Diallyl Trisulfide Induces ROS-Mediated Mitotic Arrest and Apoptosis and Inhibits HNSCC Tumor Growth and Cancer Stemness
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Cell Lines
2.2. Cell Viability Assay
2.3. Cell Cycle Analysis
2.4. Annexin V FITC Apoptosis Assay
2.5. Flow Cytometric Analysis of Mitotic Marker Phospho-(Ser10)-Histone H3
2.6. Detection of ROS by DCFDA Assay
2.7. Reactive Oxygen Species Staining by MitoSOX
2.8. Comet Assay
2.9. Western Blot Analysis
2.10. Flow Cytometric Analysis of CD44 and CD133 Cancer Stem Cell Markers
2.11. Aldehyde Dehydrogenase Assay
2.12. Spheroid Formation Assay
2.13. RNA Extraction and Quantitative Real-Time PCR
2.14. Tumor Xenograft Study
2.15. Statistical and Densitometry Analyses
3. Results
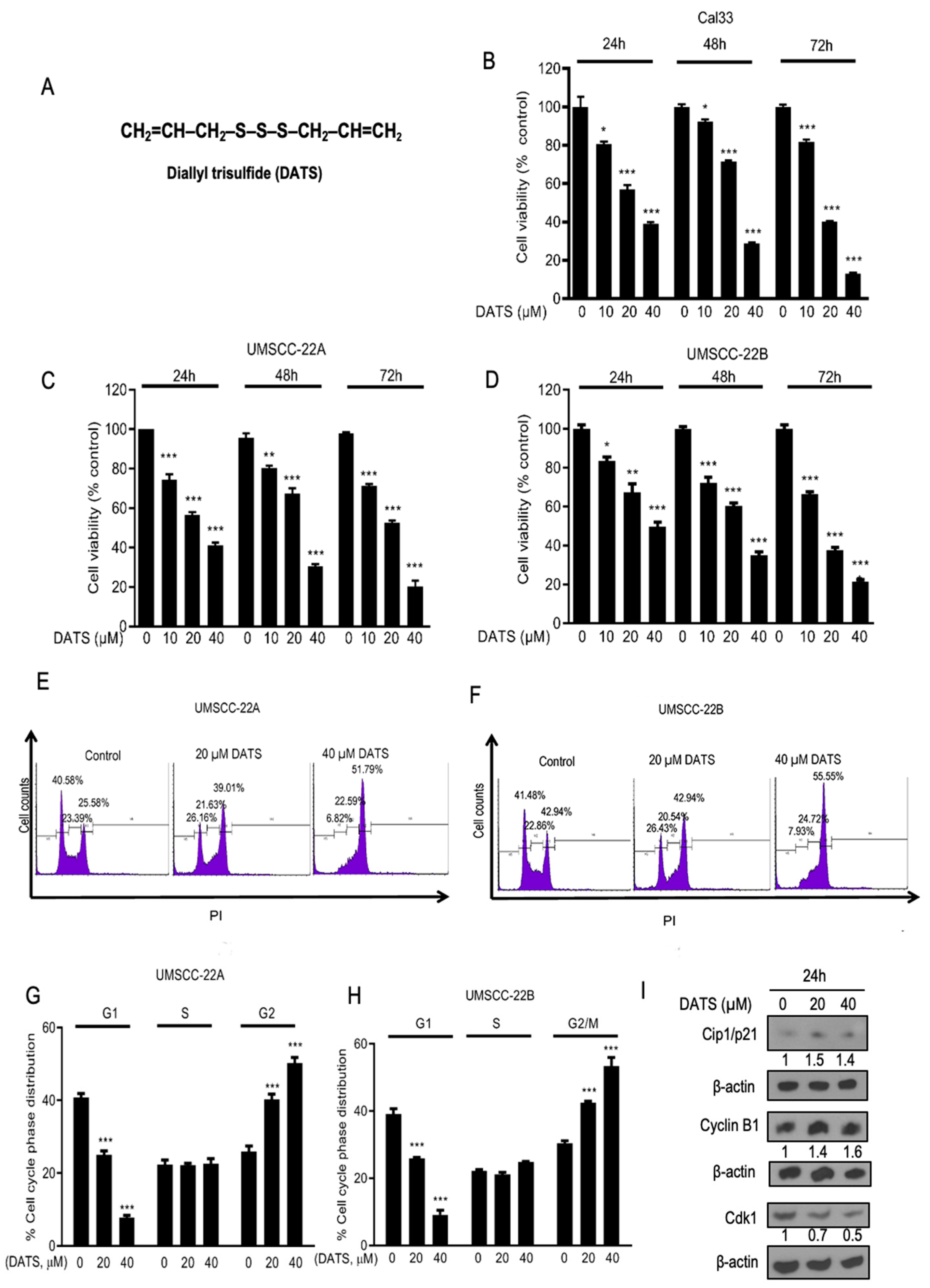
3.1. DATS Strongly Inhibited the Growth and Proliferation of Head and Neck Cancer Cells and Induced G2/M Phase Cell Cycle Arrest
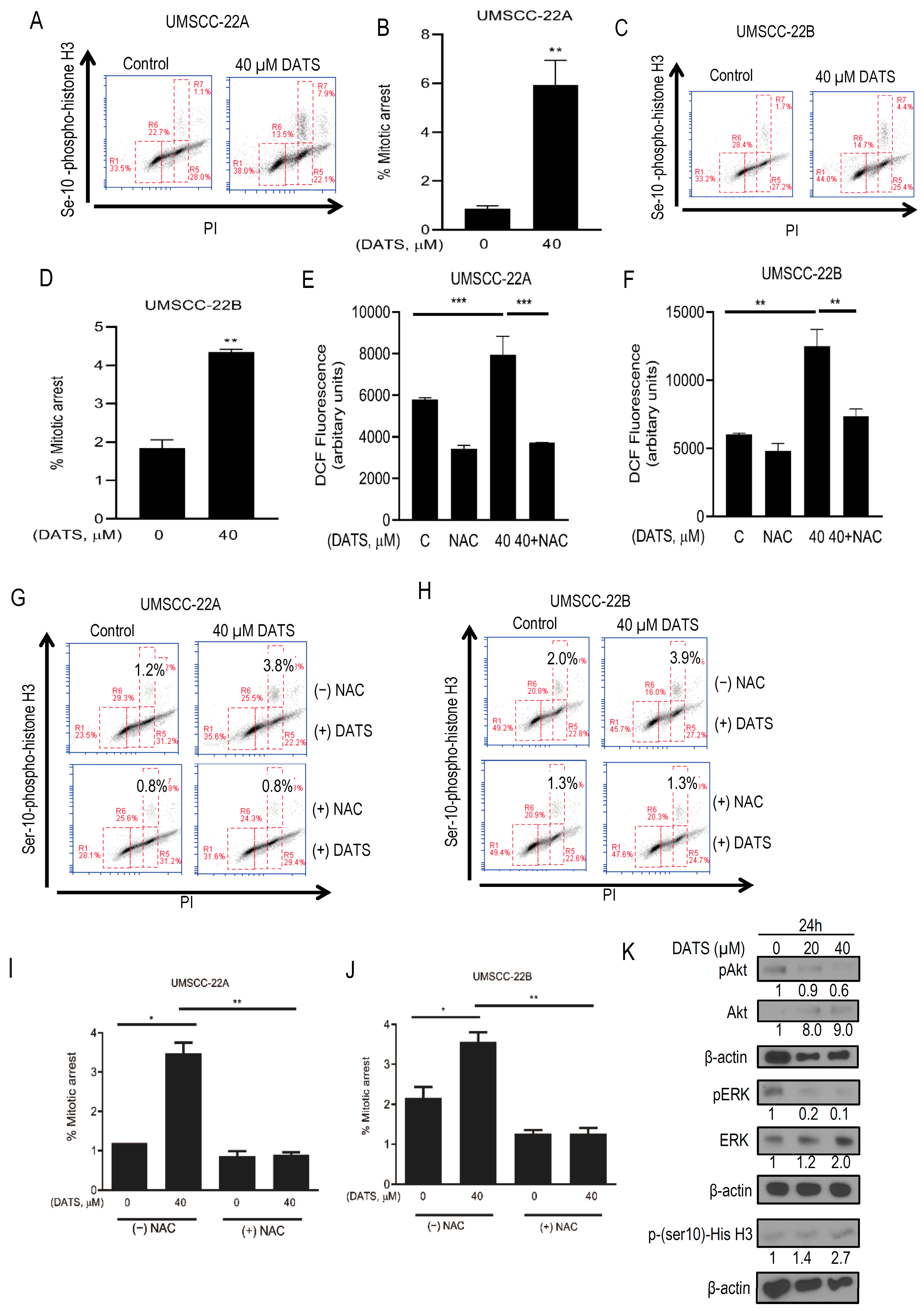
3.2. DATS Induced ROS-Mediated Mitotic Arrest and Altered G2/M Regulatory Proteins in HNSCC Cells
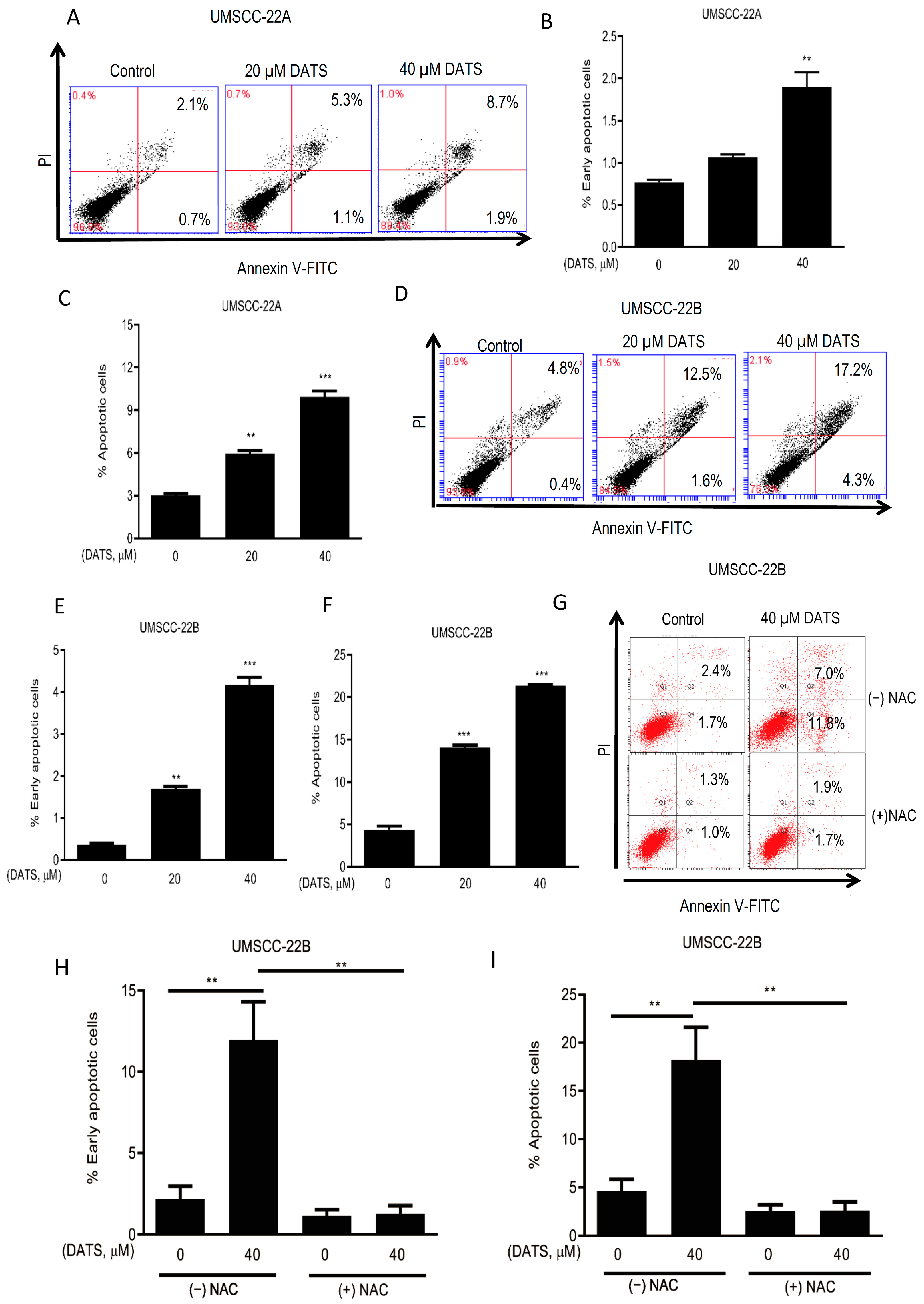
3.3. DATS Induced ROS-Mediated Apoptosis in HNSCC Cells
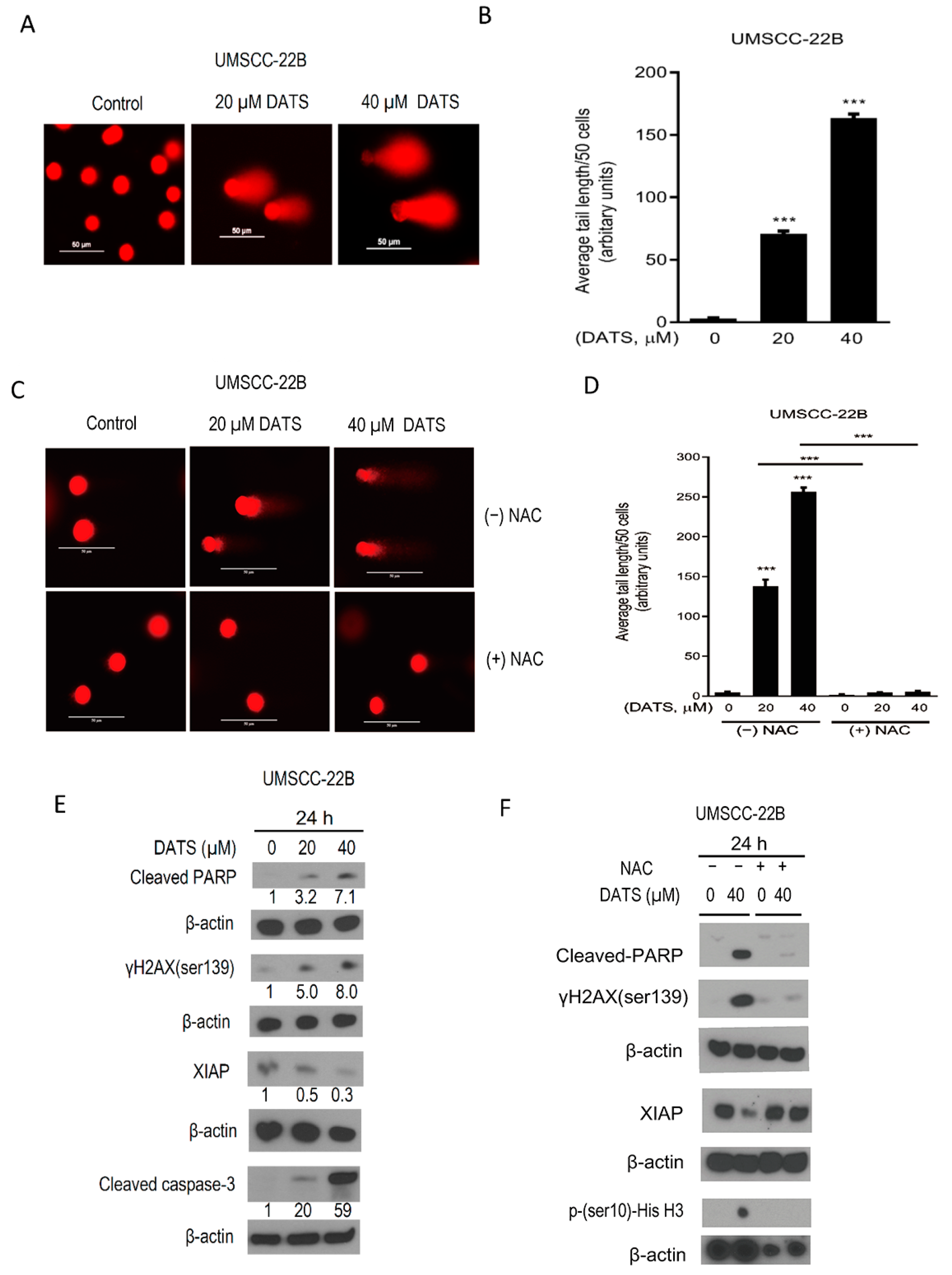
3.4. DATS Induced ROS-Mediated DNA Damage and Altered the Levels of Apoptotic Regulatory Proteins
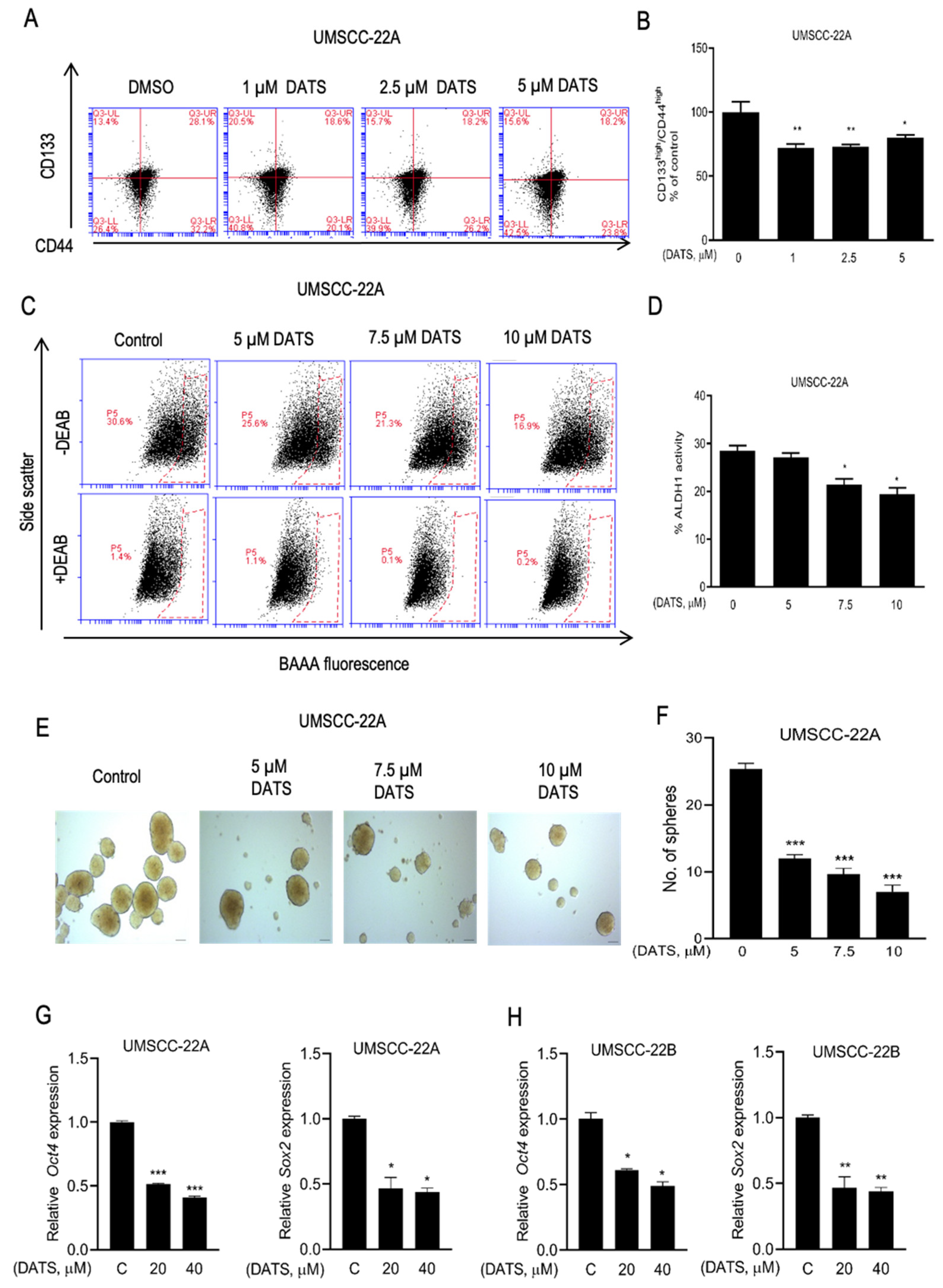
3.5. DATS Treatment Decreased HNSCC Stem Cell Population
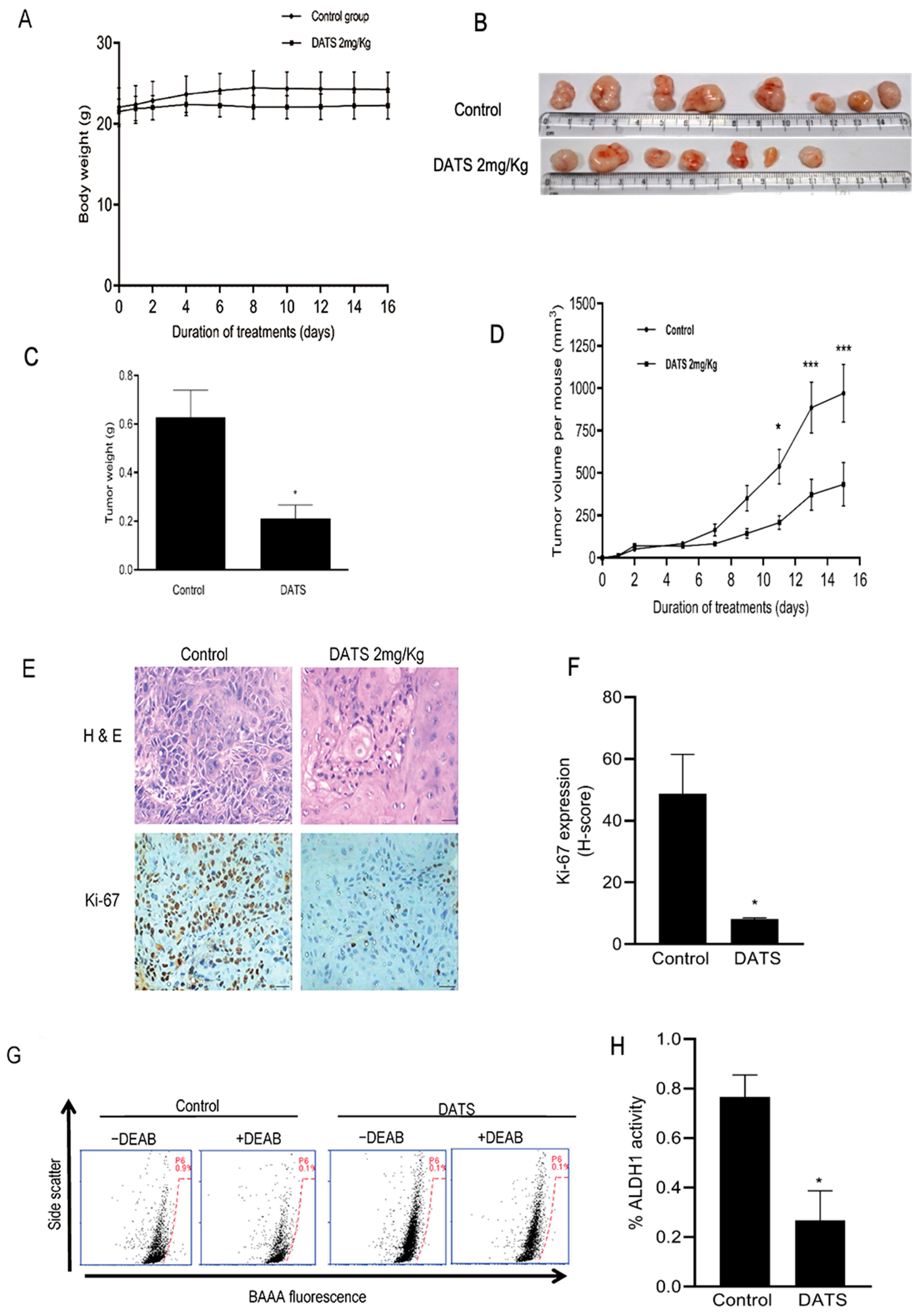
3.6. DATS Treatment Inhibited HNSCC Xenograft Growth as Well as CSC Fraction In Vivo
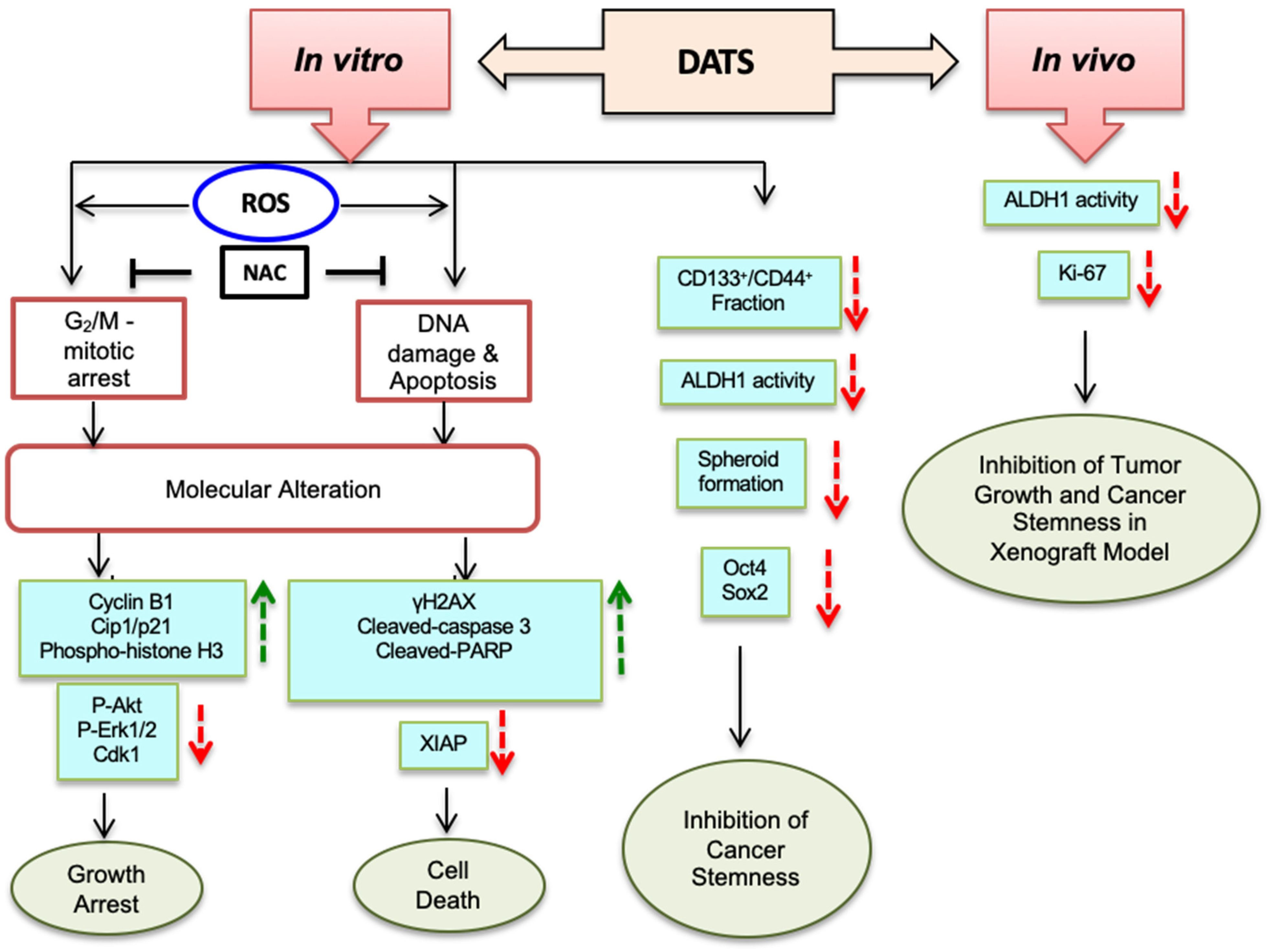
