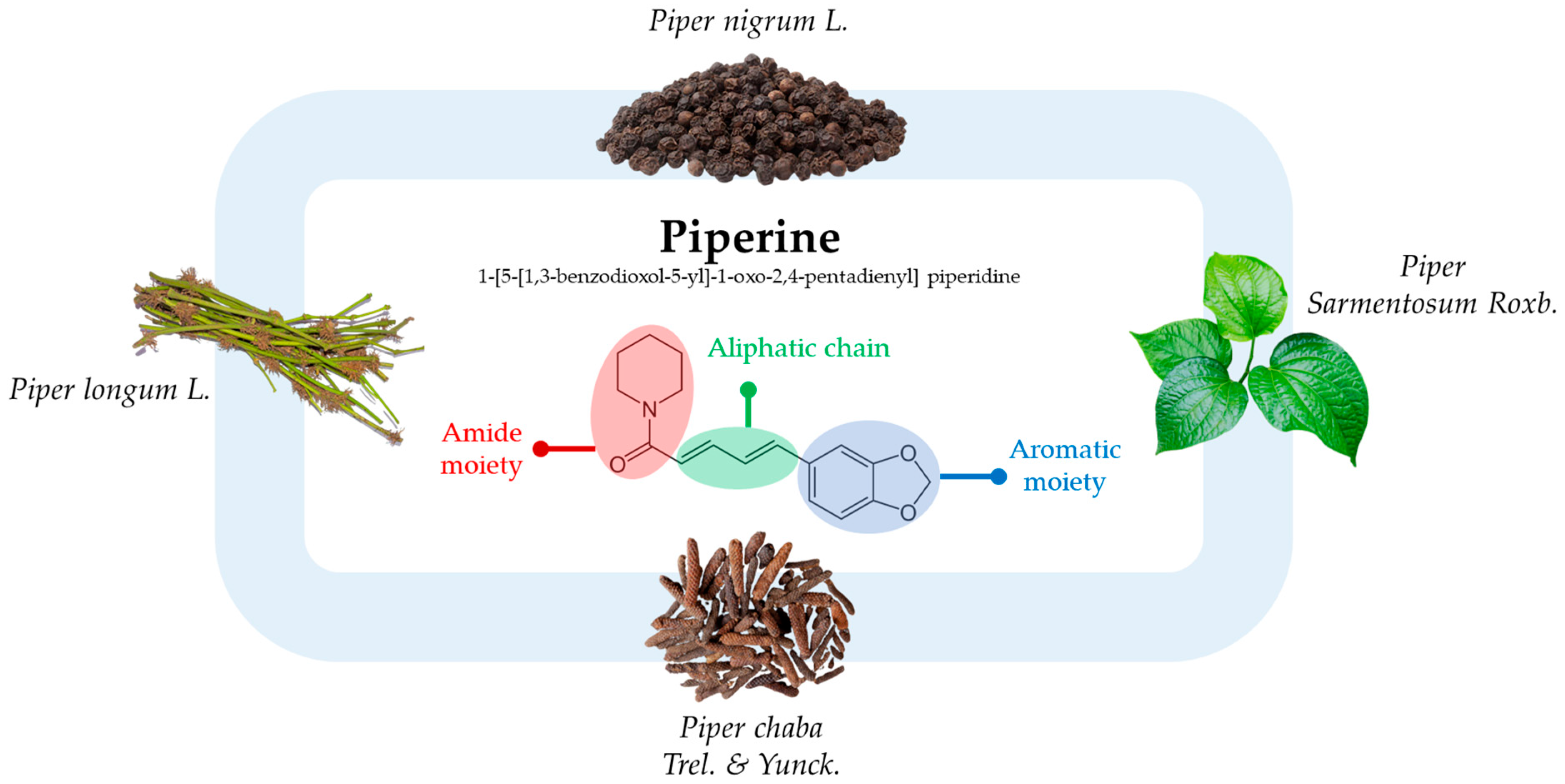
The Promise of Piperine in Cancer Chemoprevention
Abstract
Simple Summary
Abstract
1. Introduction
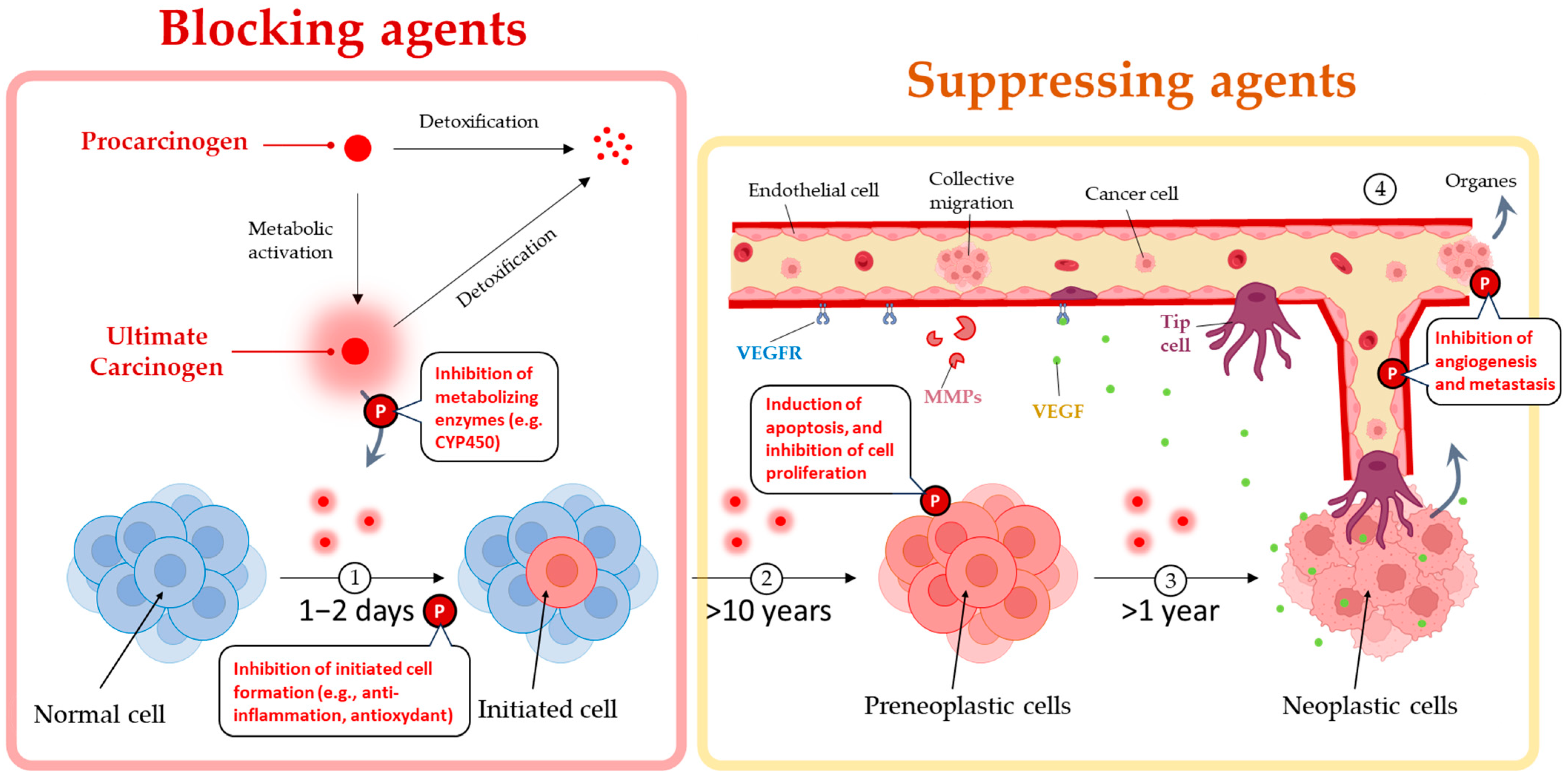
2. Piperine’s Dual Mechanisms of Prevention and Destruction of Cancer
2.1. Piperine Reduces Inflammation
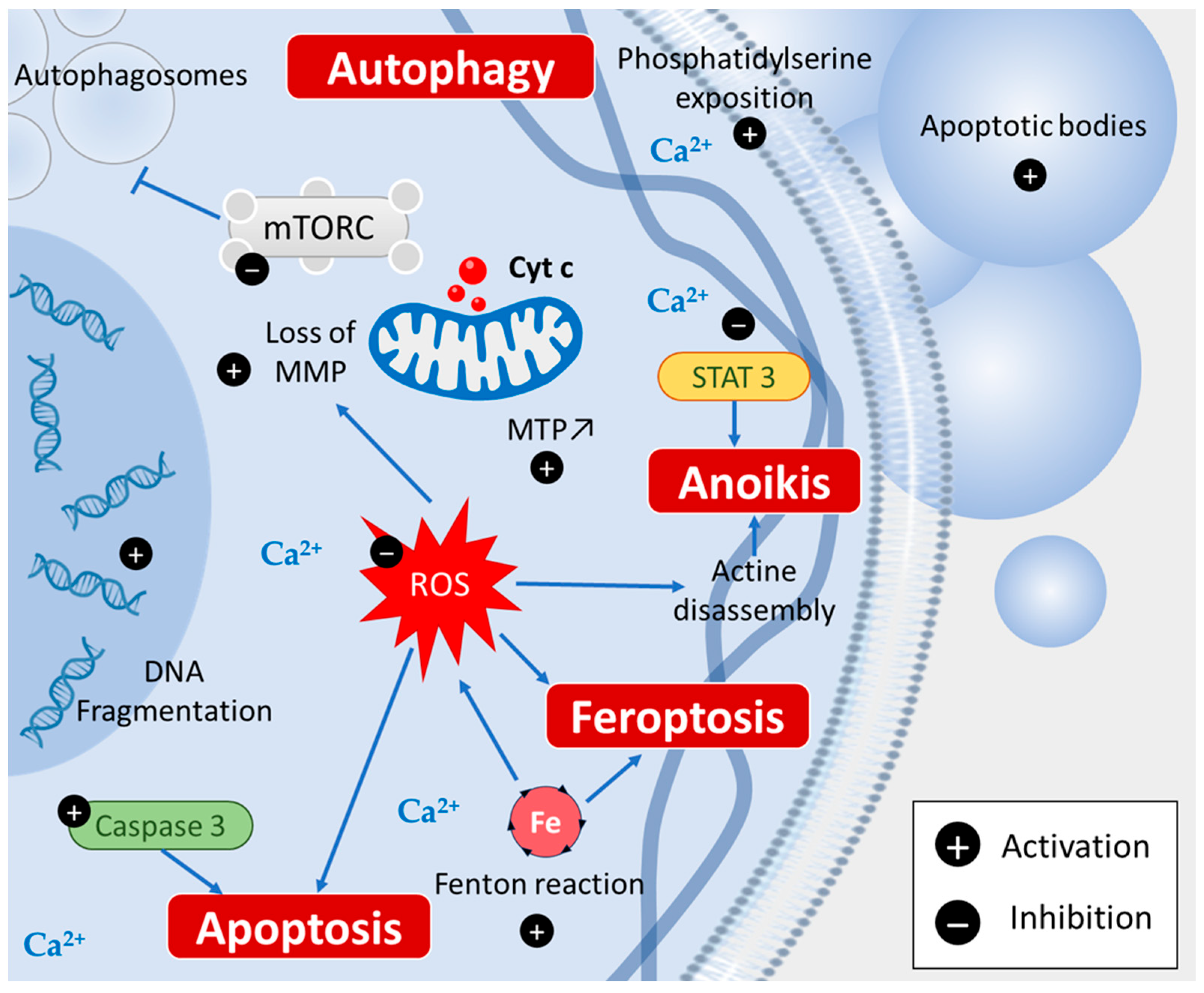
2.2. Piperine Induces Various Cell Death Types
2.2.1. Apoptosis
2.2.2. Autophagy
2.2.3. Ferroptosis
2.2.4. Anoikis
2.3. Piperine Inhibits Cancer Stem Cells
2.4. Piperine Induces Cell Cycle Arrest
2.5. Piperine Selectively Inhibits the Growth of Cancer Cells
2.6. Piperine Inhibits Cancer Invasion and Metastasis Process
2.6.1. Anti-Angiogenic Effects of Piperine
2.6.2. Anti-Metastatic Activity of Piperine
| Targets | Model | Mechanisms | References |
|---|---|---|---|
| In vitro | |||
| Inflammation |
|
| [26,27,28,29] |
| Cell death |
|
| [15,32,35,40,41,43,44,45,46] |
| Cancer stem cells |
|
| [32,39,51,52] |
| Cell cycle |
|
| [18,55,56,57] |
| Cancer cells growth |
|
| [16,18,41] |
| Invasion and metastasis |
|
| [56,60,61,62,63,64,65] |
| In vivo | |||
| Inflammation |
|
| [30,31,32] |
| Cell death (apoptosis, autophagy) |
|
| [36,42,66] |
| Invasion and metastasis |
|
| [63,64] |
3. Piperine Enlightens the Dark Side of Cancer Therapy
3.1. Radiosensitization and UV-Phototherapy
3.2. TRAIL-Based Therapy
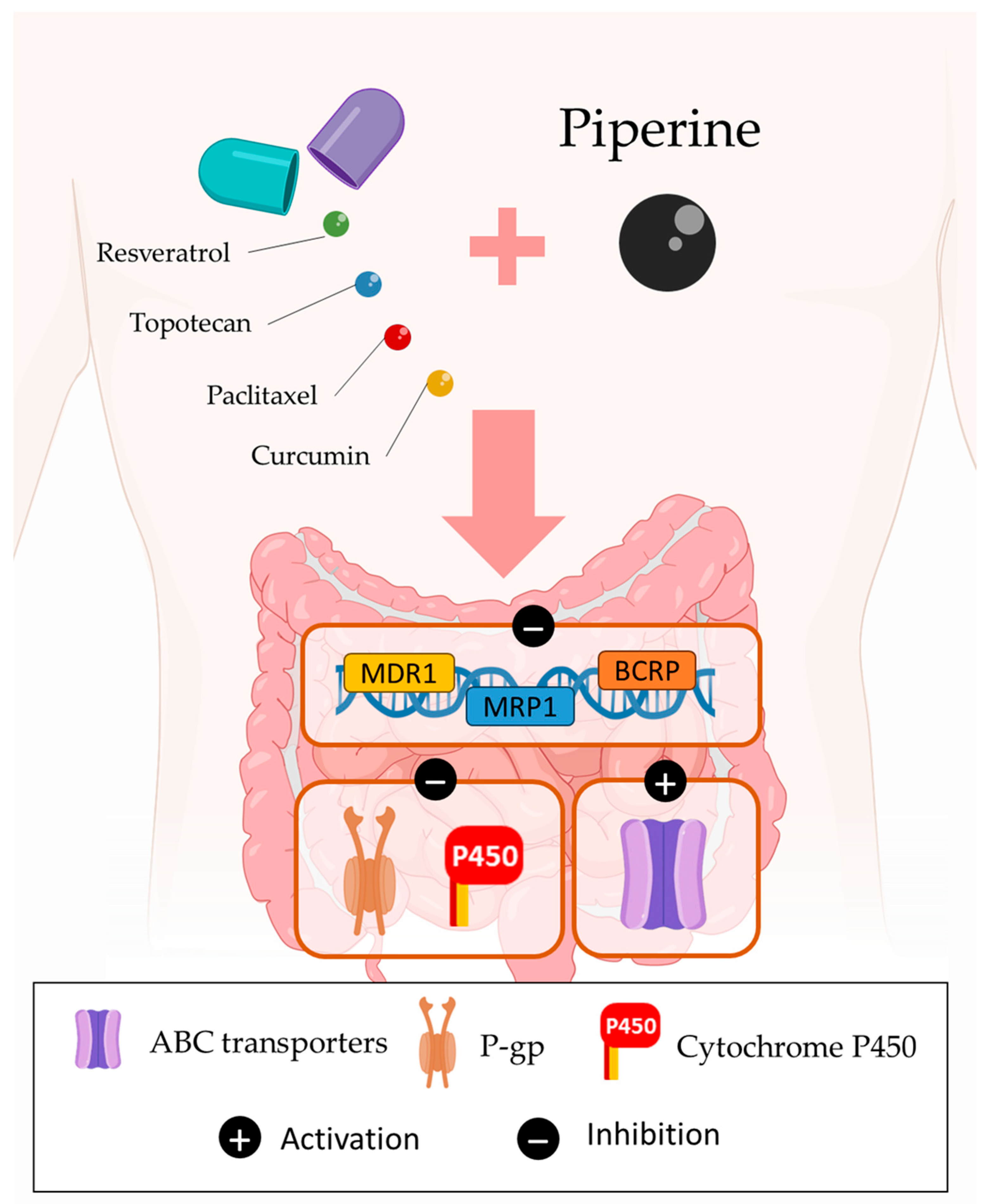
3.3. Bioavailability of Drugs
3.4. Multidrug Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.J.; Liu, C.J.; Ying, T.H.; Wu, P.J.; Wang, J.W.; Ting, Y.H.; Hsieh, Y.H.; Wang, S.C. Timosaponin AIII Inhibits Migration and Invasion Abilities in Human Cervical Cancer Cells through Inactivation of p38 MAPK-Mediated uPA Expression In Vitro and In Vivo. Cancers 2022, 15, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yokoyama, N.N.; Song, L.; Xie, J.; Sadeghi, Z.; Wu, Y.X.; Yee, S.; Wu, X.R.; Wang, B.; Uchio, E.; et al. The Potent Anti-Tumor Effects of Rhodiola Drinking Are Associated with the Inhibition of the mTOR Pathway and Modification of Tumor Metabolism in the UPII-Mutant Ha-Ras Model. Cancers 2023, 15, 3086. [Google Scholar] [CrossRef]
- Madka, V.; Patlolla, J.M.R.; Venkatachalam, K.; Zhang, Y.; Pathuri, G.; Stratton, N.; Lightfoot, S.; Janakiram, N.B.; Mohammed, A.; Rao, C.V. Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats. Cancers 2023, 15, 4001. [Google Scholar] [CrossRef]