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jawa, Y.; Yadav, P.; Gupta, S.; Mathan, S.V.; Pandey, J.; Saxena, A.K.; Kateriya, S.; Tiku, A.B.; Mondal, N.; Bhattacharya, J.; et al. Current Insights and Advancements in Head and Neck Cancer: Emerging Biomarkers and Therapeutics with Cues from Single Cell and 3D Model Omics Profiling. Front Oncol. 2021, 11, 676948. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Seethala, R.R.; Singh, S.V.; Freilino, M.L.; Bednash, J.S.; Thomas, S.M.; Panahandeh, M.C.; Gooding, W.E.; Joyce, S.C.; Lingen, M.W. Inhibition of EGFR-STAT3 signaling with erlotinib prevents carcinogenesis in a chemically-induced mouse model of oral squamous cell carcinoma. Cancer Prev. Res. 2011, 4, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Leeman-Neill, R.J.; Wheeler, S.E.; Singh, S.V.; Thomas, S.M.; Seethala, R.R.; Neill, D.B.; Panahandeh, M.C.; Hahm, E.-R.; Joyce, S.C.; Sen, M. Guggulsterone enhances head and neck cancer therapies via inhibition of signal transducer and activator of transcription-3. Carcinogenesis 2009, 30, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.W.; Wentzel, A.L.; Xiao, D.; Lew, K.L.; Singh, S.V.; Grandis, J.R. Requirement of a carbon spacer in benzyl isothiocyanate-mediated cytotoxicity and MAPK activation in head and neck squamous cell carcinoma. Carcinogenesis 2003, 24, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Mathan, S.V.; Rajput, M.; Singh, R.P. Chapter 14—Chemotherapy and radiation therapy for cancer. In Understanding Cancer; Jain, B., Pandey, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 217–236. [Google Scholar]
- Nör, C.; Zhang, Z.; Warner, K.A.; Bernardi, L.; Visioli, F.; Helman, J.I.; Roesler, R.; Nör, J.E. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 2014, 16, 137–146. [Google Scholar] [CrossRef]
- Gao, C.M.; Takezaki, T.; Ding, J.H.; Li, M.S.; Tajima, K. Protective effect of allium vegetables against both esophageal and stomach cancer: A simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Jpn. J. Cancer Res. Gann. 1999, 90, 614–621. [Google Scholar] [CrossRef]
- Dorant, E.; van den Brandt, P.A.; Goldbohm, R.A.; Sturmans, F. Consumption of onions and a reduced risk of stomach carcinoma. Gastroenterology 1996, 110, 12–20. [Google Scholar] [CrossRef]
- Chan, J.M.; Wang, F.; Holly, E.A. Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2093–2097. [Google Scholar] [CrossRef]
- Hsing, A.W.; Chokkalingam, A.P.; Gao, Y.-T.; Madigan, M.P.; Deng, J.; Gridley, G.; Fraumeni Jr, J.F. Allium vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer Inst. 2002, 94, 1648–1651. [Google Scholar]
- Galeone, C.; Pelucchi, C.; Dal Maso, L.; Negri, E.; Montella, M.; Zucchetto, A.; Talamini, R.; La Vecchia, C. Allium vegetables intake and endometrial cancer risk. Public Health Nutr. 2009, 12, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Pelucchi, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Giacosa, A.; La Vecchia, C. Onion and garlic use and human cancer. Am. J. Clin. Nutr. 2006, 84, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Mathan, S.V.; Singh, V.S.; Singh, R.P. Fighting cancer with phytochemicals from allium vegetables. Mol. Cancer Biol. 2017. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Herman-Antosiewicz, A.; Marynowski, S.W.; Singh, S.V. C-Jun NH2-terminal kinase signaling axis regulates diallyl trisulfide–induced generation of reactive oxygen species and cell cycle arrest in human prostate cancer cells. Cancer Res. 2006, 66, 5379–5386. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Singh, S.V. Diallyl trisulfide inhibits estrogen receptor-α activity in human breast cancer cells. Breast Cancer Res. Treat. 