- Ismail, B.; Ghezali, L.; Gueye, R.; Limami, Y.; Pouget, C.; Leger, D.Y.; Martin, F.; Beneytout, J.L.; Duroux, J.L.; Diab-Assaf, M.; et al. Novel methylsulfonyl chalcones as potential antiproliferative drugs for human prostate cancer: Involvement of the intrinsic pathway of apoptosis. Int. J. Oncol. 2013, 43, 1160–1168. [Google Scholar] [CrossRef][Green Version]
- Soltana, H.; Pinon, A.; Limami, Y.; Zaid, Y.; Khalki, L.; Zaid, N.; Salah, D.; Sabitaliyevich, U.Y.; Simon, A.; Liagre, B.; et al. Antitumoral activity of Ficus carica L. on colorectal cancer cell lines. Cell. Mol. Biol. 2019, 65, 6–11. [Google Scholar] [CrossRef]
- Hassan, L.; Pinon, A.; Limami, Y.; Seeman, J.; Fidanzi-Dugas, C.; Martin, F.; Badran, B.; Simon, A.; Liagre, B. Resistance to ursolic acid-induced apoptosis through involvement of melanogenesis and COX-2/PGE2 pathways in human M4Beu melanoma cancer cells. Exp. Cell Res. 2016, 345, 60–69. [Google Scholar] [CrossRef]
- da Silva Fernandes, A.; de Oliveira, C.G.; Evangelista, H.; Ds, M.S.; Araujo-Lima, C.F.; Felzenszwalb, I. In vitro chemopreventive and cytotoxic effects of Amazon mosses Leucobryum martianum (Hornsch.) and Leucobryum laevifolium (Broth) extracts. Mutagenesis 2023, gead028. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Aziz, N.S.; Sofian-Seng, N.-S.; Mohd Razali, N.S.; Lim, S.J.; Mustapha, W.A.W. A review on conventional and biotechnological approaches in white pepper production. J. Sci. Food Agric. 2019, 99, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and anti-inflammatory effects of piperine on UV-B-irradiated human HaCaT keratinocyte cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Q.; Liu, C.; Tang, Y.; Sun, C.; Zhuang, J. Nanoformulations of Ursolic Acid: A Modern Natural Anticancer Molecule. Front. Pharmacol. 2021, 12, 706121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Malhotra, S.; Prasad, A.K.; Van der Eycken, E.V.; Bracke, M.E.; Stetler-Stevenson, W.G.; Parmar, V.S.; Ghosh, B. Anti-inflammatory and antioxidant properties of Piper species: A perspective from screening to molecular mechanisms. Curr. Top. Med. Chem. 2015, 15, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Qian, J.; Xu, Z.; Meng, C.; Liu, J.; Shan, W.; Zhu, W.; Wang, Y.; Yang, Y.; Zhang, W.; et al. Piperlonguminine and Piperine Analogues as TrxR Inhibitors that Promote ROS and Autophagy and Regulate p38 and Akt/mTOR Signaling. J. Nat. Prod. 2020, 83, 3041–3049. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 105, 106–118. [Google Scholar] [CrossRef]