2014, 144, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.L.; Singh, S.V. Molecular mechanisms and targets of cancer chemoprevention by garlic-derived bioactive compound diallyl trisulfide. Indian J. Exp. Biol. 2011, 49, 805–816. [Google Scholar] [PubMed]
- Xiao, D.; Choi, S.; Johnson, D.E.; Vogel, V.G.; Johnson, C.S.; Trump, D.L.; Lee, Y.J.; Singh, S.V. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene 2004, 23, 5594–5606. [Google Scholar] [CrossRef] [PubMed]
- Tailor, D.; Hahm, E.R.; Kale, R.K.; Singh, S.V.; Singh, R.P. Sodium butyrate induces DRP1-mediated mitochondrial fusion and apoptosis in human colorectal cancer cells. Mitochondrion 2014, 16, 55–64. [Google Scholar] [CrossRef]
- Hahm, E.R.; Kim, S.H.; Pore, S.K.; Mathan, S.V.; Singh, R.P.; Singh, S.V. Mechanism of the synergistic inhibitory effect of benzyl isothiocyanate and zoledronic acid combination on breast cancer induction of osteoclast differentiation. Mol. Carcinog. 2023. [Google Scholar] [CrossRef]
- Herman-Antosiewicz, A.; Singh, S.V. Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic arrest in human prostate cancer cells. J. Biol. Chem. 2005, 280, 28519–28528. [Google Scholar] [CrossRef]
- Kumar, K.; Sabarwal, A.; Singh, R.P. Mancozeb selectively induces mitochondrial-mediated apoptosis in human gastric carcinoma cells through ROS generation. Mitochondrion 2019, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, e23354. [Google Scholar] [CrossRef]
- Sabarwal, A.; Agarwal, R.; Singh, R.P. Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol. Carcinog. 2017, 56, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Kim, S.H.; Mathan, S.V.; Singh, R.P.; Singh, S.V. Mechanistic Targets of Diallyl Trisulfide in Human Breast Cancer Cells Identified by RNA-seq Analysis. J. Cancer Prev. 2021, 26, 128–136. [Google Scholar] [CrossRef]
- Kim, S.H.; Kaschula, C.H.; Priedigkeit, N.; Lee, A.V.; Singh, S.V. Forkhead Box Q1 Is a Novel Target of Breast Cancer Stem Cell Inhibition by Diallyl Trisulfide. J. Biol. Chem. 2016, 291, 13495–13508. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Sharma, M.; Das, S.; Saxena, S. The lncRNA HMS recruits RNA-binding protein HuR to stabilize the 3′-UTR of HOXC10 mRNA. J. Biol. Chem. 2021, 297, 100997. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Kim, S.H.; Singh, K.B.; Singh, S.V. RNA-seq reveals novel cancer-selective and disease subtype-independent mechanistic targets of withaferin A in human breast cancer cells. Mol Carcinog. 2021, 60, 3–14. [Google Scholar] [CrossRef]
- Singh, K.B.; Hahm, E.R.; Pore, S.K.; Singh, S.V. Leelamine Is a Novel Lipogenesis Inhibitor in Prostate Cancer Cells In Vitro and In Vivo. Mol. Cancer Ther. 2019, 18, 1800–1810. [Google Scholar] [CrossRef]
- Hendzel, M.J.; Wei, Y.; Mancini, M.A.; Van Hooser, A.; Ranalli, T.; Brinkley, B.R.; Bazett-Jones, D.P.; Allis, C.D. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 1997, 106, 348–360. [Google Scholar] [CrossRef]
- Chandra-Kuntal, K.; Lee, J.; Singh, S.V. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res. Treat. 2013, 138, 69–79. [Google Scholar] [CrossRef]
- Haddad, R.I.; Shin, D.M. Recent advances in head and neck cancer. New Engl. J. Med. 2008, 359, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef]