- Doucette, C.D.; Hilchie, A.L.; Liwski, R.; Hoskin, D.W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013, 24, 231–239. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Li, H.; Li, B.; Sun, L.; Xie, T.; Zhu, T.; Zhou, H.; Ye, Z. Piperine inhibits proliferation of human osteosarcoma cells via G2/M phase arrest and metastasis by suppressing MMP-2/-9 expression. Int. Immunopharmacol. 2015, 24, 50–58. [Google Scholar] [CrossRef]
- Atal, N.; Bedi, K.L. Bioenhancers: Revolutionary concept to market. J. Ayurveda Integr. Med. 2010, 1, 96–99. [Google Scholar] [CrossRef]
- Świerczewska, M.; Sterzyńska, K.; Wojtowicz, K.; Kaźmierczak, D.; Iżycki, D.; Nowicki, M.; Zabel, M.; Januchowski, R. PTPRK Expression Is Downregulated in Drug Resistant Ovarian Cancer Cell Lines, and Especially in ALDH1A1 Positive CSCs-Like Populations. Int. J. Mol. Sci. 2019, 20, 2053. [Google Scholar] [CrossRef]
- Abdelhamed, S.; Yokoyama, S.; Refaat, A.; Ogura, K.; Yagita, H.; Awale, S.; Saiki, I. Piperine enhances the efficacy of TRAIL-based therapy for triple-negative breast cancer cells. Anticancer Res. 2014, 34, 1893–1899. [Google Scholar] [PubMed]
- Limami, Y.; Pinon, A.; Riaz, A.; Simon, A. TRAIL and targeting cancer cells: Between promises and obstacles. Cell. Mol. Biol. 2015, 61, 33–38. [Google Scholar] [PubMed]
- Wattenberg, L.W. Chemoprevention of cancer. Cancer Res. 1985, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Woo, H.M.; Kang, J.H.; Kawada, T.; Yoo, H.; Sung, M.K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Hu, Y.; Xu, B.; Zhao, J. Piperine mediates LPS induced inflammatory and catabolic effects in rat intervertebral disc. Int. J. Clin. Exp. Pathol. 2015, 8, 6203–6213. [Google Scholar]
- Chuchawankul, S.; Khorana, N.; Poovorawan, Y. Piperine inhibits cytokine production by human peripheral blood mononuclear cells. Genet. Mol. Res. GMR 2012, 11, 617–627. [Google Scholar] [CrossRef]
- Wang-Sheng, C.; Jie, A.; Jian-Jun, L.; Lan, H.; Zeng-Bao, X.; Chang-Qing, L. Piperine attenuates lipopolysaccharide (LPS)-induced inflammatory responses in BV2 microglia. Int. Immunopharmacol. 2017, 42, 44–48. [Google Scholar] [CrossRef]
- Zazeri, G.; Povinelli, A.P.R.; Le Duff, C.S.; Tang, B.; Cornelio, M.L.; Jones, A.M. Synthesis and Spectroscopic Analysis of Piperine- and Piperlongumine-Inspired Natural Product Scaffolds and Their Molecular Docking with IL-1β and NF-κB Proteins. Molecules 2020, 25, 2841. [Google Scholar] [CrossRef]