- Xiao, D.; Herman-Antosiewicz, A.; Antosiewicz, J.; Xiao, H.; Brisson, M.; Lazo, J.S.; Singh, S.V. Diallyl trisulfide-induced G-M phase cell cycle arrest in human prostate cancer cells is caused by reactive oxygen species-dependent destruction and hyperphosphorylation of Cdc 25 C. Oncogene 2005, 24, 6256–6268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Bommareddy, A.; Singh, S.V. Garlic constituent diallyl trisulfide suppresses x-linked inhibitor of apoptosis protein in prostate cancer cells in culture and in vivo. Cancer Pre Res. 2011, 4, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Singh, S.V. Monocarboxylate transporter 1 is a novel target for breast cancer stem like-cell inhibition by diallyl trisulfide. Mol. Carcinog. 2022, 61, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zeng, Y.; Hahm, E.R.; Kim, Y.A.; Ramalingam, S.; Singh, S.V. Diallyl trisulfide selectively causes Bax-and Bak-mediated apoptosis in human lung cancer cells. Environ. Mol. Mutagen 2009, 50, 201–212. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Hu, X.; Xia, H.; Zaren, H.A.; Chatterjee, M.L.; Agarwal, R.; Singh, S.V. Mechanism of differential efficacy of garlic organosulfides in preventing benzo (a) pyrene-induced cancer in mice. Cancer Lett. 1997, 118, 61–67. [Google Scholar]
- Sherwood, S.W.; Rush, D.F.; Kung, A.L.; Schimke, R.T. Cyclin B1 expression in HeLa S3 cells studied by flow cytometry. Exp Cell Res. 1994, 211, 275–281. [Google Scholar] [CrossRef]
- King, R.W.; Deshaies, R.J.; Peters, J.M.; Kirschner, M.W. How proteolysis drives the cell cycle. Science 1996, 274, 1652–1659. [Google Scholar] [CrossRef]
- Choi, Y.H. Diallyl trisulfide induces apoptosis and mitotic arrest in AGS human gastric carcinoma cells through reactive oxygen species-mediated activation of AMP-activated protein kinase. BiomedPharmacother. 2017, 94, 63–71. [Google Scholar]
- Hemmings, B.A.; Restuccia, D.F. Pi3k-pkb/akt pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Rad. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Sehrawat, A.; Singh, S.V. Dietary chemopreventative benzyl isothiocyanate inhibits breast cancer stem cells in vitro and in vivo. Cancer Prev. Res. 2013, 6, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Singh, S.V. The role of polycomb group protein Bmi-1 and Notch4 in breast cancer stem cell inhibition by benzyl isothiocyanate. Breast Cancer Res. Treat. 2015, 149, 681–692. [Google Scholar] [CrossRef]
- Kim, S.H.; Singh, S.V. Role of Krüppel-like Factor 4-p21(CIP1) Axis in Breast Cancer Stem-like Cell Inhibition by Benzyl Isothiocyanate. Cancer Prev. Res. 2019, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.R.; Moura, M.B.; Hahm, E.R.; Singh, K.B.; Singh, S.V. Sulforaphane Inhibits c-Myc-Mediated Prostate Cancer Stem-Like Traits. J. Cell Biochem. 2016, 117, 2482–2495. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Singh, S.V. Mammary cancer chemoprevention by withaferin A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev Res. 2014, 7, 738–747. [Google Scholar] [CrossRef]
- Sun, X.; Guo, T.; He, J.; Zhao, M.; Yan, M.; Cui, F.; Deng, Y. Determination of the concentration of diallyl trisulfide in rat whole blood using gas chromatography with electron-capture detection and identification of its major metabolite with gas chromatography mass spectrometry. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2006, 126, 521–527. [Google Scholar] [CrossRef]
- Hahm, E.R.; Mathan, S.V.; Singh, R.P.; Singh, S.V. Breast Cancer Selective Disruption of Actin Cytoskeleton by Diallyl Trisulfide. J. Cancer Prev. 2022, 27, 101–111. [Google Scholar] [CrossRef]