- Sireeratawong, S.; Itharat, A.; Lerdvuthisopon, N.; Piyabhan, P.; Khonsung, P.; Boonraeng, S.; Jaijoy, K. Anti-Inflammatory, Analgesic, and Antipyretic Activities of the Ethanol Extract of Piper interruptum Opiz. and Piper chaba Linn. ISRN Pharmacol. 2012, 2012, 480265. [Google Scholar] [CrossRef]
- Bang, J.S.; Oh, D.H.; Choi, H.M.; Sur, B.-J.; Lim, S.-J.; Kim, J.Y.; Yang, H.-I.; Yoo, M.C.; Hahm, D.-H.; Kim, K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1β-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009, 11, R49. [Google Scholar] [CrossRef] [PubMed]
- Tawani, A.; Amanullah, A.; Mishra, A.; Kumar, A. Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences. Sci. Rep. 2016, 6, 39239. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.-C.; O’Halloran, P.J.; Connolly, N.M.C.; Murphy, B.M. Targeting the apoptosis pathway to treat tumours of the paediatric nervous system. Cell Death Dis. 2022, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Jafri, A.; Siddiqui, S.; Rais, J.; Ahmad, M.S.; Kumar, S.; Jafar, T.; Afzal, M.; Arshad, M. Induction of apoptosis by piperine in human cervical adenocarcinoma via ROS mediated mitochondrial pathway and caspase-3 activation. EXCLI J. 2019, 18, 154–164. [Google Scholar] [CrossRef]
- Qi, Y.; Yao, L.; Liu, J.; Wang, W. Piperine improves the sensitivity of osteosarcoma cells to doxorubicin by inducing apoptosis and inhibiting the PI3K/AKT/GSK-3β pathway. J. Orthop. Surg. Res. 2023, 18, 180. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Chen, H.Y.; White, E. Role of autophagy in cancer prevention. Cancer Prev. Res. 2011, 4, 973–983. [Google Scholar] [CrossRef]
- Alvarez-Meythaler, J.G.; Garcia-Mayea, Y.; Mir, C.; Kondoh, H.; ME, L.L. Autophagy Takes Center Stage as a Possible Cancer Hallmark. Front. Oncol. 2020, 10, 586069. [Google Scholar] [CrossRef]
- Kaur, H.; He, B.; Zhang, C.; Rodriguez, E.; Hage, D.S.; Moreau, R. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: Implications for the inhibition of protein synthesis and TNFα signaling. J. Nutr. Biochem. 2018, 57, 276–286. [Google Scholar] [CrossRef]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine inhibits the proliferation of human prostate cancer cells via induction of cell cycle arrest and autophagy. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Choi, E.Y.; Jeon, S.J.; Lee, S.W.; Moon, J.M.; Jung, S.H.; Jung, J.Y. Piperine Induces Apoptosis and Autophagy in HSC-3 Human Oral Cancer Cells by Regulating PI3K Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 13949. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Gupta, R.L. In vitro antioxidant activity of piperine. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 271–274. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kasukabe, T.; Kumakura, S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int. J. Oncol. 2018, 52, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Fofaria, N.M.; Srivastava, S.K. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis 2015, 36, 142–150. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kaklamani, V.G.; Siziopikou, K. The role of cancer stem cells in breast cancer initiation and progression: Potential cancer stem cell-directed therapies. Oncologist 2012, 17, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef] [PubMed]
- Samiry, I.; Pinon, A.; Limami, Y.; Rais, S.; Zaid, Y.; Oudghiri, M.; Liagre, B.; Mtairag, E.M. Antitumoral activity of Caralluma europaea on colorectal and prostate cancer cell lines. J. Toxicol. Environ. Health Part A 2023, 86, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Fofaria, N.M.; Kim, S.H.; Srivastava, S.K. Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS ONE 2014, 9, e94298. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Pressete, C.G.; Viegas, F.P.; Campos, T.G.; Caixeta, E.S.; Hanemann, J.A.; Ferreira-Silva, G.Á.; Zavan, B.; Aissa, A.F.; Miyazawa, M.; Viegas, C.; et al. Piperine–Chlorogenic Acid Hybrid Inhibits the Proliferation of the SK-MEL-147 Melanoma Cells by Modulating Mitotic Kinases. Pharmaceuticals 2023, 16, 145. [Google Scholar] [CrossRef]
- Suter, T.M.; Ewer, M.S. Cancer drugs and the heart: Importance and management. Eur. Heart J. 2013, 34, 1102–1111. [Google Scholar] [CrossRef]
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef]
- Tobelem, G. Tumor angiogenesis. Nouv. Rev. Fr. D’hematol. 1990, 32, 405–406. [Google Scholar]
- Qi, Y.B.; Yang, W.; Si, M.; Nie, L. Wnt/β-catenin signaling modulates piperine-mediated antitumor effects on human osteosarcoma cells. Mol. Med. Rep. 2020, 21, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Tina Nayerpour, D.; Armaghan, L.; Zakieh Sadat, S.; Mohammad, P.; Fahimeh, H.; Vajiheh, N.; Omid, A.; Mojtaba, A.; Parisa, K. The Effect of Piperine on MMP-9, VEGF, and E-cadherin Expression in Breast Cancer MCF-7 Cell Line. Basic Clin. Cancer Res. 2020, 12, 112–119. [Google Scholar] [CrossRef]