- Marni, R.; Kundrapu, D.B.; Chakraborti, A.; Malla, R. Insight into drug sensitizing effect of diallyl disulfide and diallyl trisulfide from Allium sativum L. on paclitaxel-resistant triple-negative breast cancer cells. J. Ethnopharmacol. 2022, 296, 115452. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Xiao, D.; Xiao, H.; Powolny, A.A.; Lew, K.L.; Reilly, M.L.; Zeng, Y.; Wang, Z.; Singh, S.V. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol. Cancer Ther. 2007, 6, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.H.; Zhang, G.M.; Hao, T.L.; Zhou, B.; Zhang, H.; Jiang, Z.Y. Effect of diallyl trisulfide on the activation of T cell and macrophage-mediated cytotoxicity. J. Tongji Med. Univ. 1994, 14, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.Q.; Wang, Y.; Xu, H.X.; Fan, W.T.; Wang, M.L.; Sun, P.H.; Xie, X.Y. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin. Med. J. 2004, 117, 1155–1160. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathan, S.V.; Singh, R.; Kim, S.-H.; Singh, S.V.; Singh, R.P. Diallyl Trisulfide Induces ROS-Mediated Mitotic Arrest and Apoptosis and Inhibits HNSCC Tumor Growth and Cancer Stemness. Cancers 2024, 16, 378. https://doi.org/10.3390/cancers16020378
Mathan SV, Singh R, Kim S-H, Singh SV, Singh RP. Diallyl Trisulfide Induces ROS-Mediated Mitotic Arrest and Apoptosis and Inhibits HNSCC Tumor Growth and Cancer Stemness. Cancers. 2024; 16(2):378. https://doi.org/10.3390/cancers16020378
Chicago/Turabian StyleMathan, Sivapar V., Ragini Singh, Su-Hyeong Kim, Shivendra V. Singh, and Rana P. Singh. 2024. "Diallyl Trisulfide Induces ROS-Mediated Mitotic Arrest and Apoptosis and Inhibits HNSCC Tumor Growth and Cancer Stemness" Cancers 16, no. 2: 378. https://doi.org/10.3390/cancers16020378