- Lai, L.H.; Fu, Q.H.; Liu, Y.; Jiang, K.; Guo, Q.M.; Chen, Q.Y.; Yan, B.; Wang, Q.Q.; Shen, J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012, 33, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Gasser, A.B.; Depierre, D.; Courvoisier, B. Total urinary and free serum hydroxyproline in metastatic bone disease. Br. J. Cancer 1979, 39, 280–283. [Google Scholar] [CrossRef]
- Lin, M.T.; Lin, B.R.; Chang, C.C.; Chu, C.Y.; Su, H.J.; Chen, S.T.; Jeng, Y.M.; Kuo, M.L. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int. J. Cancer 2007, 120, 2600–2608. [Google Scholar] [CrossRef]
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2021, 84, 153484. [Google Scholar] [CrossRef]
- Bassam, H. Plants and Cancer Treatment. In Medicinal Plants; Bassam Abdul Rasool, H., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 9. [Google Scholar] [CrossRef]
- Wahnou, H.; Youlyouz-Marfak, I.; Liagre, B.; Sol, V.; Oudghiri, M.; Duval, R.E.; Limami, Y. Shining a Light on Prostate Cancer: Photodynamic Therapy and Combination Approaches. Pharmaceutics 2023, 15, 1767. [Google Scholar] [CrossRef]
- Connell, P.P.; Hellman, S. Advances in radiotherapy and implications for the next century: A historical perspective. Cancer Res. 2009, 69, 383–392. [Google Scholar] [CrossRef]
- Kim, B.M.; Hong, Y.; Lee, S.; Liu, P.; Lim, J.H.; Lee, Y.H.; Lee, T.H.; Chang, K.T.; Hong, Y. Therapeutic Implications for Overcoming Radiation Resistance in Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 26880–26913. [Google Scholar] [CrossRef]
- Nambiar, D.; Rajamani, P.; Singh, R.P. Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat. Res. 2011, 728, 139–157. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.K.; Lee, J.H.; Park, J.W. Resveratrol and piperine enhance radiosensitivity of tumor cells. BMB Rep. 2012, 45, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Eli, R.; Fasciano, J.A. An adjunctive preventive treatment for cancer: Ultraviolet light and ginkgo biloba, together with other antioxidants, are a safe and powerful, but largely ignored, treatment option for the prevention of cancer. Med. Hypotheses 2006, 66, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kushwaha, H.N.; Srivastava, A.K.; Srivastava, S.; Jamal, N.; Srivastava, K.; Ray, R.S. Piperine attenuates UV-R induced cell damage in human keratinocytes via NF-kB, Bax/Bcl-2 pathway: An application for photoprotection. J. Photochem. Photobiol. B Biol. 2017, 172, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Bolati, D.; Adijiang, A.; Muteliefu, G.; Enomoto, A.; Nishijima, F.; Dateki, M.; Niwa, T. NF-κB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am. J. Physiol. Cell Physiol. 2011, 301, C1201–C1212. [Google Scholar] [CrossRef] [PubMed]
- Mérino, D.; Lalaoui, N.; Morizot, A.; Solary, E.; Micheau, O. TRAIL in cancer therapy: Present and future challenges. Expert Opin. Ther. Targets 2007, 11, 1299–1314. [Google Scholar] [CrossRef]
- Ismail, B.; Fagnere, C.; Limami, Y.; Ghezali, L.; Pouget, C.; Fidanzi, C.; Ouk, C.; Gueye, R.; Beneytout, J.L.; Duroux, J.L.; et al. 2′-Hydroxy-4-methylsulfonylchalcone enhances TRAIL-induced apoptosis in prostate cancer cells. Anti-Cancer Drugs 2015, 26, 74–84. [Google Scholar] [CrossRef]
- Chawla-Sarkar, M.; Bae, S.I.; Reu, F.J.; Jacobs, B.S.; Lindner, D.J.; Borden, E.C. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 2004, 11, 915–923. [Google Scholar] [CrossRef]
- Premkumar, D.R.; Jane, E.P.; Foster, K.A.; Pollack, I.F. Survivin Inhibitor YM-155 Sensitizes Tumor Necrosis Factor–Related Apoptosis-Inducing Ligand-Resistant Glioma Cells to Apoptosis through Mcl-1 Downregulation and by Engaging the Mitochondrial Death Pathway. J. Pharmacol. Exp. Ther. 2013, 346, 201–210. [Google Scholar] [CrossRef]
- Pond, S.M.; Tozer, T.N. First-pass elimination. Basic concepts and clinical consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Burton, P.S.; Goodwin, J.T.; Vidmar, T.J.; Amore, B.M. Predicting drug absorption: How nature made it a difficult problem. J. Pharmacol. Exp. Ther. 2002, 303, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Heimberg, K.; Lampen, A.; Hirsch-Ernst, K.I. Safety Aspects of the Use of Isolated Piperine Ingested as a Bolus. Foods 2021, 10, 2121. [Google Scholar] [CrossRef]
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Sampath, D.; Cortes, J.; Estrov, Z.; Du, M.; Shi, Z.; Andreeff, M.; Gandhi, V.; Plunkett, W. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood 2006, 107, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Xue, M.; Cheng, J.; Zhao, J.; Zhang, S.; Jian, J.; Qiao, Y.; Liu, B. Outcomes of 219 chronic myeloid leukaemia patients with additional chromosomal abnormalities and/or tyrosine kinase domain mutations. Int. J. Lab. Hematol. 2019, 41, 94–101. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Del Río Hernández, A. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Wink, M. Molecular modes of action of cytotoxic alkaloids: From DNA intercalation, spindle poisoning, topoisomerase inhibition to apoptosis and multiple drug resistance. Alkaloids. Chem. Biol. 2007, 64, 1–47. [Google Scholar] [CrossRef]
- Wink, M.; Ashour, M.L.; El-Readi, M.Z. Secondary Metabolites from Plants Inhibiting ABC Transporters and Reversing Resistance of Cancer Cells and Microbes to Cytotoxic and Antimicrobial Agents. Front. Microbiol. 2012, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, K.; Sterzyńska, K.; Świerczewska, M.; Nowicki, M.; Zabel, M.; Januchowski, R. Piperine Targets Different Drug Resistance Mechanisms in Human Ovarian Cancer Cell Lines Leading to Increased Sensitivity to Cytotoxic Drugs. Int. J. Mol. Sci. 2021, 22, 4243. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomed. Int. J. Phytother. Phytopharm. 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.K.; Jaiswar, S.P.; Srivastav, A.K.; Goyal, S.; Dwivedi, A.; Verma, A.; Singh, J.; Pathak, A.K.; Sankhwar, P.L.; Ray, R.S. Synergistic effect of piperine and paclitaxel on cell fate via cyt-c, Bax/Bcl-2-caspase-3 pathway in ovarian adenocarcinomas SKOV-3 cells. Eur. J. Pharmacol. 2016, 791, 751–762. [Google Scholar] [CrossRef]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benayad, S.; Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Oudghiri, M.; Saad, E.M.; Duval, R.E.; Limami, Y. The Promise of Piperine in Cancer Chemoprevention. Cancers 2023, 15, 5488. https://doi.org/10.3390/cancers15225488
Benayad S, Wahnou H, El Kebbaj R, Liagre B, Sol V, Oudghiri M, Saad EM, Duval RE, Limami Y. The Promise of Piperine in Cancer Chemoprevention. Cancers. 2023; 15(22):5488. https://doi.org/10.3390/cancers15225488
Chicago/Turabian StyleBenayad, Salma, Hicham Wahnou, Riad El Kebbaj, Bertrand Liagre, Vincent Sol, Mounia Oudghiri, El Madani Saad, Raphaël Emmanuel Duval, and Youness Limami. 2023. "The Promise of Piperine in Cancer Chemoprevention" Cancers 15, no. 22: 5488. https://doi.org/10.3390/cancers15225488
APA StyleBenayad, S., Wahnou, H., El Kebbaj, R., Liagre, B., Sol, V., Oudghiri, M., Saad, E. M., Duval, R. E., & Limami, Y. (2023). The Promise of Piperine in Cancer Chemoprevention. Cancers, 15(22), 5488. https://doi.org/10.3390/cancers15225488









